Eag1 Gene and Protein Expression in Human Retinoblastoma Tumors and its Regulation by pRb in HeLa Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line and Reagents
2.2. Cell Transfection
2.3. Human Tumor Samples and Primary Cultures
2.4. Real-Time PCR
2.5. Cell Proliferation
2.6. Immunochemistry
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
3.1. RB1 Transfection Decreased Eag1 Expression in HeLa Cells
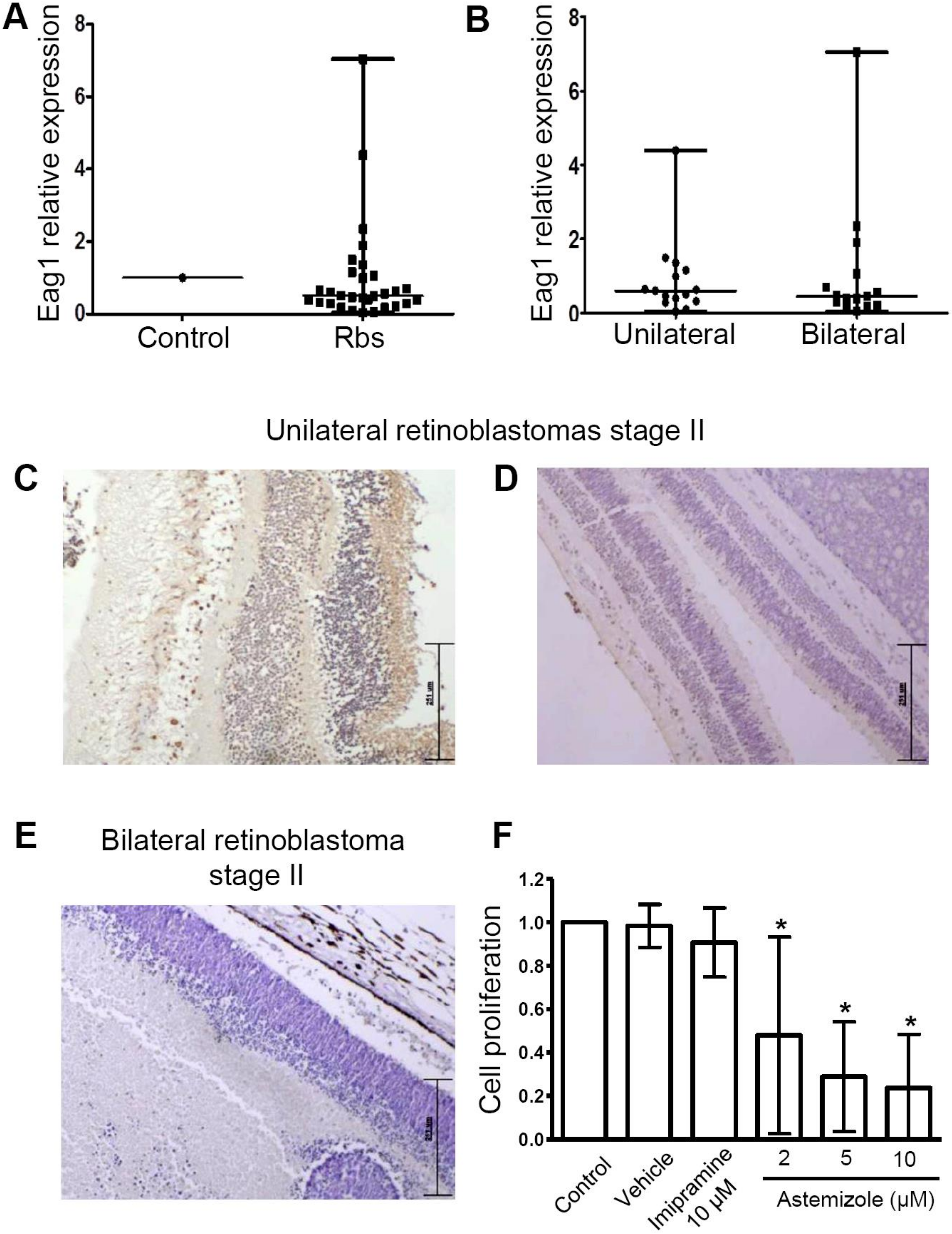
3.2. Heterogeneous Eag1 mRNA and Protein Expression in Human Retinoblastoma Samples
3.3. Astemizole Decreased the Cell Proliferation in Primary Retinoblastoma Cultures
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wong, J.R.; Tucker, M.A.; Kleinerman, R.A.; Devesa, S.S. Retinoblastoma incidence patterns in the US Surveillance, Epidemiology, and End Results program. JAMA Ophthalmol. 2014, 132, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Naseripour, M. “Retinoblastoma survival disparity”: The expanding horizon in developing countries. Saudi J. Ophthalmol. 2012, 26, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.H.; Kleinerman, R.A.; Seddon, J.M.; Abramson, D.H. Increased risk of secondary uterine leiomyosarcoma in hereditary retinoblastoma. Gynecol. Oncol. 2012, 124, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Kleinerman, R.A.; Schonfeld, S.J.; Tucker, M.A. Sarcomas in hereditary retinoblastoma. Clin. Sarcoma Res. 2012, 2, 15. [Google Scholar] [CrossRef]
- Knudson, A.G., Jr. Mutation and cancer: Statistical study of retinoblastoma. Proc. Natl. Acad. Sci. USA 1971, 68, 820–823. [Google Scholar] [CrossRef]
- Hirsch, H.A.; Gu, L.; Henry, R.W. The retinoblastoma tumor suppressor protein targets distinct general transcription factors to regulate RNA polymerase III gene expression. Mol. Cell. Biol. 2000, 20, 9182–9191. [Google Scholar] [CrossRef][Green Version]
- Dunaief, J.L.; Strober, B.E.; Guha, S.; Khavari, P.A.; Alin, K.; Luban, J.; Begemann, M.; Crabtree, G.R.; Goff, S.P. The retinoblastoma protein and BRG1 form a complex and cooperate to induce cell cycle arrest. Cell 1994, 79, 119–130. [Google Scholar] [CrossRef]
- Zhang, J.; Benavente, C.A.; McEvoy, J.; Flores-Otero, J.; Ding, L.; Chen, X.; Ulyanov, A.; Wu, G.; Wilson, M.; Wang, J.; et al. A novel retinoblastoma therapy from genomic and epigenetic analyses. Nature 2012, 481, 329–334. [Google Scholar] [CrossRef]
- Manning, A.L.; Dyson, N.J. RB: Mitotic implications of a tumour suppressor. Nat. Rev. Cancer 2012, 12, 220–226. [Google Scholar] [CrossRef]
- Castro-Magdonel, B.E.; Orjuela, M.; Camacho, J.; García-Chéquer, A.J.; Cabrera-Muñoz, M.d.L.; Sadowinski-Pine, S.; Durán-Figueroa, N.; Orozco, M.d.J.; Velazquez, A.C.; Hernández-Ángeles, A.; et al. miRNOME landscape analysis reveals a 30 miRNA core in retinoblastoma. BMC Cancer 2017, 17, 458. [Google Scholar] [CrossRef]
- Pardo, L.A.; del Camino, D.; Sanchez, A.; Alves, F.; Bruggemann, A.; Beckh, S.; Stühmer, W. Oncogenic potential of EAG K(+) channels. EMBO J. 1999, 18, 5540–5547. [Google Scholar] [CrossRef] [PubMed]
- Occhiodoro, T.; Bernheim, L.; Liu, J.H.; Bijlenga, P.; Sinnreich, M.; Bader, C.R.; Fischer-Lougheed, J. Cloning of a human ether-a-go-go potassium channel expressed in myoblasts at the onset of fusion. FEBS Lett. 1998, 434, 177–182. [Google Scholar] [CrossRef]
- Hemmerlein, B.; Weseloh, R.M.; Mello de Queiroz, F.; Knotgen, H.; Sanchez, A.; Rubio, M.E.; Martin, S.; Schliephacke, T.; Jenke, M.; Heinz Joachim, R.; et al. Overexpression of Eag1 potassium channels in clinical tumours. Mol. Cancer 2006, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Frings, S.; Brull, N.; Dzeja, C.; Angele, A.; Hagen, V.; Kaupp, U.B.; Baumann, A. Characterization of ether-a-go-go channels present in photoreceptors reveals similarity to IKx, a K+ current in rod inner segments. J. Gen. Physiol. 1998, 111, 583–599. [Google Scholar] [CrossRef]
- Available online: http://www.proteinatlas.org/ENSG00000143473-KCNH1/tissue (accessed on 29 September 2017).
- Farias, L.M.; Ocana, D.B.; Diaz, L.; Larrea, F.; Avila-Chavez, E.; Cadena, A.; Hinojosa, L.M.; Lara, G.; Villanueva, L.A.; Vargas, C.; et al. Ether à-go-go potassium channels as human cervical cancer markers. Cancer Res. 2004, 64, 6996–7001. [Google Scholar] [CrossRef]
- Ousingsawat, J.; Spitzner, M.; Puntheeranurak, S.; Terracciano, L.; Tornillo, L.; Bubendorf, L.; Kunzelmann, K. Expression of voltage-gated potassium channels in human and mouse colonic carcinoma. Clin. Cancer Res. 2007, 13, 824–831. [Google Scholar] [CrossRef]
- De Guadalupe Chavez-Lopez, M.; Hernandez-Gallegos, E.; Vazquez-Sanchez, A.Y.; Gariglio, P.; Camacho, J. Antiproliferative and proapoptotic effects of astemizole on cervical cancer cells. Int. J. Gynecol. Cancer 2014, 24, 824–828. [Google Scholar] [CrossRef]
- De Guadalupe Chavez-Lopez, M.; Perez-Carreon, J.I.; Zuniga-Garcia, V.; Diaz-Chavez, J.; Herrera, L.A.; Caro-Sanchez, C.H.; Acuna-Macias, I.; Gariglio, P.; Hernandez-Gallegos, E.; Chiliquinga, A.J.; et al. Astemizole-based anticancer therapy for hepatocellular carcinoma (HCC), and Eag1 channels as potential early-stage markers of HCC. Tumor Biol. 2015, 36, 6149–6158. [Google Scholar] [CrossRef]
- Wu, W.; Sanguinetti, M.C. Molecular Basis of Cardiac Delayed Rectifier Potassium Channel Function and Pharmacology. Card. Electrophysiol. Clin. 2016, 8, 275–284. [Google Scholar] [CrossRef]
- Fortunato, P.; Pillozzi, S.; Tamburini, A.; Pollazzi, L.; Franchi, A.; La Torre, A.; Arcangeli, A. Irresponsiveness of two retinoblastoma cases to conservative therapy correlates with up- regulation of hERG1 channels and of the VEGF-A pathway. BMC Cancer 2010, 10, 504. [Google Scholar] [CrossRef]
- Pardo, L.A.; Stühmer, W. The roles of K(+) channels in cancer. Nat. Rev. Cancer 2014, 14, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Downie, B.R.; Sanchez, A.; Knotgen, H.; Contreras-Jurado, C.; Gymnopoulos, M.; Weber, C.; Stühmer, W.; Pardo, L.A. Eag1 expression interferes with hypoxia homeostasis and induces angiogenesis in tumors. J. Biol. Chem. 2008, 283, 36234–36240. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Quiroz, J.; Camacho, J. Astemizole: An old anti-histamine as a new promising anti-cancer drug. Anti-Cancer Agents Med. Chem. 2011, 11, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Y.; Zhang, Y.; Guo, S.; Mo, L.; An, H. Eag1 Voltage-Dependent Potassium Channels: Structure, Electrophysiological Characteristics, and Function in Cancer. J. Membr. Biol. 2017, 250, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.; Ceja-Ochoa, I.; Restrepo-Angulo, I.; Larrea, F.; Avila-Chavez, E.; Garcia-Becerra, R.; Borja-Cacho, E.; Barrera, D.; Ahumada, E.; Gariglio, P.; et al. Estrogens and human papilloma virus oncogenes regulate human ether-a-go-go-1 potassium channel expression. Cancer Res. 2009, 69, 3300–3307. [Google Scholar] [CrossRef]
- Lin, H.; Li, Z.; Chen, C.; Luo, X.; Xiao, J.; Dong, D.; Lu, Y.; Yang, B.; Wang, Z. Transcriptional and post-transcriptional mechanisms for oncogenic overexpression of ether a go-go K+ channel. PLoS ONE 2011, 6, e20362. [Google Scholar] [CrossRef]
- Urrego, D.; Movsisyan, N.; Ufartes, R.; Pardo, L.A. Periodic expression of Kv10.1 driven by pRb/E2F1 contributes to G2/M progression of cancer and non-transformed cells. Cell Cycle 2016, 15, 799–811. [Google Scholar] [CrossRef]
- Orjuela, M.A.; Cabrera-Munoz, L.; Paul, L.; Ramirez-Ortiz, M.A.; Liu, X.; Chen, J.; Mejia-Rodriguez, F.; Medina-Sanson, A.; Diaz-Carreño, S.; Suen, I.H.; et al. Risk of retinoblastoma is associated with a maternal polymorphism in dihydrofolatereductase (DHFR) and prenatal folic acid intake. Cancer 2012, 118, 5912–5919. [Google Scholar] [CrossRef]
- Ortiz, C.S.; Montante-Montes, D.; Saqui-Salces, M.; Hinojosa, L.M.; Gamboa-Dominguez, A.; Hernandez-Gallegos, E.; Martínez-Benítez, B.; Solís-Pancoatl, M.R.; Garcia-Villa, E.; Ramírez, A.; et al. Eag1 potassium channels as markers of cervical dysplasia. Oncol. Rep. 2011, 26, 1377–1383. [Google Scholar]
- Davarinejad, H. Quantifications of Western Blots with ImageJ. Available online: www.yorku.ca/yisheng/Internal/Protocols/ImageJ.pdf (accessed on 20 January 2020).
- Napp, J.; Monje, F.; Stümer, W.; Pardo, L.A. Glycosylation of Eag1 (Kv10.1) potassium channels: Intracellular trafficking and functional consequences. J. Biol. Chem. 2005, 280, 29506–29512. [Google Scholar] [CrossRef]
- Greenbaum, D.; Colangelo, C.; Williams, K.; Gerstein, M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003, 4, 117. [Google Scholar] [CrossRef] [PubMed]
- Ufartes, R.; Schneider, T.; Mortensen, L.S.; de Juan Romero, C.; Hentrich, K.; Knoetgen, H.; Beilinson, V.; Moebius, W.; Tarabykin, V.; Alves, F.; et al. Behavioural and functional characterization of Kv10.1 (Eag1) knockout mice. Hum. Mol. Genet. 2013, 22, 2247–2262. [Google Scholar] [CrossRef] [PubMed]
- García-Quiroz, J.; García-Becerra, R.; Barrera, D.; Santos, N.; Avila, E.; Ordaz-Rosado, D.; Rivas-Suárez, M.; Halhali, A.; Rodríguez, P.; Gamboa-Domínguez, A.; et al. Astemizole Synergizes Calcitriol Antiproliferative Activity by Inhibiting CYP24A1 and Upregulating VDR: A Novel Approach for Breast Cancer Therapy. PLoS ONE 2012, 7, e45063. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Ramos, G.; Hernández-Gallegos, E.; Vera, E.; Chávez-López, M.G.; Zúñiga-García, V.; Sánchez-Pérez, Y.; Garrido, E.; Camacho, J. Astemizole inhibits cell proliferation in human prostate tumorigenic cells expressing ether à-go-go-1 potassium channels. Cell. Mol. Biol. 2017, 63, 11–13. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez-López, M.d.G.; Zúñiga-García, V.; Castro-Magdonel, B.E.; Vera, E.; Garrido, E.; Sánchez-Ramos, J.; Ponce-Castañeda, M.V.; Cabrera-Muñoz, M.d.L.; Escobar, Y.; Ortiz, C.S.; et al. Eag1 Gene and Protein Expression in Human Retinoblastoma Tumors and its Regulation by pRb in HeLa Cells. Genes 2020, 11, 119. https://doi.org/10.3390/genes11020119
Chávez-López MdG, Zúñiga-García V, Castro-Magdonel BE, Vera E, Garrido E, Sánchez-Ramos J, Ponce-Castañeda MV, Cabrera-Muñoz MdL, Escobar Y, Ortiz CS, et al. Eag1 Gene and Protein Expression in Human Retinoblastoma Tumors and its Regulation by pRb in HeLa Cells. Genes. 2020; 11(2):119. https://doi.org/10.3390/genes11020119
Chicago/Turabian StyleChávez-López, María de Guadalupe, Violeta Zúñiga-García, Blanca Elena Castro-Magdonel, Eunice Vera, Efraín Garrido, Janet Sánchez-Ramos, M. Verónica Ponce-Castañeda, M. de Lourdes Cabrera-Muñoz, Yesenia Escobar, Cindy Sharon Ortiz, and et al. 2020. "Eag1 Gene and Protein Expression in Human Retinoblastoma Tumors and its Regulation by pRb in HeLa Cells" Genes 11, no. 2: 119. https://doi.org/10.3390/genes11020119
APA StyleChávez-López, M. d. G., Zúñiga-García, V., Castro-Magdonel, B. E., Vera, E., Garrido, E., Sánchez-Ramos, J., Ponce-Castañeda, M. V., Cabrera-Muñoz, M. d. L., Escobar, Y., Ortiz, C. S., Hernández-Gallegos, E., Avalos-Fuentes, A., & Camacho, J. (2020). Eag1 Gene and Protein Expression in Human Retinoblastoma Tumors and its Regulation by pRb in HeLa Cells. Genes, 11(2), 119. https://doi.org/10.3390/genes11020119



