Multi-Omics Database Analysis of Aminoacyl-tRNA Synthetases in Cancer
Abstract
:1. Introduction
2. Materials and Methods
3. Results
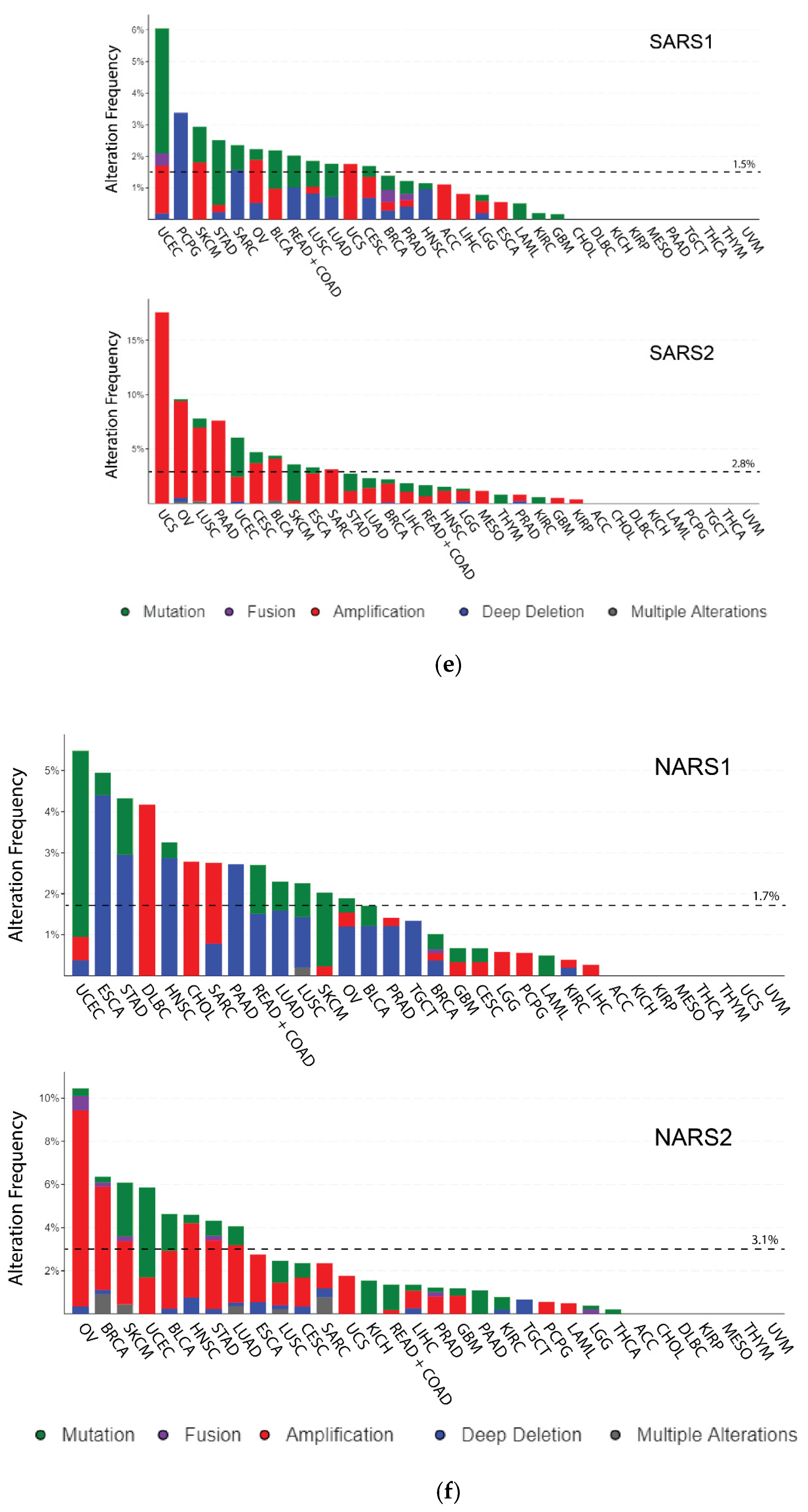
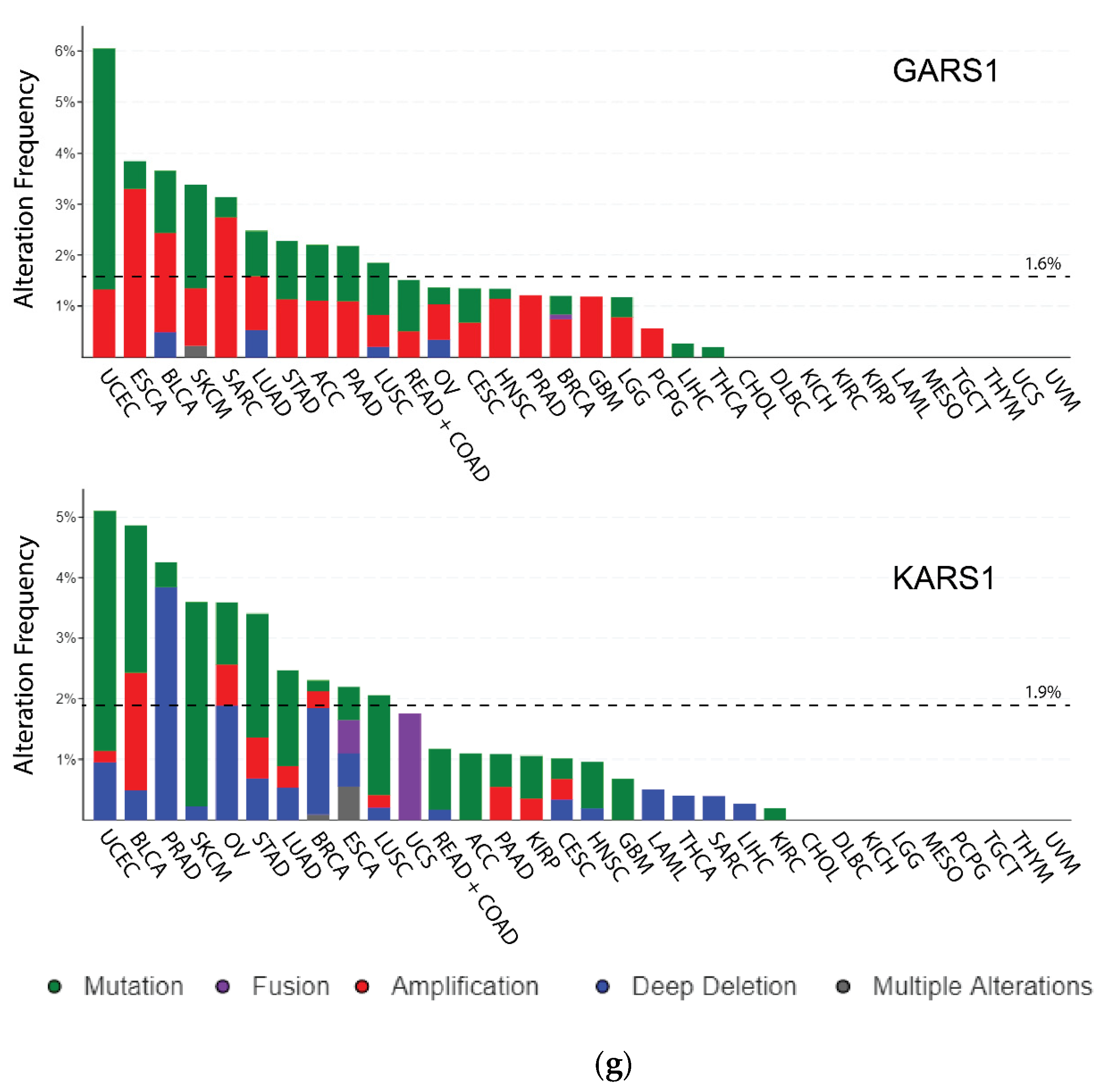
3.1. Genomic Variations (DNA)
3.2. Mutations
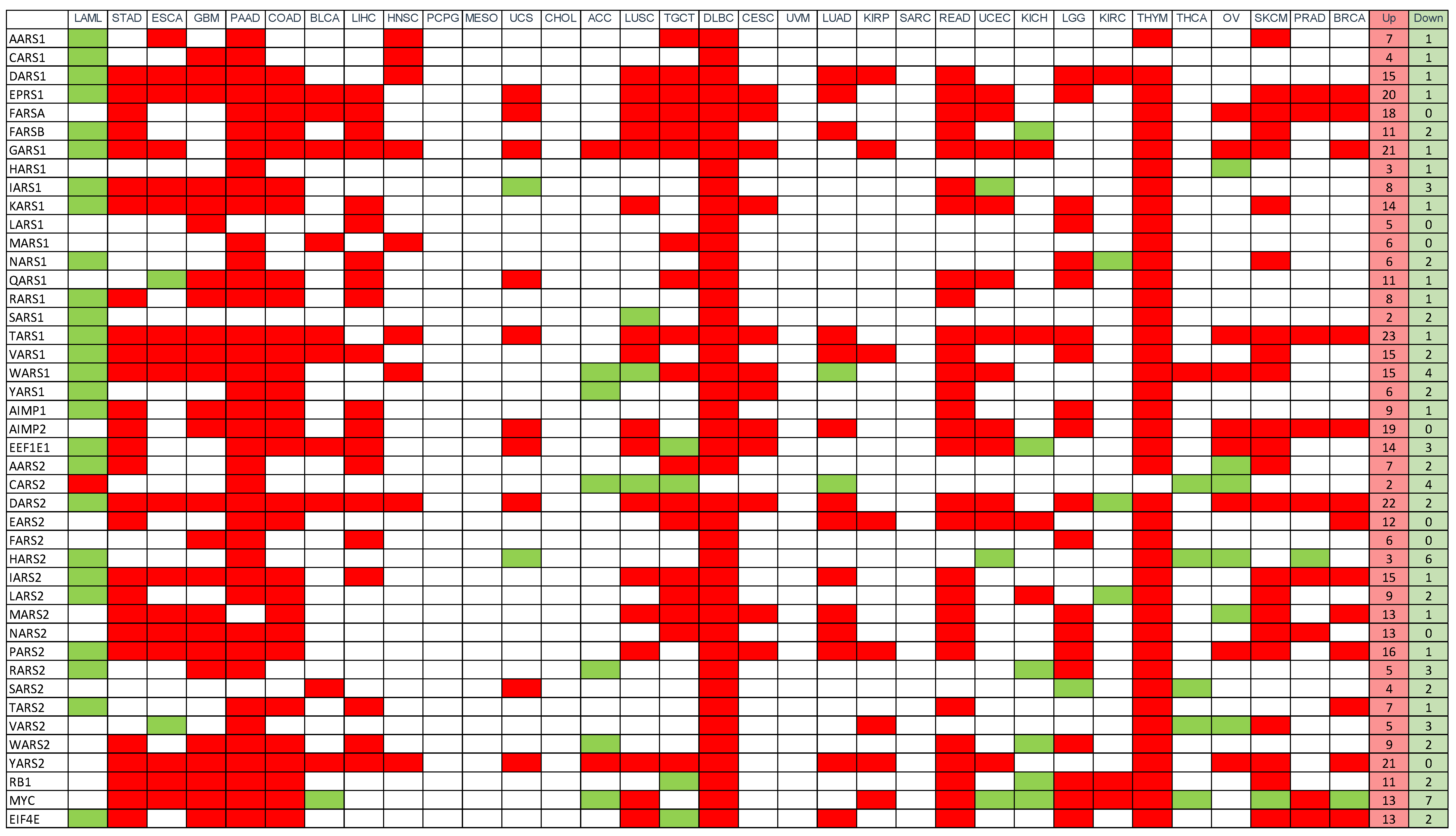
3.3. Gene Expression (mRNA)
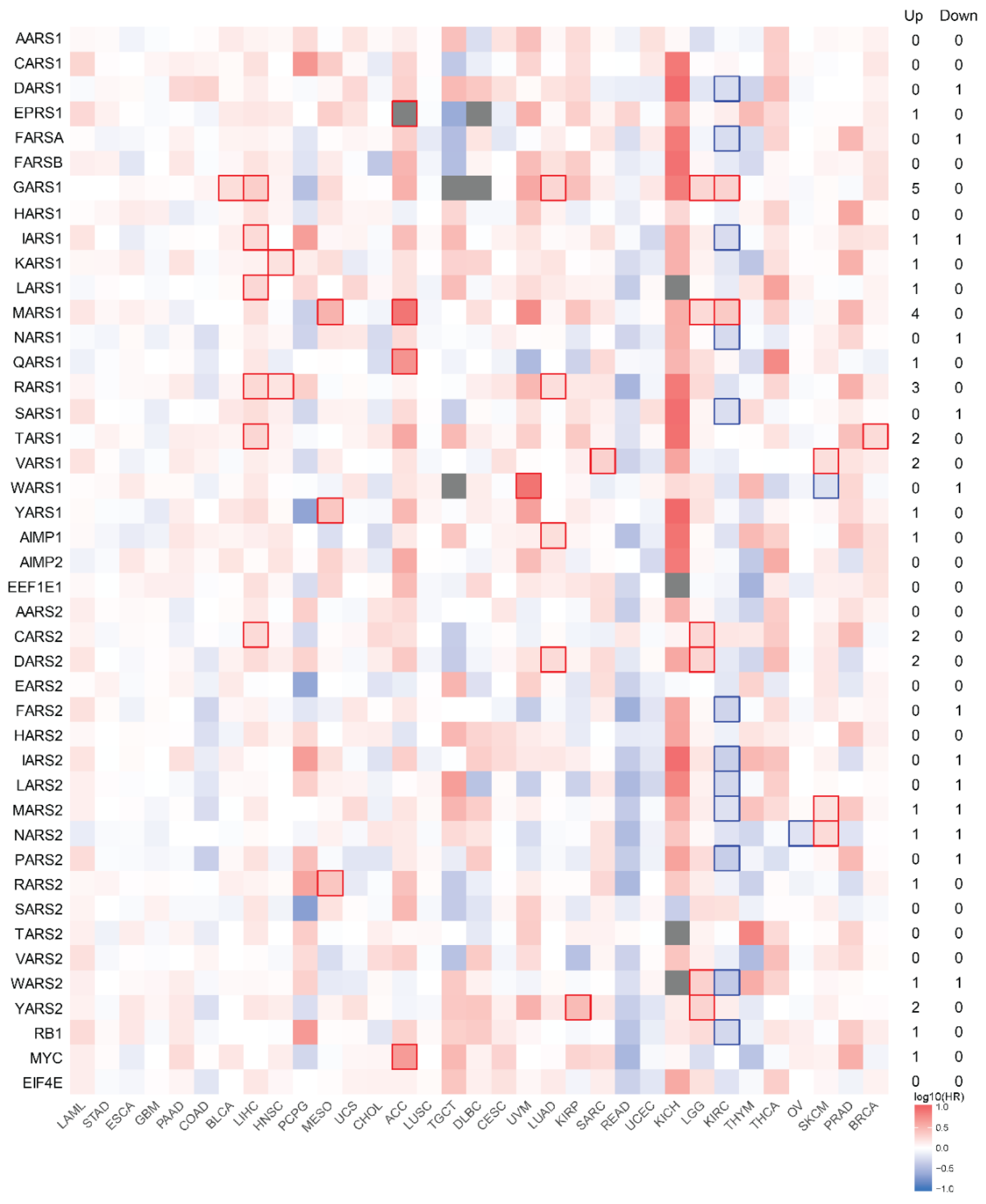
3.4. Survival Analysis with mRNA Expression
3.5. Proteomic Analysis
4. Discussion
4.1. Potential Cancer-Inhibiting aaRSs
4.2. An Example of Potential Cancer-Promoting aaRSs
4.3. Unexpected Cancer Association of AIMP2
4.4. Disconnect between FARSA and FARSB
4.5. EIF4E
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, S.; You, S.; Hwang, D. Aminoacyl-tRNA synthetases and tumorigenesis: More than housekeeping. Nat. Rev. Cancer 2011, 11, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kwon, N.H.; Kim, S. Association of Aminoacyl-tRNA Synthetases with Cancer. Photoinduced Phenom. Nucleic Acids II 2013, 344, 207–245. [Google Scholar] [CrossRef]
- Guo, M.; Yang, X.-L.; Schimmel, P. New functions of aminoacyl-tRNA synthetases beyond translation. Nat. Rev. Mol. Cell Biol. 2010, 11, 668–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, M.; Schimmel, P.; Yang, X.-L. Functional expansion of human tRNA synthetases achieved by structural inventions. FEBS Lett. 2010, 584, 434–442. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.Y.; Maeng, S.J.; Cho, H.J.; Choi, Y.S.; Chung, J.M.; Lee, S.; Kim, H.K.; Kim, J.H.; Eom, C.-Y.; Kim, Y.-G.; et al. Assembly of Multi-tRNA Synthetase Complex via Heterotetrameric Glutathione Transferase-homology Domains. J. Biol. Chem. 2015, 290, 29313–29328. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.-L. Structural Disorder in Expanding the Functionome of Aminoacyl-tRNA Synthetases. Chem. Biol. 2013, 20, 1093–1099. [Google Scholar] [CrossRef] [Green Version]
- Kyriacou, S.V.; Deutscher, M.P. An important role for the multienzyme aminoacyl-tRNA synthetase complex in mammalian translation and cell growth. Mol. Cell 2008, 29, 419–427. [Google Scholar]
- Ray, P.S.; Arif, A.; Fox, P.L. Macromolecular complexes as depots for releasable regulatory proteins. Trends Biochem. Sci. 2007, 32, 158–164. [Google Scholar] [CrossRef]
- Nam, S.H.; Kim, D.; Lee, D.; Lee, H.-M.; Song, D.-G.; Jung, J.W.; Kim, J.E.; Kim, H.-J.; Kwon, N.H.; Jo, E.-K.; et al. Lysyl-tRNA synthetase–expressing colon spheroids induce M2 macrophage polarization to promote metastasis. J. Clin. Investig. 2018, 128, 5034–5055. [Google Scholar] [CrossRef]
- Kwon, N.H.; Lee, J.Y.; Ryu, Y.-L.; Kim, C.; Kong, J.; Oh, S.; Kang, B.S.; Ahn, H.W.; Ahn, S.G.; Jeong, J.; et al. Stabilization of Cyclin-Dependent Kinase 4 by Methionyl-tRNA Synthetase in p16INK4a-Negative Cancer. ACS Pharmacol. Transl. Sci. 2018, 1, 21–31. [Google Scholar] [CrossRef]
- Katsyv, I.; Wang, M.; Song, W.M.; Zhou, X.; Zhao, Y.; Park, S.; Zhu, J.; Zhang, B.; Irie, H.Y. EPRS is a critical regulator of cell proliferation and estrogen signaling in ER+ breast cancer. Oncotarget 2016, 7, 69592–69605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyeon, D.Y.; Kim, J.H.; Ahn, T.J.; Cho, Y.; Hwang, D.; Kim, S. Evolution of the multi-tRNA synthetase complex and its role in cancer. J. Biol. Chem. 2019, 294, 5340–5351. [Google Scholar] [CrossRef] [Green Version]
- Otani, A.; Slike, B.M.; Dorrell, M.I.; Hood, J.; Kinder, K.; Ewalt, K.L.; Cheresh, D.; Schimmel, P.; Friedlander, M. A fragment of human TrpRS as a potent antagonist of ocular angiogenesis. Proc. Natl. Acad. Sci. USA 2002, 99, 178–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyanokoshi, M.; Yokosawa, T.; Wakasugi, K. Tryptophanyl-tRNA synthetase mediates high-affinity tryptophan uptake into human cells. J. Biol. Chem. 2018, 293, 8428–8438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, Y.H.; Park, S.; Choi, J.J.; Park, B.-K.; Rhee, K.H.; Kang, E.; Ahn, S.; Lee, C.-H.; Lee, J.S.; Inn, K.-S.; et al. Secreted tryptophanyl-tRNA synthetase as a primary defence system against infection. Nat. Microbiol. 2017, 2, 16191. [Google Scholar] [CrossRef]
- Lee, C.-W.; Chang, K.-P.; Chen, Y.-Y.; Liang, Y.; Hsueh, C.; Yu, J.-S.; Chang, Y.-S.; Yu, C.-J. Overexpressed tryptophanyl-tRNA synthetase, an angiostatic protein, enhances oral cancer cell invasiveness. Oncotarget 2015, 6, 21979–21992. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Wang, L.J.; Lombardo, K.; Kwak, Y.; Kim, W.H.; Resnick, M.B. Expression of Indoleamine 2, 3-dioxygenase 1 (IDO1) and Tryptophanyl-tRNA Synthetase (WARS) in Gastric Cancer Molecular Subtypes. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 360–368. [Google Scholar] [CrossRef]
- Yang, P.-P.; Yu, X.-H.; Zhou, J. Tryptophanyl-tRNA synthetase (WARS) expression in uveal melanoma—Possible contributor during uveal melanoma progression. Biosci. Biotechnol. Biochem. 2019, 84, 471–480. [Google Scholar] [CrossRef]
- Knudsen, E.S.; Pruitt, S.C.; Hershberger, P.A.; Witkiewicz, A.K.; Goodrich, D.W. Cell Cycle and Beyond: Exploiting New RB1 Controlled Mechanisms for Cancer Therapy. Trends Cancer 2019, 5, 308–324. [Google Scholar] [CrossRef]
- Siddiqui, N.; Sonenberg, N. Signalling to eIF4E in cancer. Biochem. Soc. Trans. 2015, 43, 763–772. [Google Scholar] [CrossRef]
- Shlien, A.; Malkin, D. Copy number variations and cancer. Genome Med. 2009, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data: Figure 1. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Lichtenberg, T.; Hoadley, K.A.; Poisson, L.M.; Lazar, A.J.; Cherniack, A.D.; Kovatich, A.J.; Benz, C.C.; Levine, D.A.; Lee, A.V.; et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 2018, 173, 400–416. [Google Scholar] [CrossRef] [Green Version]
- Williams, T.F.; Mirando, A.C.; Wilkinson, B.; Francklyn, C.S.; Lounsbury, K.M. Secreted Threonyl-tRNA synthetase stimulates endothelial cell migration and angiogenesis. Sci. Rep. 2013, 3, 1317. [Google Scholar] [CrossRef] [Green Version]
- Wellman, T.L.; Eckenstein, M.; Wong, C.; Rincon, M.; Ashikaga, T.; Mount, S.L.; Francklyn, C.S.; Lounsbury, K.M. Threonyl-tRNA synthetase overexpression correlates with angiogenic markers and progression of human ovarian cancer. BMC Cancer 2014, 14, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Shi, Y.; Zhang, H.-M.; Swindell, E.C.; Marshall, A.G.; Guo, M.; Kishi, S.; Yang, X.-L. Unique domain appended to vertebrate tRNA synthetase is essential for vascular development. Nat. Commun. 2012, 3, 681. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Xu, X.; Zhang, Q.; Fu, G.; Mo, Z.; Wang, G.S.; Kishi, S.; Yang, X.L. tRNA synthetase counteracts c-Myc to develop functional vasculature. Elife 2014, 3, e02349. [Google Scholar] [CrossRef]
- Ku, S.Y.; Rosario, S.; Wang, Y.; Mu, P.; Seshadri, M.; Goodrich, Z.W.; Goodrich, M.M.; Labbé, D.P.; Gomez, E.C.; Wang, J.; et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 2017, 355, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Marshall, A.E.; Roes, M.V.; Passos, D.T.; Deweerd, M.C.; Chaikovsky, A.C.; Sage, J.; Howlett, C.J.; Dick, F.A. RB1 Deletion in Retinoblastoma Protein Pathway-Disrupted Cells Results in DNA Damage and Cancer Progression. Mol. Cell. Biol. 2019, 39. [Google Scholar] [CrossRef] [Green Version]
- Azad, A.K.; Stanford, D.R.; Sarkar, S.; Hopper, A.K. Role of Nuclear Pools of Aminoacyl-tRNA Synthetases in tRNA Nuclear Export. Mol. Biol. Cell 2001, 12, 1381–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brydges, S.D.; Carruthers, V.B. Mutation of an unusual mitochondrial targeting sequence of SODB2 produces multiple targeting fates in Toxoplasma gondii. J. Cell Sci. 2003, 116, 4675–4685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diodato, D.; Ghezzi, D.; Tiranti, V. The Mitochondrial Aminoacyl tRNA Synthetases: Genes and Syndromes. Int. J. Cell Biol. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzima, E.; Reader, J.; Irani-Tehrani, M.; Ewalt, K.L.; Schwartz, M.A.; Schimmel, P. VE-cadherin Links tRNA Synthetase Cytokine to Anti-angiogenic Function. J. Biol. Chem. 2005, 280, 2405–2408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, B.-J.; Kang, J.W.; Lee, S.W.; Choi, S.-J.; Shin, Y.K.; Ahn, Y.H.; Choi, Y.H.; Choi, D.; Lee, K.S.; Kim, S. The Haploinsufficient Tumor Suppressor p18 Upregulates p53 via Interactions with ATM/ATR. Cell 2005, 120, 209–221. [Google Scholar] [CrossRef] [Green Version]
- Nagy, Á.; Lánczky, A.; Menyhárt, O.; Győrffy, B. Validation of miRNA prognostic power in hepatocellular carcinoma using expression data of independent datasets. Sci. Rep. 2018, 8, 9227. [Google Scholar]
- Uhlén, M.; Björling, E.; Agaton, C.; Szigyarto, C.A.-K.; Amini, B.; Andersen, E.; Andersson, A.-C.; Angelidou, P.; Asplund, A.; Asplund, C.; et al. A Human Protein Atlas for Normal and Cancer Tissues Based on Antibody Proteomics. Mol. Cell. Proteom. 2005, 4, 1920–1932. [Google Scholar] [CrossRef] [Green Version]
- Mo, Z.; Zhang, Q.; Liu, Z.; Lauer, J.; Yi, S.; Sun, L.; Griffin, P.R.; Yang, X.-L. Neddylation requires glycyl-tRNA synthetase to protect activated E2. Nat. Struct. Mol. Biol. 2016, 23, 730–737. [Google Scholar] [CrossRef]
- Yu, M.; Luo, C.; Huang, X.; Chen, D.; Li, S.; Qi, H.; Gao, X. Amino acids stimulate glycyl-tRNA synthetase nuclear localization for mammalian target of rapamycin expression in bovine mammary epithelial cells. J. Cell. Physiol. 2019, 234, 7608–7621. [Google Scholar] [CrossRef]
- Park, M.C.; Kang, T.; Jin, D.; Han, J.M.; Kim, S.B.; Park, Y.J.; Cho, K.; Guo, M.; He, W.; Yang, X.-L.; et al. Secreted human glycyl-tRNA synthetase implicated in defense against ERK-activated tumorigenesis. Proc. Natl. Acad. Sci. USA 2012, 109, E640–E647. [Google Scholar] [CrossRef] [Green Version]
- Jain, M.; Nilsson, R.; Sharma, S.; Madhusudhan, N.; Kitami, T.; Souza, A.L.; Kafri, R.; Kirschner, M.W.; Clish, C.B.; Mootha, V.K. Metabolite Profiling Identifies a Key Role for Glycine in Rapid Cancer Cell Proliferation. Science 2012, 336, 1040–1044. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.G.; Lee, J.Y.; Lee, J.H.; Cho, H.Y.; Kang, B.S.; Jang, S.Y.; Kim, M.H.; Guo, M.; Han, J.M.; Kim, S.J.; et al. Oncogenic Mutation of AIMP2/p38 Inhibits Its Tumor-Suppressive Interaction with Smurf2. Cancer Res. 2016, 76, 3422–3436. [Google Scholar] [CrossRef] [PubMed]
- Han, J.M.; Park, B.-J.; Park, S.G.; Oh, Y.S.; Choi, S.J.; Lee, S.W.; Hwang, S.-K.; Chang, S.-H.; Cho, M.-H.; Kim, S. AIMP2/p38, the scaffold for the multi-tRNA synthetase complex, responds to genotoxic stresses via p53. Proc. Natl. Acad. Sci. USA 2008, 105, 11206–11211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Chung, H.-J.; Vogt, M.; Jin, Y.; Malide, D.; He, L.; Dundr, M.; Levens, D.L. JTV1 co-activates FBP to induce USP29 transcription and stabilize p53 in response to oxidative stress. EMBO J. 2011, 30, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Kim, D.G.; Park, M.C.; Um, J.Y.; Han, J.M.; Park, S.G.; Choi, E.-C.; Kim, S. AIMP2 promotes TNF -dependent apoptosis via ubiquitin-mediated degradation of TRAF2. J. Cell Sci. 2009, 122, 2710–2715. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.W.; Kim, D.G.; Lee, A.-E.; Kim, H.R.; Lee, J.Y.; Kwon, N.H.; Shin, Y.K.; Hwang, S.-K.; Chang, S.-H.; Cho, M.-H.; et al. Cancer-Associated Splicing Variant of Tumor Suppressor AIMP2/p38: Pathological Implication in Tumorigenesis. PLoS Genet. 2011, 7, e1001351. [Google Scholar] [CrossRef]
- Choi, J.W.; Lee, J.-W.; Kim, J.K.; Jeon, H.-K.; Choi, J.-J.; Kim, D.G.; Kim, B.-G.; Nam, D.-H.; Kim, H.J.; Yun, S.H.; et al. Splicing variant of AIMP2 as an effective target against chemoresistant ovarian cancer. J. Mol. Cell Biol. 2012, 4, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Zhang, J.; Zhang, T. AIMP2-DX2 Promotes the Proliferation, Migration, and Invasion of Nasopharyngeal Carcinoma Cells. BioMed Res. Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Kim, D.G.; Kim, K.; Kim, T.; Lim, S.; Kong, H.; Kim, S.; Suh, Y.-G. 2-Aminophenylpyrimidines as Novel Inhibitors of Aminoacyl-tRNA Synthetase Interacting Multifunctional Protein 2 (AIMP2)-DX2 for Lung Cancer Treatment. J. Med. Chem. 2020, 63, 3908–3914. [Google Scholar] [CrossRef]
- Lim, S.; Cho, H.Y.; Kim, D.G.; Roh, Y.; Son, S.-Y.; Mushtaq, A.U.; Kim, M.; Bhattarai, D.; Sivaraman, A.; Lee, Y.; et al. Targeting the interaction of AIMP2-DX2 with HSP70 suppresses cancer development. Nat. Chem. Biol. 2019, 16, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Lo, W.-S.; Beck, D.B.; Schuch, L.A.; Oláhová, M.; Kopajtich, R.; Chong, Y.E.; Alston, C.L.; Seidl, E.; Zhai, L.; et al. Bi-allelic Mutations in Phe-tRNA Synthetase Associated with a Multi-system Pulmonary Disease Support Non-translational Function. Am. J. Hum. Genet. 2018, 103, 100–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Abronzo, L.S.; Ghosh, P.M. eIF4E Phosphorylation in Prostate Cancer. Neoplasia 2018, 20, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Arif, A.; Jia, J.; Mukhopadhyay, R.; Willard, B.; Kinter, M.; Fox, P.L. Two-Site Phosphorylation of EPRS Coordinates Multimodal Regulation of Noncanonical Translational Control Activity. Mol. Cell 2009, 35, 164–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.G.; Choi, J.W.; Lee, J.Y.; Kim, H.; Oh, Y.S.; Lee, J.W.; Tak, Y.K.; Song, J.M.; Razin, E.; Yun, S.H.; et al. Interaction of two translational components, lysyl-tRNA synthetase and p40/37LRP, in plasma membrane promotes laminin-dependent cell migration. FASEB J. 2012, 26, 4142–4159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, N.H.; Kang, T.; Lee, J.Y.; Kim, H.H.; Hong, J.; Oh, Y.S.; Han, J.M.; Ku, M.J.; Lee, S.Y.; Kim, S. Dual role of methionyl-tRNA synthetase in the regulation of translation and tumor suppressor activity of aminoacyl-tRNA synthetase-interacting multifunctional protein-3. Proc. Natl. Acad. Sci. USA 2011, 108, 19635–19640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adam, I.; Dewi, D.L.; Mooiweer, J.; Sadik, A.; Mohapatra, S.R.; Berdel, B.; Keil, M.; Sonner, J.K.; Thedieck, K.; Rose, A.J.; et al. Upregulation of tryptophanyl-tRNA synthethase adapts human cancer cells to nutritional stress caused by tryptophan degradation. OncoImmunology 2018, 7, e1486353. [Google Scholar] [CrossRef] [Green Version]



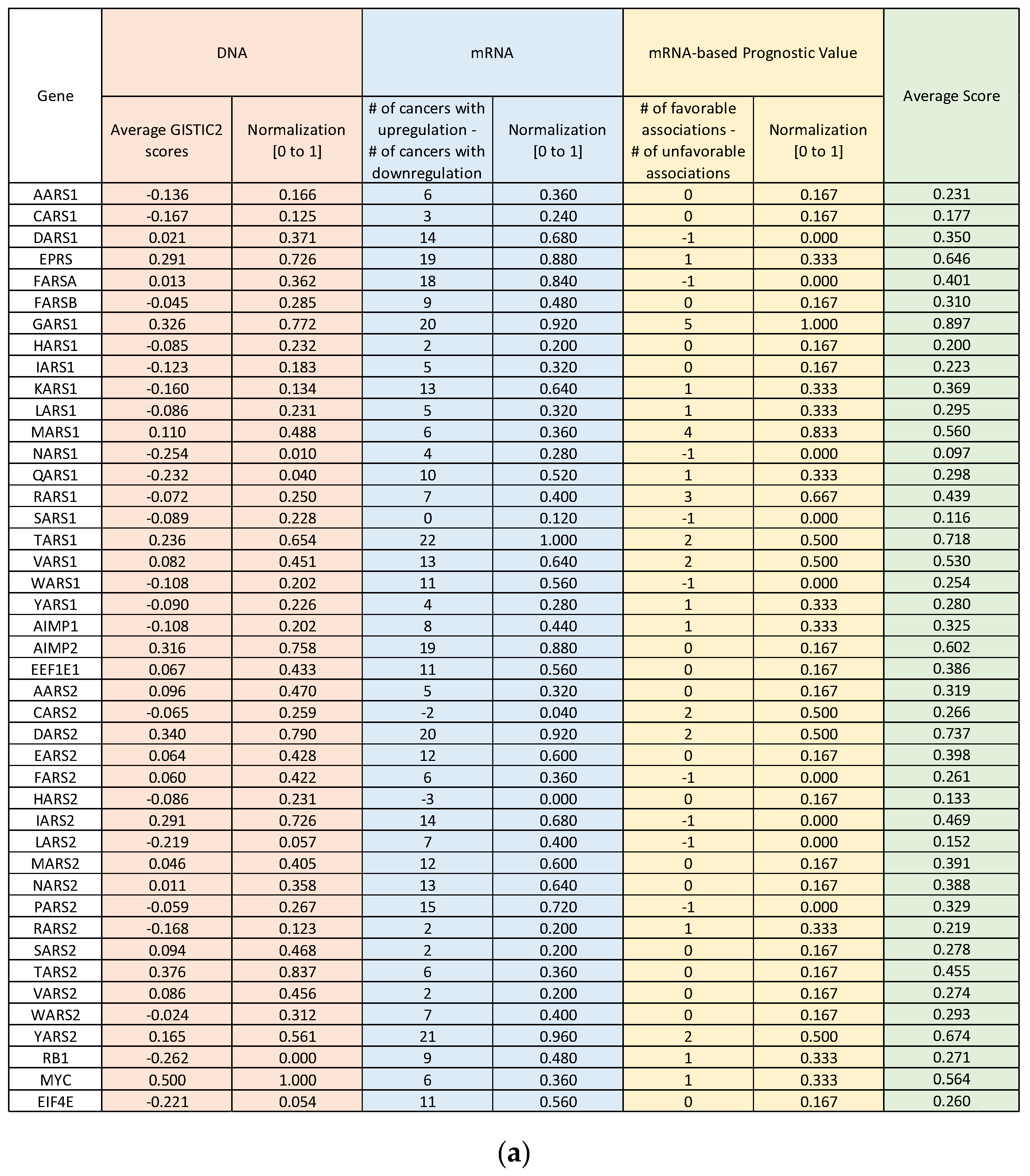
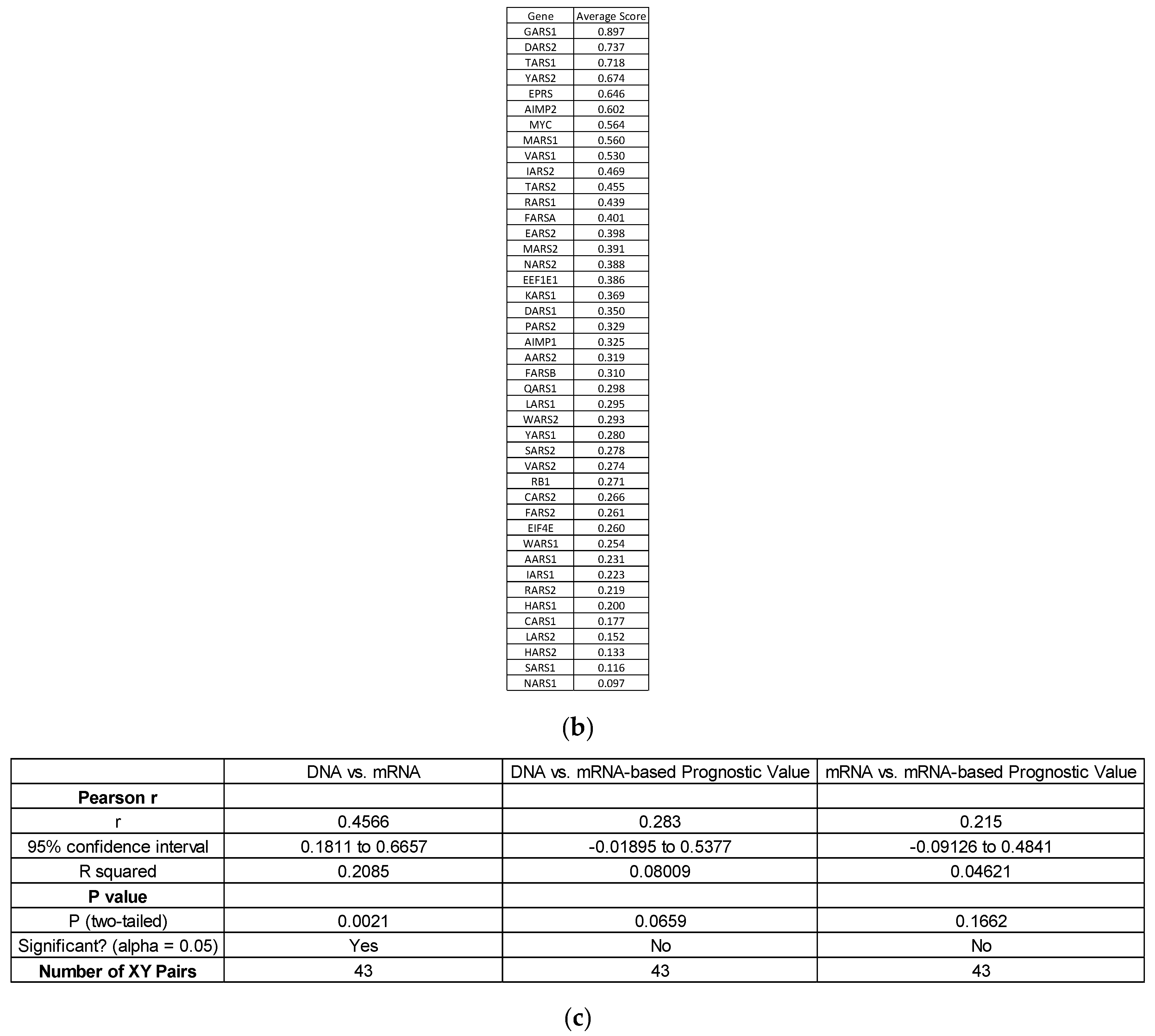
| Abbreviation | Cancer Type | OS Median Time to Event (Months) [24] |
|---|---|---|
| ACC | Adrenocortical carcinoma | 18.1 |
| BLCA | Bladder Urothelial Carcinoma | 13.5 |
| BRCA | Breast invasive carcinoma | 41.8 |
| CESC | Cervical squamous cell carcinoma and endocervical adenocarcinoma | 19.9 |
| CHOL | Cholangio carcinoma | 18 |
| COAD | Colon adenocarcinoma | 13.3 |
| DLBC | Lymphoid Neoplasm Diffuse Large B-cell Lymphoma | 19.5 |
| ESCA | Esophageal carcinoma | 11.5 |
| GBM | Glioblastoma multiforme | 12.6 |
| HNSC | Head and Neck squamous cell carcinoma | 14.1 |
| KICH | Kidney Chromophobe | 24.3 |
| KIRC | Kidney renal clear cell carcinoma | 26.9 |
| KIRP | Kidney renal papillary cell carcinoma | 21.1 |
| LAML | Acute Myeloid Leukemia | 9 |
| LGG | Brain Lower Grade Glioma | 26.7 |
| LIHC | Liver hepatocellular carcinoma | 13.7 |
| LUAD | Lung adenocarcinoma | 20.3 |
| LUSC | Lung squamous cell carcinoma | 18.1 |
| MESO | Mesothelioma | 15 |
| OV | Ovarian serous cystadenocarcinoma | 35.3 |
| PAAD | Pancreatic adenocarcinoma | 12.9 |
| PCPG | Pheochromocytoma and Paraganglioma | 14.9 |
| PRAD | Prostate adenocarcinoma | 36.2 |
| READ | Rectum adenocarcinoma | 22 |
| SARC | Sarcoma | 21.3 |
| SKCM | Skin Cutaneous Melanoma | 35.3 |
| STAD | Stomach adenocarcinoma | 11.3 |
| TGCT | Testicular Germ Cell Tumors | 18.6 |
| THCA | Thyroid carcinoma | 33.5 |
| THYM | Thymoma | 28 |
| UCEC | Uterine Corpus Endometrial Carcinoma | 23.3 |
| UCS | Uterine Carcinosarcoma | 17.1 |
| UVM | Uveal Melanoma | 19.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Vallee, I.; Dutta, A.; Wang, Y.; Mo, Z.; Liu, Z.; Cui, H.; Su, A.I.; Yang, X.-L. Multi-Omics Database Analysis of Aminoacyl-tRNA Synthetases in Cancer. Genes 2020, 11, 1384. https://doi.org/10.3390/genes11111384
Wang J, Vallee I, Dutta A, Wang Y, Mo Z, Liu Z, Cui H, Su AI, Yang X-L. Multi-Omics Database Analysis of Aminoacyl-tRNA Synthetases in Cancer. Genes. 2020; 11(11):1384. https://doi.org/10.3390/genes11111384
Chicago/Turabian StyleWang, Justin, Ingrid Vallee, Aditi Dutta, Yu Wang, Zhongying Mo, Ze Liu, Haissi Cui, Andrew I. Su, and Xiang-Lei Yang. 2020. "Multi-Omics Database Analysis of Aminoacyl-tRNA Synthetases in Cancer" Genes 11, no. 11: 1384. https://doi.org/10.3390/genes11111384
APA StyleWang, J., Vallee, I., Dutta, A., Wang, Y., Mo, Z., Liu, Z., Cui, H., Su, A. I., & Yang, X.-L. (2020). Multi-Omics Database Analysis of Aminoacyl-tRNA Synthetases in Cancer. Genes, 11(11), 1384. https://doi.org/10.3390/genes11111384





