Genes, Pathways, and Mechanisms Involved in the Virulence of Mucorales
Abstract
1. Introduction
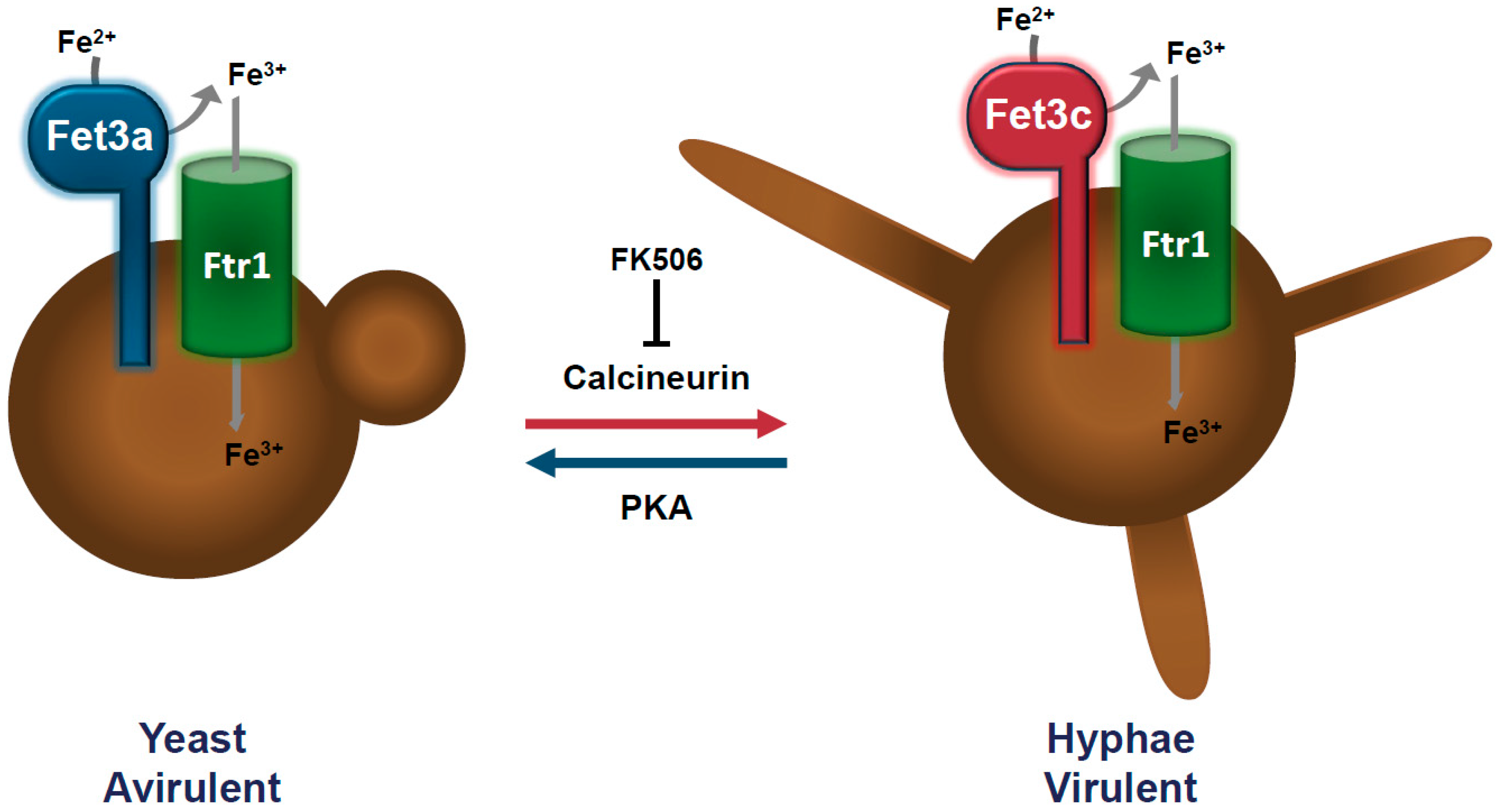
2. Host Iron Uptake is A Key Element in The Pathogenicity of Mucorales
3. Dimorphism Controls the Pathogenic Potential of Mucor circinelloides
4. RNAi in Mucorales and Its Role in Their Antifungal Drug Resistance
5. Azole Resistance in Mucorales
6. Omic Technologies to Find New Virulence Factors
7. Mucoralean Gene Response to Host Innate Immunity
8. cotH Gene Family, A Distinctive Virulence Factor in Mucorales
9. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dannaoui, E. Antifungal resistance in mucorales. Int. J. Antimicrob. Agents 2017, 50, 617–621. [Google Scholar] [CrossRef]
- Petrikkos, G.; Skiada, A.; Lortholary, O.; Roilides, E.; Walsh, T.J.; Kontoyiannis, D.P. Epidemiology and Clinical Manifestations of Mucormycosis. Clin. Infect. Dis. 2012, 54, S23–S34. [Google Scholar] [CrossRef] [PubMed]
- Prakash, H.; Chakrabarti, A. Global epidemiology of mucormycosis. J. Fungi 2019, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Chayakulkeeree, M.; Ghannoum, M.A.; Perfect, J.R. Zygomycosis: The re-emerging fungal infection. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Sridhara, S.R.; Paragache, G.; Panda, N.K.; Chakrabarti, A. Mucormycosis in immunocompetent individuals: An increasing trend. J. Otolaryngol. 2005, 34, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, A.; López-García, S.; Garre, V. High reliability transformation of the basal fungus Mucor circinelloides by electroporation. J. Microbiol. Methods 2011, 84, 442–446. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, Z.; Du, G.; Zhou, J.; Chen, J. Efficient transformation of Rhizopus delemar by electroporation of germinated spores. J. Microbiol. Methods 2014, 103, 58–63. [Google Scholar] [CrossRef]
- Baldin, C.; Ibrahim, A.S. Molecular mechanisms of mucormycosis—The bitter and the sweet. PLoS Pathog. 2017, 13, e1006408. [Google Scholar] [CrossRef]
- Hassan, M.I.A.; Voigt, K. Pathogenicity patterns of mucormycosis: Epidemiology, interaction with immune cells and virulence factors. Med. Mycol. 2019, 57, S245–S256. [Google Scholar] [CrossRef]
- Ruiz-Vázquez, R.M.; Nicolás, F.E.; Torres-Martínez, S.; Garre, V. Distinct RNAi Pathways in the Regulation of Physiology and Development in the Fungus Mucor circinelloides. Adv. Genet. 2015, 91, 55–102. [Google Scholar]
- Calo, S.; Shertz-Wall, C.; Lee, S.C.; Bastidas, R.J.; Nicolás, F.E.; Granek, J.A.; Mieczkowski, P.; Torres-Martínez, S.; Ruiz-Vázquez, R.M.; Cardenas, M.E.; et al. Antifungal drug resistance evoked via RNAi-dependent epimutations. Nature 2014, 513, 555–558. [Google Scholar] [CrossRef]
- Trieu, T.A.; Navarro-Mendoza, M.I.; Pérez-Arques, C.; Sanchis, M.; Capilla, J.; Navarro-Rodriguez, P.; Lopez-Fernandez, L.; Torres-Martínez, S.; Garre, V.; Ruiz-Vázquez, R.M.; et al. RNAi-Based Functional Genomics Identifies New Virulence Determinants in Mucormycosis. PLoS Pathog. 2017, 13, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Arques, C.; Navarro-Mendoza, M.I.; Murcia, L.; Lax, C.; Martínez-García, P.; Heitman, J.; Nicolás, F.E.; Garre, V. Mucor circinelloides thrives inside the phagosome through an Atf-mediated germination pathway. MBio 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bullen, J.J. Natural resistance, iron and infection: A challenge for clinical medicine. J. Med. Microbiol. 2006, 55, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Gebremariam, T.; Lin, L.; Liu, M.; Kontoyiannis, D.P.; French, S.; Edwards, J.E.; Filler, S.G.; Ibrahim, A.S. Bicarbonate correction of ketoacidosis alters host-pathogen interactions and alleviates mucormycosis. J. Clin. Investig. 2016, 126, 2280–2294. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.S.; Grieve, C.L.; Murugathasan, I.; Bennet, A.J.; Czekster, C.M.; Liu, H.; Naismith, J.; Moore, M.M. The rhizoferrin biosynthetic gene in the fungal pathogen Rhizopus delemar is a novel member of the NIS gene family. Int. J. Biochem. Cell Biol. 2017, 89, 136–146. [Google Scholar] [CrossRef]
- Navarro-Mendoza, M.I.; Pérez-Arques, C.; Murcia, L.; Martínez-García, P.; Lax, C.; Sanchis, M.; Capilla, J.; Nicolás, F.E.; Garre, V. Components of a new gene family of ferroxidases involved in virulence are functionally specialized in fungal dimorphism. Sci. Rep. 2018, 8, 7660. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Gebremariam, T.; Lin, L.; Luo, G.; Husseiny, M.I.; Skory, C.D.; Fu, Y.; French, S.W.; Edwards, J.E.; Spellberg, B. The high affinity iron permease is a key virulence factor required for Rhizopus oryzae pathogenesis. Mol. Microbiol. 2010, 77, 587–604. [Google Scholar] [CrossRef]
- Schwartze, V.U.; Winter, S.; Shelest, E.; Marcet-Houben, M.; Horn, F.; Wehner, S.; Linde, J.; Valiante, V.; Sammeth, M.; Riege, K.; et al. Gene Expansion Shapes Genome Architecture in the Human Pathogen Lichtheimia corymbifera: An Evolutionary Genomics Analysis in the Ancient Terrestrial Mucorales (Mucoromycotina). PLoS Genet. 2014, 10, e1004496. [Google Scholar] [CrossRef]
- Shirazi, F.; Kontoyiannis, D.P.; Ibrahim, A.S. Iron starvation induces apoptosis in Rhizopus oryzae in vitro. Virulence 2015, 6, 121–126. [Google Scholar] [CrossRef]
- Haas, H. Molecular genetics of fungal siderophore biosynthesis and uptake: The role of siderophores in iron uptake and storage. Appl. Microbiol. Biotechnol. 2003, 62, 316–330. [Google Scholar] [CrossRef]
- Thieken, A.; Winkelmann, G. Rhizoferrin: A complexone type siderophore of the Mucorales and entomophthorales (Zygomycetes). FEMS Microbiol. Lett. 1992, 73, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lin, L.; Gebremariam, T.; Luo, G.; Skory, C.D.; French, S.W.; Chou, T.F.; Edwards, J.E.; Ibrahim, A.S. Fob1 and Fob2 Proteins Are Virulence Determinants of Rhizopus oryzae via Facilitating Iron Uptake from Ferrioxamine. PLoS Pathog. 2015, 11, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.S.; Gebermariam, T.; Fu, Y.; Lin, L.; Husseiny, M.I.; French, S.W.; Schwartz, J.; Skory, C.D.; Edwards, J.E.; Spellberg, B.J. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J. Clin. Investig. 2007, 117, 2649–2657. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, P.; Cornely, O.A.; Dannaoui, E. Antifungal combinations in Mucorales: A microbiological perspective. Mycoses 2019, 3, 746–760. [Google Scholar] [CrossRef] [PubMed]
- Kousser, C.; Clark, C.; Sherrington, S.; Voelz, K.; Hall, R.A. Pseudomonas aeruginosa inhibits Rhizopus microsporus germination through sequestration of free environmental iron. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, M. Mucor dimorphism. Microbiol. Rev. 1991, 55, 234–258. [Google Scholar] [CrossRef]
- Wolff, A.M.; Appel, K.F.; Petersen, J.B.; Poulsen, U.; Arnau, J. Identification and analysis of genes involved in the control of dimorphism in Mucor circinelloides (syn. racemosus). FEMS Yeast Res. 2002, 2, 203–213. [Google Scholar]
- Lee, S.C.; Li, A.; Calo, S.; Heitman, J. Calcineurin plays key roles in the dimorphic transition and virulence of the human pathogenic zygomycete Mucor circinelloides. PLoS Pathog. 2013, 9, e1003625. [Google Scholar] [CrossRef]
- Ruiz-Herrera, J. Polyamines, DNA methylation, and fungal differentiation. Crit. Rev. Microbiol. 1994, 20, 143–150. [Google Scholar] [CrossRef]
- Binder, U.; Navarro-Mendoza, M.I.; Naschberger, V.; Bauer, I.; Nicolas, F.E.; Pallua, J.D.; Lass-Flörl, C.; Garre, V. Generation of a mucor circinelloides reporter strain—A promising new tool to study antifungal drug efficacy and mucormycosis. Genes 2018, 9, 613. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Mendoza, M.I.; Pérez-Arques, C.; Panchal, S.; Nicolás, F.E.; Mondo, S.J.; Ganguly, P.; Pangilinan, J.; Grigoriev, I.V.; Heitman, J.; Sanyal, K.; et al. Early Diverging Fungus Mucor circinelloides Lacks Centromeric Histone CENP-A and Displays a Mosaic of Point and Regional Centromeres. Curr. Biol. 2019, 29, 3791–3802. [Google Scholar] [CrossRef] [PubMed]
- Nicolás, F.; Ruiz-Vázquez, R. Functional Diversity of RNAi-Associated sRNAs in Fungi. Int. J. Mol. Sci. 2013, 14, 15348–15360. [Google Scholar] [CrossRef] [PubMed]
- Nicolás, F.E.; Navarro-Mendoza, M.I.; Pérez-Arques, C.; López-García, S.; Navarro, E.; Torres-Martínez, S.; Garre, V. Molecular tools for carotenogenesis analysis in the mucoral Mucor circinelloides. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2018; Volume 1852, pp. 221–237. [Google Scholar]
- Lee, S.C.; Li, A.; Calo, S.; Inoue, M.; Tonthat, N.K.; Bain, J.M.; Louw, J.; Shinohara, M.L.; Erwig, L.P.; Schumacher, M.A.; et al. Calcineurin orchestrates dimorphic transitions, antifungal drug responses and host-pathogen interactions of the pathogenic mucoralean fungus Mucor circinelloides. Mol. Microbiol. 2015, 97, 844–865. [Google Scholar] [CrossRef]
- Boyce, K.J.; Andrianopoulos, A. Fungal dimorphism: The switch from hyphae to yeast is a specialized morphogenetic adaptation allowing colonization of a host. FEMS Microbiol. Rev. 2015, 39, 797–811. [Google Scholar] [CrossRef]
- Li, C.H.; Cervantes, M.; Springer, D.J.; Boekhout, T.; Ruiz-Vazquez, R.M.; Torres-Martinez, S.R.; Heitman, J.; Lee, S.C. Sporangiospore size dimorphism is linked to virulence of Mucor circinelloides. PLoS Pathog. 2011, 7, e1002086. [Google Scholar] [CrossRef]
- Ocampo, J.; Nuñez, L.F.; Silva, F.; Pereyra, E.; Moreno, S.; Garre, V.; Rossi, S. A subunit of protein kinase a regulates growth and differentiation in the fungus Mucor circinelloides. Eukaryot Cell 2009, 8, 933–944. [Google Scholar] [CrossRef]
- Patiño-Medina, J.A.; Reyes-Mares, N.Y.; Valle-Maldonado, M.I.; Jácome-Galarza, I.E.; Pérez-Arques, C.; Nuñez-Anita, R.E.; Campos-García, J.; Anaya-Martínez, V.; Ortiz-Alvarado, R.; Ramírez-Díaz, M.I.; et al. Heterotrimeric G-alpha subunits Gpa11 and Gpa12 define a transduction pathway that control spore size and virulence in Mucor circinelloides. PLoS ONE 2019, 14, e0226682. [Google Scholar] [CrossRef]
- Valle-Maldonado, M.I.; Jácome-Galarza, I.E.; Díaz-Pérez, A.L.; Martínez-Cadena, G.; Campos-García, J.; Ramírez-Díaz, M.I.; Reyes-De la Cruz, H.; Riveros-Rosas, H.; Díaz-Pérez, C.; Meza-Carmen, V. Phylogenetic analysis of fungal heterotrimeric G protein-encoding genes and their expression during dimorphism in Mucor circinelloides. Fungal Biol. 2015, 119, 1179–1193. [Google Scholar] [CrossRef]
- Chang, Z.; Billmyre, R.B.; Lee, S.C.; Heitman, J. Broad antifungal resistance mediated by RNAi-dependent epimutation in the basal human fungal pathogen Mucor circinelloides. PLoS Genet. 2019, 15, e1007957. [Google Scholar] [CrossRef]
- Calo, S.; Nicolas, F.E.; Vila, A.; Torres-Martinez, S.; Ruiz-Vazquez, R.M. Two distinct RNA-dependent RNA polymerases are required for initiation and amplification of RNA silencing in the basal fungus Mucor circinelloides. Mol. Microbiol. 2012, 83, 379–394. [Google Scholar] [CrossRef]
- De Haro, J.P.; Calo, S.; Cervantes, M.; Nicolás, F.E.; Torres-Martínez, S.; Ruiz-Vázquez, R.M. A Single dicer Gene Is Required for Efficient Gene Silencing Associated with Two Classes of Small Antisense RNAs in Mucor circinelloides. Eukaryot Cell 2009, 8, 1486–1497. [Google Scholar] [CrossRef]
- Cervantes, M.; Vila, A.; Nicolás, F.E.; Moxon, S.; de Haro, J.P.; Dalmay, T.; Torres-Martínez, S.; Ruiz-Vázquez, R.M. A Single Argonaute Gene Participates in Exogenous and Endogenous RNAi and Controls Cellular Functions in the Basal Fungus Mucor circinelloides. PLoS ONE 2013, 8, e69283. [Google Scholar] [CrossRef]
- Nicolas, F.E.; Moxon, S.; de Haro, J.P.; Calo, S.; Grigoriev, I.V.; Torres-Martinez, S.; Moulton, V.; Ruiz-Vazquez, R.M.; Dalmay, T. Endogenous short RNAs generated by Dicer 2 and RNA-dependent RNA polymerase 1 regulate mRNAs in the basal fungus Mucor circinelloides. Nucleic Acids Res. 2010, 38, 5535–5541. [Google Scholar] [CrossRef]
- Nicolás, F.E.; Vila, A.; Moxon, S.; Cascales, M.D.; Torres-Martínez, S.; Ruiz-Vázquez, R.M.; Garre, V. The RNAi machinery controls distinct responses to environmental signals in the basal fungus Mucor circinelloides. BMC Genom. 2015, 16, 237. [Google Scholar] [CrossRef]
- Calo, S.; Nicolás, F.E.; Lee, S.C.; Vila, A.; Cervantes, M.; Torres-Martinez, S.; Ruiz-Vazquez, R.M.; Cardenas, M.E.; Heitman, J. A non-canonical RNA degradation pathway suppresses RNAi-dependent epimutations in the human fungal pathogen Mucor circinelloides. PLoS Genet. 2017, 13, e1006686. [Google Scholar] [CrossRef]
- Trieu, T.A.; Calo, S.; Nicolás, F.E.; Vila, A.; Moxon, S.; Dalmay, T.; Torres-Martínez, S.; Garre, V.; Ruiz-Vázquez, R.M. A Non-canonical RNA Silencing Pathway Promotes mRNA Degradation in Basal Fungi. PLoS Genet. 2015, 11, e1005168. [Google Scholar] [CrossRef]
- Chang, Z.; Heitman, J. Drug-resistant epimutants exhibit organ-specific stability and induction during murine infections caused by the human fungal pathogen Mucor circinelloides. MBio 2019, 10. [Google Scholar] [CrossRef]
- Groll, A.H.; Gea-Banacloche, J.C.; Glasmacher, A.; Just-Nuebling, G.; Maschmeyer, G.; Walsh, T.J. Clinical pharmacology of antifungal compounds. Infect. Dis. Clin. N. Am. 2003, 17, 159–191. [Google Scholar] [CrossRef]
- Lass-Flörl, C. Triazole antifungal agents in invasive fungal infections: A comparative review. Drugs 2011, 71, 2405–2419. [Google Scholar] [CrossRef]
- Watson, P.F.; Rose, M.E.; Ellis, S.W.; England, H.; Kelly, S.L. Defective sterol C5-6 desaturation and azole resistance: A new hypothesis for the mode of action of azole antifungals. Biochem. Biophys. Res. Commun. 1989, 164, 1170–1175. [Google Scholar] [CrossRef]
- Vitale, R.G.; De Hoog, G.S.; Schwarz, P.; Dannaoui, E.; Deng, S.; Machouart, M.; Voigt, K.; Van De Sande, W.W.J.; Dolatabadi, S.; Meis, J.F.; et al. Antifungal susceptibility and phylogeny of opportunistic members of the order Mucorales. J. Clin. Microbiol. 2012, 50, 66–75. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Chakrabarti, A.; Chowdhary, A.; Cordoba, S.; Dannaoui, E.; Dufresne, P.; Fothergill, A.; Ghannoum, M.; Gonzalez, G.M.; Guarro, J.; et al. Multicenter evaluation of MIC distributions for epidemiologic cutoff value definition to detect amphotericin B, posaconazole, and itraconazole resistance among the most clinically relevant species of Mucorales. Antimicrob. Agents Chemother. 2015, 59, 1745–1750. [Google Scholar] [CrossRef]
- Maurer, E.; Binder, U.; Sparber, M.; Lackner, M.; Caramalho, R.; Lass-Flörl, C. Susceptibility profiles of amphotericin B and posaconazole against clinically relevant mucorales species under hypoxic conditions. Antimicrob. Agents Chemother. 2015, 59, 1344–1346. [Google Scholar] [CrossRef][Green Version]
- Chowdhary, A.; Singh, P.K.; Kathuria, S.; Hagen, F.; Meis, J.F. Comparison of the EUCAST and CLSI broth microdilution methods for testing isavuconazole, posaconazole, and amphotericin b against molecularly identified Mucorales species. Antimicrob. Agents Chemother. 2015, 59, 7882–7887. [Google Scholar] [CrossRef]
- Luo, G.; Gebremariam, T.; Lee, H.; French, S.W.; Wiederhold, N.P.; Patterson, T.F.; Filler, S.G.; Ibrahim, A.S. Efficacy of liposomal amphotericin B and posaconazole in intratracheal models of murine mucormycosis. Antimicrob. Agents Chemother. 2013, 57, 3340–3347. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Jensen, R.H.; Meletiadis, J. In vitro activity of isavuconazole and comparators against clinical isolates of the Mucorales order. Antimicrob. Agents Chemother. 2015, 59, 7735–7742. [Google Scholar] [CrossRef]
- Luo, G.; Gebremariam, T.; Lee, H.; Edwards, J.E.; Kovanda, L.; Ibrahim, A.S. Isavuconazole therapy protects immunosuppressed mice from mucormycosis. Antimicrob. Agents Chemother. 2014, 58, 2450–2453. [Google Scholar] [CrossRef]
- Amphotericin B nephrotoxicity|Journal of Antimicrobial Chemotherapy|Oxford Academic. Available online: https://academic.oup.com/jac/article/49/suppl_1/37/2473434 (accessed on 3 March 2020).
- Nishimoto, A.T.; Sharma, C.; Rogers, P.D. Molecular and genetic basis of azole antifungal resistance in the opportunistic pathogenic fungus Candida albicans. J. Antimicrob. Chemother. 2019, 75, 257–270. [Google Scholar] [CrossRef]
- Chowdhary, A.; Sharma, C.; Hagen, F.; Meis, J.F. Exploring azole antifungal drug resistance in Aspergillus fumigatus with special reference to resistance mechanisms. Future Microbiol. 2014, 9, 697–711. [Google Scholar] [CrossRef]
- Prasad, R.; De Wergifosse, P.; Goffeau, A.; Balzi, E. Molecular cloning and characterization of a novel gene of Candida albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr. Genet. 1995, 27, 320–329. [Google Scholar] [CrossRef]
- Sanglard, D.; Kuchler, K.; Ischer, F.; Pagani, J.L.; Monod, M.; Bille, J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 1995, 39, 2378–2386. [Google Scholar] [CrossRef]
- Franz, R.; Kelly, S.L.; Lamb, D.C.; Kelly, D.E.; Ruhnke, M.; Morschhäuser, J. Multiple molecular mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob. Agents Chemother. 1998, 42, 3065–3072. [Google Scholar] [CrossRef]
- Dunkel, N.; Blass, J.; Rogers, P.D.; Morschhäuser, J. Mutations in the multi-drug resistance regulator MRR1, followed by loss of heterozygosity, are the main cause of MDR1 overexpression in fluconazole-resistant Candida albicans strains. Mol. Microbiol. 2008, 69, 827–840. [Google Scholar] [CrossRef]
- White, T.C. The presence of an R467K amino acid substitution and loss of allelic variation correlate with an azole-resistant lanosterol 14alpha demethylase in Candida albicans. Antimicrob. Agents Chemother. 1997, 41, 1488–1494. [Google Scholar] [CrossRef]
- Sanglard, D.; Ischer, F.; Koymans, L.; Bille, J. Amino acid substitutions in the cytochrome P-450 lanosterol 14α-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob. Agents Chemother. 1998, 42, 241–253. [Google Scholar]
- Leonardelli, F.; Macedo, D.; Dudiuk, C.; Cabeza, M.S.; Gamarra, S.; Garcia-Effron, G. Aspergillus fumigatus Intrinsic Fluconazole Resistance Is Due to the Naturally Occurring T301I Substitution in Cyp51Ap. Antimicrob. Agents Chemother. 2016, 60, 5420–5426. [Google Scholar] [CrossRef]
- Snelders, E.; Karawajczyk, A.; Schaftenaar, G.; Verweij, P.E.; Melchers, W.J.G. Azole resistance profile of amino acid changes in Aspergillus fumigatus CYP51A based on protein homology modeling. Antimicrob. Agents Chemother. 2010, 54, 2425–2430. [Google Scholar] [CrossRef]
- Diaz-Guerra, T.M.; Mellado, E.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L. A point mutation in the 14α-sterol demethylase gene cyp51a contributes to itraconazole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2003, 47, 1120–1224. [Google Scholar] [CrossRef]
- Abdolrasouli, A.; Rhodes, J.; Beale, M.A.; Hagen, F.; Rogers, T.R.; Chowdhary, A.; Meis, J.F.; Armstrong-James, D.; Fisher, M.C. Genomic context of azole resistance mutations in Aspergillus fumigatus determined using whole-genome sequencing. MBio 2015, 6, e00536. [Google Scholar] [CrossRef]
- Hagiwara, D.; Watanabe, A.; Kamei, K.; Goldman, G.H. Epidemiological and Genomic Landscape of Azole Resistance Mechanisms in Aspergillus Fungi. Front. Microbiol. 2016, 7, 1382. [Google Scholar] [CrossRef]
- Warrilow, A.G.; Nishimoto, A.T.; Parker, J.E.; Price, C.L.; Flowers, S.A.; Kelly, D.E.; David Rogers, P.; Kelly, S.L. The evolution of Azole resistance in Candida albicans Sterol 14-demethylase (CYP51) through incremental amino acid substitutions. Antimicrob. Agents Chemother. 2019, 63, e02586. [Google Scholar] [CrossRef]
- Mellado, E.; Diaz-Guerra, T.M.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L. Identification of two different 14-alpha sterol demethylase-related genes (cyp51A and cyp51B) in Aspergillus fumigatus and other Aspergillus species. J. Clin. Microbiol. 2001, 39, 2431–2438. [Google Scholar] [CrossRef]
- Caramalho, R.; Tyndall, J.D.A.; Monk, B.C.; Larentis, T.; Lass-Flörl, C.; Lackner, M. Intrinsic short-tailed azole resistance in mucormycetes is due to an evolutionary conserved aminoacid substitution of the lanosterol 14α-demethylase. Sci. Rep. 2017, 7, 15898. [Google Scholar] [CrossRef]
- Sagatova, A.A.; Keniya, M.V.; Wilson, R.K.; Sabherwal, M.; Tyndall, J.D.A.; Monk, B.C. Triazole resistance mediated by mutations of a conserved active site tyrosine in fungal lanosterol 14α-demethylase. Sci. Rep. 2016, 6, 26213. [Google Scholar] [CrossRef]
- Fu, Y.; Lee, H.; Collins, M.; Tsai, H.F.; Spellberg, B.; Edwards, J.E.; Kwon-Chung, K.J.; Ibrahim, A.S. Cloning and functional characterization of the Rhizopus oryzae high affinity iron permease (rFTR1) gene. FEMS Microbiol. Lett. 2004, 235, 169–176. [Google Scholar] [CrossRef]
- Patiño-Medina, J.A.; Maldonado-Herrera, G.; Pérez-Arques, C.; Alejandre-Castañeda, V.; Reyes-Mares, N.Y.; Valle-Maldonado, M.I.; Campos-García, J.; Ortiz-Alvarado, R.; Jácome-Galarza, I.E.; Ramírez-Díaz, M.I.; et al. Control of morphology and virulence by ADP-ribosylation factors (Arf) in Mucor circinelloides. Curr. Genet. 2018, 64, 853–869. [Google Scholar] [CrossRef]
- López-Fernández, L.; Sanchis, M.; Navarro-Rodríguez, P.; Nicolás, F.E.; Silva-Franco, F.; Guarro, J.; Garre, V.; Navarro-Mendoza, M.I.; Pérez-Arques, C.; Capilla, J. Understanding Mucor circinelloides pathogenesis by comparative genomics and phenotypical studies. Virulence 2018, 9, 707–720. [Google Scholar] [CrossRef]
- Chibucos, M.C.; Soliman, S.; Gebremariam, T.; Lee, H.; Daugherty, S.; Orvis, J.; Shetty, A.C.; Crabtree, J.; Hazen, T.H.; Etienne, K.A.; et al. An integrated genomic and transcriptomic survey of mucormycosis-causing fungi. Nat. Commun. 2016, 7, 1–11. [Google Scholar] [CrossRef]
- Ma, L.-J.; Ibrahim, A.S.; Skory, C.; Grabherr, M.G.; Burger, G.; Butler, M.; Elias, M.; Idnurm, A.; Lang, B.F.; Sone, T.; et al. Genomic Analysis of the Basal Lineage Fungus Rhizopus oryzae Reveals a Whole-Genome Duplication. PLoS Genet. 2009, 5, e1000549. [Google Scholar] [CrossRef]
- Corrochano, L.M.; Kuo, A.; Marcet-Houben, M.; Polaino, S.; Salamov, A.; Villalobos-Escobedo, J.M.; Grimwood, J.; Álvarez, M.I.; Avalos, J.; Bauer, D.; et al. Expansion of Signal Transduction Pathways in Fungi by Extensive Genome Duplication. Curr. Biol. 2016, 26, 1577–1584. [Google Scholar] [CrossRef]
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H.; et al. Epidemiology and Outcome of Zygomycosis: A Review of 929 Reported Cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Spellberg, B.; Walsh, T.J.; Kontoyiannis, D.P. Pathogenesis of mucormycosis. Clin. Infect. Dis. 2012, 54, S16–S22. [Google Scholar] [CrossRef]
- Inglesfield, S.; Jasiulewicz, A.; Hopwood, M.; Tyrrell, J.; Youlden, G.; Mazon-Moya, M.; Millington, O.R.; Mostowy, S.; Jabbari, S.; Voelz, K. Robust phagocyte recruitment controls the opportunistic fungal pathogen Mucor circinelloides in innate granulomas In Vivo. MBio 2018, 9, e02010–e02017. [Google Scholar] [CrossRef]
- Sheldom, W.H.; Bauer, H. The development of the acute inflammatory response to experimental cutaneous mucormycosis in normal and diabetic rabbits. J. Exp. Med. 1959, 110, 845–852. [Google Scholar] [CrossRef]
- Waldorf, A.R.; Ruderman, N.; Diamond, R.D. Specific susceptibility to mucormycosis in murine diabetes and bronchoalveolar macrophage defense against Rhizopus. J. Clin. Investig. 1984, 74, 150–160. [Google Scholar] [CrossRef]
- Voelz, K.; Gratacap, R.L.; Wheeler, R.T. A zebrafish larval model reveals early tissue-specific innate immune responses to Mucor circinelloides. Dis. Model. Mech. 2015, 8, 1375–1388. [Google Scholar] [CrossRef]
- Waldorf, A.R.; Levitz, S.M.; Diamond, R.D. In vivo bronchoalveolar macrophage defense against Rhizopus oryzae and Aspergillus fumigatus. J. Infect. Dis. 1984, 150, 752–760. [Google Scholar] [CrossRef]
- Andrianaki, A.M.; Kyrmizi, I.; Thanopoulou, K.; Baldin, C.; Drakos, E.; Soliman, S.S.M.; Shetty, A.C.; McCracken, C.; Akoumianaki, T.; Stylianou, K.; et al. Iron restriction inside macrophages regulates pulmonary host defense against Rhizopus species. Nat. Commun. 2018, 9, 3333. [Google Scholar] [CrossRef]
- Kraibooj, K.; Park, H.-R.; Dahse, H.-M.; Skerka, C.; Voigt, K.; Figge, M.T. Virulent strain of Lichtheimia corymbifera shows increased phagocytosis by macrophages as revealed by automated microscopy image analysis. Mycoses 2014, 57, 56–66. [Google Scholar] [CrossRef]
- Westermann, A.J.; Barquist, L.; Vogel, J. Resolving host–pathogen interactions by dual RNA-seq. PLoS Pathog. 2017, 13, e1006033. [Google Scholar] [CrossRef]
- López-Muñoz, A.; Nicolás, F.E.; García-Moreno, D.; Pérez-Oliva, A.B.; Navarro-Mendoza, M.I.; Hernández-Oñate, M.A.; Herrera-Estrella, A.; Torres-Martínez, S.; Ruiz-Vázquez, R.M.; Garre, V.; et al. An Adult Zebrafish Model Reveals that Mucormycosis Induces Apoptosis of Infected Macrophages. Sci. Rep. 2018, 8, 12802. [Google Scholar] [CrossRef]
- McKenney, P.T.; Driks, A.; Eichenberger, P. The Bacillus subtilis endospore: Assembly and functions of the multilayered coat. Nat. Rev. Microbiol. 2013, 11, 33–44. [Google Scholar] [CrossRef]
- Nguyen, K.B.; Sreelatha, A.; Durrant, E.S.; Lopez-Garrido, J.; Muszewska, A.; Dudkiewicz, M.; Grynberg, M.; Yee, S.; Pogliano, K.; Tomchick, D.R.; et al. Phosphorylation of spore coat proteins by a family of atypical protein kinases. Proc. Natl. Acad. Sci. USA 2016, 113, E3482–E3491. [Google Scholar] [CrossRef]
- Saggese, A.; Scamardella, V.; Sirec, T.; Cangiano, G.; Isticato, R.; Pane, F.; Amoresano, A.; Ricca, E.; Baccigalupi, L. Antagonistic role of CotG and CotH on spore germination and coat formation in Bacillus subtilis. PLoS ONE 2014, 9, e104900. [Google Scholar] [CrossRef]
- Gebremariam, T.; Liu, M.; Luo, G.; Bruno, V.; Phan, Q.T.; Waring, A.J.; Edwards, J.E.; Filler, S.G.; Yeaman, M.R.; Ibrahim, A.S. CotH3 mediates fungal invasion of host cells during mucormycosis. J. Clin. Investig. 2014, 124, 237–250. [Google Scholar] [CrossRef]
- Lebreton, A.; Meslet-Cladière, L.; Morin-Sardin, S.; Coton, E.; Jany, J.L.; Barbier, G.; Corre, E. Comparative analysis of five Mucor species transcriptomes. Genomics 2019, 111, 1306–1314. [Google Scholar] [CrossRef]
- Challa, S. Mucormycosis: Pathogenesis and Pathology. Curr. Fungal Infect. Rep. 2019, 13, 11–20. [Google Scholar] [CrossRef]
- Liu, H.; Lee, M.J.; Solis, N.V.; Phan, Q.T.; Swidergall, M.; Ralph, B.; Ibrahim, A.S.; Sheppard, D.C.; Filler, S.G. Aspergillus fumigatus CalA binds to integrin α5β1 and mediates host cell invasion. Nat. Microbiol. 2016, 2, 16211. [Google Scholar] [CrossRef]
- Ibrahim, I.M.; Abdelmalek, D.H.; Elfiky, A.A. GRP78: A cell’s response to stress. Life Sci. 2019, 226, 156–163. [Google Scholar] [CrossRef]
- Liu, M.; Spellberg, B.; Phan, Q.T.; Fu, Y.; Fu, Y.; Lee, A.S.; Edwards, J.E.; Filler, S.G.; Ibrahim, A.S. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J. Clin. Investig. 2010, 120, 1914–1924. [Google Scholar] [CrossRef] [PubMed]
- Alspaugh, J.A. Hostile takeover: Fungal protein promotes host cell invasion. J. Clin. Investig. 2014, 124, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Gebremariam, T.; Alkhazraji, S.; Soliman, S.S.M.; Gu, Y.; Jeon, H.H.; Zhang, L.; French, S.W.; Stevens, D.A.; Edwards, J.E.; Filler, S.G.; et al. Anti-CotH3 antibodies protect mice from mucormycosis by prevention of invasion and augmenting opsonophagocytosis. Sci. Adv. 2019, 5, eaaw1327. [Google Scholar] [CrossRef]
- Baldin, C.; Soliman, S.S.M.; Jeon, H.H.; Alkhazraji, S.; Gebremariam, T.; Gu, Y.; Bruno, V.M.; Cornely, O.A.; Leather, H.L.; Sugrue, M.W.; et al. PCR-based approach targeting mucorales-specific gene family for diagnosis of mucormycosis. J. Clin. Microbiol. 2018, 56, e00746. [Google Scholar] [CrossRef] [PubMed]
- Van Heeswijck, R.; Roncero, M.I.G. High frequency transformation of Mucor with recombinant plasmid DNA. Carlsberg Res. Commun. 1984, 49, 691. [Google Scholar] [CrossRef]
- Reed, C.; Ibrahim, A.; Edwards, J.E.; Walot, I.; Spellberg, B. Deferasirox, an iron-chelating agent, as salvage therapy for rhinocerebral mucormycosis. Antimicrob. Agents Chemother. 2006, 50, 3968–3969. [Google Scholar] [CrossRef]
- Hooks, M.A. Tacrolimus, a new immunosuppressant—A review of the literature. Ann. Pharmacother. 1994, 28, 501–511. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lax, C.; Pérez-Arques, C.; Navarro-Mendoza, M.I.; Cánovas-Márquez, J.T.; Tahiri, G.; Pérez-Ruiz, J.A.; Osorio-Concepción, M.; Murcia-Flores, L.; Navarro, E.; Garre, V.; et al. Genes, Pathways, and Mechanisms Involved in the Virulence of Mucorales. Genes 2020, 11, 317. https://doi.org/10.3390/genes11030317
Lax C, Pérez-Arques C, Navarro-Mendoza MI, Cánovas-Márquez JT, Tahiri G, Pérez-Ruiz JA, Osorio-Concepción M, Murcia-Flores L, Navarro E, Garre V, et al. Genes, Pathways, and Mechanisms Involved in the Virulence of Mucorales. Genes. 2020; 11(3):317. https://doi.org/10.3390/genes11030317
Chicago/Turabian StyleLax, Carlos, Carlos Pérez-Arques, María Isabel Navarro-Mendoza, José Tomás Cánovas-Márquez, Ghizlane Tahiri, José Antonio Pérez-Ruiz, Macario Osorio-Concepción, Laura Murcia-Flores, Eusebio Navarro, Victoriano Garre, and et al. 2020. "Genes, Pathways, and Mechanisms Involved in the Virulence of Mucorales" Genes 11, no. 3: 317. https://doi.org/10.3390/genes11030317
APA StyleLax, C., Pérez-Arques, C., Navarro-Mendoza, M. I., Cánovas-Márquez, J. T., Tahiri, G., Pérez-Ruiz, J. A., Osorio-Concepción, M., Murcia-Flores, L., Navarro, E., Garre, V., & Nicolás, F. E. (2020). Genes, Pathways, and Mechanisms Involved in the Virulence of Mucorales. Genes, 11(3), 317. https://doi.org/10.3390/genes11030317







