Selection and Validation of Reference Genes for qRT-PCR in Lentinula edodes under Different Experimental Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain, Culture Conditions and Sample Collection
2.2. RNA Isolation and cDNA Synthesis
2.3. Selection of Candidate Reference Genes and Primer Design
2.4. qRT-PCR, Amplification Efficiency and Data Analysis
2.5. Validation of Reference Genes
2.6. Analysis of Candidate Reference Genes under Different Conditions Based on RNA-Seq Data
3. Results
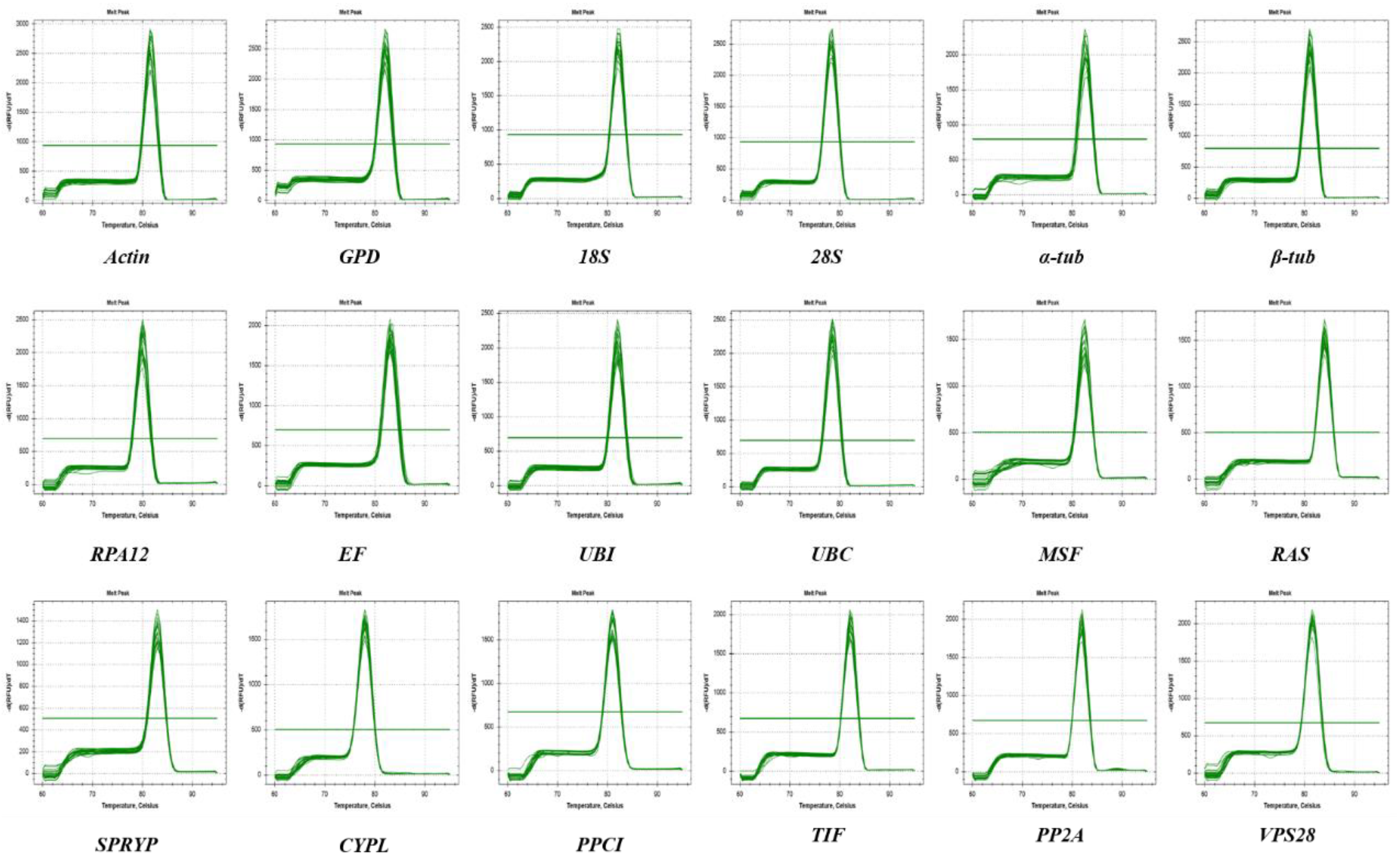
3.1. Selection of Candidate Reference Genes and Primer Design
3.2. Expression Profile of Candidate Reference Genes in L. edodes
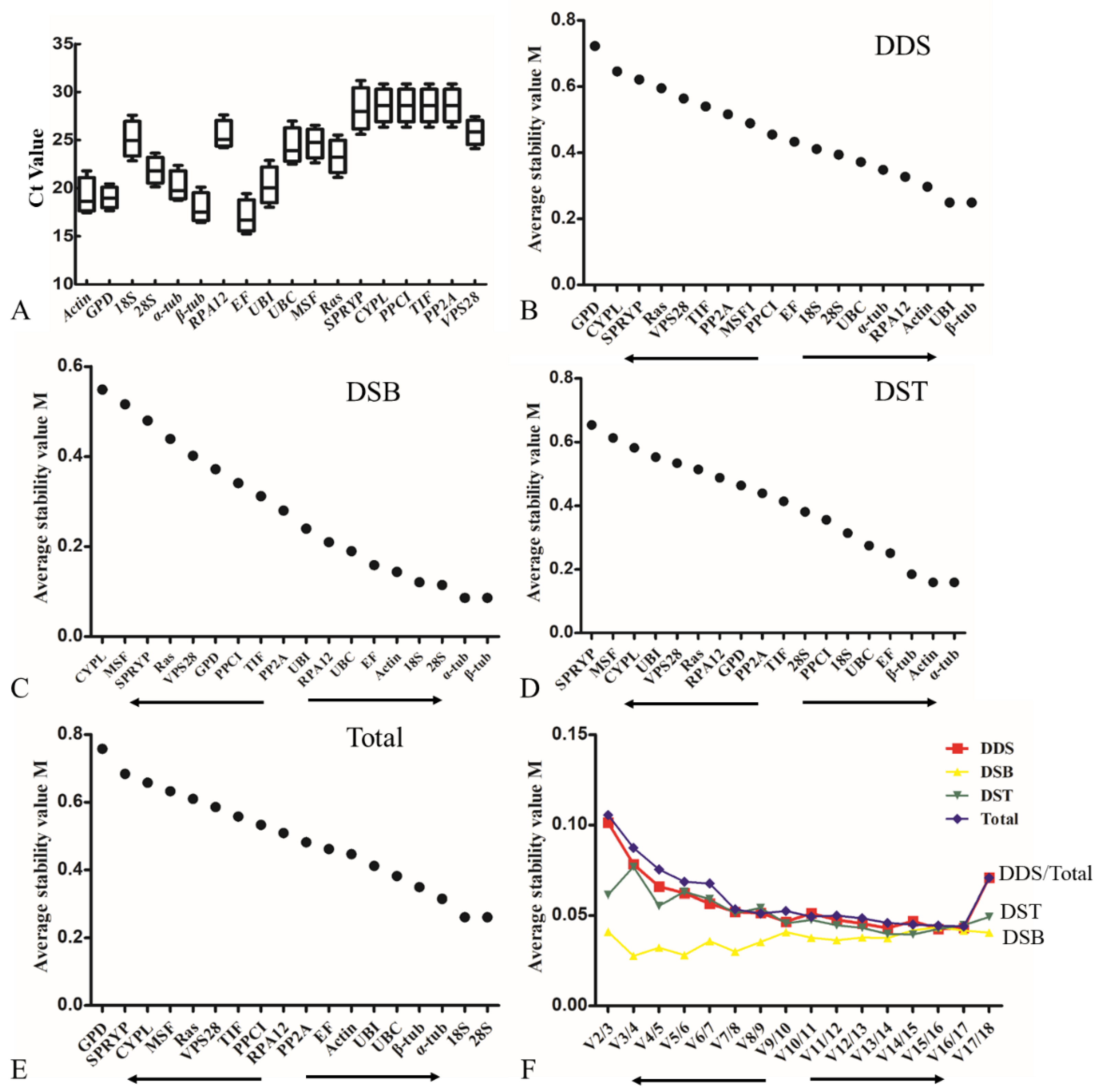
3.3. Evaluation of Expression Stability of the Eighteen Candidate Reference Genes in L. edodes
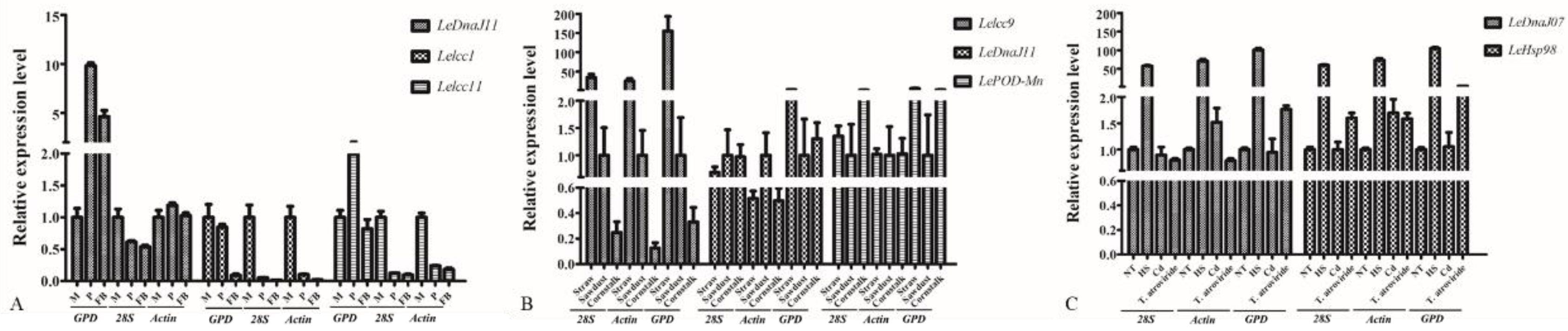
3.4. Validation of Selected Reference Genes in L. edodes
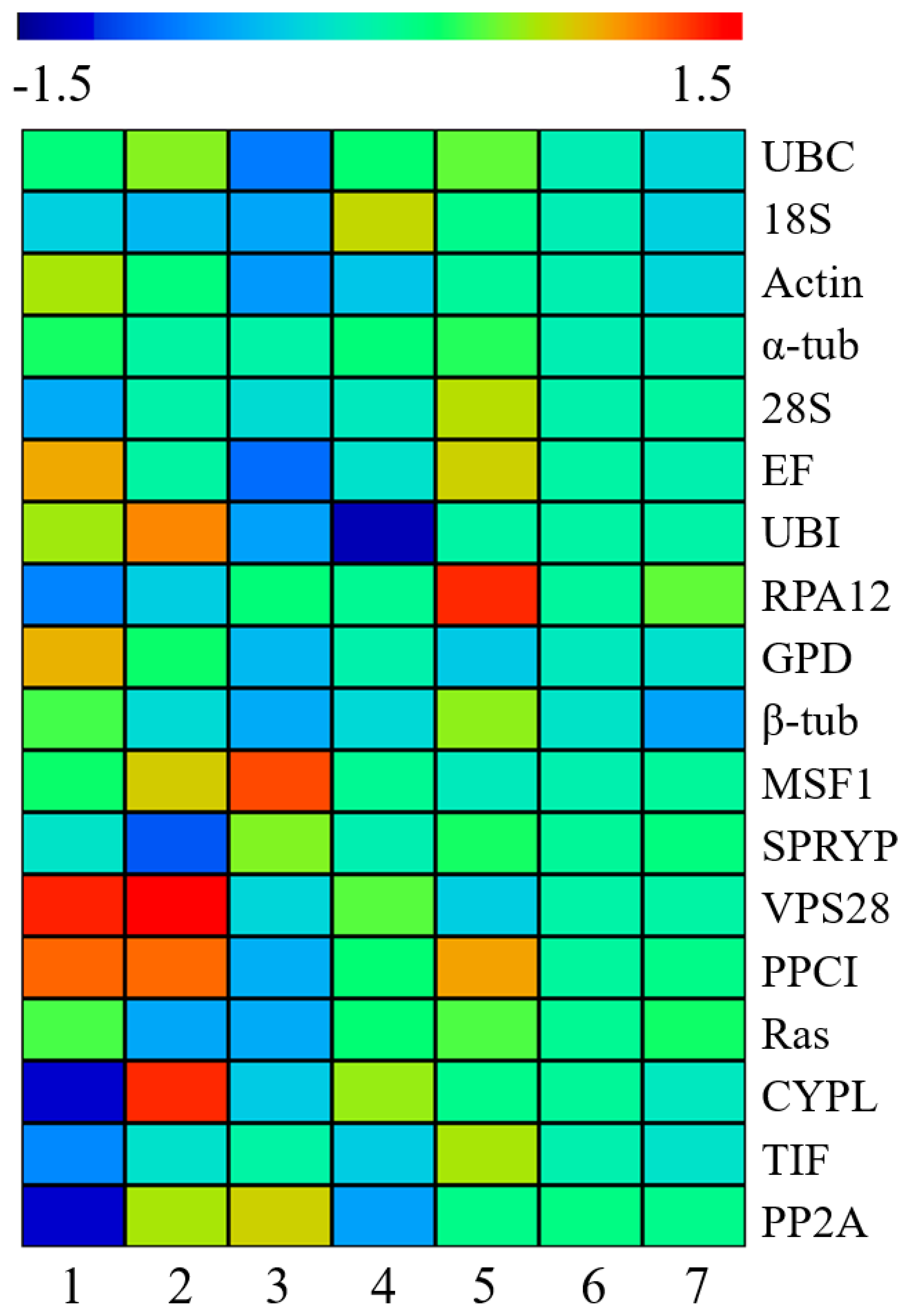
3.5. Evaluation of the Expression Stability of Candidate Reference Genes in Different L. edodes Strains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bustin, S.A.; Benes, V.; Nolan, T.; Pfaffl, M.W. Quantitative real-time RT-PCR—A perspective. J. Mol. Endocrinol. 2005, 34, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Engel, H.; Kueppers, C.; Koenig, M.; Loeffert, D. Successful gene expression analysis by multiplex, real-time, one-step RT-PCR, irrespective of the targets amplified. BioTechniques 2007, 43, 230–231. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.K.; Mafra, V.; De Souza, W.R.; Ribeiro, A.P.; Vinecky, F.; Basso, M.F.; Andrade, B.; Andrade Dias Brito da Cunha, B.; Kobayashi, A.; Correa Molinari, H.B. Selection of reliable reference genes for qRT-PCR analysis during developmental stages and abiotic stress in Setaria viridis. Sci. Rep. UK 2016, 6, 28348. [Google Scholar] [CrossRef] [PubMed]
- Angela Pérez-Novo, C.; Claeys, C.; Speleman, F.; Van Cauwenberge, P.; Bachert, C.; Vandesompele, J. Impact of RNA quality on reference gene expression stability. Biotechniques 2005, 39, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Huggett, J.; Dheda, K.; Bustin, S.; Zumla, A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun. 2005, 6, 279. [Google Scholar] [CrossRef] [PubMed]
- VanGuilder, H.D.; Vrana, K.E.; Freeman, W.M. Twenty-five years of quantitative PCR for gene expression analysis. Biotechniques 2008, 44, 619–626. [Google Scholar] [CrossRef]
- Zampieri, D.; Nora, L.C.; Basso, V.; Camassola, M.; Dillon, A.J. Validation of reference genes in Penicillium echinulatum to enable gene expression study using real-time quantitative RT-PCR. Curr. Genet. 2014, 60, 231–236. [Google Scholar] [CrossRef]
- Hao, X.; Horvath, D.P.; Chao, W.S.; Yang, Y.; Wang, X.; Xiao, B. Identification and evaluation of reliable reference genes for quantitative real-time PCR analysis in tea plant (Camellia sinensis (L.) O. Kuntze). Int. J. Mol. Sci. 2014, 15, 22155–22172. [Google Scholar] [CrossRef]
- Park, S.J.; Kwon, S.G.; Hwang, J.H.; Park, D.H.; Kim, T.W.; Kim, C.W. Selection of appropriate reference genes for QRT-PCR analysis in Berkshire, Duroc, Landrace, and Yorkshire pigs. Gene 2015, 558, 152–158. [Google Scholar] [CrossRef]
- Dheda, K.; Huggett, J.F.; Chang, J.S.; Kim, L.U.; Bustin, S.A.; Johnson, M.A.; Rook, G.A.; Zumla, A. The implications of using an inappropriate reference gene for real-time reverse transcription PCR data normalization. Anal. Biochem. 2005, 344, 141–143. [Google Scholar] [CrossRef]
- Lull, C.; Wichers, H.J.; Savelkoul, H.F. Antiinflammatory and immunomodulating properties of fungal metabolites. Mediat. Inflamm. 2005, 2005, 63–80. [Google Scholar] [CrossRef]
- Philippoussis, A.; Diamantopoulou, P.; Israilides, C. Productivity of agricultural residues used for the cultivation of the medicinal fungus Lentinula edodes. Int. Biodeter. Biodegr. 2007, 59, 216–219. [Google Scholar] [CrossRef]
- Finimundy, T.C.; Dillon, A.J.P.; Henriques, J.A.P.; Ely, M.R. A review on general nutritional compounds and pharmacological properties of the Lentinula edodes mushroom. Food Sci. Nutr. 2014, 5, 1095. [Google Scholar]
- Cai, Y.; Gong, Y.; Liu, W.; Hu, Y.; Chen, L.; Yan, L.; Zhou, Y.; Bian, Y. Comparative secretomic analysis of lignocellulose degradation by Lentinula edodes grown on microcrystalline cellulose, lignosulfonate and glucose. J. Proteom. 2017, 163, 92–101. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Nakade, K.; Yoshida, K.; Natsume, S.; Miyazaki, K.; Sato, S.; van Peer, A.F.; Konno, N. Grouping of multicopper oxidases in Lentinula edodes by sequence similarities and expression patterns. AMB Express 2015, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gong, Y.; Cai, Y.; Liu, W.; Zhou, Y.; Xiao, Y.; Xu, Z.; Liu, Y.; Lei, X.; Wang, G. Genome sequence of the edible cultivated mushroom Lentinula edodes (Shiitake) reveals insights into lignocellulose degradation. PLoS ONE 2016, 11, e0160336. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, X.; Liu, W. De novo transcriptomic analysis during Lentinula edodes fruiting body growth. Gene 2018, 641, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.H.; Jian, H.H.; Song, C.Y.; Bao, D.P.; Shang, X.D.; Wu, D.Q.; Tan, Q.; Zhang, X.H. Transcriptome analysis of candidate genes and signaling pathways associated with light-induced brown film formation in Lentinula edodes. Appl. Microbiol. Biot. 2013, 97, 4977–4989. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Nakade, K.; Sato, S.; Yoshida, K.; Miyazaki, K.; Natsume, S.; Konno, N. Lentinula edodes genome survey and postharvest transcriptome analysis. Appl. Environ. Microb. 2017, 83, e02990–e03016. [Google Scholar] [CrossRef]
- Wang, G.Z.; Ma, C.J.; Luo, Y.; Zhou, S.S.; Zhou, Y.; Ma, X.L.; Cai, Y.L.; Yu, J.J.; Bian, Y.B.; Gong, Y.H. Proteome and transcriptome reveal involvement of heat shock proteins and indoleacetic acid metabolism process in Lentinula edodes Thermotolerance. Cell Physiol. Biochem. 2018, 50, 1617–1637. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, S.; Luo, Y.; Ma, C.; Gong, Y.; Zhou, Y.; Gao, S.; Huang, Z.; Yan, L.; Hu, Y.; et al. The heat shock protein 40 LeDnaJ regulates stress resistance and indole-3-acetic acid biosynthesis in Lentinula edodes. Fungal. Genet. Biol. 2018, 118, 37–44. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Y.; Zhao, L.; Liu, Y.; Zhao, M. Selection of reliable reference genes for qRT-PCR during methyl jasmonate, salicylic acid and hydrogen peroxide treatments in Ganoderma lucidum. World J. Microb. Biot. 2018, 34, 92. [Google Scholar] [CrossRef]
- Qian, J.; Gao, Y.; Wáng, Y.; Wu, Y.; Wāng, Y.; Zhao, Y.; Chen, H.; Bao, D.; Xu, J.; Bian, X. Selection and evaluation of appropriate reference genes for qRT-PCR normalization of Volvariella volvacea gene expression under different conditions. Biomed. Res. Int. 2018, 2018, 6125706. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, W.; Cai, Y.; Lan, A.; Bian, Y. Validation of internal control genes for quantitative real-time PCR gene expression analysis in Morchella. Molecules 2018, 23, 2331. [Google Scholar] [CrossRef]
- Castanera, R.; López-Varas, L.; Pisabarro, A.G.; Ramírez, L. Validation of reference genes for transcriptional analyses in Pleurotus ostreatus using qRT-PCR. Appl. Environ. Microb. 2015, 81, 4120–4129. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, H.; Chen, M.; Song, X.; Yu, C.; Zhao, Y.; Wu, Y. Reference gene selection for quantitative real-time PCR of mycelia from Lentinula edodes under high-temperature stress. Biomed. Res. Int. 2018, 2018, 1670328. [Google Scholar] [CrossRef]
- Xiang, Q.; Li, J.; Qin, P.; He, M.; Yu, X.; Zhao, K.; Zhang, X.; Ma, M.; Chen, Q.; Chen, X. Identification and evaluation of reference genes for qRT-PCR studies in Lentinula edodes. PLoS ONE 2018, 13, e0190226. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Jatoi, W.N.; Adkins, S.; Saud, S. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Haldar, S.; Ghosh, T.K.; Bedford, M.R. Effects of yeast (Saccharomyces cerevisiae) and yeast protein concentrate on production performance of broiler chickens exposed to heat stress and challenged with Salmonella enteritidis. Anim. Feed Sci. Technol. 2011, 168, 61–71. [Google Scholar] [CrossRef]
- Lu, Z.; Kong, X.; Lu, Z.; Xiao, M.; Chen, M.; Zhu, L.; Shen, Y.; Hu, X.; Song, S. Para-aminobenzoic acid (PABA) synthase enhances thermotolerance of mushroom Agaricus bisporus. PLoS ONE 2014, 9, e91298. [Google Scholar] [CrossRef]
- Cao, X.T.; Bian, Y.B.; Xiao, X.J.; Wang, G.Z. Effect of heat stress on Lentinula edodes mycelial growth recovery and resistance to Trichoderma harzianum. Acta Edulis Fungi 2015, 22, 81–85. [Google Scholar]
- Wang, G.; Cao, X.; Ma, X.; Guo, M.; Liu, C.; Yan, L.; Bian, Y. Diversity and effect of Trichoderma spp. associated with green mold disease on Lentinula edodes in China. Microbiologyopen 2016, 5, 709–718. [Google Scholar] [CrossRef]
- Chen, G.; Zeng, G.; Tang, L.; Du, C.; Jiang, X.; Huang, G.; Liu, H.; Shen, G. Cadmium removal from simulated wastewater to biomass byproduct of Lentinus edodes. Bioresource Technol. 2008, 99, 7034–7040. [Google Scholar] [CrossRef]
- Qiao, X.; Huang, W.; Bian, Y. Effective removal of cadmium ions from a simulated gastrointestinal fluid by Lentinus edodes. Int. J. Environ. Res. Pub. Health 2014, 11, 12486–12498. [Google Scholar] [CrossRef]
- Willems, E.; Mateizel, I.; Kemp, C.; Cauffman, G.; Sermon, K.; Leyns, L. Selection of reference genes in mouse embryos and in differentiating human and mouse ES cells. Int. J. Dev. Biol. 2004, 50, 627–635. [Google Scholar] [CrossRef]
- Radonić, A.; Thulke, S.; Mackay, I.M.; Landt, O.; Siegert, W.; Nitsche, A. Guideline to reference gene selection for quantitative real-time PCR. Biochem. Bioph. Res. Co. 2004, 313, 856–862. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034-1. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Die, J.V.; Román, B.; Nadal, S.; González-Verdejo, C.I. Evaluation of candidate reference genes for expression studies in Pisum sativum under different experimental conditions. Planta 2010, 232, 145–153. [Google Scholar] [CrossRef]
- Tao, Y.; Van Peer, A.F.; Huang, Q.; Shao, Y.; Zhang, L.; Xie, B.; Jiang, Y.; Zhu, J.; Xie, B. Identification of novel and robust internal control genes from Volvariella volvacea that are suitable for QRT-PCR in filamentous fungi. Sci. Rep. UK 2016, 6, 29236. [Google Scholar] [CrossRef]
- Newton, J.R.; De Santis, C.; Jerry, D.R. The gene expression response of the catadromous perciform barramundi Lates calcarifer to an acute heat stress. J. Fish. Biol. 2012, 81, 81–93. [Google Scholar] [CrossRef]
- Wang, P.; Xiong, A.; Gao, Z.; Yu, X.; Li, M.; Hou, Y.; Sun, C.; Qu, S. Selection of suitable reference genes for qRT-PCR normalization under abiotic stresses and hormone stimulation in persimmon (Diospyros kaki thunb). PLoS ONE 2016, 11, e0160885. [Google Scholar]
- Wan, H.; Zhao, Z.; Qian, C.; Sui, Y.; Malik, A.A.; Chen, J. Selection of appropriate reference genes for gene expression studies by quantitative real-time polymerase chain reaction in cucumber. Anal Biochem. 2010, 399, 257–261. [Google Scholar] [CrossRef]
- Schmidt, G.W.; Delaney, S.K. Stable internal reference genes for normalization of real-time RT-PCR in tobacco (Nicotiana tabacum) during development and abiotic stress. Mol. Genet. Genom. 2010, 283, 233–241. [Google Scholar] [CrossRef]
- Niu, X.; Qi, J.; Zhang, G.; Xu, J.; Tao, A.; Fang, P.; Su, J. Selection of reliable reference genes for quantitative real-time PCR gene expression analysis in Jute (Corchorus capsularis) under stress treatments. Front. Plant Sci. 2015, 6, 848. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Zhang, Y.J.; Luo, Z.B.; Fan, Y.H.; Tang, G.R.; Liu, L.J.; Pei, Y. Selection of optimal reference genes for expression analysis in the entomopathogenic fungus Beauveria bassiana during development, under changing nutrient conditions, and after exposure to abiotic stresses. Appl. Microbiol. Biot. 2012, 93, 679–685. [Google Scholar] [CrossRef]
- Reid, K.E.; Olsson, N.; Schlosser, J.; Peng, F.; Lund, S.T. An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biol. 2006, 6, 27. [Google Scholar] [CrossRef]
- Li, Q.F.; Sun, S.S.M.; Yuan, D.Y.; Yu, H.X.; Gu, M.H.; Liu, Q.Q. Validation of candidate reference genes for the accurate normalization of real-time quantitative RT-PCR data in rice during seed development. Plant Mol. Biol. Rep. 2010, 28, 49–57. [Google Scholar] [CrossRef]
| Symbol | Gene ID | Gene Name | Forward Primer Sequence | Reverse Primer Sequence | Size (bp) | Efficiency (%) | R2 |
|---|---|---|---|---|---|---|---|
| Actin | LE01Gene01050 | Actin | GCATCCTGTCCTTCTTACCGAG | AAGAGCGAAACCCTCGTAGATG | 214 | 96.1 | 0.998 |
| GPD | LE01Gene07114 | Glyceraldehyde-3-phosphate dehydrogenase | CCTTCCGCTGATGCACCTAT | GTTGACAGAACGACCGCCAC | 240 | 94.1 | 0.998 |
| 18S | LE01Gene00881 | 18S ribosomal RNA | ATGCTGGCTCCGTTCGC | AGGTGCTCCCCGCTTCTTA | 168 | 97.5 | 0.988 |
| 28S | LE01Gene02296 | 28S ribosomal RNA | GCCTGCCACAAAGGATGAAA | GCTCCAAGCCTAAGAACAGTCCA | 157 | 90.6 | 0.992 |
| β-tub | LE01Gene08622 | β-tubulin | CAGACCCAAGACACGGACG | TGGCAGTAGAGTTACCCAGGAA | 213 | 93.2 | 0.998 |
| α-tub | LE01Gene01435 | α-tubulin | TCCAACTTGAACAGGCTTATCG | AACGGAAAGTGAATACGAGGGA | 134 | 94.9 | 0.994 |
| RPA12 | LE01Gene06896 | DNA-directed RNA polymerase I subunit RPA12 | TCCGAAAGATAGCGAACCGAA | GGAGACCAAATCGCCCAAGT | 184 | 97.2 | 0.998 |
| EF | LE01Gene03252 | Elongation factor | ACTTCCCAGGCTGATTGTGCT | TCGCTCCATTTGGTGGTGTC | 170 | 96.6 | 1 |
| UBI | LE01Gene06776 | UBI Ubiquitin-protein ligase E3 | ATCACGGTCACGAAAGAACAACT | CTTACATTCCAAAACTCGCACAGA | 171 | 96 | 0.998 |
| UbC | LE01Gene00040 | Ubiquitin-conjugating | GGCGGTCCAGTTTGTTGTCA | CGGTCGGTGTTTCTCCTTGC | 178 | 95.6 | 0.993 |
| MSF1 | LE01Gene08077 | MSF1-domain-containing protein | TATTCGCCTTCGTCAACACCT | ACCGCTGAGCCATCCACCT | 118 | 102.3 | 0.997 |
| Ras | LE01Gene13143 | Ras protein | AGGTCGGGATGAATGAGGG | CTTCGTCGTTTGGATCTTTGC | 222 | 99.5 | 0.999 |
| SPRYP | LE01Gene00010 | SPRY-domain-containing protein | ATGTCAAACTGTCCCGTCTTCC | CCATAAGGTGTTCCGTTTCGTT | 103 | 91.2 | 0.998 |
| CYPL | LE01Gene07769 | Cyclophilin-like protein | AGTGGTGTACTCCCTGATTTTGTC | GGTCTGCGTCGCCCTTTT | 100 | 92.9 | 0.997 |
| PPCI | LE01Gene06576 | Peptidyl-prolyl cis-trans isomerase | AGGACGAATTGCATCCAGAAC | GGGTAGGACCAAGAGTCAAGAAG | 111 | 97.6 | 0.997 |
| TIF | LE01Gene09672 | Translation initiation factor | ACCGCCGTAAAACGAGTAGC | CCTGGTTGCGAGGTGAATG | 203 | 101.5 | 0.998 |
| PP2A | LE01Gene09754 | Protein phosphatase 2A regulatory subunit B | TCGGCTGACGATTTGCG | GAGTAGTGGGGTCTTCCTCTTCTT | 253 | 100.9 | 0.998 |
| VPS28 | LE01Gene10038 | Vacuolar protein sorting-associated protein 28 | CTTCAAGGGCAGCAAGGATT | TGGCGTGACTGTTCTTCGGTA | 108 | 100.9 | 0.999 |
| Rank | Different Substrates | Different Development Stages | Different Stresses | Total | ||||
|---|---|---|---|---|---|---|---|---|
| NormFinder | Stability Value | NormFinder | Stability Value | NormFinder | Stability Value | NormFinder | Stability Value | |
| 1 | UBI | 0.077 | RPA12 | 0.042 | α-tub | 0.124 | α-tub | 0.161 |
| 2 | RPA12 | 0.09 | α-tub | 0.15 | 18S | 0.172 | 28S | 0.204 |
| 3 | 18S | 0.152 | Actin | 0.186 | PPCI | 0.184 | β-tub | 0.231 |
| 4 | 28S | 0.165 | β-tub | 0.188 | 28S | 0.21 | 18S | 0.242 |
| 5 | EF | 0.172 | UBI | 0.198 | TIF | 0.23 | PP2A | 0.292 |
| 6 | UBC | 0.19 | PPCI | 0.254 | Actin | 0.234 | RPA12 | 0.303 |
| 7 | TIF | 0.209 | 28S | 0.314 | β-tub | 0.246 | PPCI | 0.306 |
| 8 | Actin | 0.228 | MSF | 0.314 | PP2A | 0.284 | Actin | 0.309 |
| 9 | PPCI | 0.235 | UBC | 0.318 | GPD | 0.317 | UBI | 0.314 |
| 10 | α-tub | 0.238 | EF | 0.319 | UBC | 0.321 | UBC | 0.316 |
| 11 | β-tub | 0.248 | PP2A | 0.339 | Ras | 0.332 | EF | 0.352 |
| 12 | PP2A | 0.26 | 18S | 0.351 | EF | 0.353 | TIF | 0.357 |
| 13 | VPS28 | 0.329 | VPS28 | 0.395 | VPS28 | 0.368 | VPS28 | 0.38 |
| 14 | GPD | 0.355 | TIF | 0.424 | RPA12 | 0.38 | Ras | 0.431 |
| 15 | Ras | 0.401 | SPRYP | 0.46 | UBI | 0.399 | MSF | 0.445 |
| 16 | SPRYP | 0.443 | CYPL | 0.501 | CYPL | 0.452 | CYPL | 0.488 |
| 17 | MSF | 0.478 | Ras | 0.504 | MSF | 0.521 | SPRYP | 0.536 |
| 18 | CYPL | 0.502 | GPD | 0.88 | SPRYP | 0.61 | GPD | 0.879 |
| Actin | 18S | 28S | RPA12 | UBC | UBI | EF | α-tub | β-tub | PPCI | |
|---|---|---|---|---|---|---|---|---|---|---|
| Different substrates | ||||||||||
| std dev [± Ct] | 0.54 | 0.35 | 0.42 | 0.39 | 0.31 | 0.48 | 0.52 | 0.52 | 0.57 | 0.39 |
| coeff. of corr. [R] | 0.998 | 0.485 | 0.965 | 0.995 | 0.897 | 1 | 0.977 | 0.964 | 0.997 | 0.757 |
| Ranking | 6 | 10 | 2 | 1 | 8 | 3 | 4 | 5 | 7 | 9 |
| Different development stages | ||||||||||
| std dev [± Ct] | 0.34 | 0.92 | 0.87 | 0.42 | 0.44 | 0.72 | 0.44 | 0.68 | 0.64 | 0.57 |
| coeff. of corr. [R] | 0.994 | 0.979 | 0.995 | 0.924 | 0.947 | 0.971 | 0.806 | 0.96 | 0.994 | 0.889 |
| Ranking | 1 | 8 | 7 | 2 | 3 | 6 | 10 | 5 | 4 | 9 |
| Difference stresses | ||||||||||
| std dev [± Ct] | 1.27 | 0.86 | 0.84 | 0.95 | 1.34 | 1.33 | 1.39 | 1.17 | 1.28 | 0.93 |
| coeff. of corr. [R] | 0.999 | 0.283 | 0.998 | 0.928 | 0.964 | 0.969 | 0.986 | 1 | 0.992 | 0.993 |
| Ranking | 5 | 10 | 1 | 3 | 8 | 7 | 9 | 4 | 6 | 2 |
| Total samples | ||||||||||
| std dev [± Ct] | 1.00 | 0.94 | 0.98 | 0.78 | 1.03 | 1.19 | 1.02 | 1.11 | 1.14 | 0.85 |
| coeff. of corr. [R] | 0.957 | 0.638 | 0.967 | 0.943 | 0.973 | 0.977 | 0.954 | 0.991 | 0.991 | 0.937 |
| p-value | 0.001 | 0.089 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| Ranking | 4 | 10 | 3 | 1 | 6 | 9 | 5 | 7 | 8 | 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Wang, G.; Wang, C.; Gong, Y.; Bian, Y.; Zhou, Y. Selection and Validation of Reference Genes for qRT-PCR in Lentinula edodes under Different Experimental Conditions. Genes 2019, 10, 647. https://doi.org/10.3390/genes10090647
Luo Y, Wang G, Wang C, Gong Y, Bian Y, Zhou Y. Selection and Validation of Reference Genes for qRT-PCR in Lentinula edodes under Different Experimental Conditions. Genes. 2019; 10(9):647. https://doi.org/10.3390/genes10090647
Chicago/Turabian StyleLuo, Yi, Gangzheng Wang, Chen Wang, Yuhua Gong, Yinbing Bian, and Yan Zhou. 2019. "Selection and Validation of Reference Genes for qRT-PCR in Lentinula edodes under Different Experimental Conditions" Genes 10, no. 9: 647. https://doi.org/10.3390/genes10090647
APA StyleLuo, Y., Wang, G., Wang, C., Gong, Y., Bian, Y., & Zhou, Y. (2019). Selection and Validation of Reference Genes for qRT-PCR in Lentinula edodes under Different Experimental Conditions. Genes, 10(9), 647. https://doi.org/10.3390/genes10090647





