Intracellular Communication among Morphogen Signaling Pathways during Vertebrate Body Plan Formation
Abstract
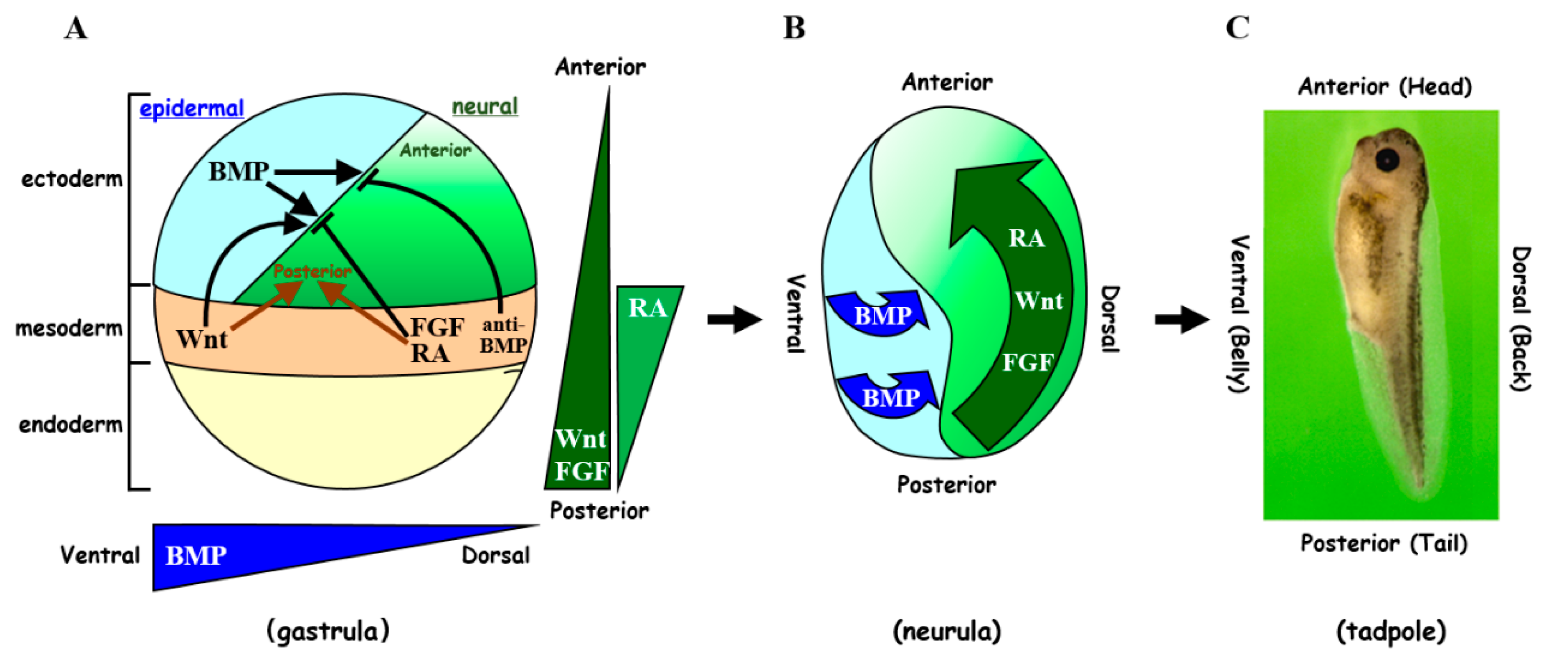
1. Introduction
2. Phosphorylation of Smad
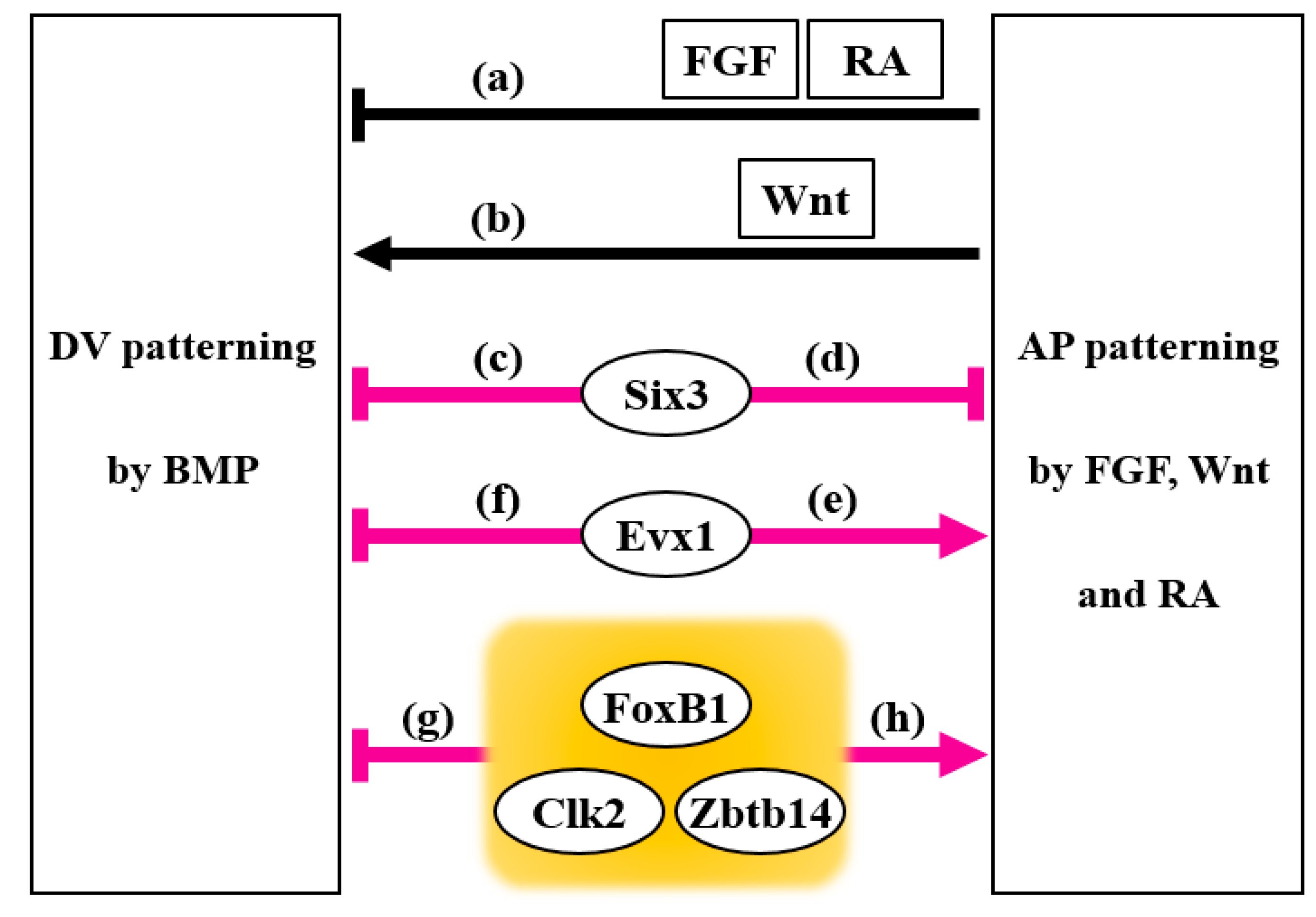
3. Six3
4. Evx1
5. FoxB1
6. Zbtb14
7. Clk2
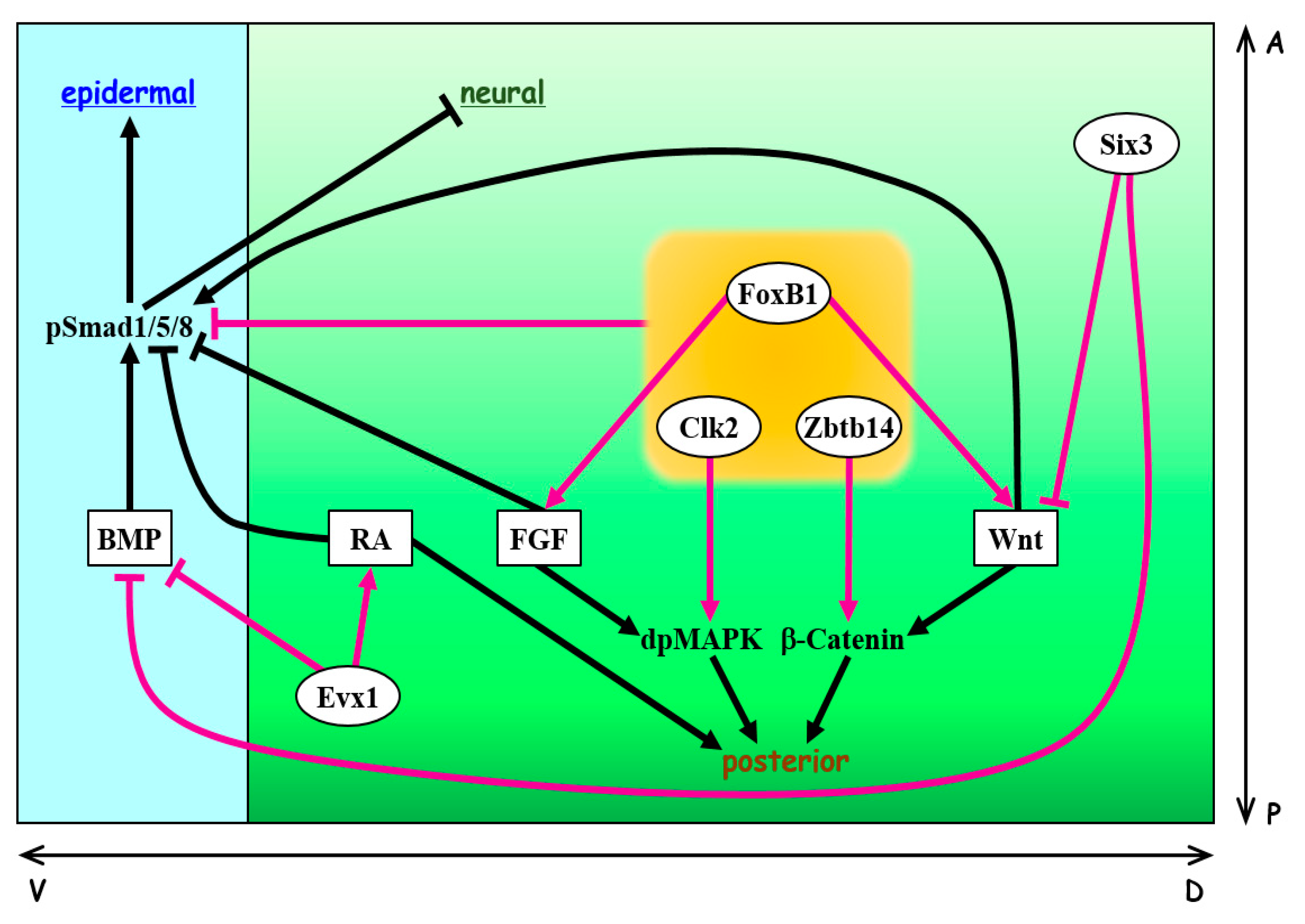
8. Conclusion and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gurdon, J.B.; Bourillot, P.-Y. Morphogen gradient interpretation. Nature 2001, 413, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, D.; Rembold, M.; Leptin, M. From morphogen to morphogenesis and back. Nature 2017, 541, 311–320. [Google Scholar] [CrossRef] [PubMed]
- De Robertis, E.M.; Larraín, J.; Oelgeschläger, M.; Wessely, O. The establishment of Spemann’s organizer and patterning of the vertebrate embryo. Nat. Rev. Genet. 2000, 1, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Harland, R.; Gerhart, J. Formation and function of Spemann’s organizer. Annu. Rev. Cell Dev. Biol. 1997, 13, 611–667. [Google Scholar] [CrossRef] [PubMed]
- Heasman, J. Patterning the early Xenopus embryo. Development 2006, 133, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Kishigami, S.; Mishina, Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev. 2005, 16, 265–278. [Google Scholar] [CrossRef]
- Muñoz-Sanjuán, I.; H.-Brivanlou, A. Early posterior/ventral fate specification in the vertebrate embryo. Dev. Biol. 2001, 237, 1–17. [Google Scholar] [CrossRef]
- Niehrs, C. On growth and form: A Cartesian coordinate system of Wnt and BMP signaling specifies bilaterian body axes. Development 2010, 137, 845–857. [Google Scholar] [CrossRef]
- Stern, C.D. Neural induction: Old problem, new findings, yet more questions. Development 2005, 132, 2007–2021. [Google Scholar] [CrossRef]
- Whitman, M. TGF-β family signaling in Xenopus and zebrafish embryos. In The TGF-β Family; Derynck, R., Miyazono, K., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2008; Volume 50, pp. 547–584. ISBN 9780879697525. [Google Scholar]
- Wu, M.Y.; Hill, C.S. TGF-β superfamily signaling in embryonic development and homeostasis. Dev. Cell 2009, 16, 329–343. [Google Scholar] [CrossRef]
- Dorey, K.; Amaya, E. FGF signalling: Diverse roles during early vertebrate embryogenesis. Development 2010, 137, 3731–3742. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, R.T.; Niehrs, C. Fibroblast growth factor signaling during early vertebrate development. Endocr. Rev. 2005, 26, 63–77. [Google Scholar] [CrossRef] [PubMed]
- De Robertis, E.M.; Kuroda, H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu. Rev. Cell Dev. Biol. 2004, 20, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Sanjuán, I.; Brivanlou, A.H. Neural induction, the default model and embryonic stem cells. Nat. Rev. Neurosci. 2002, 3, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Durston, A.J.; van der Wees, J.; Pijnappel, W.W.M.; Schilthuis, J.G.; Godsave, S.F. Retinoid signalling and axial patterning during early vertebrate embryogenesis. Cell. Mol. Life Sci. 1997, 53, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Duester, G. Retinoic acid synthesis and signaling during early organogenesis. Cell 2008, 134, 921–931. [Google Scholar] [CrossRef]
- Kam, R.K.T.; Deng, Y.; Chen, Y.; Zhao, H. Retinoic acid synthesis and functions in early embryonic development. Cell Biosci. 2012, 2, 11. [Google Scholar] [CrossRef]
- Rhinn, M.; Dollé, P. Retinoic acid signalling during development. Development 2012, 139, 843–858. [Google Scholar] [CrossRef]
- Struhl, G.; Struhl, K.; Macdonald, P.M. The gradient morphogen bicoid is a concentration-dependent transcriptional activator. Cell 1989, 57, 1259–1273. [Google Scholar] [CrossRef]
- Khokha, M.K.; Yeh, J.; Grammer, T.C.; Harland, R.M. Depletion of three BMP antagonists from Spemann’s organizer leads to a catastrophic loss of dorsal structures. Dev. Cell 2005, 8, 401–411. [Google Scholar] [CrossRef]
- Schmidt, J.E.; Suzuki, A.; Ueno, N.; Kimelman, D. Localized BMP-4 mediates dorsal/ventral patterning in the early Xenopus embryo. Dev. Biol. 1995, 169, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Reversade, B.; Kuroda, H.; Lee, H.; Mays, A.; De Robertis, E.M. Depletion of Bmp2, Bmp4, Bmp7 and Spemann organizer signals induces massive brain formation in Xenopus embryos. Development 2005, 132, 3381–3392. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.W.; Godson, C.; Brazil, D.P.; Martin, F. Extracellular BMP-antagonist regulation in development and disease: Tied up in knots. Trends Cell Biol. 2010, 20, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.A.; Lagna, G.; Suzuki, A.; Hemmati-Brivanlou, A. Concentration-dependent patterning of the Xenopus ectoderm by BMP4 and its signal transducer Smad1. Development 1997, 124, 3177–3184. [Google Scholar] [PubMed]
- Katagiri, T.; Watabe, T. Bone morphogenetic proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a021899. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Budi, E.H. Specificity, versatility, and control of TGF-β family signaling. Sci. Signal. 2019, 12, eaav5183. [Google Scholar] [CrossRef]
- Xu, P.; Lin, X.; Feng, X.-H. Posttranslational regulation of Smads. Cold Spring Harb. Perspect. Biol 2016, 6, a022087. [Google Scholar] [CrossRef]
- Feng, X.-H.; Derynck, R. Specificity and versatility in TGF-β signaling through Smads. Annu. Rev. Cell Dev. Biol. 2005, 21, 659–693. [Google Scholar] [CrossRef]
- Massagué, J.; Chen, Y.-G. Controlling TGF-β signaling. Genes Dev. 2000, 14, 627–644. [Google Scholar] [CrossRef]
- Moustakas, A.; Heldin, C.-H. The regulation of TGFβ signal transduction. Development 2009, 136, 3699–3714. [Google Scholar] [CrossRef]
- Gaarenstroom, T.; Hill, C.S. TGF-β signaling to chromatin: How Smads regulate transcription during self-renewal and differentiation. Semin. Cell Dev. Biol. 2014, 32, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Feledy, J.A.; Beanan, M.J.; Sandoval, J.J.; Goodrich, J.S.; Lim, J.H.; Matsuo-Takasaki, M.; Sato, S.M.; Sargent, T.D. Inhibitory patterning of the anterior neural plate in Xenopus by homeodomain factors Dlx3 and Msx1. Dev. Biol. 1999, 212, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Matsuo-Takasaki, M.; Sargent, T.D. Distinct roles for Distal-less genes Dlx3 and Dlx5 in regulating ectodermal development in Xenopus. Mol. Reprod. Dev. 2001, 60, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Matsuo-Takasaki, M.; Thomas, M.L.; Weeks, D.L.; Sargent, T.D. Transcription factor AP-2 is an essential and direct regulator of epidermal development in Xenopus. Dev. Biol. 2002, 245, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Onichtchouk, D.; Gawantka, V.; Dosch, R.; Delius, H.; Hirschfeld, K.; Blumenstock, C.; Niehrs, C. The Xvent-2 homeobox gene is part of the BMP-4 signalling pathway controlling dorsoventral patterning of Xenopus mesoderm. Development 1996, 122, 3045–3053. [Google Scholar] [PubMed]
- Pera, E.; Stein, S.; Kessel, M. Ectodermal patterning in the avian embryo: Epidermis versus neural plate. Development 1999, 126, 63–73. [Google Scholar] [PubMed]
- Suzuki, A.; Ueno, N.; Hemmati-Brivanlou, A. Xenopus msx1 mediates epidermal induction and neural inhibition by BMP4. Development 1997, 124, 3037–3044. [Google Scholar] [PubMed]
- Gamse, J.; Sive, H. Vertebrate anteroposterior patterning: The Xenopus neurectoderm as a paradigm. BioEssays 2000, 22, 976–986. [Google Scholar] [CrossRef]
- Maden, M. Retinoid signalling in the development of the central nervous system. Nat. Rev. Neurosci. 2002, 3, 843–853. [Google Scholar] [CrossRef]
- Niehrs, C. Regionally specific induction by the Spemann-Mangold organizer. Nat. Rev. Genet. 2004, 5, 425–434. [Google Scholar] [CrossRef]
- Stern, C.D.; Charité, J.; Deschamps, J.; Duboule, D.; Durston, A.J.; Kmita, M.; Nicolas, J.-F.; Palmeirim, I.; Smith, J.C.; Wolpert, L. Head-tail patterning of the vertebrate embryo: One, two or many unresolved problems? Int. J. Dev. Biol. 2006, 50, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, H.V.; Pownal, M.E.; Slack, J.M.W. eFGF regulates Xbra expression during Xenopus gastrulation. EMBO J. 1994, 13, 4469–4481. [Google Scholar] [CrossRef] [PubMed]
- Keenan, I.D.; Sharrard, R.M.; Isaacs, H.V. FGF signal transduction and the regulation of Cdx gene expression. Dev. Biol. 2006, 299, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Northrop, J.L.; Kimelman, D. Dorsal-ventral differences in Xcad-3 expression in response to FGF-mediated induction in Xenopus. Dev. Biol. 1994, 161, 490–503. [Google Scholar] [CrossRef]
- Pownall, M.E.; Tucker, A.S.; Slack, J.M.W.; Isaacs, H.V. eFGF, Xcad3 and Hox genes form a molecular pathway that establishes the anteroposterior axis in Xenopus. Development 1996, 122, 3881–3892. [Google Scholar] [PubMed]
- Haremaki, T.; Tanaka, Y.; Hongo, I.; Yuge, M.; Okamoto, H. Integration of multiple signal transducing pathways on Fgf response elements of the Xenopus caudal homologue Xcad3. Development 2003, 130, 4907–4917. [Google Scholar] [CrossRef]
- He, X.; Semenov, M.; Tamai, K.; Zeng, X. LDL receptor-related proteins 5 and 6 in Wnt/β-catenin signaling: Arrows point the way. Development 2004, 131, 1663–1677. [Google Scholar] [CrossRef]
- Clevers, H. Wnt/β-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef]
- Hikasa, H.; Sokol, S.Y. Wnt signaling in vertebrate axis specification. Cold Spring Harb. Perspect. Biol. 2013, 5, a007955. [Google Scholar] [CrossRef]
- Logan, C.Y.; Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/β-Catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Elkouby, Y.M.; Elias, S.; Casey, E.S.; Blythe, S.A.; Tsabar, N.; Klein, P.S.; Root, H.; Liu, K.J.; Frank, D. Mesodermal Wnt signaling organizes the neural plate via Meis3. Development 2010, 137, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, H.V.; Pownall, M.E.; Slack, J.M.W. Regulation of Hox gene expression and posterior development by the Xenopus caudal homologue Xcad3. EMBO J. 1998, 17, 3413–3427. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, T.J.; Duester, G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat. Rev. Mol. Cell Biol. 2015, 16, 110–123. [Google Scholar] [CrossRef] [PubMed]
- White, R.J.; Schilling, T.F. How degrading: Cyp26s in hindbrain development. Dev. Dyn. 2008, 237, 2775–2790. [Google Scholar] [CrossRef] [PubMed]
- Maden, M.; Gale, E.; Kostetskii, I.; Zile, M. Vitamin A-deficient quail embryos have half a hindbrain and other neural defects. Curr. Biol. 1996, 6, 417–426. [Google Scholar] [CrossRef]
- Gavalas, A.; Krumlauf, R. Retinoid signalling and hindbrain patterning. Curr. Opin. Genet. Dev. 2000, 10, 380–386. [Google Scholar] [CrossRef]
- Tuazon, F.B.; Mullins, M.C. Temporally coordinated signals progressively pattern the anteroposterior and dorsoventral body axes. Semin. Cell Dev. Biol. 2015, 42, 118–133. [Google Scholar] [CrossRef]
- Carron, C.; Shi, D.-L. Specification of anteroposterior axis by combinatorial signaling during Xenopus development. WIREs. Dev. Biol. 2016, 5, 150–168. [Google Scholar] [CrossRef]
- Kudoh, T.; Wilson, S.W.; Dawid, I.B. Distinct roles for Fgf, Wnt and retinoic acid in posteriorizing the neural ectoderm. Development 2002, 129, 4335–4346. [Google Scholar] [PubMed]
- Shiotsugu, J.; Katsuyama, Y.; Arima, K.; Baxter, A.; Koide, T.; Song, J.; Chandraratna, R.A.S.; Blumberg, B. Multiple points of interaction between retinoic acid and FGF signaling during embryonic axis formation. Development 2004, 131, 2653–2667. [Google Scholar] [CrossRef] [PubMed]
- Nordström, U.; Jessell, T.M.; Edlund, T. Progressive induction of caudal neural character by graded Wnt signaling. Nat. Neurosci. 2002, 5, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, A.; Souchelnytskyi, S.; Heldin, C.-H. Smad regulation in TGF-β signal transduction. J. Cell Sci. 2001, 114, 4359–4369. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, M.; Doody, J.; Massagué, J. Opposing BMP and EGF signalling pathways converge on the TGF-β family mediator Smad1. Nature 1997, 389, 618–622. [Google Scholar] [CrossRef]
- Kuroda, H.; Fuentealba, L.; Ikeda, A.; Reversade, B.; De Robertis, E.M. Default neural induction, neuralization of dissociated Xenopus cells is mediated by Ras/MAPK activation. Genes Dev. 2005, 19, 1022–1027. [Google Scholar] [CrossRef]
- Pera, E.M.; Ikeda, A.; Eivers, E.; De Robertis, E.M. Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes Dev. 2003, 17, 3023–3028. [Google Scholar] [CrossRef]
- Wilson, S.I.; Rydström, A.; Trimborn, T.; Willert, K.; Nusse, R.; Jessell, T.M.; Edlund, T. The status of Wnt signalling regulates neural and epidermal fates in the chick embryo. Nature 2001, 411, 325–330. [Google Scholar] [CrossRef]
- Glinka, A.; Wu, W.; Delius, H.; Monaghan, A.P.; Blumenstock, C.; Niehrs, C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 1998, 391, 357–362. [Google Scholar] [CrossRef]
- Hashimoto, H.; Itoh, M.; Yamanaka, Y.; Yamashita, S.; Shimizu, T.; Solnica-Krezel, L.; Hibi, M.; Hirano, T. Zebrafish Dkk1 functions in forebrain specification and axial mesendoderm formation. Dev. Biol. 2000, 217, 138–152. [Google Scholar] [CrossRef]
- Eivers, E.; Fuentealba, L.C.; De Robertis, E.M. Integrating positional information at the level of Smad1/5/8. Curr. Opin. Genet. Dev. 2008, 18, 304–310. [Google Scholar] [CrossRef]
- Sapkota, G.; Alarcón, C.; Spagnoli, F.M.; Brivanlou, A.H.; Massagué, J. Balancing BMP signaling through integrated inputs into the Smad1 linker. Mol. Cell 2007, 25, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Fuentealba, L.C.; Eivers, E.; Ikeda, A.; Hurtado, C.; Kuroda, H.; Pera, E.M.; De Robertis, E.M. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell 2007, 131, 980–993. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Kavsak, P.; Abdollah, S.; Wrana, J.L.; Thomsen, G.H. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature 1999, 400, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chang, C.; Gehling, D.J.; Hemmati-Brivanlou, A.; Derynck, R. Regulation of Smad degradation and activity by Smurf2, an E3 ubiquitin ligase. Proc. Natl. Acad. Sci. USA 2001, 98, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Podos, S.D.; Hanson, K.K.; Wang, Y.-C.; Ferguson, E.L. The DSmurf ubiquitin-protein ligase restricts BMP signaling spatially and temporally during Drosophila embryogenesis. Dev. Cell 2001, 1, 567–578. [Google Scholar] [CrossRef]
- Sagner, A.; Briscoe, J. Morphogen interpretation: Concentration, time, competence, and signaling dynamics. WIREs. Dev. Biol. 2017, 6, e271. [Google Scholar] [CrossRef]
- Wilson, P.A.; Hemmati-Brivanlou, A. Vertebrate neural induction: Inducers, inhibitors, and a new synthesis. Neuron 1997, 18, 699–710. [Google Scholar] [CrossRef]
- Tozer, S.; Le Dréau, G.; Marti, E.; Briscoe, J. Temporal control of BMP signalling determines neuronal subtype identity in the dorsal neural tube. Development 2013, 140, 1467–1474. [Google Scholar] [CrossRef]
- Duval, N.; Vaslin, C.; Barata, T.C.; Frarma, Y.; Contremoulins, V.; Baudin, X.; Nedelec, S.; Ribes, V.C. BMP4 patterns Smad activity and generates stereotyped cell fate organisation in spinal organoids. Development 2019, 146, dev175430. [Google Scholar] [CrossRef]
- Sheng, N.; Xie, Z.; Wang, C.; Bai, G.; Zhang, K.; Zhu, Q.; Song, J.; Guillemot, F.; Chen, Y.-G.; Lin, A.; et al. Retinoic acid regulates bone morphogenic protein signal duration by promoting the degradation of phosphorylated Smad1. Proc. Natl. Acad. Sci. USA 2010, 107, 18886–18891. [Google Scholar] [CrossRef]
- Eivers, E.; Demagny, H.; Choi, R.H.; De Robertis, E.M. Phosphorylation of Mad controls competition between Wingless and BMP signaling. Sci. Signal. 2011, 4, ra68. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wei, C.-Y.; Wang, H.-P.; Zhu, Z.-Y.; Sun, Y.-H. Transcriptional factors Smad1 and Smad9 act redundantly to mediate zebrafish ventral specification downstream of Smad5. J. Biol. Chem. 2014, 289, 6604–6618. [Google Scholar] [CrossRef] [PubMed]
- le Dréau, G.; Garcia-Campmany, L.; Angeles Rabadán, M.; Ferronha, T.; Tozer, S.; Briscoe, J.; Martí, E. Canonical BMP7 activity is required for the generation of discrete neuronal populations in the dorsal spinal cord. Development 2012, 139, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, K.D.; Dunn, N.R.; Robertson, E.J. Mouse embryos lacking Smad1 signals display defects in extra-embryonic tissues and germ cell formation. Development 2001, 128, 3609–3621. [Google Scholar] [PubMed]
- Chang, H.; Huylebroeck, D.; Verschueren, K.; Guo, Q.; Matzuk, M.M.; Zwijsen, A. Smad5 knockout mice die at mid-gestation due to multiple embryonic and extraembryonic defects. Development 1999, 126, 1631–1642. [Google Scholar] [PubMed]
- Arnold, S.J.; Maretto, S.; Islam, A.; Bikoff, E.K.; Robertson, E.J. Dose-dependent Smad1, Smad5 and Smad8 signaling in the early mouse embryo. Dev. Biol. 2006, 296, 104–118. [Google Scholar] [CrossRef]
- Aubin, J.; Davy, A.; Soriano, P. In vivo convergence of BMP and MAPK signaling pathways: Impact of differential Smad1 phosphorylation on development and homeostasis. Genes Dev. 2004, 18, 1482–1494. [Google Scholar] [CrossRef]
- Oliver, G.; Mailhos, A.; Wehr, R.; Copeland, N.G.; Jenkins, N.A.; Gruss, P. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development 1995, 121, 4045–4055. [Google Scholar] [PubMed]
- Zhou, X.; Hollemann, T.; Pieler, T.; Gruss, P. Cloning and expression of xSix3, the Xenopus homologue of murine Six3. Mech. Dev. 2000, 91, 327–330. [Google Scholar] [CrossRef]
- Ghanbari, H.; Seo, H.-C.; Fjose, A.; Brändli, A.W. Molecular cloning and embryonic expression of Xenopus Six homeobox genes. Mech. Dev. 2001, 101, 271–277. [Google Scholar] [CrossRef]
- Kumar, J.P. The sine oculis homeobox (SIX) family of transcription factors as regulators of development and disease. Cell. Mol. Life Sci. 2009, 66, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Sato, S.; Ozaki, H.; Ikeda, K. Six family genes-structure and function as transcription factors and their roles in development. BioEssays 2000, 22, 616–626. [Google Scholar] [CrossRef]
- Lagutin, O.V.; Zhu, C.C.; Kobayashi, D.; Topczewski, J.; Shimamura, K.; Puelles, L.; Russell, H.R.C.; McKinnon, P.J.; Solnica-Krezel, L.; Oliver, G. Six3 repression of Wnt signaling in the anterior neuroectoderm is essential for vertebrate forebrain development. Genes Dev. 2003, 17, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lagutin, O.V.; Mende, M.; Streit, A.; Oliver, G. Six3 activation of Pax6 expression is essential for mammalian lens induction and specification. EMBO J. 2006, 25, 5383–5395. [Google Scholar] [CrossRef]
- Gestri, G.; Carl, M.; Appolloni, I.; Wilson, S.W.; Barsacchi, G.; Andreazzoli, M. Six3 functions in anterior neural plate specification by promoting cell proliferation and inhibiting Bmp4 expression. Development 2005, 132, 2401–2413. [Google Scholar] [CrossRef]
- Braun, M.M.; Etheridge, A.; Bernard, A.; Robertson, C.P.; Roelink, H. Wnt signaling is required at distinct stages of development for the induction of the posterior forebrain. Development 2003, 130, 5579–5587. [Google Scholar] [CrossRef]
- Sun, X.; Meyers, E.N.; Lewandoski, M.; Martin, G.R. Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev. 1999, 13, 1834–1846. [Google Scholar] [CrossRef]
- Takata, N.; Sakakura, E.; Eiraku, M.; Kasukawa, T.; Sasai, Y. Self-patterning of rostral-caudal neuroectoderm requires dual role of Fgf signaling for localized Wnt antagonism. Nat. Commun. 2017, 8, 1339. [Google Scholar] [CrossRef]
- Avaron, F.; Thaëron-Antono, C.; Beck, C.W.; Borday-Birraux, V.; Géraudie, J.; Casane, D.; Laurenti, P. Comparison of even-skipped related gene expression pattern in vertebrates shows an association between expression domain loss and modification of selective constraints on sequences. Evol. Dev. 2003, 5, 145–156. [Google Scholar] [CrossRef]
- Bastian, H.; Gruss, P. A murine even-skipped homologue, Evx 1, is expressed during early embryogenesis and neurogenesis in a biphasic manner. EMBO J. 1990, 9, 1839–1852. [Google Scholar] [CrossRef]
- Dush, M.K.; Martin, G.R. Analysis of mouse Evx genes: Evx-1 displays graded expression in the primitive streak. Dev. Biol. 1992, 151, 273–287. [Google Scholar] [CrossRef]
- Ruiz i Altaba, A.; Melton, D.A. Involvement of the Xenopus homeobox gene Xhox3 in pattern formation along the anterior-posterior axis. Cell 1989, 57, 317–326. [Google Scholar] [CrossRef]
- Agathon, A.; Thisse, C.; Thisse, B. The molecular nature of the zebrafish tail organizer. Nature 2003, 424, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.; Maegawa, S.; Weinberg, E.S.; Wilson, S.W.; Dawid, I.B.; Kudoh, T. Induction and patterning of trunk and tail neural ectoderm by the homeobox gene eve1 in zebrafish embryos. Proc. Natl. Acad. Sci. USA 2010, 107, 3564–3569. [Google Scholar] [CrossRef]
- Barro, O.; Vriz, S.; Joly, J.-S.; Joly, C.; Condamine, H.; Boulekbache, H. Widespread expression of the eve1 gene in zebrafish embryos affects the anterior-posterior axis pattern. Dev. Genet. 1995, 17, 117–128. [Google Scholar] [CrossRef]
- Ruiz i Altaba, A.; Choi, T.; Melton, D.A. Expression of the Xhox3 homeobox protein in Xenopus embryos: Blocking its early function suggests the requirement of Xhox3 for normal posterior development. Dev. Growth Differ. 1991, 33, 651–669. [Google Scholar] [CrossRef]
- Kalisz, M.; Winzi, M.; Bisgaard, H.C.; Serup, P. EVEN-SKIPPED HOMEOBOX 1 controls human ES cell differentiation by directly repressing GOOSECOID expression. Dev. Biol. 2012, 362, 94–103. [Google Scholar] [CrossRef]
- Moran-Rivard, L.; Kagawa, T.; Saueressig, H.; Gross, M.K.; Burrill, J.; Goulding, M. Evx1 is a postmitotic determinant of V0 interneuron identity in the spinal cord. Neuron 2001, 29, 385–399. [Google Scholar] [CrossRef]
- Tanibe, M.; Michiue, T.; Yukita, A.; Danno, H.; Ikuzawa, M.; Ishiura, S.; Asashima, M. Retinoic acid metabolizing factor xCyp26c is specifically expressed in neuroectoderm and regulates anterior neural patterning in Xenopus laevis. Int. J. Dev. Biol. 2008, 52, 893–901. [Google Scholar] [CrossRef]
- Kudoh, T.; Concha, M.L.; Houart, C.; Dawid, I.B.; Wilson, S.W. Combinatorial Fgf and Bmp signalling patterns the gastrula ectoderm into prospective neural and epidermal domains. Development 2004, 131, 3581–3592. [Google Scholar] [CrossRef]
- Griffin, K.; Patient, R.; Holder, N. Analysis of FGF function in normal and no tail zebrafish embryos reveals separate mechanisms for formation of the trunk and the tail. Development 1995, 121, 2983–2994. [Google Scholar] [PubMed]
- Ramel, M.-C.; Lekven, A.C. Repression of the vertebrate organizer by Wnt8 is mediated by Vent and Vox. Development 2004, 131, 3991–4000. [Google Scholar] [CrossRef] [PubMed]
- Ueno, S.; Weidinger, G.; Osugi, T.; Kohn, A.D.; Golob, J.L.; Pabon, L.; Reinecke, H.; Moon, R.T.; Murry, C.E. Biphasic role for Wnt/β-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 9685–9690. [Google Scholar] [CrossRef]
- Carlsson, P.; Mahlapuu, M. Forkhead transcription factors: Key players in development and metabolism. Dev. Biol. 2002, 250, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Weigel, D.; Jäckle, H. The fork head domain: A novel DNA binding motif of eukaryotic transcription factors? Cell 1990, 63, 455–456. [Google Scholar] [CrossRef]
- Alvarez-Bolado, G.; Zhou, X.; Voss, A.K.; Thomas, T.; Gruss, P. Winged helix transcription factor Foxb1 is essential for access of mammillothalamic axons to the thalamus. Development 2000, 127, 1029–1038. [Google Scholar] [PubMed]
- Dou, C.; Ye, X.; Stewart, C.; Lai, E.; Li, S.C. TWH regulates the development of subsets of spinal cord neurons. Neuron 1997, 18, 539–551. [Google Scholar] [CrossRef][Green Version]
- Kloetzli, J.M.; Fontaine-Glover, I.A.; Brown, E.R.; Kuo, M.; Labosky, P.A. The winged helix gene, Foxb1, controls development of mammary glands and regions of the CNS that regulate the milk-ejection reflex. Genesis 2001, 29, 60–71. [Google Scholar] [CrossRef]
- Labosky, P.A.; Winnier, G.E.; Jetton, T.L.; Hargett, L.; Ryan, A.K.; Rosenfeld, M.G.; Parlow, A.F.; Hogan, B.L. The winged helix gene, Mf3, is required for normal development of the diencephalon and midbrain, postnatal growth and the milk-ejection reflex. Development 1997, 124, 1263–1274. [Google Scholar] [PubMed]
- Radyushkin, K.; Anokhin, K.; Meyer, B.I.; Jiang, Q.; Alvarez-Bolado, G.; Gruss, P. Genetic ablation of the mammillary bodies in the Foxb1 mutant mouse leads to selective deficit of spatial working memory. Eur. J. Neurosci. 2005, 21, 219–229. [Google Scholar] [CrossRef]
- Wehr, R.; Mansouri, A.; de Maeyer, T.; Gruss, P. Fkh5-deficient mice show dysgenesis in the caudal midbrain and hypothalamic mammillary body. Development 1997, 124, 4447–4456. [Google Scholar] [PubMed]
- Gamse, J.T.; Sive, H. Early anteroposterior division of the presumptive neurectoderm in Xenopus. Mech. Dev. 2001, 104, 21–36. [Google Scholar] [CrossRef]
- Takebayashi-Suzuki, K.; Kitayama, A.; Terasaka-Iioka, C.; Ueno, N.; Suzuki, A. The forkhead transcription factor FoxB1 regulates the dorsal-ventral and anterior-posterior patterning of the ectoderm during early Xenopus embryogenesis. Dev. Biol. 2011, 360, 11–29. [Google Scholar] [CrossRef] [PubMed]
- David, C.J.; Massagué, J. Contextual determinants of TGFβ action in development, immunity and cancer. Nat. Rev. Mol. Cell Biol. 2018, 19, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.-H.; Moustakas, A. Role of Smads in TGFβ signaling. Cell Tissue Res. 2012, 347, 21–36. [Google Scholar] [CrossRef]
- Lee, K.-H.; Kwak, Y.-D.; Kim, D.-H.; Chang, M.-Y.; Lee, Y.-S.; Lee, Y.-S. Human zinc finger protein 161, a novel transcriptional activator of the dopamine transporter. Biochem. Biophys. Res. Commun. 2004, 313, 969–976. [Google Scholar] [CrossRef]
- Numoto, M.; Niwa, O.; Kaplan, J.; Wong, K.-K.; Merrell, K.; Kamiya, K.; Yanagihara, K.; Calame, K. Transcriptional repressor ZF5 identifies a new conserved domain in zinc finger proteins. Nucleic Acids Res. 1993, 21, 3767–3775. [Google Scholar] [CrossRef]
- Numoto, M.; Yokoro, K.; Koshi, J. ZF5, which is a Kruppel-type transcriptional repressor, requires the zinc finger domain for self-association. Biochem. Biophys. Res. Commun. 1999, 256, 573–578. [Google Scholar] [CrossRef]
- Yokoro, K.; Yanagidani, A.; Obata, T.; Yamamoto, S.; Numoto, M. Genomic cloning and characterization of the mouse POZ/zinc-finger protein ZF5. Biochem. Biophys. Res. Commun. 1998, 246, 668–674. [Google Scholar] [CrossRef]
- Takebayashi-Suzuki, K.; Konishi, H.; Miyamoto, T.; Nagata, T.; Uchida, M.; Suzuki, A. Coordinated regulation of the dorsal-ventral and anterior-posterior patterning of Xenopus embryos by the BTB/POZ zinc finger protein Zbtb14. Dev. Growth Differ. 2018, 60, 158–173. [Google Scholar] [CrossRef]
- Mathew, R.; Seiler, M.P.; Scanlon, S.T.; Mao, A.-p.; Constantinides, M.G.; Bertozzi-Villa, C.; Singer, J.D.; Bendelac, A. BTB-ZF factors recruit the E3 ligase cullin 3 to regulate lymphoid effector programs. Nature 2012, 491, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Siggs, O.M.; Beutler, B. The BTB-ZF transcription factors. Cell Cycle 2012, 11, 3358–3369. [Google Scholar] [CrossRef] [PubMed]
- Perez-Torrado, R.; Yamada, D.; Defossez, P.A. Born to bind: The BTB protein-protein interaction domain. BioEssays 2006, 28, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Calabrese, M.F.; Liu, J.; Waddell, M.B.; Nourse, A.; Hammel, M.; Miller, D.J.; Walden, H.; Duda, D.M.; Seyedin, S.N.; et al. Structures of SPOP-substrate complexes: Insights into molecular architectures of BTB-Cul3 ubiquitin ligases. Mol. Cell 2009, 36, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Pintard, L.; Willis, J.H.; Willems, A.; Johnson, J.-L.F.; Srayko, M.; Kurz, T.; Glaser, S.; Mains, P.E.; Tyers, M.; Bowerman, B.; et al. The BTB protein MEL-26 is a substrate-specific adaptor of the CUL-3 ubiquitin-ligase. Nature 2003, 425, 311–316. [Google Scholar] [CrossRef]
- Xu, L.; Wei, Y.; Reboul, J.; Vaglio, P.; Shin, T.-H.; Vidal, M.; Elledge, S.J.; Harper, J.W. BTB proteins are substrate-specific adaptors in an SCF-like modular ubiquitin ligase containing CUL-3. Nature 2003, 425, 316–321. [Google Scholar] [CrossRef]
- Li, Y.; Klena, N.T.; Gabriel, G.C.; Liu, X.; Kim, A.J.; Lemke, K.; Chen, Y.; Chatterjee, B.; Devine, W.; Damerla, R.R.; et al. Global genetic analysis in mice unveils central role for cilia in congenital heart disease. Nature 2015, 521, 520–524. [Google Scholar] [CrossRef]
- San Agustin, J.T.; Klena, N.; Granath, K.; Panigrahy, A.; Stewart, E.; Devine, W.; Strittmatter, L.; Jonassen, J.A.; Liu, X.; Lo, C.W.; et al. Genetic link between renal birth defects and congenital heart disease. Nat. Commun. 2016, 7, 11103. [Google Scholar] [CrossRef]
- Ille, F.; Sommer, L. Wnt signaling: Multiple functions in neural development. Cell. Mol. Life Sci. 2005, 62, 1100–1108. [Google Scholar] [CrossRef]
- Jessell, T.M.; Sanes, J.R. The decade of the developing brain. Curr. Opin. Neurobiol. 2000, 10, 599–611. [Google Scholar] [CrossRef]
- Paul, B.M.; Vanden Heuvel, G.B. Kidney: Polycystic kidney disease. WIREs. Dev. Biol. 2014, 3, 465–487. [Google Scholar] [CrossRef] [PubMed]
- Perantoni, A.O. Renal development: Perspectives on a Wnt-dependent process. Semin. Cell Dev. Biol. 2003, 14, 201–208. [Google Scholar] [CrossRef]
- Schedl, A.; Hastie, N.D. Cross-talk in kidney development. Curr. Opin. Genet. Dev. 2000, 10, 543–549. [Google Scholar] [CrossRef]
- Brade, T.; Pane, L.S.; Moretti, A.; Chien, K.R.; Laugwitz, K.-L. Embryonic heart progenitors and cardiogenesis. Cold Spring Harb. Perspect. Med. 2013, 3, a013847. [Google Scholar] [CrossRef] [PubMed]
- Garside, V.C.; Chang, A.C.; Karsan, A.; Hoodless, P.A. Co-ordinating Notch, BMP, and TGF-β signaling during heart valve development. Cell. Mol. Life Sci. 2013, 70, 2899–2917. [Google Scholar] [CrossRef]
- Klaus, A.; Birchmeier, W. Developmental signaling in myocardial progenitor cells: A comprehensive view of Bmp- and Wnt/β-catenin signaling. Pediatr. Cardiol. 2009, 30, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Virgirinia, R.P.; Jahan, N.; Okada, M.; Takebayashi-Suzuki, K.; Yoshida, H.; Nakamura, M.; Akao, H.; Yoshimoto, Y.; Fatchiyah, F.; Ueno, N.; et al. Cdc2-like kinase 2 (Clk2) promotes early neural development in Xenopus embryos. Dev. Growth Differ. 2019, 61, 365–377. [Google Scholar] [CrossRef]
- Lindberg, R.A.; Quinn, A.M.; Hunter, T. Dual-specificity protein kinases: Will any hydroxyl do? Trends Biochem. Sci. 1992, 17, 114–119. [Google Scholar] [CrossRef]
- Duncan, P.I.; Howell, B.W.; Marius, R.M.; Drmanic, S.; Douville, E.M.J.; Bell, J.C. Alternative splicing of STY, a nuclear dual specificity kinase. J. Biol. Chem. 1995, 270, 21524–21531. [Google Scholar] [CrossRef]
- Park, S.Y.; Piao, Y.; Thomas, C.; Fuller, G.N.; de Groot, J.F. Cdc2-like kinase 2 is a key regulator of the cell cycle via FOXO3a/p27 in glioblastoma. Oncotarget 2016, 7, 26793–26805. [Google Scholar] [CrossRef]
- Rodgers, J.T.; Haas, W.; Gygi, S.P.; Puigserver, P. Cdc2-like kinase 2 is an insulin-regulated suppressor of hepatic gluconeogenesis. Cell Metab. 2010, 11, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Bidinosti, M.; Botta, P.; Krüttner, S.; Proenca, C.C.; Stoehr, N.; Bernhard, M.; Fruh, I.; Mueller, M.; Bonenfant, D.; Voshol, H.; et al. CLK2 inhibition ameliorates autistic features associated with SHANK3 deficiency. Science 2016, 351, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, G.; Zarbalis, K.S. Prenatal neurogenesis in autism spectrum disorders. Front. Chem. 2016, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.P.; Murphy, M.B.; Landreth, G. The dual-specificity CLK kinase induces neuronal differentiation of PC12 cells. Mol. Cell. Biol. 1994, 14, 6954–6961. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Petsalaki, E.; Zachos, G. Clks 1, 2 and 4 prevent chromatin breakage by regulating the Aurora B-dependent abscission checkpoint. Nat. Commun. 2016, 7, 11451. [Google Scholar] [CrossRef]
- Tabata, M.; Rodgers, J.T.; Hall, J.A.; Lee, Y.; Jedrychowski, M.P.; Gygi, S.P.; Puigserver, P. Cdc2-like kinase 2 suppresses hepatic fatty acid oxidation and ketogenesis through disruption of the PGC-1α and MED1 complex. Diabetes 2014, 63, 1519–1532. [Google Scholar] [CrossRef]
- Xiong, F.; Tentner, A.R.; Huang, P.; Gelas, A.; Mosaliganti, K.R.; Souhait, L.; Rannou, N.; Swinburne, I.A.; Obholzer, N.D.; Cowgill, P.D.; et al. Specified neural progenitors sort to form sharp domains after noisy Shh signaling. Cell 2013, 153, 550–561. [Google Scholar] [CrossRef]
- Akieda, Y.; Ogamino, S.; Furuie, H.; Ishitani, S.; Akiyoshi, R.; Nogami, J.; Masuda, T.; Shimizu, N.; Ohkawa, Y.; Ishitani, T. Cell competition corrects noisy Wnt morphogen gradients to achieve robust patterning in the zebrafish embryo. Nat. Commun. 2019, 10, 4710. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takebayashi-Suzuki, K.; Suzuki, A. Intracellular Communication among Morphogen Signaling Pathways during Vertebrate Body Plan Formation. Genes 2020, 11, 341. https://doi.org/10.3390/genes11030341
Takebayashi-Suzuki K, Suzuki A. Intracellular Communication among Morphogen Signaling Pathways during Vertebrate Body Plan Formation. Genes. 2020; 11(3):341. https://doi.org/10.3390/genes11030341
Chicago/Turabian StyleTakebayashi-Suzuki, Kimiko, and Atsushi Suzuki. 2020. "Intracellular Communication among Morphogen Signaling Pathways during Vertebrate Body Plan Formation" Genes 11, no. 3: 341. https://doi.org/10.3390/genes11030341
APA StyleTakebayashi-Suzuki, K., & Suzuki, A. (2020). Intracellular Communication among Morphogen Signaling Pathways during Vertebrate Body Plan Formation. Genes, 11(3), 341. https://doi.org/10.3390/genes11030341




