Sensitivity to Dietary Wheat Gluten in Atlantic Salmon Indicated by Gene Expression Changes in Liver and Intestine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Customised Salmon Diets
2.2. Fish and Feeding Trial
2.3. Measurements of Blood Parameters
2.4. Chemical Analysis of Liver Fat
2.5. RNA Extraction
2.6. Microarray
2.7. Data Analysis
2.8. Ethical Statement
3. Results
3.1. Growth Performance
3.2. Blood Serum Analysis
3.3. Hepatosomatic Index and Liver Fat Content
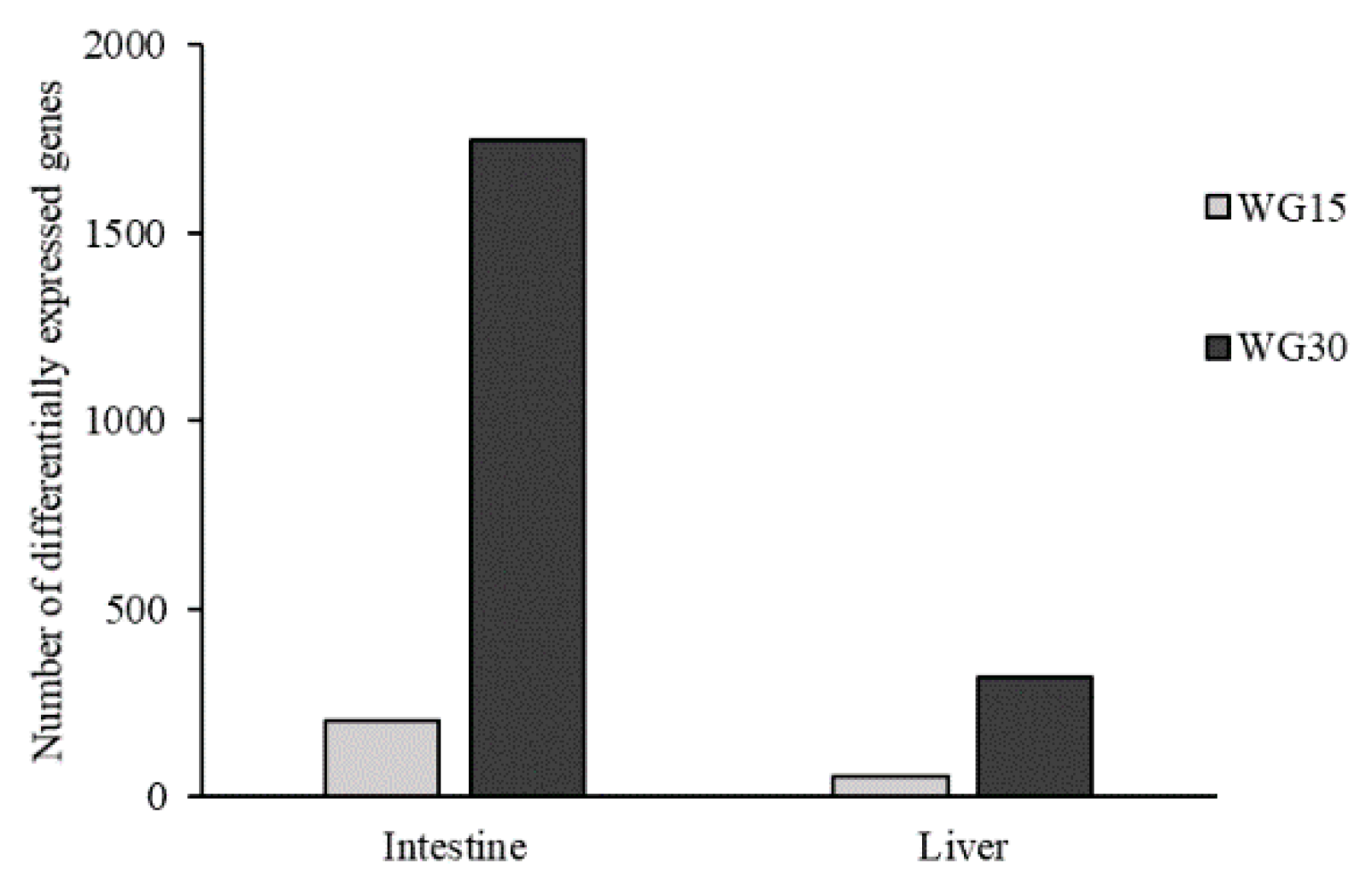
3.4. Gene Expression Profiling
3.5. Intestinal Gene Expression
3.6. Hepatic Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. The State of Food Security and Nutrition in the World 2018: Building Climate Resilience for food Security and Nutrition; Food and Agriculture Organization: Rome, Italy, 2018. [Google Scholar]
- Seafood.org. Available online: https://seafoodfromnorway.us/origin/Norway-the-worlds-leader-in-aquaculture/the-truth-about-norwegian-farm-raised-salmon/ (accessed on 14 March 2020).
- Skåre, J.U.; Brantsæter, A.L.; Frøyland, L.; Hemre, G.I.; Knutsen, H.K.; Lillegaard, I.T.; Andreassen, Å.K.; Elvevoll, E.O.; Andersen, L.F.; Hjeltnes, B.; et al. Benefit-Risk Assessment of Fish and Fish Products in the Norwegian Diet—An Update. Opinion of the Scientific Steering Committee of the Norwegian Scientific Committee for Food Safety; VKM Report; Norwegian Scientific Committee for Food Safety: Oslo, Norway, 2014.
- Ytrestøyl, T.; Aas, T.S.; Åsgård, T. Utilisation of feed resources in production of Atlantic salmon (Salmo salar) in Norway. Aquaculture 2015, 448, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Aas, T.S.; Ytrestøyl, T.; Åsgård, T. Utilization of feed resources in the production of Atlantic salmon (Salmo salar) in Norway: An update for 2016. Aquac. Rep. 2019, 15, 100216. [Google Scholar] [CrossRef]
- Tacon, A.G.J.; Metian, M. Global overview on the use of fish meal and fish oil in industrially compounded aquaculture: Trends and future prospects. Aquaculture 2008, 285, 146–158. [Google Scholar] [CrossRef]
- Carter, C.G.; Hauler, R.C. Fish meal replacement by plant meals in extruded feeds for Atlantic salmon (Salmo salar) L. Aquaculture 2000, 185, 299–311. [Google Scholar] [CrossRef] [Green Version]
- Morken, T.; Kraugerud, O.F.; Sørensen, M.; Storebakken, T.; Hillestad, M.; Christiansen, R.; Øverland, M. Effects of feed processing conditions and acid salts on nutrient digestibility and physical quality of soy-based diets for Atlantic salmon (Salmo salar). Aquac. Nutr. 2010, 18, 21–34. [Google Scholar] [CrossRef]
- Refstie, S.; Storebakken, T.; Baeverfjord, G.; Roem, A.J. Long-term protein and lipid growth of Atlantic salmon (Salmo salar) fed diets with partial replacement of fish meal by soy protein products at medium or high lipid level. Aquaculture 2001, 193, 91–106. [Google Scholar] [CrossRef]
- Penn, M.H.; Bendiksen, E.Å.; Campbell, P.; Krogdahl, Å. High level of dietary pea protein concentrate induces enteropathy in Atlantic salmon (Salmo salar L.). Aquaculture 2011, 310, 267–273. [Google Scholar] [CrossRef]
- Apper-Bossard, E.; Feneuil, A.; Wagner, A.; Respondek, F. Use of vital wheat gluten in aquaculture feeds. Aquat. Biosyst. 2013, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Storebakken, T.; Shearer, K.D.; Baeverfjord, G.; Nielsen, B.G.; Åsgård, T.; Scott, T.; De Laporte, A. Digestibility of macronutrients, energy and amino acids, absorption of elements and absence of intestinal enteritis in Atlantic salmon (Salmo salar) fed diets with wheat gluten. Aquaculture 2000, 184, 115–132. [Google Scholar] [CrossRef]
- Francis, G.; Makkar, H.P.; Becker, K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Wieser, H. Chemistry of gluten proteins. Food Microbiol. 2007, 24, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Dale, H.F.; Biesiekierski, J.R.; Lied, G.A. Non-coeliac gluten sensitivity and the spectrum of gluten-related disorders: An updated overview. Nutr. Res. Rev. 2019, 32, 28–37. [Google Scholar] [CrossRef]
- Sugiura, S.H.; Dong, F.M.; Rathbone, C.K.; Hardy, R.W. Apparent protein digestibility and mineral availabilities in various feed ingredients for salmonid feeds. Aquaculture 1998, 159, 177–202. [Google Scholar] [CrossRef]
- Schneider, O.; Amirkolaie, A.K.; Vera-Cartas, J.; Eding, E.H.; Schrama, J.W.; Verreth, J.A. Digestibility, faeces recovery, and related carbon, nitrogen and phosphorus balances of five feed ingredients evaluated as fishmeal alternatives in Nile tilapia (Oreochromis niloticus L.). Aquac. Res. 2004, 35, 1370–1379. [Google Scholar] [CrossRef]
- Volkoff, H. The role of neuropeptide Y, orexins, cocaine and amphedetamide related transcript, cholecystokinin, amlyn and leptin in the regulation of feeding in fish. Comp. Biochem. Physiol. A 2006, 144, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Aslaksen, M.A.; Kraugerud, O.F.; Penn, M.; Svihus, B.; Denstadli, V.; Jørgensen, H.Y.; Hillestad, M.; Krogdahl, Å.; Storebakken, T. Screening of nutrient digestibilities and intestinal pathologies in Atlantic salmon (Salmo salar) fed diets with legumes, oilseeds, or cereals. Aquaculture 2007, 272, 541–555. [Google Scholar] [CrossRef]
- De Santis, C.; Bartie, K.L.; Olsen, R.E.; Taggart, J.B.; Tocher, D.R. Nutrigenomic profiling of transcriptional processes affected in liver and distal intestine in response to a soybean meal-induced nutritional stress in Atlantic salmon (Salmo salar). Comp. Biochem. Physiol. Part D Genom. Proteom. 2015, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.K.; Kortner, T.M.; Krasnov, A.; Björkhem, I.; Penn, M.; Krogdahl, Å. Choline supplementation prevents diet induced gut mucosa lipid accumulation in post-smolt Atlantic salmon (Salmo salar L.). BMC Vet. Res. 2020, 16, 32. [Google Scholar] [CrossRef] [Green Version]
- Kortner, T.M.; Gu, J.; Krogdahl, Å.; Bakke, A.M. Transcriptional regulation of cholesterol and bile acid metabolism after dietary soyabean meal treatment in Atlantic salmon (Salmo salar L.). Br. J. Nutr. 2013, 109, 593–604. [Google Scholar] [CrossRef] [Green Version]
- Gu, M.; Kortner, T.M.; Penn, M.; Hansen, A.K.; Krogdahl, Å. Effects of dietary plant meal and soya-saponin supplementation on intestinal and hepatic lipid droplet accumulation and lipoprotein and sterol metabolism in Atlantic salmon (Salmo salar L.). Br. J. Nutr. 2014, 111, 432–444. [Google Scholar] [CrossRef] [Green Version]
- Balakireva, A.V.; Zamyatnin, A.A. Properties of gluten intolerance: Gluten structure, evolution, pathogenicity and detoxification capabilities. Nutrients 2016, 8, 644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhanasiri, A.K.; Johny, A.; Xue, X.; Berge, G.M.; Bogevik, A.S.; Rise, M.L.; Fæste, C.K.; Fernandes, J.M. Plant-based diets induce transcriptomic changes in muscle of zebrafish and Atlantic salmon. Front. Genet. 2020, 11, 1288. [Google Scholar] [CrossRef]
- Kortner, T.M.; Penn, M.H.; Bjӧrkhem, I.; Måsøval, K.; Krogdahl, Å. Bile components and lecithin supplemented to plant based diets do not diminish diet related intestinal inflammation in Atlantic salmon. BMC Vet. Res. 2016, 12, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubitz, R.; Dröge, C.; Stindt, J.; Weissenberger, K.; Häussinger, D. The bile salt export pump (BSEP) in health and disease. Clin. Res. Hepatol. Gastroenterol. 2012, 36, 536–553. [Google Scholar] [CrossRef]
- Chawla, A.; Repa, J.J.; Evans, R.M.; Mangelsdorf, D.J. Nuclear receptors and lipid physiology: Opening the X-files. Science 2001, 294, 1866–1870. [Google Scholar] [CrossRef] [Green Version]
- Liland, N.S.; Espe, M.; Rosenlund, G.; Waagbø, R.; Hjelle, J.I.; Lie, Ø.; Fontanillas, R.; Torstensen, B.E. High levels of dietary phytosterols affect lipid metabolism and increase liver and plasma TAG in Atlantic salmon (Salmo salar L.). Br. J. Nutr. 2013, 110, 1958–1967. [Google Scholar] [CrossRef] [Green Version]
- Miyazawa, S.; Hashimoto, T.; Yokota, S. Identity of long-chain acyl-coenzyme a synthetase of microsomes, mitochondria, and peroxisomes in rat liver. J. Biochem. 1985, 98, 723–733. [Google Scholar] [CrossRef] [Green Version]
- Everard, A.; Plovier, H.; Rastelli, M.; Van Hul, M.; de Wouters d’Oplinter, A.; Geurts, L.; Druart, C.; Robine, S.; Delzenne, N.M.; Muccioli, G.G.; et al. Intestinal epithelial N-acylphosphatidylethanolamine phospholipase D links dietary fat to metabolic adaptations in obesity and steatosis. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Jiang, Z.G.; Robson, S.C.; Yao, Z. Lipoprotein metabolism in nonalcoholic fatty liver disease. J. Biomed. Res. 2013, 27, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Torstensen, B.E.; Espe, M.; Stubhaug, I.; Lie, Ø. Dietary plant proteins and vegetable oil blends increase adiposity and plasma lipids in Atlantic salmon (Salmo salar L.). Br. J. Nutr. 2011, 106, 633–647. [Google Scholar] [CrossRef] [Green Version]
- Romano, A.; Barca, A.; Storelli, C.; Verri, T. Teleost fish models in membrane transport research: The PEPT1 (SLC15A1) H+–oligopeptide transporter as a case study. Physiol. J. 2014, 592, 881–897. [Google Scholar] [CrossRef]
- Terova, G.; Robaina, L.; Izquierdo, M.; Cattaneo, A.; Molinari, S.; Bernardini, G.; Saroglia, M. PepT1 mRNA expression levels in sea bream (Sparus aurata) fed different plant protein sources. SpringerPlus 2013, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Kortner, T.M.; Skugor, S.; Penn, M.H.; Mydland, L.T.; Djordjevic, B.; Hillestad, M.; Krasnov, A.; Krogdahl, Å. Dietary soyasaponin supplementation to pea protein concentrate reveals nutrigenomic interactions underlying enteropathy in Atlantic salmon (Salmo salar). BMC Vet. Res. 2012, 8, 101. [Google Scholar] [CrossRef] [Green Version]
- Sahlmann, C.; Sutherland, B.J.; Kortner, T.M.; Koop, B.F.; Krogdahl, Å.; Bakke, A.M. Early response of gene expression in the distal intestine of Atlantic salmon (Salmo salar L.) during the development of soybean meal induced enteritis. Fish Shellfish Immun. 2013, 34, 599–609. [Google Scholar] [CrossRef]
- Skugor, S.; Grisdale-Helland, B.; Refstie, S.; Afanasyev, S.; Vielma, J.; Krasnov, A. Gene expression responses to restricted feeding and extracted soybean meal in Atlantic salmon (Salmo salar L.). Aquac. Nutr. 2011, 17, 505–517. [Google Scholar] [CrossRef]
- Jamnik, J.; García-Bailo, B.; Borchers, C.H.; El-Sohemy, A. Gluten Intake is Positively Associated with Plasma α2-Macroglobulin in Young Adults. J Nutr. 2015, 145, 1256–1262. [Google Scholar] [CrossRef]
- Johny, A.; Fæste, C.K.; Bogevik, A.S.; Berge, G.M.; Fernandes, J.M.; Ivanova, L. Development and validation of a liquid chromatography high-resolution mass spectrometry method for the simultaneous determination of mycotoxins and phytoestrogens in plant-based fish feed and exposed fish. Toxins 2019, 11, 222. [Google Scholar] [CrossRef] [Green Version]
- Helland, S.J.; Grisdale-Helland, B.; Nerland, S. A simple method for the measurement of daily feed intake of groups of fish in tanks. Aquaculture 1996, 139, 157–163. [Google Scholar] [CrossRef]
- Floch, J. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Krasnov, A.; Timmerhaus, G.; Afanasyev, S.; Jørgensen, S.M. Development and assessment of oligonucleotide microarrays for Atlantic salmon (Salmo salar L.). Comp. Biochem. Physiol. Part D Genom. Proteom. 2011, 6, 31–38. [Google Scholar] [CrossRef]
- Commission, E. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 53, 33–79. [Google Scholar]
- Molberg, Ø.; Mcadam, S.N.; Körner, R.; Quarsten, H.; Kristiansen, C.; Madsen, L.; Fugger, L.; Scott, H.; Norén, O.; Roepstorff, P.; et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat. Med. 1998, 4, 713–717. [Google Scholar] [CrossRef]
- Caputo, I.; D’Amato, A.; Troncone, R.; Auricchio, S.; Esposito, C. Transglutaminase 2 in celiac disease: Mini review article. Amino Acids. 2004, 26, 381–386. [Google Scholar] [CrossRef]
- Dhanasiri, A.K.; Chen, X.; Dahle, D.; Siriyappagouder, P.; Fæste, C.K.; Fernandes, J.M. Dietary inclusion of plant ingredients induces epigenetic changes in the intestine of zebrafish. Epigenetics 2020, 15, 1035–1051. [Google Scholar] [CrossRef]
- Hadjivassiliou, M.; Sanders, D.S.; Grünewald, R.A.; Woodroofe, N.; Boscolo, S.; Aeschlimann, D. Gluten sensitivity: From gut to brain. Lancet Neurol. 2010, 9, 318–330. [Google Scholar] [CrossRef]
- Neunlist, M.; Schemann, M. Nutrient-induced changes in the phenotype and function of the enteric nervous system. J. Physiol. 2014, 592, 2959–2965. [Google Scholar] [CrossRef]


| FM (Control) | WG15 | WG30 | |
|---|---|---|---|
| Diet composition (g/100 g) | |||
| Fishmeal | 63.35 | 48.35 | 33.35 |
| Wheat | 12.0 | 12.0 | 12.0 |
| Wheat gluten * | - | 15.0 | 30.0 |
| Fish oil | 20.0 | 20.0 | 20.0 |
| Additives # | 4.65 | 4.65 | 4.65 |
| Total protein | 45.2 | 46.7 | 48.1 |
| Total lipids | 26.5 | 25.4 | 24.3 |
| Chemical content (% in diet) | |||
| Dry matter | 93.6 | 93.9 | 94.1 |
| Protein | 45.2 | 46.7 | 48.1 |
| Lipid | 26.5 | 25.4 | 24.3 |
| Ash | 13.5 | 11.0 | 8.6 |
| Energy (MJ/kg) | 21.3 | 21.2 | 21.1 |
| Yttrium | 0.007 | 0.007 | 0.007 |
| FM | WG15 | WG30 | p-Value | |
|---|---|---|---|---|
| Initial wt (g) | 225 ± 2 | 219 ± 4 | 223 ± 0 | 0.31 |
| Final wt (g) | 548 ± 34 | 563 ± 4 | 513 ± 8 | 0.28 |
| Feed intake (g) | 8251 ± 214 a | 7602 ± 12 b | 6620 ± 108 c | 0.0005 |
| FCR | 0.80 ± 0.02 | 0.74 ± 0.01 | 0.76 ± 0.01 | 0.08 |
| SGR (% d−1) | 1.53 ± 0.03 a | 1.54 ± 0.04 a | 1.36 ± 0.03 b | 0.02 |
| TGC | 3.40 ± 0.06 a | 3.41 ± 0.10 a | 2.97 ± 0.07 b | 0.01 |
| Liver fat (%) | 4.9 ± 0.0 b | 5.6 ± 0.2 ab | 6.9 ± 0.7 a | 0.04 |
| CF | 1.31 ± 0.02 | 1.32 ± 0.02 | 1.30 ± 0.02 | 0.83 |
| HSI | 1.23 ± 0.03 b | 1.21 ± 0.02 b | 1.64 ± 0.09 a | 0.01 |
| Digestibility | FM | WG15 | WG30 | p-Value |
|---|---|---|---|---|
| Lipid | 97.2 ± 0.7 | 97.1 ± 0.2 | 96.8 ± 0.4 | 0.86 |
| Nitrogen | 86.4 ± 0.7 c | 89.1 ± 0.1 b | 91.8 ± 0.3 a | 0.0005 |
| Energy | 88.6 ± 0.7 | 88.5 ± 0.1 | 88.5 ± 0.5 | 0.97 |
| FM | WG15 | WG30 | p-Value | |
|---|---|---|---|---|
| FFA (mmol/L) | 0.41 ± 0.02 a | 0.29 ± 0.01 b | 0.31 ± 0.02 b | 0.01 |
| Tprot (g/L) | 42.9 ± 1.1 b | 40.7 ± 1.0 b | 50.3 ± 1.8 a | 0.02 |
| TG (mmol/L) | 3.75 ± 0.22 | 3.48 ± 0.33 | 5.15 ± 0.67 | 0.31 |
| ALT (U/L) | 36.7 ± 2.7 b | 26.1 ± 2.3 b | 81.3 ± 17.8 a | 0.04 |
| AST (U/L) | 1082 ± 143 | 763 ± 100 | 3116 ± 827 | 0.09 |
| GO Categories | Features | p-Value | GO Categories | Features | p-Value |
|---|---|---|---|---|---|
| Mid-intestine | |||||
| Antioxidant activity | 13/57 | <0.001 | Lipid transport | 25/207 | <0.001 |
| Autophagy | 23/324 | 0.020 | Lipoxygenase pathway | 5/38 | 0.032 |
| Cadherin binding | 76/1263 | 0.002 | Liver development | 31/481 | 0.027 |
| Cholesterol biosynthetic process | 16/109 | <0.001 | Long-chain fatty-acyl-CoA biosynthetic process | 11/63 | <0.001 |
| Cholesterol efflux | 18/52 | <0.001 | MHC class II protein complex | 5/24 | 0.001 |
| Cholesterol homeostasis | 34/250 | <0.001 | MHC class II protein complex binding | 8/54 | 0.001 |
| Cholesterol metabolic process | 26/230 | <0.001 | Muscle organ development | 25/287 | 0.048 |
| Defence response | 19/223 | 0.004 | Myosin filament | 15/174 | 0.011 |
| Defence response to virus | 39/535 | 0.001 | Phospholipase A2 activity | 11/55 | <0.001 |
| Fatty acid biosynthetic process | 12/131 | 0.015 | Phospholipid metabolic process | 17/158 | <0.001 |
| Glycolipid biosynthetic process | 8/51 | <0.001 | Retinoid metabolic process | 22/196 | <0.001 |
| Immune response | 59/971 | 0.006 | Steroid binding | 10/95 | 0.009 |
| Innate immune response | 74/1196 | 0.001 | Sterol metabolic process | 6/54 | 0.043 |
| Interferon-γ-mediated signalling pathway | 35/241 | <0.001 | Transforming growth factor β receptor binding | 10/116 | 0.046 |
| Iron ion binding | 29/403 | 0.006 | Triglyceride catabolic process | 19/63 | <0.001 |
| Keratinisation | 16/199 | 0.018 | Triglyceride metabolic process | 10/77 | 0.001 |
| Linoleic acid metabolic process | 12/82 | <0.001 | Ubiquitin protein ligase activity | 45/657 | 0.001 |
| Lipid catabolic process | 32/235 | <0.001 | Ubiquitin-dependent protein catabolic process | 51/865 | 0.022 |
| Lipid homeostasis | 18/159 | <0.001 | Very-low-density lipoprotein particle | 10/52 | <0.001 |
| Lipid metabolic process | 36/572 | 0.023 | Wound healing | 25/361 | 0.021 |
| Lipid particle | 26/300 | <0.001 | Xenobiotic metabolic process | 21/228 | <0.001 |
| Liver | |||||
| Cholesterol biosynthetic process | 12/109 | 12/109 | Fatty acid biosynthetic process | 6/131 | <0.001 |
| Iron ion binding | 8/403 | 0.010 | Lipid metabolic process | 10/572 | 0.011 |
| Liver development | 8/481 | 0.040 | Peptidase inhibitor activity | 7/153 | <0.001 |
| Receptor-mediated endocytosis | 7/394 | 0.039 | Sterol biosynthetic process | 5/35 | <0.001 |
| Triglyceride metabolic process | 5/77 | <0.001 | Xenobiotic metabolic process | 6/228 | 0.004 |
| KEGG Pathways | Features | p-Value | KEGG Pathways | Features | p-Value |
|---|---|---|---|---|---|
| Mid-intestine | |||||
| α-Linolenic acid metabolism | 13/32 | <0.001 | GnRH signalling pathway | 21/288 | 0.020 |
| Arachidonic acid metabolism | 10/90 | 0.005 | Linoleic acid metabolism | 10/38 | <0.001 |
| Biosynthesis of unsaturated fatty acids | 8/40 | <0.001 | mTOR signalling pathway | 12/148 | 0.043 |
| Ether lipid metabolism | 12/64 | <0.001 | PPAR signalling pathway | 19/164 | <0.001 |
| Fc epsilon RI signalling pathway | 15/180 | 0.015 | Retinol metabolism | 18/98 | <0.001 |
| Focal adhesion | 46/716 | 0.006 | Steroid hormone biosynthesis | 7/70 | 0.049 |
| Glycerophospholipid metabolism | 20/196 | <0.001 | Vascular smooth muscle contraction | 29/418 | 0.011 |
| Liver | |||||
| Arginine and proline metabolism | 6/196 | 0.001 | p53 signalling pathway | 6/150 | <0.001 |
| Cell cycle | 6/273 | 0.016 | PPAR signalling pathway | 6/164 | <0.001 |
| Drug metabolism—other enzymes | 5/78 | <0.001 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johny, A.; Berge, G.M.; Bogevik, A.S.; Krasnov, A.; Ruyter, B.; Fæste, C.K.; Østbye, T.-K.K. Sensitivity to Dietary Wheat Gluten in Atlantic Salmon Indicated by Gene Expression Changes in Liver and Intestine. Genes 2020, 11, 1339. https://doi.org/10.3390/genes11111339
Johny A, Berge GM, Bogevik AS, Krasnov A, Ruyter B, Fæste CK, Østbye T-KK. Sensitivity to Dietary Wheat Gluten in Atlantic Salmon Indicated by Gene Expression Changes in Liver and Intestine. Genes. 2020; 11(11):1339. https://doi.org/10.3390/genes11111339
Chicago/Turabian StyleJohny, Amritha, Gerd Marit Berge, André S. Bogevik, Aleksei Krasnov, Bente Ruyter, Christiane Kruse Fæste, and Tone-Kari Knutsdatter Østbye. 2020. "Sensitivity to Dietary Wheat Gluten in Atlantic Salmon Indicated by Gene Expression Changes in Liver and Intestine" Genes 11, no. 11: 1339. https://doi.org/10.3390/genes11111339
APA StyleJohny, A., Berge, G. M., Bogevik, A. S., Krasnov, A., Ruyter, B., Fæste, C. K., & Østbye, T.-K. K. (2020). Sensitivity to Dietary Wheat Gluten in Atlantic Salmon Indicated by Gene Expression Changes in Liver and Intestine. Genes, 11(11), 1339. https://doi.org/10.3390/genes11111339






