Differential Dorsolateral Prefrontal Cortex Proteomic Profiles of Suicide Victims with Mood Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Tissue Collection
2.3. Sample Preparation: Tissue lysis
2.4. SDS-PAGE
2.5. Trypsin Digest
2.6. Label-Free Proteomics Acquisition: Nano-Flow Liquid Chromatography–Electrospray Tandem Mass Spectrometry (nanoLC–ESI–MS/MS)
2.7. Data Analysis
2.8. Pathway Analysis
2.9. Western Blots
3. Results
3.1. Demographics
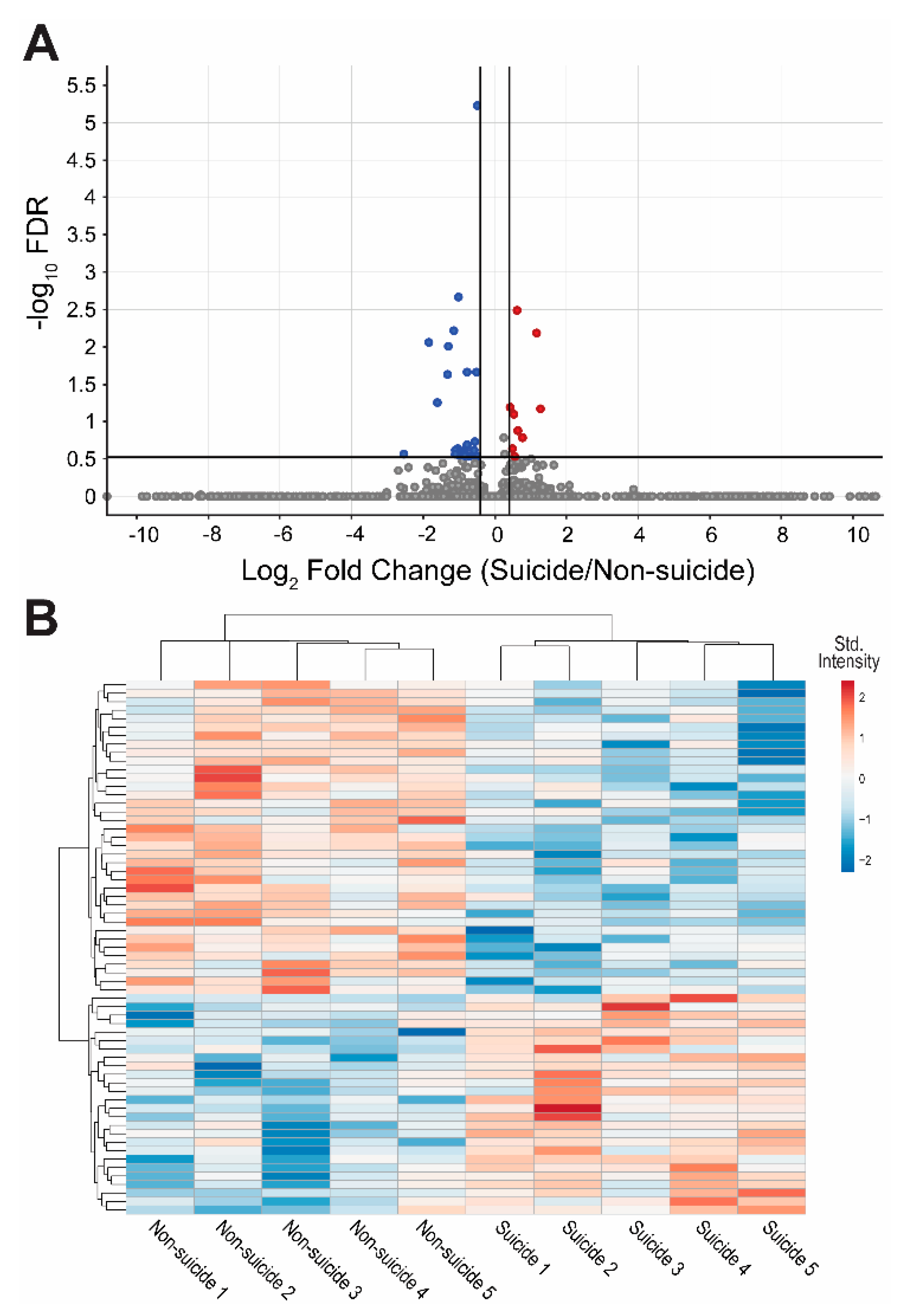
3.2. Proteomics Analysis
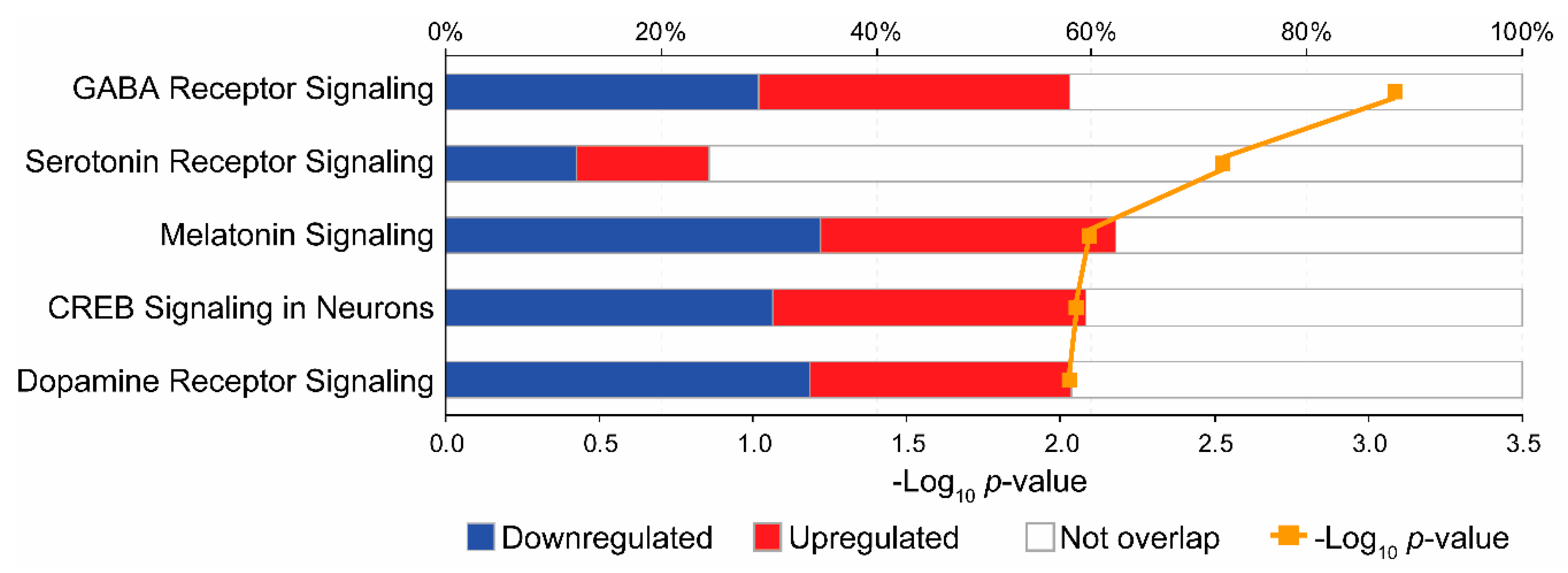
3.3. Pathway Analysis
3.4. Validation of Top Protein Changes in DLPFC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hedegaard, H.; Curtin, S.C.; Warner, M. Suicide Mortality in the United States, 1999–2017. NCHS Data Brief 2018, 330, 1–8. Available online: https://www.cdc.gov/nchs/products/databriefs/db330.htm (accessed on 23 February 2020).
- World Health Organization. Preventing Suicide: A Global Imperative; World Health Organization: Geneva, Switzerland, 2014; Volume 7. [Google Scholar]
- Van Heeringen, K.; Mann, J.J. The neurobiology of suicide. Lancet. Psychiatry 2014, 1, 63–72. [Google Scholar] [CrossRef]
- Benedetti, F.; Radaelli, D.; Poletti, S.; Locatelli, C.; Falini, A.; Colombo, C.; Smeraldi, E. Opposite effects of suicidality and lithium on gray matter volumes in bipolar depression. J. Affect. Disord. 2011, 135, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.; Schultz, C.C.; Koch, K.; Schachtzabel, C.; Sauer, H.; Schlosser, R.G. Prefrontal cortical thickness in depressed patients with high-risk for suicidal behavior. J. Psychiatr. Res. 2012, 46, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Rizk, M.M.; Rubin-Falcone, H.; Lin, X.; Keilp, J.G.; Miller, J.M.; Milak, M.S.; Sublette, M.E.; Oquendo, M.A.; Ogden, R.T.; Abdelfadeel, N.A.; et al. Gray matter volumetric study of major depression and suicidal behavior. Psychiatry Res. Neuroimaging 2018, 283, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.D.; Kassir, S.A.; Bakalian, M.J.; Galfalvy, H.; Mann, J.J.; Arango, V. Neuron density and serotonin receptor binding in prefrontal cortex in suicide. Int. J. Neuropsychopharmacol. 2012, 15, 435–447. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kekesi, K.A.; Juhasz, G.; Simor, A.; Gulyassy, P.; Szego, E.M.; Hunyadi-Gulyas, E.; Darula, Z.; Medzihradszky, K.F.; Palkovits, M.; Penke, B.; et al. Altered functional protein networks in the prefrontal cortex and amygdala of victims of suicide. PLoS ONE 2012, 7, e50532. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Verwer, R.W.H.; van Wamelen, D.J.; Qi, X.R.; Gao, S.F.; Lucassen, P.J.; Swaab, D.F. Prefrontal changes in the glutamate-glutamine cycle and neuronal/glial glutamate transporters in depression with and without suicide. J. Psychiatr. Res. 2016, 82, 8–15. [Google Scholar] [CrossRef]
- Mann, J.J.; Currier, D.M. Stress, genetics and epigenetic effects on the neurobiology of suicidal behavior and depression. Eur. Psychiatry J. Assoc. Eur. Psychiatr. 2010, 25, 268–271. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Sanchez, J.C.; Gooley, A.A.; Appel, R.D.; Humphery-Smith, I.; Hochstrasser, D.F.; Williams, K.L. Progress with proteome projects: Why all proteins expressed by a genome should be identified and how to do it. Biotechnol. Genet. Eng. Rev. 1996, 13, 19–50. [Google Scholar] [CrossRef]
- Martins-de-Souza, D.; Harris, L.W.; Guest, P.C.; Turck, C.W.; Bahn, S. The role of proteomics in depression research. Eur. Arch. Psychiatry Clin. Neurosci. 2010, 260, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Turecki, G. The molecular bases of the suicidal brain. Nat. Rev. Neurosci. 2014, 15, 802–816. [Google Scholar] [CrossRef] [PubMed]
- Frye, M.A.; Nassan, M.; Jenkins, G.D.; Kung, S.; Veldic, M.; Palmer, B.A.; Feeder, S.E.; Tye, S.J.; Choi, D.S.; Biernacka, J.M. Feasibility of investigating differential proteomic expression in depression: Implications for biomarker development in mood disorders. Transl. Psychiatry 2015, 5, e689. [Google Scholar] [CrossRef] [PubMed]
- Burger, B.; Hernandez Sanchez, L.F.; Lereim, R.R.; Barsnes, H.; Vaudel, M. Analyzing the Structure of Pathways and Its Influence on the Interpretation of Biomedical Proteomics Data Sets. J. Proteome Res. 2018, 17, 3801–3809. [Google Scholar] [CrossRef] [PubMed]
- Nussbeck, S.; Wemheuer, W.; Beier, K. Why brain banking should be regarded as a special type of biobanking: Ethical, practical, and data-management challenges. J. Biorepository Sci. Appl. Med. 2015, 3, 3–14. [Google Scholar]
- Ho, A.M.; Winham, S.J.; Armasu, S.M.; Blacker, C.J.; Millischer, V.; Lavebratt, C.; Overholser, J.C.; Jurjus, G.J.; Dieter, L.; Mahajan, G.; et al. Genome-wide DNA methylomic differences between dorsolateral prefrontal and temporal pole cortices of bipolar disorder. J. Psychiatr. Res. 2019, 117, 45–54. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- Cox, J.; Neuhauser, N.; Michalski, A.; Scheltema, R.A.; Olsen, J.V.; Mann, M. Andromeda: A peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 2011, 10, 1794–1805. [Google Scholar] [CrossRef]
- Consortium, T.U. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2018, 47, D506–D515. [Google Scholar] [CrossRef]
- Martins-de-Souza, D.; Guest, P.C.; Harris, L.W.; Vanattou-Saifoudine, N.; Webster, M.J.; Rahmoune, H.; Bahn, S. Identification of proteomic signatures associated with depression and psychotic depression in post-mortem brains from major depression patients. Transl. Psychiatry 2012, 2, e87. [Google Scholar] [CrossRef]
- Oliveros, A.; Starski, P.; Lindberg, D.; Choi, S.; Heppelmann, C.J.; Dasari, S.; Choi, D.S. Label-Free Neuroproteomics of the Hippocampal-Accumbal Circuit Reveals Deficits in Neurotransmitter and Neuropeptide Signaling in Mice Lacking Ethanol-Sensitive Adenosine Transporter. J. Proteome Res. 2017, 16, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Pinacho, R.; Villalmanzo, N.; Meana, J.J.; Ferrer, I.; Berengueras, A.; Haro, J.M.; Villen, J.; Ramos, B. Altered CSNK1E, FABP4 and NEFH protein levels in the dorsolateral prefrontal cortex in schizophrenia. Schizophr. Res. 2016, 177, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.S.; Pan, Z.; Shi, W.; Brown, B.S.; Wymore, R.S.; Cohen, I.S.; Dixon, J.E.; McKinnon, D. KCNQ2 and KCNQ3 potassium channel subunits: Molecular correlates of the M-channel. Science 1998, 282, 1890–1893. [Google Scholar] [CrossRef] [PubMed]
- Manville, R.W.; Papanikolaou, M.; Abbott, G.W. Direct neurotransmitter activation of voltage-gated potassium channels. Nat. Commun 2018, 9, 1847. [Google Scholar] [CrossRef]
- Okada, M.; Zhu, G.; Hirose, S.; Ito, K.I.; Murakami, T.; Wakui, M.; Kaneko, S. Age-dependent modulation of hippocampal excitability by KCNQ-channels. Epilepsy Res. 2003, 53, 81–94. [Google Scholar] [CrossRef]
- Ogdie, M.N.; Fisher, S.E.; Yang, M.; Ishii, J.; Francks, C.; Loo, S.K.; Cantor, R.M.; McCracken, J.T.; McGough, J.J.; Smalley, S.L.; et al. Attention deficit hyperactivity disorder: Fine mapping supports linkage to 5p13, 6q12, 16p13, and 17p11. Am. J. Hum. Genet. 2004, 75, 661–668. [Google Scholar] [CrossRef][Green Version]
- Martinez, M.; Goldin, L.R.; Cao, Q.; Zhang, J.; Sanders, A.R.; Nancarrow, D.J.; Taylor, J.M.; Levinson, D.F.; Kirby, A.; Crowe, R.R.; et al. Follow-up study on a susceptibility locus for schizophrenia on chromosome 6q. Am. J. Med. Genet. 1999, 88, 337–343. [Google Scholar] [CrossRef]
- Dick, D.M.; Foroud, T.; Flury, L.; Bowman, E.S.; Miller, M.J.; Rau, N.L.; Moe, P.R.; Samavedy, N.; El-Mallakh, R.; Manji, H.; et al. Genomewide linkage analyses of bipolar disorder: A new sample of 250 pedigrees from the National Institute of Mental Health Genetics Initiative. Am. J. Hum. Genet. 2003, 73, 107–114. [Google Scholar] [CrossRef]
- Gargus, J.J. Ion channel functional candidate genes in multigenic neuropsychiatric disease. Biol. Psychiatry 2006, 60, 177–185. [Google Scholar] [CrossRef]
- Mooney, D.M.; Zhang, L.; Basile, C.; Senatorov, V.V.; Ngsee, J.; Omar, A.; Hu, B. Distinct forms of cholinergic modulation in parallel thalamic sensory pathways. Proc. Natl. Acad. Sci. USA 2004, 101, 320–324. [Google Scholar] [CrossRef]
- Krishnan, V.; Han, M.H.; Graham, D.L.; Berton, O.; Renthal, W.; Russo, S.J.; Laplant, Q.; Graham, A.; Lutter, M.; Lagace, D.C.; et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 2007, 131, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.K.; Juarez, B.; Ku, S.M.; Zhang, H.; Calizo, R.C.; Walsh, J.J.; Chaudhury, D.; Zhang, S.; Hawkins, A.; Dietz, D.M.; et al. KCNQ channel openers reverse depressive symptoms via an active resilience mechanism. Nat. Commun 2016, 7, 11671. [Google Scholar] [CrossRef] [PubMed]
- Stafstrom, C.E.; Grippon, S.; Kirkpatrick, P. Ezogabine (retigabine). Nat. Rev. Drug Discov. 2011, 10, 729–730. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Bartolomé-Martín, D.; Rotem, N.; Rozas, C.; Dellal, S.S.; Chacon, M.A.; Kadriu, B.; Gulinello, M.; Khodakhah, K.; Faber, D.S. Rescue of homeostatic regulation of striatal excitability and locomotor activity in a mouse model of Huntington’s disease. Proc. Natl. Acad. Sci. USA 2015, 112, 2239–2244. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Costi, S.; Morris, L.S.; Van Dam, N.T.; Kautz, M.; Whitton, A.E.; Friedman, A.K.; Collins, K.A.; Ahle, G.; Chadha, N.; et al. Effects of the KCNQ channel opener ezogabine on functional connectivity of the ventral striatum and clinical symptoms in patients with major depressive disorder. Mol. Psychiatry 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.Y.; Wang, X.; Xu, M.; Maheshwari, A.; Curry, D.; Lam, S.; Adesina, A.M.; Noebels, J.L.; Sun, Q.Q.; Cooper, E.C. Ankyrin-G isoform imbalance and interneuronopathy link epilepsy and bipolar disorder. Mol. Psychiatry 2017, 22, 1464–1472. [Google Scholar] [CrossRef]
- Judy, J.T.; Seifuddin, F.; Pirooznia, M.; Mahon, P.B.; Jancic, D.; Goes, F.S.; Schulze, T.; Cichon, S.; Noethen, M.; Rietschel, M.; et al. Converging Evidence for Epistasis between ANK3 and Potassium Channel Gene KCNQ2 in Bipolar Disorder. Front. Genet. 2013, 4, 87. [Google Scholar] [CrossRef][Green Version]
- Judy, J.T.; Zandi, P.P. A review of potassium channels in bipolar disorder. Front. Genet. 2013, 4, 105. [Google Scholar] [CrossRef][Green Version]
- Borsotto, M.; Cavarec, L.; Bouillot, M.; Romey, G.; Macciardi, F.; Delaye, A.; Nasroune, M.; Bastucci, M.; Sambucy, J.L.; Luan, J.J.; et al. PP2A-Bgamma subunit and KCNQ2 K+ channels in bipolar disorder. Pharm. J. 2007, 7, 123–132. [Google Scholar] [CrossRef]
- Kaminsky, Z.; Jones, I.; Verma, R.; Saleh, L.; Trivedi, H.; Guintivano, J.; Akman, R.; Zandi, P.; Lee, R.S.; Potash, J.B. DNA methylation and expression of KCNQ3 in bipolar disorder. Bipolar Disord. 2015, 17, 150–159. [Google Scholar] [CrossRef]
- Manville, R.W.; Abbott, G.W. Gabapentin Is a Potent Activator of KCNQ3 and KCNQ5 Potassium Channels. Mol. Pharmacol. 2018, 94, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Leith, W.M.; Lambert, W.E.; Boehnlein, J.K.; Freeman, M.D. The association between gabapentin and suicidality in bipolar patients. Int. Clin. Psychopharmacol. 2019, 34, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Molero, Y.; Larsson, H.; D’Onofrio, B.M.; Sharp, D.J.; Fazel, S. Associations between gabapentinoids and suicidal behaviour, unintentional overdoses, injuries, road traffic incidents, and violent crime: Population based cohort study in Sweden. BMJ 2019, 365, l2147. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.A.; Cipriani, A. Lithium and suicide in mood disorders: Updated meta-review of the scientific literature. Bipolar Disord. 2017, 19, 575–586. [Google Scholar] [CrossRef]
- Roberts, E.; Cipriani, A.; Geddes, J.R.; Nierenberg, A.A.; Young, A.H. The evidence for lithium in suicide prevention. Br. J. Psychiatry 2017, 211, 396. [Google Scholar] [CrossRef]
- D’Anci, K.E.; Uhl, S.; Giradi, G.; Martin, C. Treatments for the Prevention and Management of Suicide: A Systematic Review. Ann. Intern. Med. 2019, 171, 334–342. [Google Scholar] [CrossRef]
- Wildburger, N.C.; Laezza, F. Control of neuronal ion channel function by glycogen synthase kinase-3: New prospective for an old kinase. Front. Mol. Neurosci. 2012, 5, 80. [Google Scholar] [CrossRef]
- Zhang, P.; Xiang, N.; Chen, Y.; Sliwerska, E.; McInnis, M.G.; Burmeister, M.; Zollner, S. Family-based association analysis to finemap bipolar linkage peak on chromosome 8q24 using 2,500 genotyped SNPs and 15,000 imputed SNPs. Bipolar Disord. 2010, 12, 786–792. [Google Scholar] [CrossRef]
- Zhu, B.; Chen, C.; Xue, G.; Lei, X.; Li, J.; Moyzis, R.K.; Dong, Q.; Lin, C. The GABRB1 gene is associated with thalamus volume and modulates the association between thalamus volume and intelligence. Neuroimage 2014, 102 Pt 2, 756–763. [Google Scholar] [CrossRef]
- Lynch, J.F.; Winiecki, P.; Gilman, T.L.; Adkins, J.M.; Jasnow, A.M. Hippocampal GABAB(1a) Receptors Constrain Generalized Contextual Fear. Neuropsychopharmacology 2017, 42, 914–924. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Reutiman, T.J.; Folsom, T.D.; Rooney, R.J.; Patel, D.H.; Thuras, P.D. mRNA and protein levels for GABAAalpha4, alpha5, beta1 and GABABR1 receptors are altered in brains from subjects with autism. J. Autism Dev. Disord. 2010, 40, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Duka, T.; Nikolaou, K.; King, S.L.; Banaschewski, T.; Bokde, A.L.; Buchel, C.; Carvalho, F.M.; Conrod, P.J.; Flor, H.; Gallinat, J.; et al. GABRB1 Single Nucleotide Polymorphism Associated with Altered Brain Responses (but not Performance) during Measures of Impulsivity and Reward Sensitivity in Human Adolescents. Front. Behav. Neurosci. 2017, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Poulter, M.O.; Du, L.; Zhurov, V.; Palkovits, M.; Faludi, G.; Merali, Z.; Anisman, H. Altered Organization of GABA(A) Receptor mRNA Expression in the Depressed Suicide Brain. Front. Mol. Neurosci. 2010, 3, 3. [Google Scholar] [CrossRef]
- Martins-de-Souza, D.; Guest, P.C.; Vanattou-Saifoudine, N.; Harris, L.W.; Bahn, S. Proteomic technologies for biomarker studies in psychiatry: Advances and needs. Int. Rev. Neurobiol. 2011, 101, 65–94. [Google Scholar] [CrossRef] [PubMed]
- Vatner, S.F.; Park, M.; Yan, L.; Lee, G.J.; Lai, L.; Iwatsubo, K.; Ishikawa, Y.; Pessin, J.; Vatner, D.E. Adenylyl cyclase type 5 in cardiac disease, metabolism, and aging. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1–H8. [Google Scholar] [CrossRef]
- Kheirbek, M.A.; Britt, J.P.; Beeler, J.A.; Ishikawa, Y.; McGehee, D.S.; Zhuang, X. Adenylyl cyclase type 5 contributes to corticostriatal plasticity and striatum-dependent learning. J. Neurosci. 2009, 29, 12115–12124. [Google Scholar] [CrossRef]
| Non-Suicide Death | Suicide | p-Value | |
|---|---|---|---|
| Male (n) | 5 | 5 | - |
| Age (years; median and range) † | 52 (38–63) | 56 (44–69) | 0.529 |
| Disorder (n; %) * | |||
| Bipolar Disorder (Type I) | 4 (80%) | 3 (60%) | 1.000 |
| Major Depressive Disorder | 1 (20%) | 2 (40%) | |
| Last recorded mood (n; %) * | |||
| Depression | 4 (80%) | 5 (100%) | 1.000 |
| Unknown | 1 (20%) | 0 (0%) | |
| Psychosis history (n; %) * | |||
| Psychosis | 3 (60%) | 2 (60%) | 1.000 |
| No psychosis | 2 (40%) | 3 (40%) | |
| Postmortem toxicology (n; %) * | |||
| Antidepressant medication | 0 | 1 (20%) | 1.000 |
| Antipsychotic medication | 1 (20%) | 1 (20%) | 1.000 |
| Mood stabilizer medication | 1 (20%) | 0 | 1.000 |
| Anxiolytic medication | 1 (20%) | 0 | 1.000 |
| Alcohol | 0 | 0 | - |
| Cocaine | 1 (20%) | 0 | 1.000 |
| Cannabinoids | 0 | 0 | - |
| Opiates | 0 | 1 (20%) | 1.000 |
| Other drugs | 2 (40%) | 0 | 0.444 |
| Body mass index (median and range) † | 49.4 (23–53.2) | 23.8 (17.5–34.2) | 0.047 |
| Smoker (n; %) *a | |||
| Yes (Current/Former) | 3 (75%) | 4 (80%) | 1.000 |
| No | 1 (25%) | 1 (20%) | |
| Postmortem interval (h; median and range) † | 22 (10–27.3) | 20 (17–25) | 1.000 |
| pH (median and range) † | 6.08 (5.96–6.88) | 6.69 (6.27– 7.02) | 0.222 |
| Protein Name | Gene | Intensity | Log2 FC * | p-Value ** | FDR | After BMI Adjustment | ||
|---|---|---|---|---|---|---|---|---|
| Non-suicide | Suicide | p-Value *** | FDR *** | |||||
| Potassium voltage-gated channel subfamily Q member 3 | KCNQ3 | 2.23 × 1011 | 1.6 × 1011 | −0.481 | 2.10 × 10−09 | 5.93 × 10−06 | 0.005 | 0.463 |
| Metallo-beta-lactamase domain-containing protein 1 | MBLAC1 | 6.82 × 1010 | 3.38 × 1010 | −1.011 | 1.16 × 10−06 | 0.002 | 0.028 | 0.790 |
| Tripartite motif containing 36 | TRIM36 | 9.18 × 1011 | 1.41 × 1012 | 0.614 | 2.31 × 10−06 | 0.003 | 0.004 | 0.418 |
| RNA-binding motif protein X-linked | RBMX | 3.74 × 1012 | 1.68 × 1012 | −1.152 | 5.32 × 10−06 | 0.006 | 0.046 | 0.908 |
| Adenylate cyclase 5 | ADCY5 | 1.02 × 1011 | 2.3 × 1011 | 1.176 | 7.00 × 10−06 | 0.007 | 0.014 | 0.782 |
| Ectonucleoside triphosphate diphosphohydrolase 2 | ENTPD2 | 3.96 × 1011 | 1.11 × 1011 | −1.834 | 1.09 × 10−05 | 0.009 | 0.034 | 0.827 |
| NIMA related kinase 7 | NEK7 | 1.1 × 1011 | 4.44 × 1010 | −1.305 | 1.41 × 10−05 | 0.010 | 0.068 | 0.999 |
| Sorting nexin 5 | SNX5 | 4.16 × 1012 | 2.44 × 1012 | −0.770 | 4.24 × 10−05 | 0.022 | 0.001 | 0.245 |
| Fumarylacetoacetate hydrolase | FAH | 1.09 × 1012 | 7.6 × 1011 | −0.523 | 4.16 × 10−05 | 0.022 | 0.038 | 0.890 |
| Megalencephalic leukoencephalopathy with subcortical cysts 1 | MLC1 | 4.77 × 1012 | 1.92 × 1012 | −1.315 | 5.00 × 10−05 | 0.023 | 0.044 | 0.893 |
| Muscleblind-like splicing regulator 1 | MBNL1 | 9.02 × 1010 | 2.97 × 1010 | −1.601 | 1.27 × 10−04 | 0.055 | 0.127 | 0.999 |
| Cytosolic carboxypeptidase | CBPC1 | 1.64 × 1011 | 2.22 × 1011 | 0.432 | 1.60 × 10−04 | 0.064 | 0.008 | 0.602 |
| Hyperpolarization activated cyclic nucleotide gated potassium and sodium channel 2 | HCN2 | 2.24 × 1011 | 5.45 × 1011 | 1.281 | 1.78 × 10−04 | 0.067 | 0.037 | 0.870 |
| Phospholipase C-like 1 (inactive) | PLCL1 | 3.95 × 1012 | 5.69 × 1012 | 0.524 | 2.28 × 10−04 | 0.080 | 0.088 | 0.999 |
| Dedicator of cytokinesis 1 | DOCK1 | 4.24 × 1011 | 6.58 × 1011 | 0.635 | 4.01 × 10−04 | 0.133 | 0.059 | 0.965 |
| Contactin 4 | CNTN4 | 8.76 × 1011 | 1.5 × 1012 | 0.773 | 5.57 × 10−04 | 0.165 | 0.015 | 0.749 |
| Connector enhancer of kinase suppressor of Ras 2 | CNKSR2 | 1.41 × 1012 | 9.57 × 1011 | −0.555 | 6.58 × 10−04 | 0.186 | 0.048 | 0.909 |
| Calcium/calmodulin-dependent protein kinase type II subunit delta | CAMK2D | 3.48 × 1013 | 2.03 × 1013 | −0.777 | 7.60 × 10−04 | 0.204 | 0.043 | 0.906 |
| Eukaryotic translation initiation factor 4E | EIF4E | 2.8 × 1012 | 1.36 × 1012 | −1.043 | 9.14 × 10−04 | 0.232 | 0.177 | 0.999 |
| Ubiquitination factor E4A | UBE4A | 2.5 × 1012 | 3.49 × 1012 | 0.485 | 9.44 × 10−04 | 0.232 | 0.010 | 0.680 |
| Serine racemase | SRR | 9.44 × 1011 | 4.41 × 1011 | −1.096 | 0.001 | 0.240 | 0.114 | 0.999 |
| Gamma-aminobutyric acid type A receptor beta1 subunit | GABRB1 | 7.94 × 1011 | 5.33 × 1011 | −0.575 | 0.001 | 0.244 | 0.127 | 0.999 |
| Membrane palmitoylated protein 2 | MPP2 | 1.26 × 1013 | 7.09 × 1012 | −0.835 | 0.001 | 0.244 | 0.060 | 0.970 |
| Probable glutamate-tRNA ligase | EARS2 | 1.93 × 1012 | 1.04 × 1012 | −0.893 | 0.001 | 0.263 | 0.026 | 0.776 |
| RNA-binding protein EWS | EWSR1 | 6.18 × 1011 | 3.41 × 1011 | −0.857 | 0.001 | 0.263 | 0.087 | 0.999 |
| Sorting nexin-14 | SNX14 | 2.76 × 1010 | 4.73 × 1009 | −2.544 | 0.001 | 0.271 | 0.024 | 0.761 |
| Arf-GAP with Rho-GAP domain, ANK repeat and PH domain-containing protein 1 | ARAP1 | 7.04 × 1009 | 3.3 × 1009 | −1.094 | 0.001 | 0.271 | 0.204 | 0.999 |
| Amine oxidase (flavin-containing) A | MAOA | 2.79 × 1013 | 1.84 × 1013 | −0.603 | 0.002 | 0.277 | 0.190 | 0.999 |
| Rho guanine nucleotide exchange factor 9 | ARHGEF9 | 4.82 × 1011 | 2.39 × 1011 | −1.012 | 0.002 | 0.282 | 0.061 | 0.975 |
| Apoptosis-associated speck-like protein containing a CARD | PYCARD | 8.75 × 1010 | 4.89 × 1010 | −0.840 | 0.002 | 0.284 | 0.003 | 0.385 |
| N-acetylserotonin O-methyltransferase-like protein | ASMTL | 2.35 × 1012 | 1.41 × 1012 | −0.739 | 0.002 | 0.292 | 0.191 | 0.999 |
| Vesicle transport protein GOT1B | GOLT1B | 9.75 × 1010 | 1.43 × 1011 | 0.556 | 0.002 | 0.295 | 0.001 | 0.235 |
| Guanine nucleotide exchange C9orf72 | C9orf72 | 4.16 × 1011 | 2.95 × 1011 | -0.497 | 0.002 | 0.295 | 0.031 | 0.799 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabello-Arreola, A.; Ho, A.M.-C.; Ozerdem, A.; Cuellar-Barboza, A.B.; Kucuker, M.U.; Heppelmann, C.J.; Charlesworth, M.C.; Ceylan, D.; Stockmeier, C.A.; Rajkowska, G.; et al. Differential Dorsolateral Prefrontal Cortex Proteomic Profiles of Suicide Victims with Mood Disorders. Genes 2020, 11, 256. https://doi.org/10.3390/genes11030256
Cabello-Arreola A, Ho AM-C, Ozerdem A, Cuellar-Barboza AB, Kucuker MU, Heppelmann CJ, Charlesworth MC, Ceylan D, Stockmeier CA, Rajkowska G, et al. Differential Dorsolateral Prefrontal Cortex Proteomic Profiles of Suicide Victims with Mood Disorders. Genes. 2020; 11(3):256. https://doi.org/10.3390/genes11030256
Chicago/Turabian StyleCabello-Arreola, Alejandra, Ada Man-Choi Ho, Aysegul Ozerdem, Alfredo B. Cuellar-Barboza, Mehmet U. Kucuker, Carrie J. Heppelmann, M. Cristine Charlesworth, Deniz Ceylan, Craig A. Stockmeier, Grazyna Rajkowska, and et al. 2020. "Differential Dorsolateral Prefrontal Cortex Proteomic Profiles of Suicide Victims with Mood Disorders" Genes 11, no. 3: 256. https://doi.org/10.3390/genes11030256
APA StyleCabello-Arreola, A., Ho, A. M.-C., Ozerdem, A., Cuellar-Barboza, A. B., Kucuker, M. U., Heppelmann, C. J., Charlesworth, M. C., Ceylan, D., Stockmeier, C. A., Rajkowska, G., Frye, M. A., Choi, D.-S., & Veldic, M. (2020). Differential Dorsolateral Prefrontal Cortex Proteomic Profiles of Suicide Victims with Mood Disorders. Genes, 11(3), 256. https://doi.org/10.3390/genes11030256





