Extracellular Vesicles Derived from Human Gingival Mesenchymal Stem Cells: A Transcriptomic Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. hGMSCs Culture Estabilishment
2.2. hGMSCs–Derived EVs Isolation
2.3. RNA Extraction and Transcriptomic Analysis
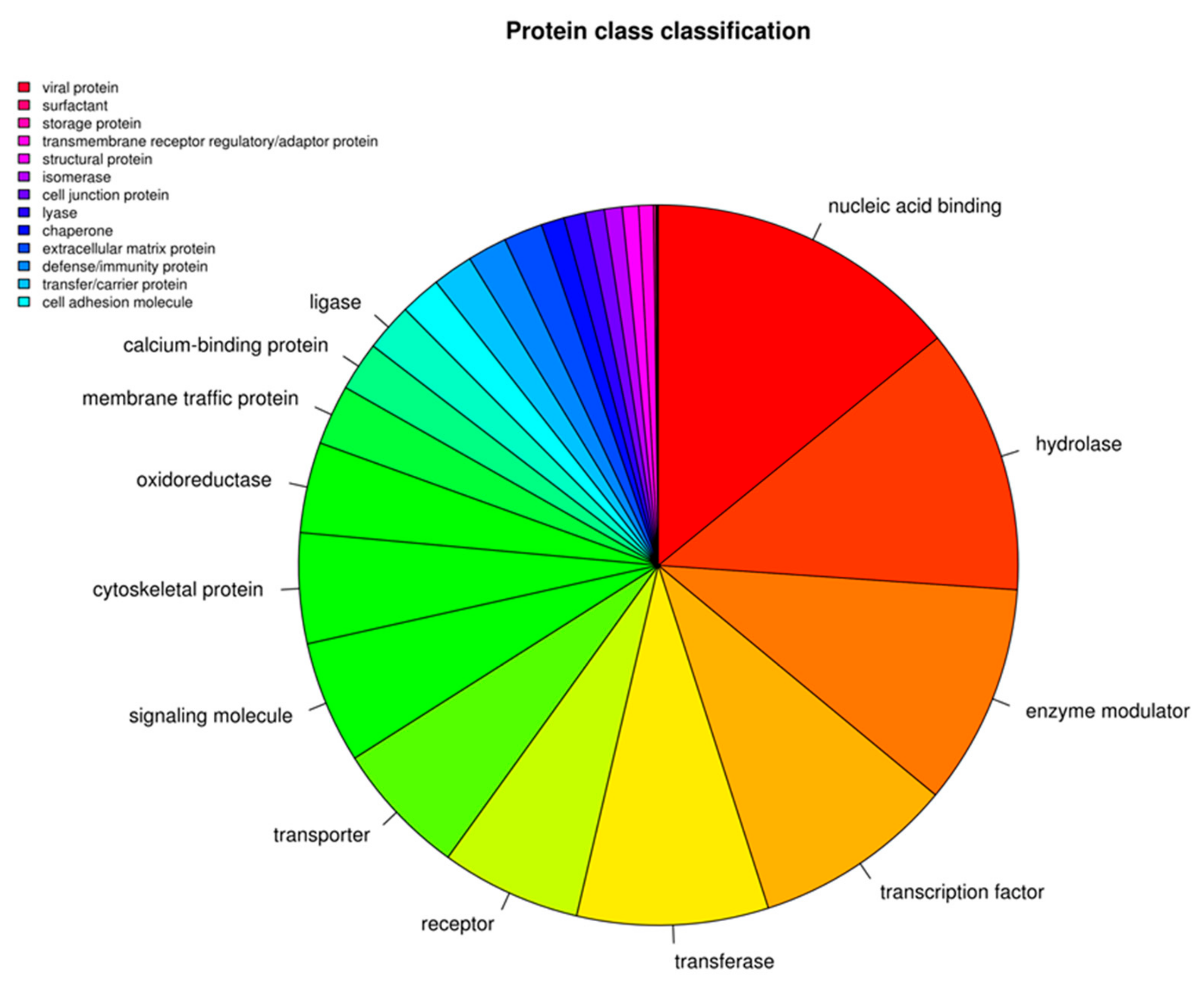
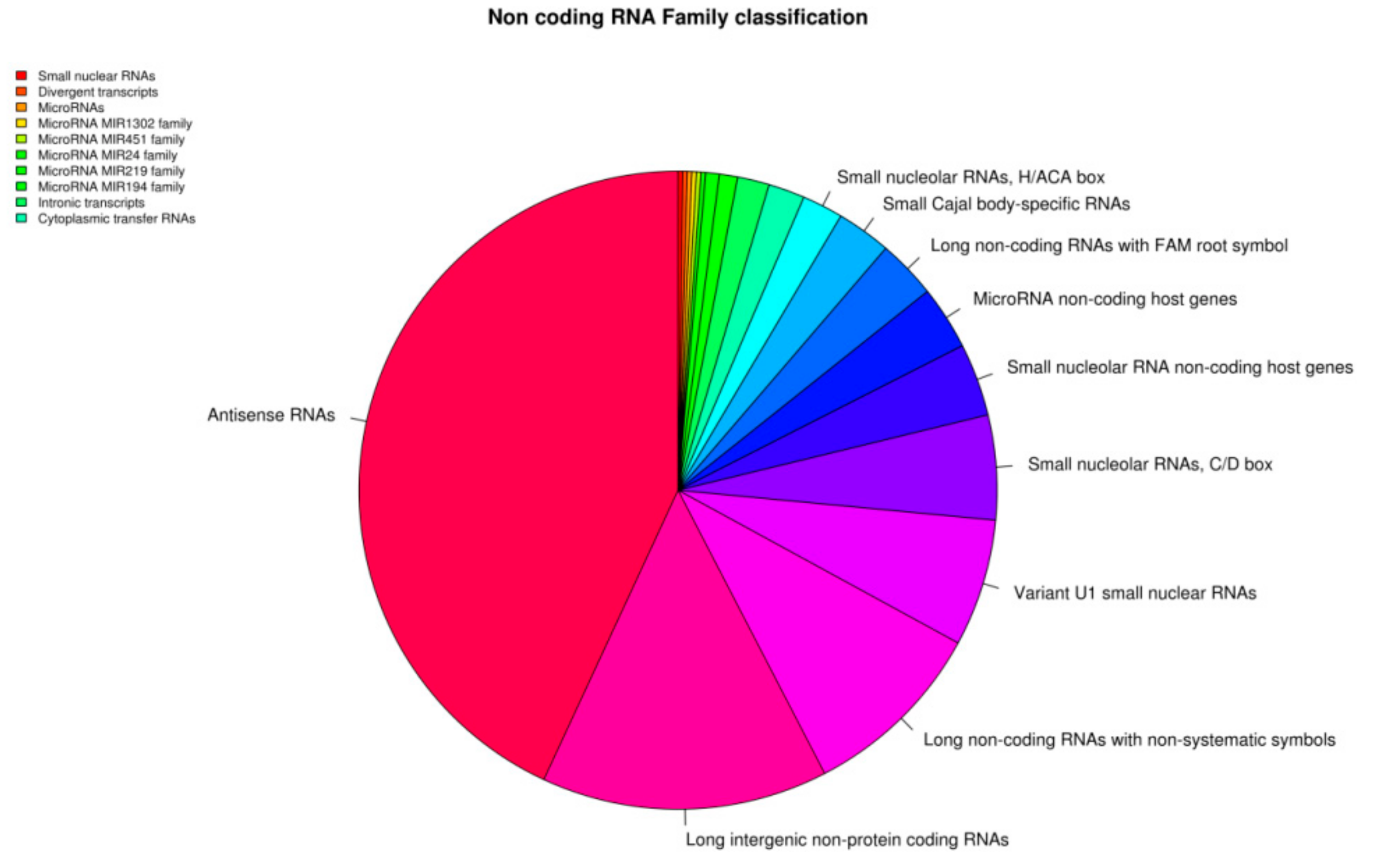
3. Results
Transcriptomic Investigation of EVs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharpe, P.T. Dental mesenchymal stem cells. Development 2016, 143, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Yang, P.; Ge, S. Isolation and characterization of human gingiva-derived mesenchymal stem cells using limiting dilution method. J. Dent. Sci. 2016, 11, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (dpscs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.-M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.-Y.; Shi, S. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. Shed: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed]
- Morsczeck, C.; Götz, W.; Schierholz, J.; Zeilhofer, F.; Kühn, U.; Möhl, C.; Sippel, C.; Hoffmann, K. Isolation of precursor cells (pcs) from human dental follicle of wisdom teeth. Matrix Biol. 2005, 24, 155–165. [Google Scholar] [CrossRef]
- Seo, B.-M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Fournier, B.; Loison-Robert, L.; Ferre, F.; Owen, G.; Larjava, H.; Häkkinen, L. Characterisation of human gingival neural crest-derived stem cells in monolayer and neurosphere cultures. Eur. Cell Mater. 2016, 31, 40–58. [Google Scholar] [CrossRef]
- Xu, X.; Chen, C.; Akiyama, K.; Chai, Y.; Le, A.D.; Wang, Z.; Shi, S. Gingivae contain neural-crest- and mesoderm-derived mesenchymal stem cells. J. Dent. Res. 2013, 92, 825–832. [Google Scholar] [CrossRef]
- Diomede, F.; Zini, N.; Pizzicannella, J.; Merciaro, I.; Pizzicannella, G.; D’Orazio, M.; Piattelli, A.; Trubiani, O. 5-aza exposure improves reprogramming process through embryoid body formation in human gingival stem cells. Front. Genet. 2018, 9, 419. [Google Scholar] [CrossRef]
- Nuti, N.; Corallo, C.; Chan, B.; Ferrari, M.; Gerami-Naini, B. Multipotent differentiation of human dental pulp stem cells: A literature review. Stem Cell Rev. Rep. 2016, 12, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.H.; Lee, J.E.; Yun, J.H.; Kim, I.; Ko, Y.; Park, J.B. Isolation and characterization of human mesenchymal stem cells from gingival connective tissue. J. Periodontal Res. 2015, 50, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Z.; Su, W.R.; Shi, S.H.; Wilder-Smith, P.; Xiang, A.P.; Wong, A.; Nguyen, A.L.; Kwon, C.W.; Le, A.D. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells 2010, 28, 1856–1868. [Google Scholar] [CrossRef] [PubMed]
- Fawzy El-Sayed, K.M.; Dörfer, C.E. Gingival mesenchymal stem/progenitor cells: A unique tissue engineering gem. Stem Cells Int. 2016, 2016. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Diomede, F.; Cardelli, P.; Bramanti, A.; Scionti, D.; Bramanti, P.; Trubiani, O.; Mazzon, E. Transcriptomic analysis of gingival mesenchymal stem cells cultured on 3 d bioprinted scaffold: A promising strategy for neuroregeneration. J. Biomed. Mater. Res. Part A 2018, 106, 126–137. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Diomede, F.; Scionti, D.; Bramanti, P.; Trubiani, O.; Mazzon, E. The role of hypoxia on the neuronal differentiation of gingival mesenchymal stem cells: A transcriptional study. Cell Transplant. 2019, 28, 538–552. [Google Scholar] [CrossRef]
- Mao, Q.; Nguyen, P.D.; Shanti, R.M.; Shi, S.; Shakoori, P.; Zhang, Q.; Le, A.D. Gingiva-derived mesenchymal stem cell-extracellular vesicles activate schwann cell repair phenotype and promote nerve regeneration. Tissue Eng. Part A 2019, 25, 887–900. [Google Scholar] [CrossRef]
- Zhang, P.; Yeo, J.C.; Lim, C.T. Advances in technologies for purification and enrichment of extracellular vesicles. SLAS Technol. Transl. Life Sci. Innov. 2019, 24, 477–488. [Google Scholar] [CrossRef]
- Camussi, G.; Deregibus, M.-C.; Bruno, S.; Grange, C.; Fonsato, V.; Tetta, C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am. J. Cancer Res. 2011, 1, 98. [Google Scholar]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mrnas and micrornas is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654. [Google Scholar] [CrossRef]
- del Conde, I.; Shrimpton, C.N.; Thiagarajan, P.; López, J.A. Tissue-factor–bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood 2005, 106, 1604–1611. [Google Scholar] [CrossRef]
- Diomede, F.; Gugliandolo, A.; Scionti, D.; Merciaro, I.; Cavalcanti, M.; Mazzon, E.; Trubiani, O. Biotherapeutic effect of gingival stem cells conditioned medium in bone tissue restoration. Int. J. Mol. Sci. 2018, 19, 329. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. Star: Ultrafast universal rna-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Trapnell, C.; Hendrickson, D.G.; Sauvageau, M.; Goff, L.; Rinn, J.L.; Pachter, L. Differential analysis of gene regulation at transcript resolution with rna-seq. Nat. Biotechnol. 2013, 31, 46–53. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Ebert, D.; Huang, X.; Thomas, P.D. Panther version 14: More genomes, a new panther go-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2019, 47, D419–D426. [Google Scholar] [CrossRef]
- Xin, H.; Li, Y.; Chopp, M. Exosomes/mirnas as mediating cell-based therapy of stroke. Front. Cell. Neurosci. 2014, 8, 377. [Google Scholar] [CrossRef]
- Ragni, E.; Banfi, F.; Barilani, M.; Cherubini, A.; Parazzi, V.; Larghi, P.; Dolo, V.; Bollati, V.; Lazzari, L. Extracellular vesicle-shuttled mrna in mesenchymal stem cell communication. Stem Cells 2017, 35, 1093–1105. [Google Scholar] [CrossRef]
- Ratajczak, J.; Wysoczynski, M.; Hayek, F.; Janowska-Wieczorek, A.; Ratajczak, M. Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia 2006, 20, 1487. [Google Scholar] [CrossRef]
- Diomede, F.; Gugliandolo, A.; Cardelli, P.; Merciaro, I.; Ettorre, V.; Traini, T.; Bedini, R.; Scionti, D.; Bramanti, A.; Nanci, A.; et al. Three-dimensional printed pla scaffold and human gingival stem cell-derived extracellular vesicles: A new tool for bone defect repair. Stem Cell Res. Ther. 2018, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Judson, R.L.; Babiarz, J.E.; Venere, M.; Blelloch, R. Embryonic stem cell-specific micrornas promote induced pluripotency. Nat. Biotechnol. 2009, 27, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dong, R.; Diao, S.; Du, J.; Fan, Z.; Wang, F. Differential long noncoding rna/mrna expression profiling and functional network analysis during osteogenic differentiation of human bone marrow mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Fatima, F.; Vallabhaneni, K.C.; Penfornis, P.; Valadi, H.; Ekstrom, K.; Kholia, S.; Whitt, J.D.; Fernandes, J.D.; Pochampally, R.; et al. Extracellular vesicles: Evolving factors in stem cell biology. Stem Cells Int. 2016, 2016, 1073140. [Google Scholar] [CrossRef]
- Mattick, J.S.; Makunin, I.V. Non-coding rna. Hum. Mol. Genet. 2006, 15. [Google Scholar] [CrossRef]
- Fatima, F.; Ekstrom, K.; Nazarenko, I.; Maugeri, M.; Valadi, H.; Hill, A.F.; Camussi, G.; Nawaz, M. Non-coding rnas in mesenchymal stem cell-derived extracellular vesicles: Deciphering regulatory roles in stem cell potency, inflammatory resolve, and tissue regeneration. Front. Genet. 2017, 8, 161. [Google Scholar] [CrossRef]
- Fatima, F.; Nawaz, M. Vesiculated long non-coding rnas: Offshore packages deciphering trans-regulation between cells, cancer progression and resistance to therapies. Non-Coding RNA 2017, 3, 10. [Google Scholar] [CrossRef]
- Goumans, M.J.; Valdimarsdottir, G.; Itoh, S.; Rosendahl, A.; Sideras, P.; ten Dijke, P. Balancing the activation state of the endothelium via two distinct tgf-β type i receptors. EMBO J. 2002, 21, 1743–1753. [Google Scholar] [CrossRef]
- Jian, H.; Shen, X.; Liu, I.; Semenov, M.; He, X.; Wang, X.F. Smad3-dependent nuclear translocation of β-catenin is required for tgf-beta1-induced proliferation of bone marrow-derived adult human mesenchymal stem cells. Genes Dev. 2006, 20, 666–674. [Google Scholar] [CrossRef]
- Li, M.O.; Flavell, R.A. Contextual regulation of inflammation: A duet by transforming growth factor-β and interleukin-10. Immunity 2008, 28, 468–476. [Google Scholar] [CrossRef]
- Rajan, T.S.; Giacoppo, S.; Diomede, F.; Ballerini, P.; Paolantonio, M.; Marchisio, M.; Piattelli, A.; Bramanti, P.; Mazzon, E.; Trubiani, O. The secretome of periodontal ligament stem cells from ms patients protects against eae. Sci. Rep. 2016, 6, 38743. [Google Scholar] [CrossRef] [PubMed]
- Rajan, T.S.; Diomede, F.; Bramanti, P.; Trubiani, O.; Mazzon, E. Conditioned medium from human gingival mesenchymal stem cells protects motor-neuron-like nsc-34 cells against scratch-injury-induced cell death. Int. J. Immunopathol. Pharmacol. 2017, 30, 383–394. [Google Scholar] [CrossRef]
- Eirin, A.; Riester, S.M.; Zhu, X.Y.; Tang, H.; Evans, J.M.; O’Brien, D.; van Wijnen, A.J.; Lerman, L.O. Microrna and mrna cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene 2014, 551, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Carreira, A.C.; Alves, G.G.; Zambuzzi, W.F.; Sogayar, M.C.; Granjeiro, J.M. Bone morphogenetic proteins: Structure, biological function and therapeutic applications. Arch. Biochem. Biophys. 2014, 561, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, M.K.; Wang, E.; Morris, E.A. Bmp-2 and bmp-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of il-1. J. Cell. Physiol. 2001, 189, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Berry-Wynne, K.M.; Asai-Coakwell, M.; Sundaresan, P.; Footz, T.; French, C.R.; Abitbol, M.; Fleisch, V.C.; Corbett, N.; Allison, W.T. Mutation of the bone morphogenetic protein gdf3 causes ocular and skeletal anomalies. Hum. Mol. Genet. 2009, 19, 287–298. [Google Scholar] [CrossRef] [PubMed]
- McPherron, A.C.; Lawler, A.M.; Lee, S.-J. Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nat. Genet. 1999, 22, 260. [Google Scholar] [CrossRef]
- Wang, J.; Yu, T.; Wang, Z.; Ohte, S.; Yao, R.E.; Zheng, Z.; Geng, J.; Cai, H.; Ge, Y.; Li, Y. A new subtype of multiple synostoses syndrome is caused by a mutation in gdf6 that decreases its sensitivity to noggin and enhances its potency as a bmp signal. J. Bone Miner. Res. 2016, 31, 882–889. [Google Scholar] [CrossRef]
- Diomede, F.; D’aurora, M.; Gugliandolo, A.; Merciaro, I.; Ettorre, V.; Bramanti, A.; Piattelli, A.; Gatta, V.; Mazzon, E.; Fontana, A. A novel role in skeletal segment regeneration of extracellular vesicles released from periodontal-ligament stem cells. Int. J. Nanomed. 2018, 13, 3805. [Google Scholar] [CrossRef]
- Westendorf, J.J.; Kahler, R.A.; Schroeder, T.M. Wnt signaling in osteoblasts and bone diseases. Gene 2004, 341, 19–39. [Google Scholar] [CrossRef]
- Jing, H.; Su, X.; Gao, B.; Shuai, Y.; Chen, J.; Deng, Z.; Liao, L.; Jin, Y. Epigenetic inhibition of wnt pathway suppresses osteogenic differentiation of bmscs during osteoporosis. Cell Death Dis. 2018, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Perrien, D.S.; Akel, N.S.; Edwards, P.K.; Carver, A.A.; Bendre, M.S.; Swain, F.L.; Skinner, R.A.; Hogue, W.R.; Nicks, K.M.; Pierson, T.M. Inhibin a is an endocrine stimulator of bone mass and strength. Endocrinology 2007, 148, 1654–1665. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. Vegf receptor signaling—In control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Grosso, A.; Burger, M.G.; Lunger, A.; Schaefer, D.J.; Banfi, A.; Di Maggio, N. It takes two to tango: Coupling of angiogenesis and osteogenesis for bone regeneration. Front. Bioeng. Biotechnol. 2017, 5, 68. [Google Scholar] [CrossRef] [PubMed]
- Pizzicannella, J.; Gugliandolo, A.; Orsini, T.; Fontana, A.; Ventrella, A.; Mazzon, E.; Bramanti, P.; Diomede, F.; Trubiani, O. Engineered extracellular vesicles from human periodontal-ligament stem cells increase vegf/vegfr2 expression during bone regeneration. Front. Physiol. 2019, 10, 512. [Google Scholar] [CrossRef] [PubMed]
- Eirin, A.; Zhu, X.Y.; Puranik, A.S.; Woollard, J.R.; Tang, H.; Dasari, S.; Lerman, A.; van Wijnen, A.J.; Lerman, L.O. Comparative proteomic analysis of extracellular vesicles isolated from porcine adipose tissue-derived mesenchymal stem/stromal cells. Sci. Rep. 2016, 6, 36120. [Google Scholar] [CrossRef] [PubMed]
- Galzie, Z.; Kinsella, A.R.; Smith, J.A. Fibroblast growth factors and their receptors. Biochem. Cell Biol. 1997, 75, 669–685. [Google Scholar] [CrossRef]
- Javerzat, S.; Auguste, P.; Bikfalvi, A. The role of fibroblast growth factors in vascular development. Trends Mol. Med. 2002, 8, 483–489. [Google Scholar] [CrossRef]
- Yun, Y.-R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.-H.; Shin, U.S.; Kim, H.-W. Fibroblast growth factors: Biology, function, and application for tissue regeneration. J. Tissue Eng. 2010, 1, 218142. [Google Scholar] [CrossRef]
- Bucan, V.; Vaslaitis, D.; Peck, C.T.; Strauss, S.; Vogt, P.M.; Radtke, C. Effect of exosomes from rat adipose-derived mesenchymal stem cells on neurite outgrowth and sciatic nerve regeneration after crush injury. Mol. Neurobiol. 2019, 56, 1812–1824. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Li, J.; Yi, Z. The role of the fibroblast growth factor family in bone-related diseases. Chem. Biol. Drug Des. 2019, 94, 1740–1749. [Google Scholar] [CrossRef] [PubMed]
- Ohbayashi, N.; Shibayama, M.; Kurotaki, Y.; Imanishi, M.; Fujimori, T.; Itoh, N.; Takada, S. Fgf18 is required for normal cell proliferation and differentiation during osteogenesis and chondrogenesis. Genes Dev. 2002, 16, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Ford-Perriss, M.; Abud, H.; Murphy, M. Fibroblast growth factors in the developing central nervous system. Clin. Exp. Pharmacol. Physiol. 2001, 28, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Murase, S.; Mosser, E.; Schuman, E.M. Depolarization drives β-catenin into neuronal spines promoting changes in synaptic structure and function. Neuron 2002, 35, 91–105. [Google Scholar] [CrossRef]
- Strand, N.S.; Hoi, K.K.; Phan, T.M.; Ray, C.A.; Berndt, J.D.; Moon, R.T. Wnt/β-catenin signaling promotes regeneration after adult zebrafish spinal cord injury. Biochem. Biophys. Res. Commun. 2016, 477, 952–956. [Google Scholar] [CrossRef]
- Yin, Z.-S.; Zu, B.; Chang, J.; Zhang, H. Repair effect of wnt3a protein on the contused adult rat spinal cord. Neurol. Res. 2008, 30, 480–486. [Google Scholar] [CrossRef]
- Rodriguez, J.; Esteve, P.; Weinl, C.; Ruiz, J.M.; Fermin, Y.; Trousse, F.; Dwivedy, A.; Holt, C.; Bovolenta, P. Sfrp1 regulates the growth of retinal ganglion cell axons through the fz2 receptor. Nat. Neurosci. 2005, 8, 1301–1309. [Google Scholar] [CrossRef]
- Garcia, A.L.; Udeh, A.; Kalahasty, K.; Hackam, A.S. A growing field: The regulation of axonal regeneration by wnt signaling. Neural Regen. Res. 2018, 13, 43–52. [Google Scholar]
- Park, H.; Poo, M.M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Binder, D.K.; Scharfman, H.E. Brain-derived neurotrophic factor. Growth Factors (ChurSwitz.) 2004, 22, 123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and functional implications of adult neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Schäbitz, W.-R.; Schwab, S.; Spranger, M.; Hacke, W. Intraventricular brain-derived neurotrophic factor reduces infarct size after focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 1997, 17, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Oo, T.F.; Kholodilov, N.; Burke, R.E. Regulation of natural cell death in dopaminergic neurons of the substantia nigra by striatal glial cell line-derived neurotrophic factor in vivo. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 5141–5148. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, M.; Gong, A.; Zhang, X.; Wu, X.; Zhu, Y.; Shi, H.; Wu, L.; Zhu, W.; Qian, H.; et al. Hucmsc-exosome mediated-wnt4 signaling is required for cutaneous wound healing. Stem Cells 2015, 33, 2158–2168. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Park, W.S.; Kim, Y.E.; Sung, D.K.; Sung, S.I.; Ahn, J.Y.; Chang, Y.S. Vascular endothelial growth factor mediates the therapeutic efficacy of mesenchymal stem cell-derived extracellular vesicles against neonatal hyperoxic lung injury. Exp. Mol. Med. 2018, 50, 26. [Google Scholar] [CrossRef]
- Sun, H.; Hu, S.; Zhang, Z.; Lun, J.; Liao, W.; Zhang, Z. Expression of exosomal micrornas during chondrogenic differentiation of human bone mesenchymal stem cells. J. Cell. Biochem. 2019, 120, 171–181. [Google Scholar] [CrossRef]
- Wang, X.; Omar, O.; Vazirisani, F.; Thomsen, P.; Ekstrom, K. Mesenchymal stem cell-derived exosomes have altered microrna profiles and induce osteogenic differentiation depending on the stage of differentiation. PLoS ONE 2018, 13, e0193059. [Google Scholar] [CrossRef]

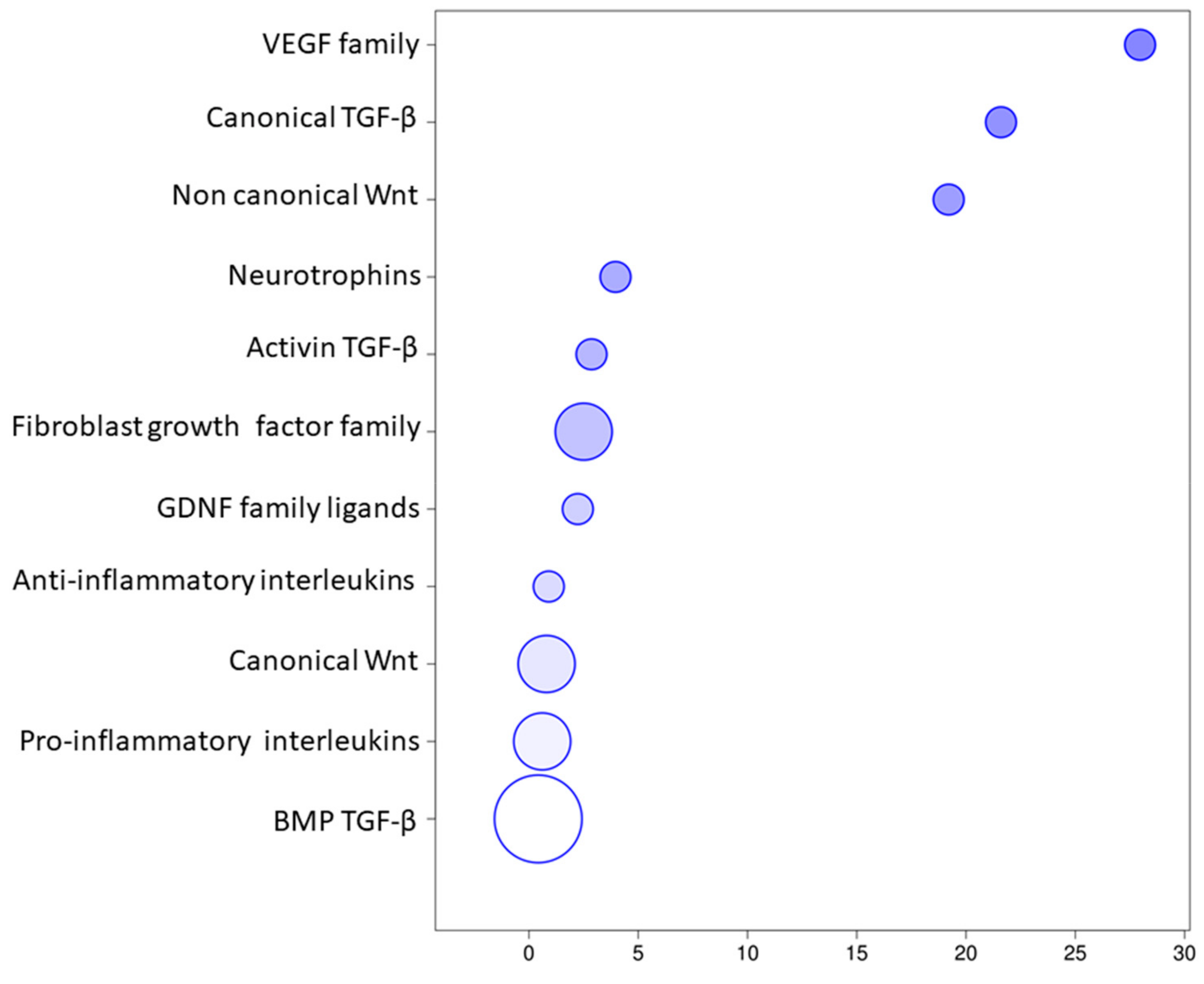
| Transcript | Name | Biological Process |
|---|---|---|
| IL19 | Interleukin 19 | Apoptotic process; immune response |
| IL37 | Interleukin 37 | Inflammatory response; immune response; neutrophil chemotaxis |
| IL21 | Interleukin 21 | Immune response; positive regulation of cell population proliferation; positive regulation of B cell proliferation; positive regulation of tissue remodeling; positive regulation of T cell proliferation |
| IL17A | Interleukin 17A | Inflammatory response; immune response; positive regulation of osteoclast differentiation; apoptotic process; fibroblast activation |
| IL15 | Interleukin 15 | Neutrophil activation; positive regulation of cell population proliferation; lymph node development; inflammatory response; immune response; positive regulation of tissue remodeling; macrophage differentiation; cell-cell signaling; cell maturation; cellular response to vitamin D |
| IL12A | Interleukin 12A | Cell cycle arrest; positive regulation of natural killer cell mediated cytotoxicity directed against tumor cell target; immune response; positive regulation of dendritic cell chemotaxis; defense response to Gram-positive bacterium |
| IL12B | Interleukin 12B | Positive regulation of lymphocyte proliferation; positive regulation of tissue remodeling; sensory perception of pain; positive regulation of osteoclast differentiation; defense response to Gram-negative bacterium; positive regulation of memory T cell differentiation |
| IL6 | Interleukin 6 | Regulation of dendritic cell differentiation; positive regulation of osteoblast differentiation; regulation of odontoblast differentiation; negative regulation of neuron apoptotic process; inflammatory response; liver regeneration; immune response; regulation of osteoclast differentiation; positive regulation of biomineral tissue development; neuron differentiation |
| IL7 | Interleukin 7 | Cell-cell signaling; positive regulation of organ growth; immune response; bone resorption; negative regulation of apoptotic process; positive regulation of T cell differentiation; positive regulation of B cell proliferation |
| IL5 | Interleukin 5 | Positive regulation of eosinophil differentiation; inflammatory response; positive regulation of B cell proliferation; positive regulation of podosome assembly |
| IL25 | Interleukin 25 | Inflammatory response to antigenic stimulus; eosinophil differentiation |
| IL24 | Interleukin 24 | Positive regulation of cell population proliferation |
| IL27 | Interleukin 27 | Inflammatory response; response to bacterium; innate immune response; regulation of T cell proliferation |
| IL32 | Interleukin 32 | Immune response; cell adhesion |
| IL1B | Interleukin 1 β | Activation of MAPK activity; positive regulation of T cell mediated immunity; inflammatory response, apoptotic process; cell-cell signaling; positive regulation of vascular endothelial growth factor production; astrocyte activation; positive regulation of glial cell proliferation |
| IL36B | Interleukin 36 β | Inflammatory response; innate immune response; neutrophil chemotaxis |
| IL16 | Interleukin 16 | Immune response; leukocyte chemotaxis |
| IL36G | Interleukin 36 γ | Inflammatory response; innate immune response; cell-cell signaling; neutrophil chemotaxis |
| IL33 | Interleukin 33 | Microglial cell activation involved in immune response; microglial cell proliferation; negative regulation of interferon-γ production |
| Transcript | Name | Biological Process |
|---|---|---|
| TGFB1 | Transforming Growth Factor β 1 | Hematopoietic progenitor cell differentiation; embryonic liver development; positive regulation of protein import into nucleus; positive regulation of vascular permeability; epithelial to mesenchymal transition; positive regulation of MAP kinase activity; positive regulation of cell population proliferation; lymph node development; heart development; positive regulation of cell division; neural tube development; BMP signaling pathway; positive regulation of bone mineralization; regulation of blood vessel remodeling; vasculogenesis; positive regulation of microglia differentiation |
| TGFB2 | Transforming Growth Factor β 2 | Negative regulation of angiogenesis; glial cell migration; kidney development; positive regulation of ossification; epithelial to mesenchymal transition; heart development; cranial skeletal system development; neuron development; BMP signaling pathway; cardiac muscle proliferation; generation of neurons |
| TGFB3 | Transforming Growth Factor β 3 | Digestive tract development; BMP signaling pathway; positive regulation of bone mineralization; negative regulation of neuron apoptotic process; positive regulation of epithelial to mesenchymal transition; ossification involved in bone remodeling |
| BMP7 | Bone Morphogenetic Protein 7 | Axon guidance; skeletal system development; ossification; hindbrain development; cellular response to BMP stimulus; positive regulation of bone mineralization; response to vitamin D; neuron projection morphogenesis; cardiac muscle tissue development; positive regulation of dendrite development |
| AMH | Anti-Mullerian Hormone | Cell-cell signaling; BMP signaling pathway |
| GDF9 | Growth Differentiation Factor 9 | Positive regulation of cell population proliferation; BMP signaling pathway; negative regulation of cell growth |
| BMP4 | Bone Morphogenetic Protein 4 | Positive regulation of kidney development; cellular response to BMP stimulus; telencephalon development; positive regulation of neuron differentiation; positive regulation of osteoblast differentiation; BMP signaling pathway involved in heart induction |
| BMP3 | Bone Morphogenetic Protein 3 | Regulation of MAPK cascade; skeletal system development; osteoblast differentiation |
| BMP8A | Bone Morphogenetic Protein 8a | Regulation of MAPK cascade, ossification |
| BMP8B | Bone Morphogenetic Protein 8b | Regulation of MAPK cascade; skeletal system development; ossification |
| BMP6 | Bone Morphogenetic Protein 6 | Positive regulation of neuron differentiation; positive regulation of osteoblast differentiation; skeletal system development; kidney development; cellular response to BMP stimulus; positive regulation of bone mineralization |
| GDF3 | Growth Differentiation Factor 3 | Negative regulation of BMP signaling pathway; skeletal system development |
| GDF2 | Growth Differentiation Factor 2 | Positive regulation of angiogenesis; blood vessel morphogenesis; ossification; osteoblast differentiation |
| GDF1 | Growth Differentiation Factor 1 | Regulation of MAPK cascade; regulation of apoptotic process; BMP signaling pathway |
| GDF6 | Growth Differentiation Factor 6 | Positive regulation of neuron differentiation; regulation of MAPK cascade |
| GDF5 | Growth Differentiation Factor 5 | Negative regulation of mesenchymal apoptotic process; positive regulation of neuron differentiation; ossification involved in bone remodeling; negative regulation of neuron apoptotic process |
| BMP2 | Bone Morphogenetic Protein 2 | Activation of MAPK activity; inflammatory response; skeletal system development; positive regulation of osteoblast proliferation; bone mineralization involved in bone maturation; telencephalon development; positive regulation of neuron differentiation; positive regulation of osteoblast differentiation; heart development |
| BMP1 | Bone Morphogenetic Protein 1 | Skeletal system development; ossification |
| BMP10 | Bone Morphogenetic Protein 10 | Positive regulation of cardiac muscle cell proliferation; regulation of MAPK cascade; positive regulation of cell proliferation involved in heart morphogenesis |
| BMP5 | Bone Morphogenetic Protein 5 | Skeletal system development; positive regulation of dendritic development |
| BMP15 | Bone Morphogenetic Protein 15 | Regulation of MAPK cascade; BMP signaling pathway |
| GDF15 | Growth Differentiation Factor 15 | Positive regulation of MAPK cascade; activation of MAPK cascade; BMP signaling pathway |
| GDF11 | Growth Differentiation Factor 11 | Regulation of MAPK cascade; skeletal system development |
| GDF10 | Growth Differentiation Factor 10 | Regulation of MAPK cascade; skeletal system development; BMP signaling pathway |
| INHA | Inhibin Subunit α | Regulation of MAPK cascade; skeletal system development; negative regulation of interferon-γ biosynthetic process |
| INHBA | Inhibin Subunit β A | Regulation of MAPK cascade; nervous system development; negative regulation of interferon-γ biosynthetic process; GABAergic neuron differentiation |
| INHBC | Inhibin Subunit β C | Regulation of MAPK cascade |
| Transcript | Name | Biological Process |
|---|---|---|
| WNT2B | Wnt Family Member 2B | Neuron differentiation; forebrain reorganization; canonical Wnt signaling |
| WNT3 | Wnt Family Member 3 | Axon guidance; canonical Wnt signaling pathway involved in osteoblast differentiation; pathway involved in midbrain dopaminergic neuron differentiation; neuron differentiation; regulation of neurogenesis |
| WNT10A | Wnt Family Member 10A | Neural crest cell differentiation; neuron differentiation; canonical Wnt signaling pathway |
| WNT10B | Wnt Family Member 10B | Positive regulation of osteoblast differentiation; regulation of skeletal muscle tissue development; canonical Wnt signaling pathway; positive regulation of bone mineralization; neuron differentiation |
| WNT4 | Wnt Family Member 4 | Kidney development; positive regulation of bone mineralization; neuron differentiation; canonical Wnt signaling pathway |
| WNT8A | Wnt Family Member 8A | Neuron differentiation; canonical Wnt signaling |
| WNT9A | Wnt Family Member 9A | Neuron differentiation; canonical Wnt signaling |
| WNT16 | Wnt Family Member 16 | Bone remodeling; neuron differentiation |
| WNT7A | Wnt Family Member 7A | Central nervous system vasculogenesis; axonogenesis; positive regulation of synapse assembly; neuron differentiation; excitatory synapse assembly; neurotransmitter secretion; dendritic spine morphogenesis; regulation of axon diameter; cell proliferation in forebrain |
| WNT11 | Wnt Family Member 11 | Neuron differentiation; artery morphogenesis; osteoblast differentiation; bone mineralization |
| WNT5B | Wnt Family Member 5B | Neuron differentiation |
| WNT5A | Wnt Family Member 5A | Axon guidance; positive regulation of angiogenesis; activation of MAPK activity; inhibitory synapse assembly; excitatory synapse assembly; positive regulation of ossification; chemoattraction of serotonergic neuron axon; chemorepulsion of dopaminergic neuron axon; positive regulation of fibroblast proliferation; positive regulation of protein localization to synapse; positive regulation of neuron projection development; neuron differentiation; Wnt signaling pathway involved in midbrain dopaminergic neuron differentiation; regulation of postsynaptic cytosolic calcium ion concentration |
| Transcript | Name | Biological Process |
|---|---|---|
| FGF10 | Fibroblast Growth Factor 10 | Activation of MAPK activity; angiogenesis; positive regulation of fibroblast proliferation; radial glial cell differentiation; positive regulation of MAPK cascade; pituitary gland development; actin cytoskeletal reorganization; tissue regeneration; blood vessel remodeling |
| FGF5 | Fibroblast Growth Factor 5 | Nervous system development; glial cell differentiation |
| FGF2 | Fibroblast Growth Factor 2 | Positive regulation of cardiac muscle cell proliferation; positive regulation of angiogenesis; activation of MAPK activity; nervous system development; negative regulation of cell death; somatic stem cell population maintenance; |
| FGF13 | Fibroblast Growth Factor 13 | Nervous system development; hippocampus development; learning; memory; cerebral cortex cell migration |
| FGF19 | Fibroblast Growth Factor 19 | MAPK cascade; nervous system development |
| FGF18 | Fibroblast Growth Factor 18 | Positive regulation of MAP kinase activity; positive regulation of angiogenesis |
| FGF1 | Fibroblast Growth Factor 1 | Positive regulation of MAP kinase activity; positive regulation of angiogenesis; lung development |
| FGF9 | Fibroblast Growth Factor 9 | Positive regulation of cardiac muscle cell proliferation; angiogenesis; osteoblast differentiation; substantia nigra development |
| FGF12 | Fibroblast Growth Factor 12 | Regulation of neuronal action potential; neuromuscular process; nervous system development; chemical synaptic transmission; heart development |
| FGF7 | Fibroblast Growth Factor 7 | Epidermis development; actin cytoskeleton reorganization |
| FGF6 | Fibroblast Growth Factor 6 | MAPK cascade; angiogenesis |
| FGF11 | Fibroblast Growth Factor 11 | Nervous system development |
| FGF4 | Fibroblast Growth Factor 4 | Stem cell population maintenance; MAPK cascade |
| FGF23 | Fibroblast Growth Factor 23 | Vitamin D catabolic process; negative regulation of osteoblast differentiation; negative regulation of bone mineralization |
| FGF20 | Fibroblast Growth Factor 20 | Positive regulation of dopaminergic neuron differentiation; negative regulation of neuron apoptotic process |
| FGF14 | Fibroblast Growth Factor 14 | Regulation of postsynaptic membrane potential; regulation of synaptic vesicle recycling; regulation of synaptic plasticity; nervous system development |
| PSPN | Persephin | Axon guidance; nervous system development |
| GDNF | Glial Cell Derived Neurotrophic Factor | Axon guidance; nervous system development |
| PGF | Placental Growth Factor | Positive regulation of angiogenesis |
| VEGFA | Vascular Endothelial Growth Factor A | Positive regulation of angiogenesis; response to hypoxia; positive regulation of MAP kinase activity; commissural neuron axon guidance; positive regulation of blood vessel endothelial cell migration; dopaminergic neuron differentiation; hearth morphogenesis; positive regulation of neuroblast differentiation; artery morphogenesis; positive regulation of axon extension involved in axon guidance |
| VEGFC | Vascular Endothelial Growth Factor C | Positive regulation of angiogenesis; response to hypoxia; positive regulation of neuroblast proliferation; negative regulation of blood pressure |
| VEGFB | Vascular Endothelial Growth Factor B | Positive regulation of angiogenesis; negative regulation of neuron apoptotic process; response to hypoxia |
| NGF | Nerve Growth Factor | Neuron projection morphogenesis; positive regulation of neuron differentiation; memory; nerve development; negative regulation of neuron apoptotic process; positive regulation of axonogenesis; peripheral nervous system development |
| NTF3 | Neurotrophin 3 | Nervous system development; neuron projection morphogenesis; regulation of neuron differentiation; memory; nerve development; negative regulation of neuron apoptotic process; peripheral nervous system development |
| NTF4 | Neurotrophin 4 | Neuron projection morphogenesis; peripheral nervous system development; regulation of neuron differentiation; nerve development; negative regulation of neuron apoptotic process; long-term memory |
| BDNF | Brain Derived Neurotrophic Factor | Nervous system development; neuron projection morphogenesis; axon guidance; regulation of neuron differentiation; memory; nerve development; negative regulation of neuron apoptotic process; positive regulation of neuron projection development |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silvestro, S.; Chiricosta, L.; Gugliandolo, A.; Pizzicannella, J.; Diomede, F.; Bramanti, P.; Trubiani, O.; Mazzon, E. Extracellular Vesicles Derived from Human Gingival Mesenchymal Stem Cells: A Transcriptomic Analysis. Genes 2020, 11, 118. https://doi.org/10.3390/genes11020118
Silvestro S, Chiricosta L, Gugliandolo A, Pizzicannella J, Diomede F, Bramanti P, Trubiani O, Mazzon E. Extracellular Vesicles Derived from Human Gingival Mesenchymal Stem Cells: A Transcriptomic Analysis. Genes. 2020; 11(2):118. https://doi.org/10.3390/genes11020118
Chicago/Turabian StyleSilvestro, Serena, Luigi Chiricosta, Agnese Gugliandolo, Jacopo Pizzicannella, Francesca Diomede, Placido Bramanti, Oriana Trubiani, and Emanuela Mazzon. 2020. "Extracellular Vesicles Derived from Human Gingival Mesenchymal Stem Cells: A Transcriptomic Analysis" Genes 11, no. 2: 118. https://doi.org/10.3390/genes11020118
APA StyleSilvestro, S., Chiricosta, L., Gugliandolo, A., Pizzicannella, J., Diomede, F., Bramanti, P., Trubiani, O., & Mazzon, E. (2020). Extracellular Vesicles Derived from Human Gingival Mesenchymal Stem Cells: A Transcriptomic Analysis. Genes, 11(2), 118. https://doi.org/10.3390/genes11020118





