Heterologous Expression of SvMBD5 from Salix viminalis L. Promotes Flowering in Arabidopsis thaliana L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Methods
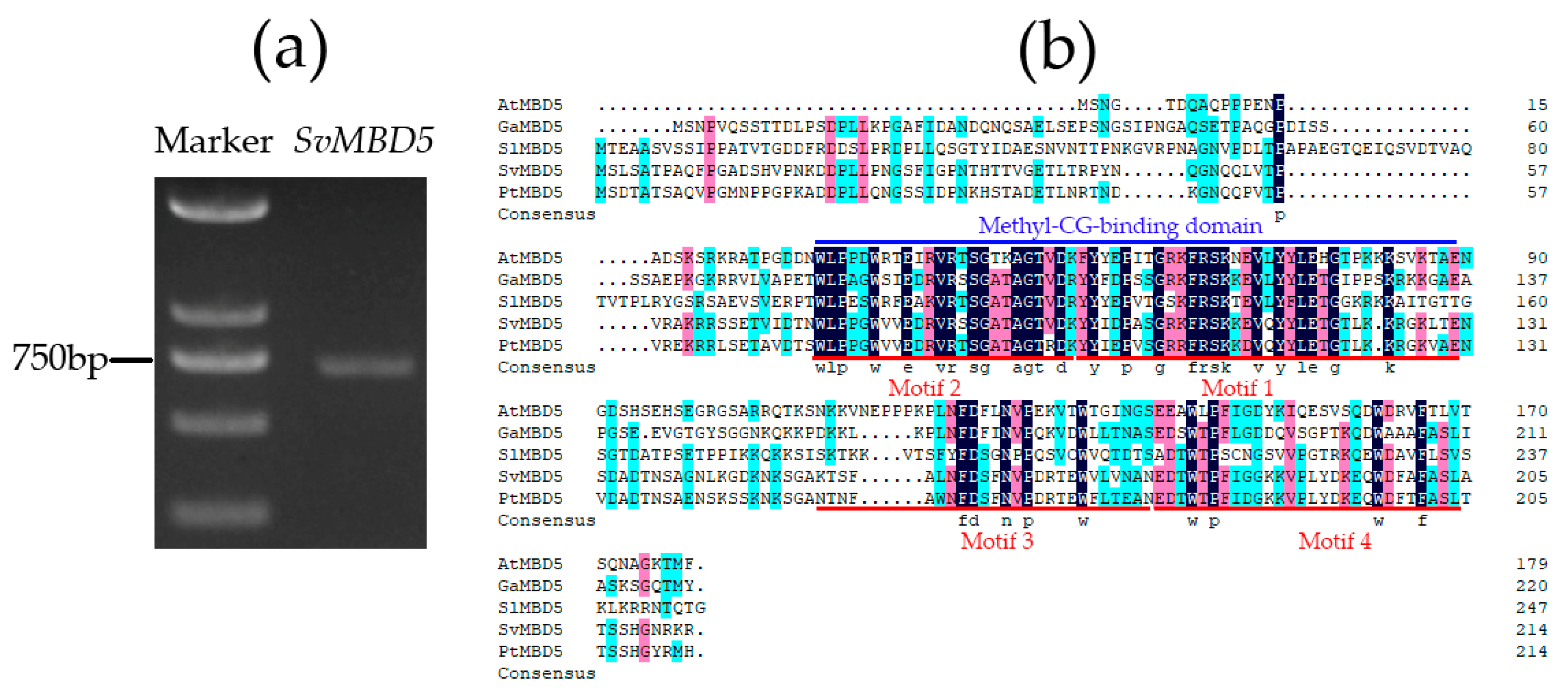
2.2.1. Molecular Cloning of SvMBD5 by Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
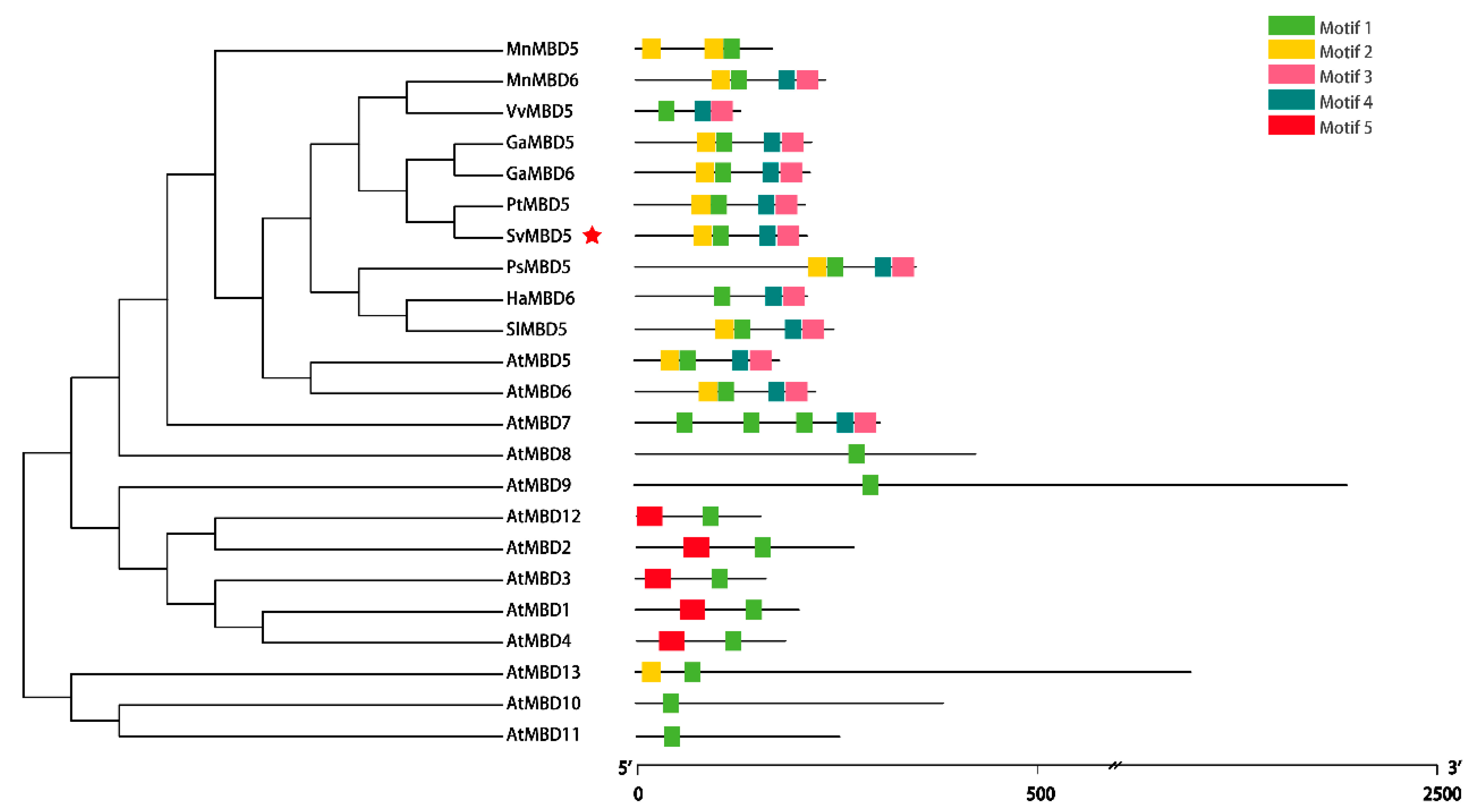
2.2.2. Conserved Motif and Phylogenetic Analyses of SvMBD5
2.2.3. Expression of SvMBD5 at Different Developmental Stages
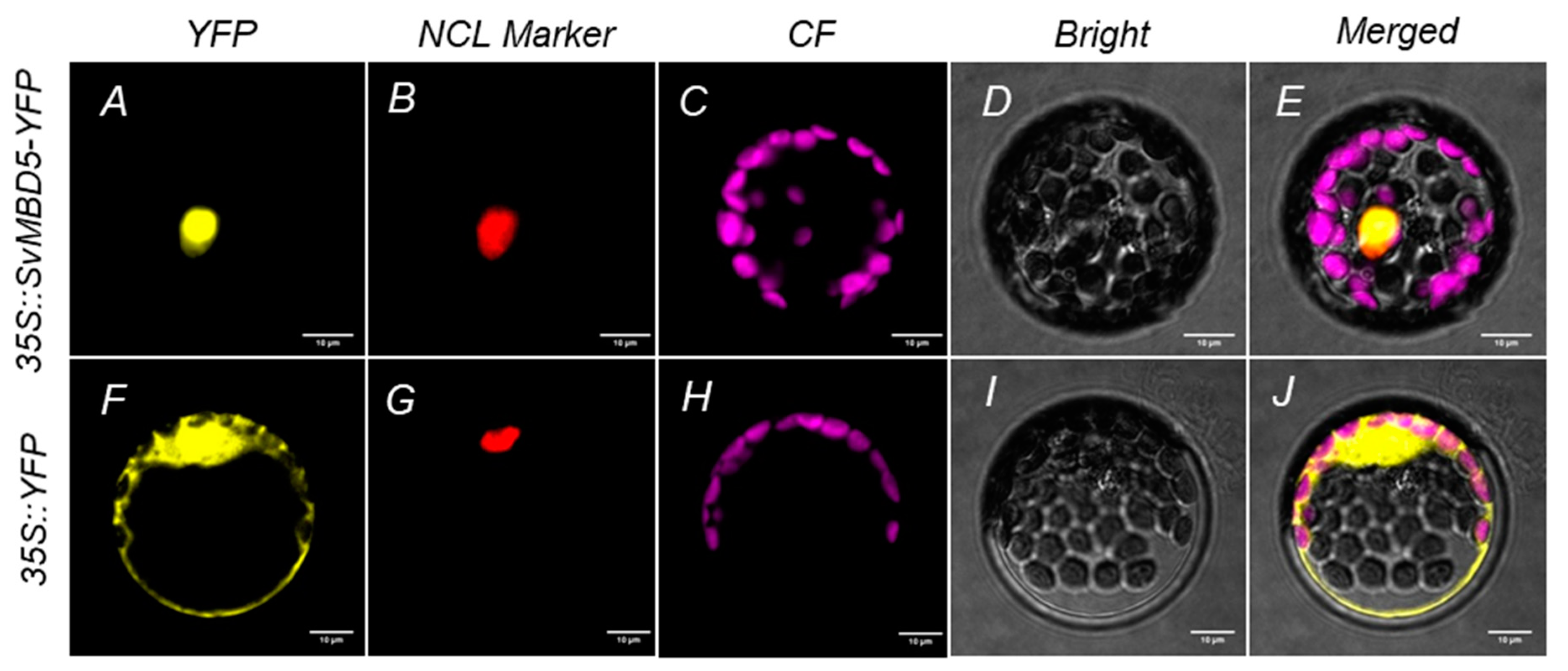
2.2.4. Subcellular Localization of SvMBD5
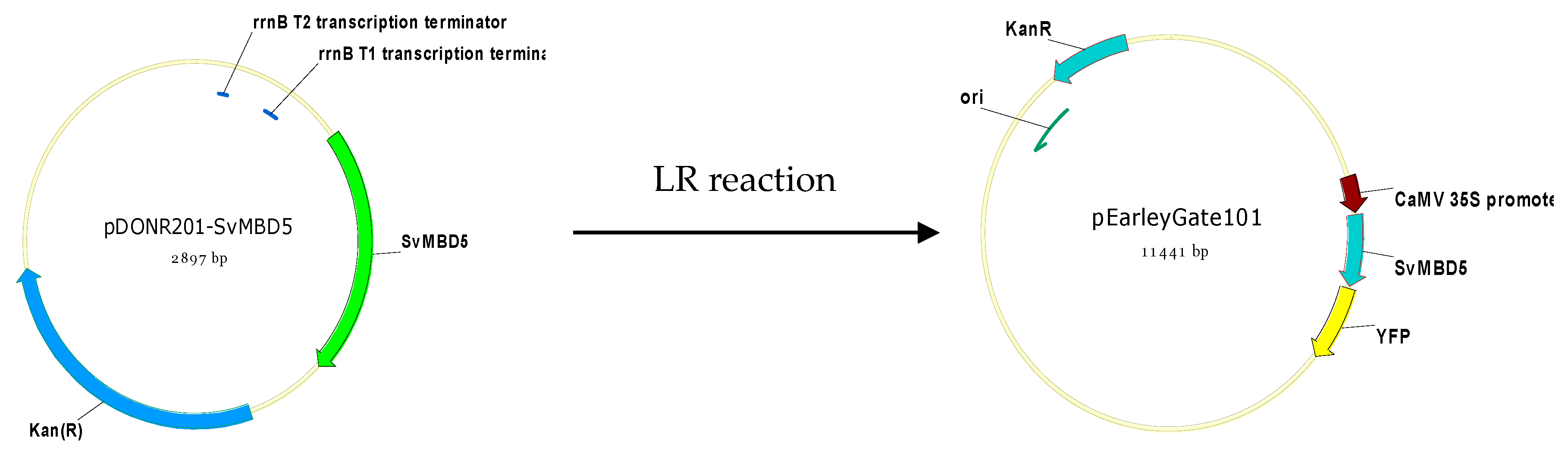
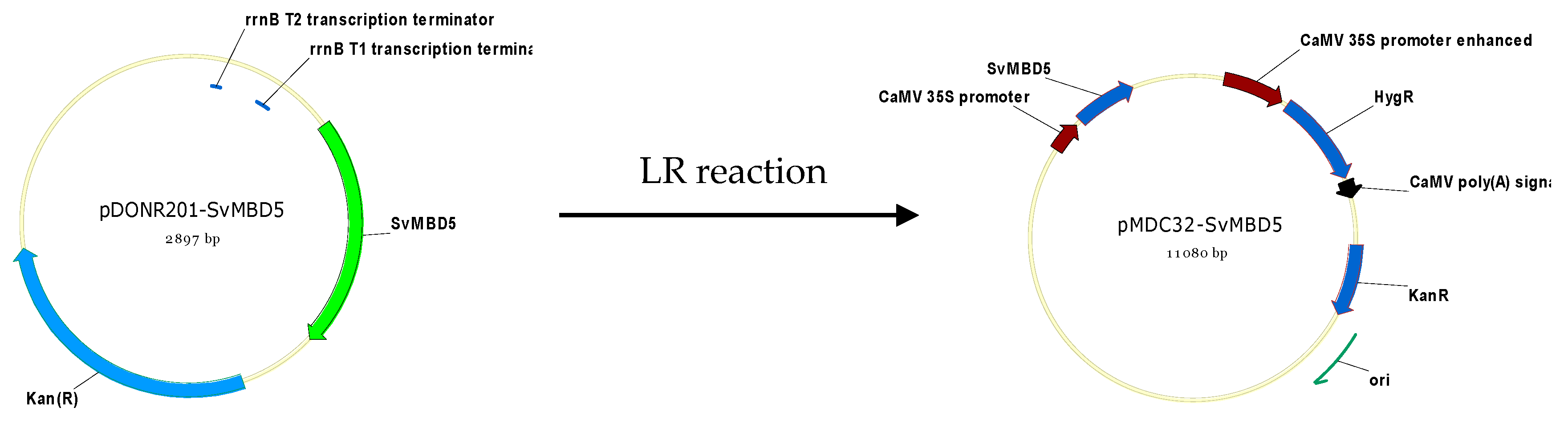
2.2.5. Generation of SvMBD5-Expressing Arabidopsis Lines
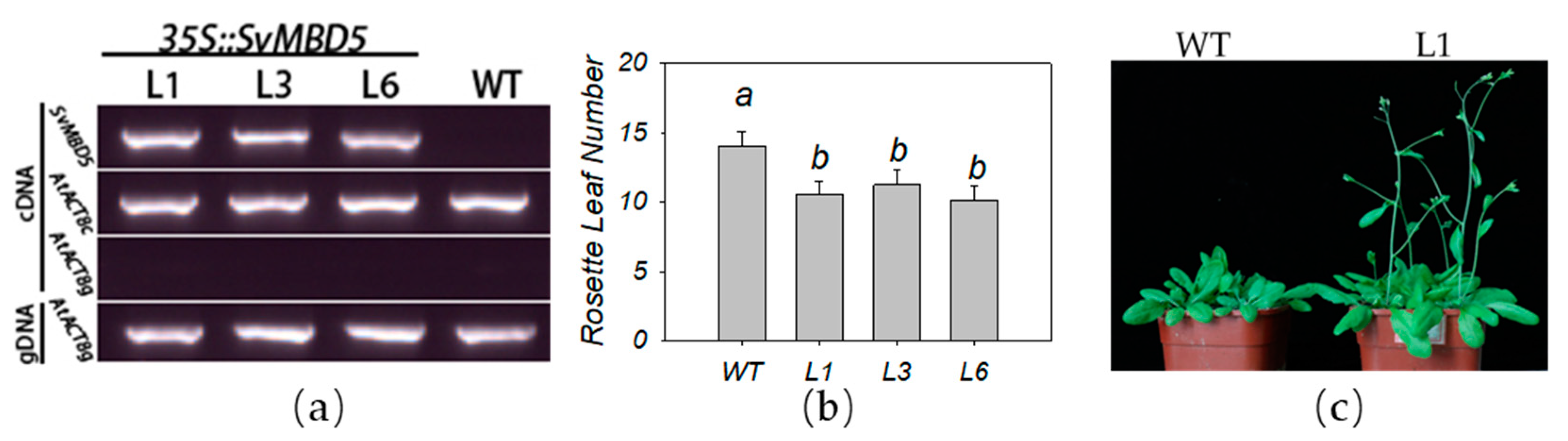
2.2.6. Transgenic Plant Detection and Phenotype Analysis
2.2.7. Semiquantitative RT-PCR and Quantitative RT-PCR
3. Results
3.1. Identification and Sequence Characteristics of SvMBD5
3.2. Expression Analysis of SvMBD5
3.3. Subcellular Localization of SvMBD5 Protein
3.4. Heterologous Expression of SvMBD5 Promotes Flowering in A. thaliana
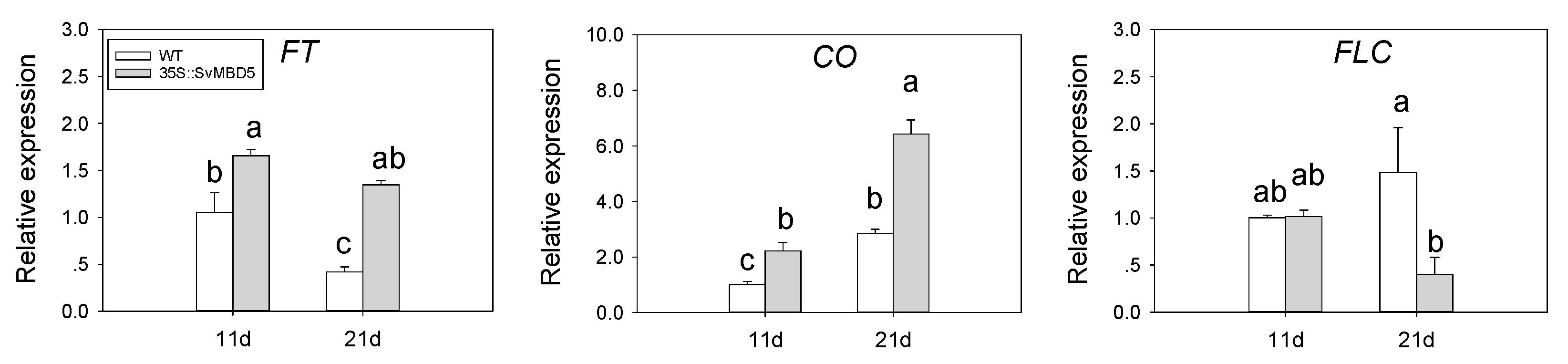
3.5. Expression of Key Flowering-Related Genes in SvMBD5 Transgenic Plants
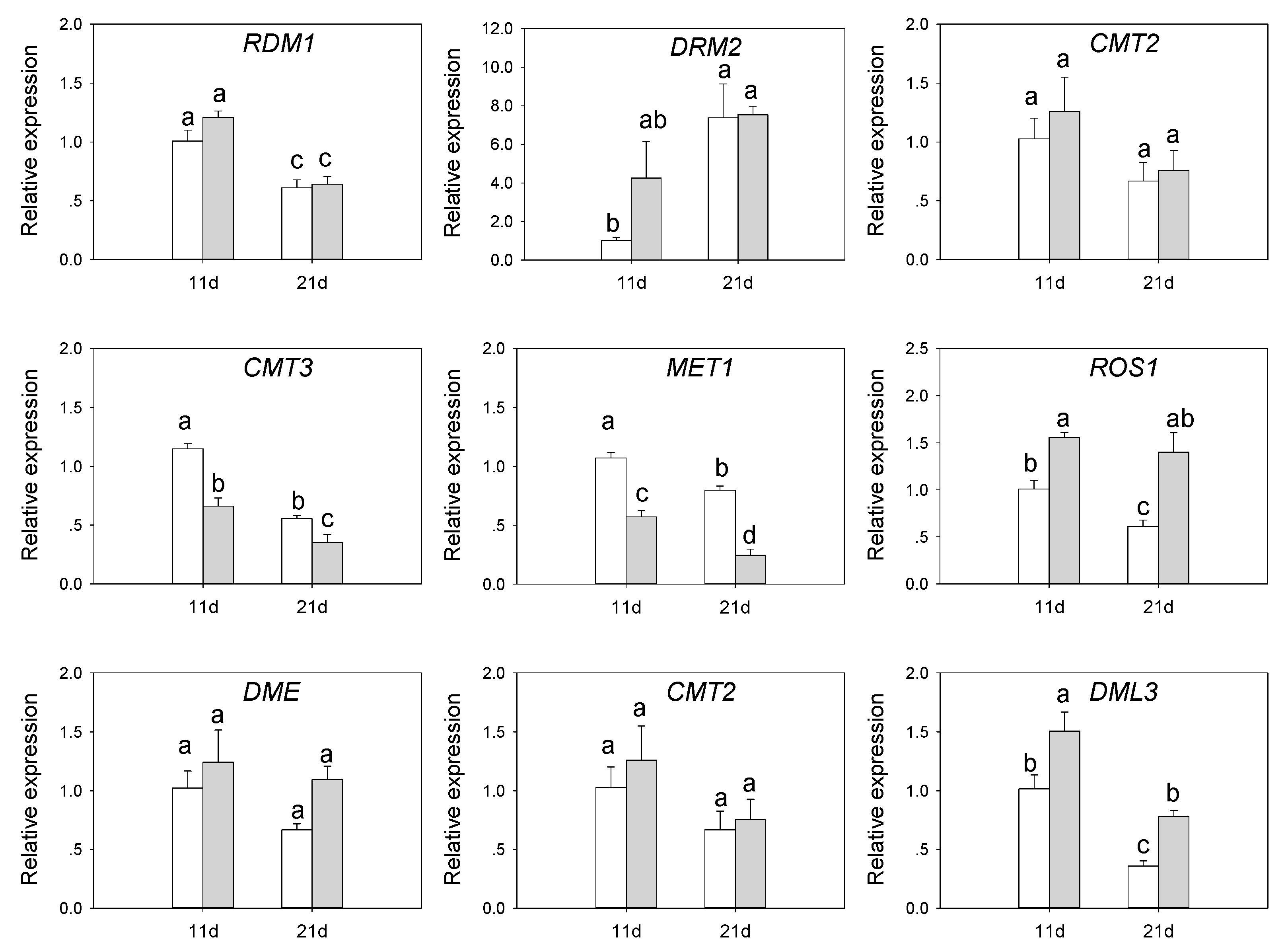
3.6. Expression of DNA Methylation-Related Genes in SvMBD5 Transgenic Plants
4. Discussion
4.1. MBD5 Might Be Involved in Demethylation
4.2. How Does the Heterologous Expression of SvMBD5 Promote Flowering?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- He, X.J.; Chen, T.; Zhu, J.K. Regulation and function of DNA methylation in plants and animals. Cell Res. 2011, 21, 442–465. [Google Scholar] [CrossRef] [PubMed]
- Law, J.A.; Jacobsen, S.E. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 2010, 11, 204–220. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Cokus, S.J.; Zhang, X.; Chen, P.Y.; Bostick, M.; Goll, M.G.; Hetzel, J.; Jain, J.; Strauss, S.H.; Halpern, M.E.; et al. Conservation and divergence of methylation patterning in plants and animals. Proc. Natl. Acad. Sci. USA 2010, 107, 8689–8694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lang, Z.; Zhu, J.-K. Dynamics and function of DNA methylation in plants. Nat. Rev. Mol. Cell Biol. 2018, 19, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Arıkan, B.; Özden, S.; Turgut-Kara, N. DNA methylation related gene expression and morphophysiological response to abiotic stresses in arabidopsis thaliana. Environ. Exp. Bot. 2018, 149, 17–26. [Google Scholar] [CrossRef]
- Wang, X.; Hu, L.; Wang, X.; Li, N.; Xu, C.; Gong, L.; Liu, B. DNA methylation affects gene alternative splicing in plants: An example from rice. Mol. Plant 2016, 9, 305–307. [Google Scholar] [CrossRef]
- Charlesworth, D. Plant sex chromosome evolution. J. Exp. Bot. 2013, 64, 405–420. [Google Scholar] [CrossRef]
- Vongs, A.; Kakutani, T.; Martienssen, R.A.; Richards, E.J. Arabidopsis thaliana DNA methylation mutants. Science 1993, 260, 1926–1928. [Google Scholar] [CrossRef]
- Zhang, X.; Yazaki, J.; Sundaresan, A.; Cokus, S.; Chan, S.W.; Chen, H.; Henderson, I.R.; Shinn, P.; Pellegrini, M.; Jacobsen, S.E.; et al. Genome-wide high-resolution mapping and functional analysis of DNA methylation in arabidopsis. Cell 2006, 126, 1189–1201. [Google Scholar] [CrossRef]
- Movahedi, A.; Sun, W.; Zhang, J.; Wu, X.; Mousavi, M.; Mohammadi, K.; Yin, T.; Zhuge, Q. Rna-directed DNA methylation in plants. Plant Cell Rep. 2015, 34, 1857–1862. [Google Scholar] [CrossRef]
- Matzke, M.A.; Kanno, T.; Matzke, A.J. Rna-directed DNA methylation: The evolution of a complex epigenetic pathway in flowering plants. Annu. Rev. Plant. Biol. 2015, 66, 243–267. [Google Scholar] [CrossRef] [PubMed]
- Stroud, H.; Do, T.; Du, J.; Zhong, X.; Feng, S.; Johnson, L.; Patel, D.J.; Jacobsen, S.E. Non-cg methylation patterns shape the epigenetic landscape in arabidopsis. Nat. Struct. Mol. Biol. 2014, 21, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Zemach, A.; Kim, M.Y.; Silva, P.; Rodrigues, J.A.; Dotson, B.; Brooks, M.D.; Zilberman, D. Local DNA hypomethylation activates genes in rice endosperm. Proc. Natl. Acad. Sci. USA 2010, 107, 18729–18734. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.M.; Du, J.; Hale, C.J.; Bischof, S.; Feng, S.; Chodavarapu, R.K.; Zhong, X.; Marson, G.; Pellegrini, M.; Segal, D.J.; et al. Sra- and set-domain-containing proteins link rna polymerase v occupancy to DNA methylation. Nature 2014, 507, 124–128. [Google Scholar] [CrossRef]
- Huettel, B.; Kanno, T.; Daxinger, L.; Aufsatz, W.; Matzke, A.J.; Matzke, M. Endogenous targets of rna-directed DNA methylation and pol iv in arabidopsis. EMBO J. 2006, 25, 2828–2836. [Google Scholar] [CrossRef]
- Zemach, A.; Kim, M.Y.; Hsieh, P.H.; Coleman-Derr, D.; Eshed-Williams, L.; Thao, K.; Harmer, S.L.; Zilberman, D. The arabidopsis nucleosome remodeler ddm1 allows DNA methyltransferases to access h1-containing heterochromatin. Cell 2013, 153, 193–205. [Google Scholar] [CrossRef]
- Gong, Z.; Morales-Ruiz, T.; Ariza, R.R.; Roldán-Arjona, T.; David, L.; Zhu, J.K. Ros1, a repressor of transcriptional gene silencing in arabidopsis, encodes a DNA glycosylase/lyase. Cell 2002, 111, 803–814. [Google Scholar] [CrossRef]
- Mary, G.; Hoe, H.J.; Tzung-Fu, H.; Jon, P.; Yeonhee, C.; Harada, J.J.; Goldberg, R.B.; Fischer, R.L. Demeter DNA glycosylase establishes medea polycomb gene self-imprinting by allele-specific demethylation. Cell 2006, 124, 495–506. [Google Scholar]
- Ortega-Galisteo, A.P.; Morales-Ruiz, T.; Ariza, R.R.; Roldán-Arjona, T. Arabidopsis demeter-like proteins dml2 and dml3 are required for appropriate distribution of DNA methylation marks. Plant. Mol. Biol. 2008, 67, 671–681. [Google Scholar] [CrossRef]
- Fernanda, A.; Avnish, K.; Jian-Kang, Z. Role of the arabidopsis DNA glycosylase/lyase ros1 in active DNA demethylation. Proc. Natl. Acad. Sci. USA 2006, 103, 11796–11801. [Google Scholar]
- Teresa, M.R.; Ana Pilar, O.G.; María Isabel, P.M.; María Isabel, M.M.; Ariza, R.R.; Teresa, R.A. Demeter and repressor of silencing 1 encode 5-methylcytosine DNA glycosylases. Proc. Natl. Acad. Sci. USA 2006, 103, 6853–6858. [Google Scholar]
- Jianhua, Z.; Avnish, K.; Sridhar, V.V.; Fernanda, A.; Jian-Kang, Z. The DNA glycosylase/lyase ros1 functions in pruning DNA methylation patterns in arabidopsis. Curr. Biol. 2007, 17, 54–59. [Google Scholar]
- Hohn, T.; Corsten, S.; Rieke, S.; Muller, M.; Rothnie, H. Methylation of coding region alone inhibits gene expression in plant protoplasts. Proc. Natl. Acad. Sci. USA 1996, 93, 8334–8339. [Google Scholar] [CrossRef] [PubMed]
- Zilberman, D.; Gehring, M.; Tran, R.K.; Ballinger, T.; Henikoff, S. Genome-wide analysis of arabidopsis thaliana DNA methylation uncovers an interdependence between methylation and transcription. Nat. Genet. 2007, 39, 61–69. [Google Scholar] [PubMed]
- Zemach, A.; Grafi, G. Characterization of arabidopsis thaliana methyl-cpg-binding domain (mbd) proteins. Plant J. 2003, 34, 565–572. [Google Scholar] [CrossRef]
- Mikako, I.; Akiko, K.; Nozomu, K.; Hiroshi, S. Methylated DNA-binding proteins from arabidopsis. Plant. Physiol. 2003, 133, 1747–1754. [Google Scholar]
- Zemach, A.; Grafi, G. Methyl-cpg-binding domain proteins in plants: Interpreters of DNA methylation. Trends Plant. Sci. 2007, 12, 80–85. [Google Scholar] [CrossRef]
- Springer, N.M.; Kaeppler, S.M. Evolutionary divergence of monocot and dicot methyl-cpg-binding domain proteins. Plant. Physiol. 2005, 138, 92–104. [Google Scholar] [CrossRef]
- Grafi, G.; Zemach, A.; Pitto, L. Methyl-cpg-binding domain (mbd) proteins in plants. Biochim. Biophys. Acta 2007, 1769, 287–294. [Google Scholar] [CrossRef]
- Berg, A. Ten members of the arabidopsis gene family encoding methyl-cpg-binding domain proteins are transcriptionally active and at least one, atmbd11, is crucial for normal development. Nucleic Acids Res. 2003, 31, 5291–5304. [Google Scholar] [CrossRef]
- Wang, C.; Dong, X.; Jin, D.; Zhao, Y.; Xie, S.; Li, X.; He, X.; Lang, Z.; Lai, J.; Zhu, J.K.; et al. Methyl-cpg-binding domain protein mbd7 is required for active DNA demethylation in arabidopsis. Plant. Physiol. 2015, 167, 905–914. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parida, A.P.; Sharma, A.; Sharma, A.K. Atmbd6, a methyl cpg binding domain protein, maintains gene silencing in arabidopsis by interacting with rna binding proteins. J. Biosci. 2017, 42, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Deng, H.; Miao, M.; Li, H.; Huang, S.; Wang, S.; Liu, Y. Tomato mbd5, a methyl cpg binding domain protein, physically interacting with uv-damaged DNA binding protein-1, functions in multiple processes. New Phytol. 2016, 210, 208–226. [Google Scholar] [CrossRef] [PubMed]
- Stangeland, B.; Rosenhave, E.M.; Winge, P.; Berg, A.; Amundsen, S.S.; Karabeg, M.; Mandal, A.; Bones, A.M.; Grini, P.E.; Aalen, R.B. Atmbd8 is involved in control of flowering time in the c24 ecotype of arabidopsis thaliana. Physiol. Plant. 2009, 136, 110–126. [Google Scholar] [CrossRef]
- Peng, M.; Cui, Y.; Bi, Y.M.; Rothstein, S.J. Atmbd9: A protein with a methyl-cpg-binding domain regulates flowering time and shoot branching in arabidopsis. Plant J. 2006, 46, 282–296. [Google Scholar] [CrossRef]
- Yaish, M.W.; Peng, M.; Rothstein, S.J. Atmbd9 modulates arabidopsis development through the dual epigenetic pathways of DNA methylation and histone acetylation. Plant J. 2010, 59, 123–135. [Google Scholar] [CrossRef]
- Bailey, T.L.; Williams, N.; Misleh, C.; Li, W.W. Meme: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006, 34, W369–W373. [Google Scholar] [CrossRef]
- Liu, Q.; Dang, H.; Chen, Z.; Wu, J.; Chen, Y.; Chen, S.; Luo, L. Genome-wide identification, expression, and functional analysis of the sugar transporter gene family in cassava (Manihot esculenta). Int. J. Mol. Sci. 2018, 19, 987. [Google Scholar] [CrossRef]
- Miao, Y.; Jiang, L. Transient expression of fluorescent fusion proteins in protoplasts of suspension cultured cells. Nat. Protoc. 2007, 2, 2348–2353. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for agrobacterium-mediated transformation of arabidopsis thaliana. Plant J. 2010, 16, 735–743. [Google Scholar] [CrossRef]
- Wang, H.; Li, K.; Sun, X.; Xie, Y.; Han, X. Isolation and characterization of larch baby boom2 and its regulation of adventitious root development. Gene 2019, 30, 90–98. [Google Scholar] [CrossRef]
- Song, Y.H.; Smith, R.W.; To, B.J.; Millar, A.J.; Imaizumi, T. Fkf1 conveys timing information for constans stabilization in photoperiodic flowering. Science 2012, 336, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Kankel, M.W.; Ramsey, D.E.; Stokes, T.L.; Flowers, S.K.; Haag, J.R.; Jeddeloh, J.A.; Riddle, N.C.; Verbsky, M.L.; Richards, E.J. Arabidopsis met1 cytosine methyltransferase mutants. Genetics 2003, 163, 1109–1122. [Google Scholar] [PubMed]
- Lindroth, A.M.; Cao, X.; Jackson, J.P.; Zilberman, D.; McCallum, C.M.; Henikoff, S.; Jacobsen, S.E. Requirement of chromomethylase3 for maintenance of cpxpg methylation. Science 2001, 292, 2077–2080. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Lang, Z.; Zhang, H.; Zhu, J.-K. The DNA demethylase ros1 targets genomic regions with distinct chromatin modifications. Nat. Plants 2016, 2, 16169. [Google Scholar] [CrossRef]
- Ruiz-García, L.; Cervera, M.T.; Martínez-Zapater, J.M. DNA methylation increases throughout arabidopsis development. Planta 2005, 222, 301–306. [Google Scholar] [CrossRef]
- Yang, H.; Chang, F.; You, C.; Cui, J.; Zhu, G.; Wang, L.; Zheng, Y.; Qi, J.; Ma, H. Whole-genome DNA methylation patterns and complex associations with gene structure and expression during flower development in arabidopsis. Plant J. 2015, 81, 268–281. [Google Scholar] [CrossRef]
- Kelly, J.; Vining, K.R.P.; Wihelm, L.J.; Priest, H.D.; Pellegrini, M.; Mockler, T.C. Michael Freitag and Steven H Strauss. Dynamic DNA cytosine methylation in the Populus trichocarpa genome: Tissue-level variation and relationship to gene expression. BMC Genom. 2012, 13, 19. [Google Scholar]
- Cheng, Y.-H.; Peng, X.-Y.; Yu, Y.-C.; Sun, Z.-Y.; Han, L. The effects of DNA methylation inhibition on flower development in the dioecious plant salix viminalis. Forests 2019, 10, 173. [Google Scholar] [CrossRef]
- Jean Finnegan, E.; Kovac, K.A.; Jaligot, E.; Sheldon, C.C.; James Peacock, W.; Dennis, E.S. The downregulation of flowering locus c (flc) expression in plants with low levels of DNA methylation and by vernalization occurs by distinct mechanisms. Plant J. 2005, 44, 420–432. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, T.; Stelly, D.M.; Chen, Z.J. Epigenomic and functional analyses reveal roles of epialleles in the loss of photoperiod sensitivity during domestication of allotetraploid cottons. Genome Biol. 2017, 18, 99. [Google Scholar] [CrossRef] [PubMed]

| Gene | Left Primer (5’→3’) | Right Primer (5’→3’) | |
|---|---|---|---|
| RT-PCR | AtACT8c | CTGGAAAGTGCTGAGGGAAG | GGACTCTGGTGATGGTGTGT |
| AtACT8g | ACCACCAATCCAGACACTGT | TCCACATGCTATCCTCCGTC | |
| SvMBD5 | TCAACGCTTTCTGTTACCAT | ATGTCATTGTCAGCAACTCC | |
| qRT-PCR | SvMBD5 | CTGTCCATCCACCTTCCCTT | TCCCGAGTCTGAAGGAGAGA |
| SvACT | AGAGGACTTCAGGACAACGG | TTTCACAACCACAGCAGAGC | |
| ACTIN8 | CTCCAGCGAATCCAGCCTTA | GCCGATGCTGATGACATTCA | |
| CMT2 | CCGAGCATACATTGTCGAAATT | CGCTTAATTGTAATTCGCCTGA | |
| CMT3 | TTCCCTCTTCCAACTTGACTAC | GATTGAGAAAGGATGAAGCGTC | |
| CO | CCATGGATGAAATGTATGCGTT | GGAGATAGAGTTGTTCCGCTTA | |
| DME | CAGCAGTTCTATCTCTCGGTAG | ACTCCTCTGAAGAATGCCTTAC | |
| DML2 | AGTTCTGATTGCTACGTGAGAA | CTAGCATAAACCCTATCGACGT | |
| DML3 | CTTGGGGAAGTTTACACAATCG | GTTGACACAAATGTTGGTCGTA | |
| DRM2 | GATGAAACACACTTCTCCACAC | ACATCAAAGGTGTAGGAGAAGG | |
| FLC | GGAGATTTGTCCAGCAGGTGA | GCCAAGAAGACCGAACTCATG | |
| FT | TGACAATTGTAGAAAACTGCGG | CTACAACTGGAACAACCTTTGG | |
| MET1 | CGACAATCATAATCCGCCAATT | CTGATGTTGAAGATCGTCCAAC | |
| RDM1 | ATACATCTCTGCTCTTCTCAGC | CAATGACAATGGAACTACGACC | |
| ROS1 | TAACAGGAGTTACAGGCACAAT | TGAACTTTGGAAACGACGTAAC |
| Motif Name | Sequence | Length (aa) | Annotation |
|---|---|---|---|
| Motif 1 |  | 21 | _ |
| Motif 2 |  | 24 | Methyl-CpG-binding domain (PF01429) |
| Motif 3 |  | 25 | _ |
| Motif 4 |  | 28 | _ |
| Motif 5 |  | 33 | _ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Cheng, L.; Cao, Q.; Zou, J.; Li, X.; Ma, X.; Zhou, J.; Zhai, F.; Sun, Z.; Lan, Y.; et al. Heterologous Expression of SvMBD5 from Salix viminalis L. Promotes Flowering in Arabidopsis thaliana L. Genes 2020, 11, 285. https://doi.org/10.3390/genes11030285
Cheng Y, Cheng L, Cao Q, Zou J, Li X, Ma X, Zhou J, Zhai F, Sun Z, Lan Y, et al. Heterologous Expression of SvMBD5 from Salix viminalis L. Promotes Flowering in Arabidopsis thaliana L. Genes. 2020; 11(3):285. https://doi.org/10.3390/genes11030285
Chicago/Turabian StyleCheng, Yunhe, Lili Cheng, Qingchang Cao, Junzhu Zou, Xia Li, Xiaodong Ma, Jingjing Zhou, Feifei Zhai, Zhenyuan Sun, Yanping Lan, and et al. 2020. "Heterologous Expression of SvMBD5 from Salix viminalis L. Promotes Flowering in Arabidopsis thaliana L." Genes 11, no. 3: 285. https://doi.org/10.3390/genes11030285
APA StyleCheng, Y., Cheng, L., Cao, Q., Zou, J., Li, X., Ma, X., Zhou, J., Zhai, F., Sun, Z., Lan, Y., & Han, L. (2020). Heterologous Expression of SvMBD5 from Salix viminalis L. Promotes Flowering in Arabidopsis thaliana L. Genes, 11(3), 285. https://doi.org/10.3390/genes11030285





