White Adipose Tissue Response of Obese Mice to Ambient Oxygen Restriction at Thermoneutrality: Response Markers Identified, but no WAT Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Study
2.2. Tissue and Blood Collection
2.3. Tissue Analysis and Histology
2.4. Serum and Tissue Adipokine/Insulin Analysis
2.5. RNA Isolation, cDNA Synthesis, and Microarray Hybridization and Analysis
2.6. Reverse Transcription Quantitative PCR
2.7. Statistical Analysis
3. Results
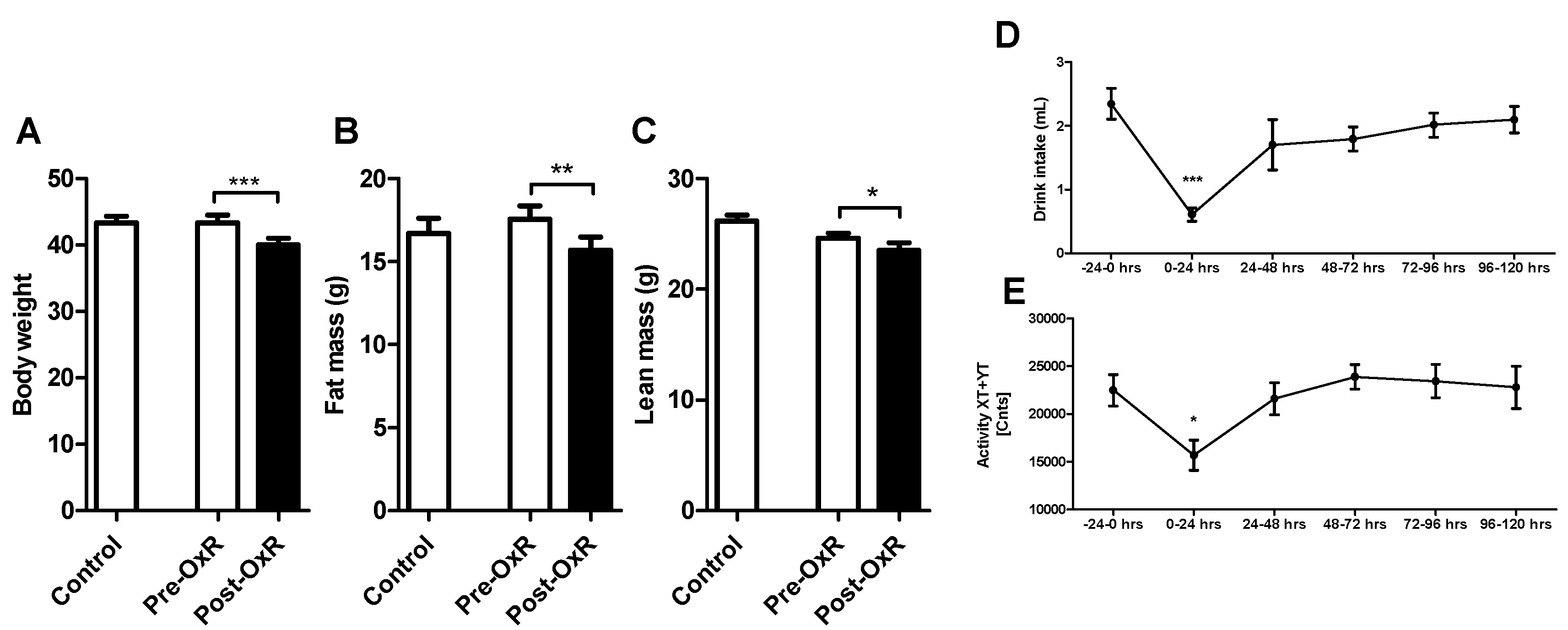
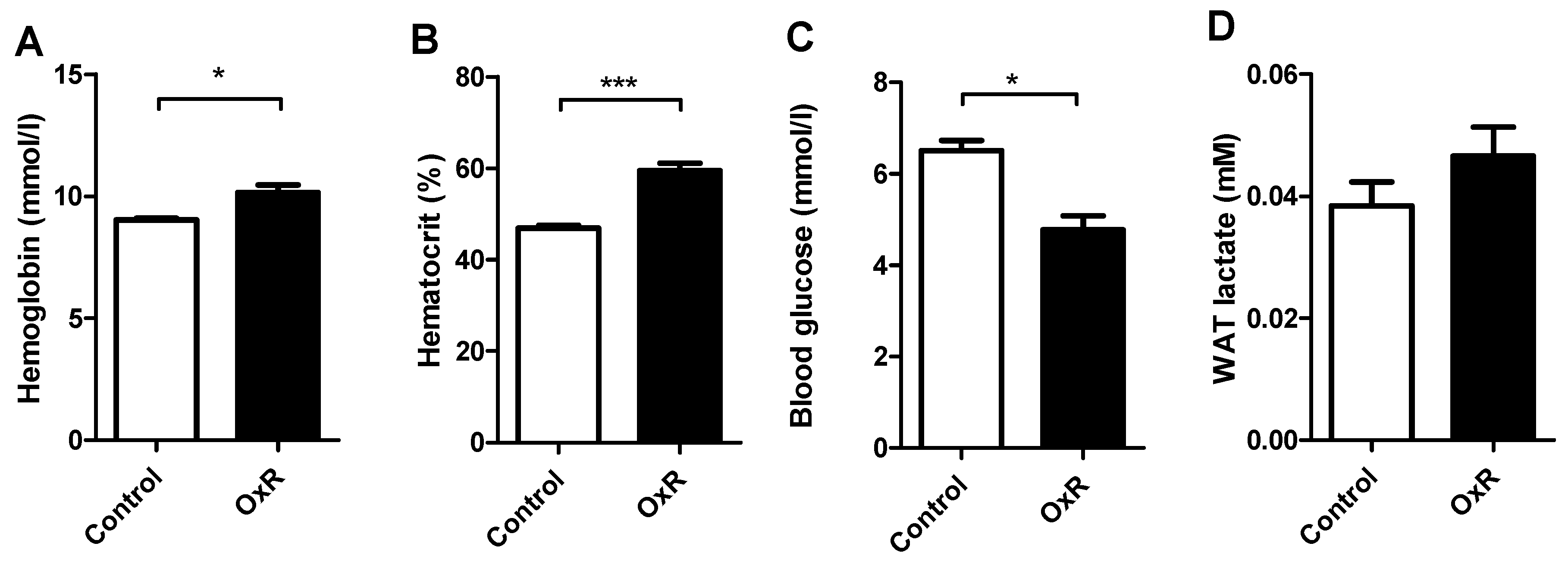
3.1. Five Days of Oxygen Restriction (OxR) Altered Physiological Parameters
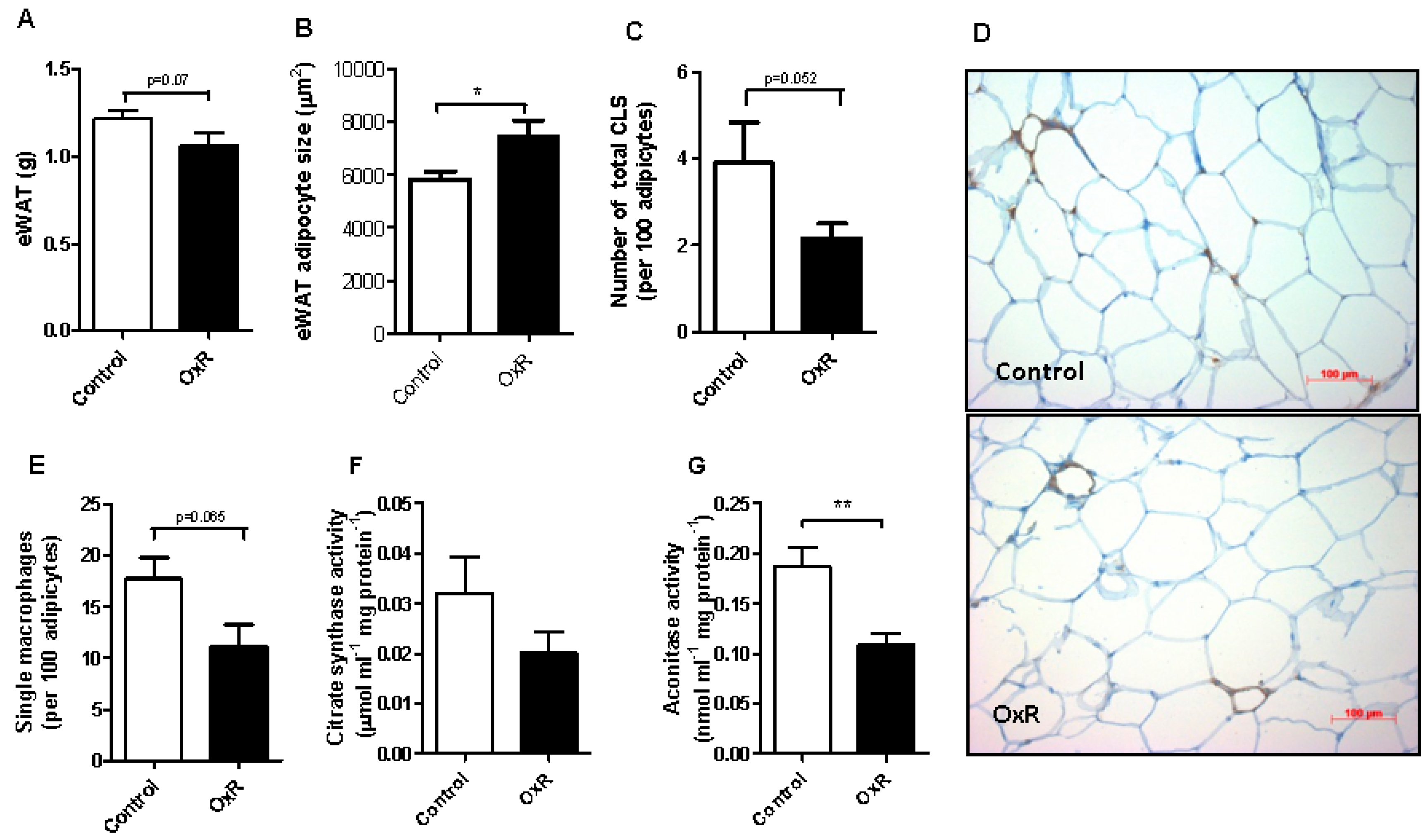
3.2. Oxygen Restriction (OxR) Did Not Increase Macrophage Infiltration in White Adipose Tissue (WAT), but Decreased WAT Mitochondrial Density
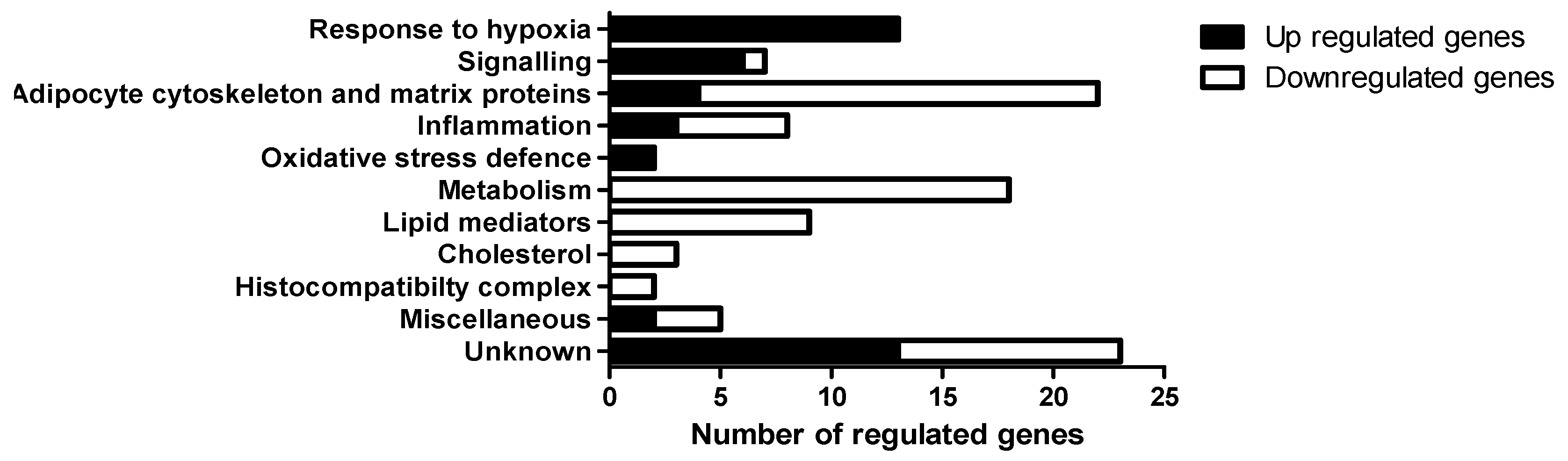
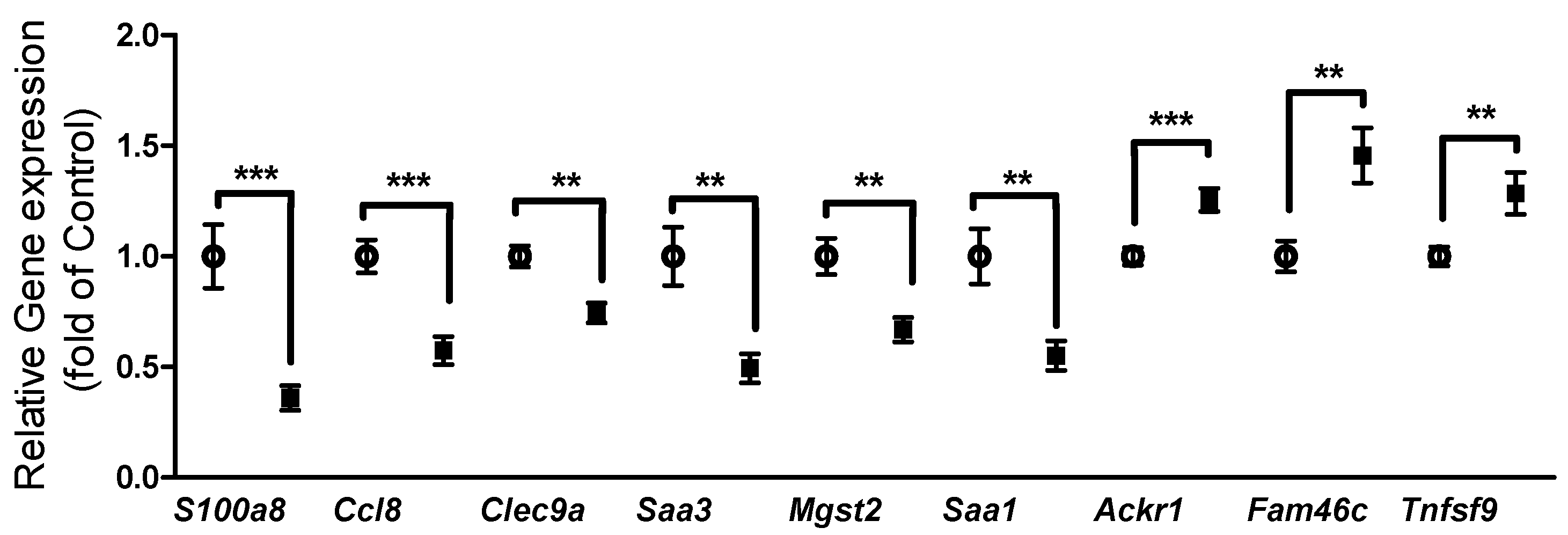
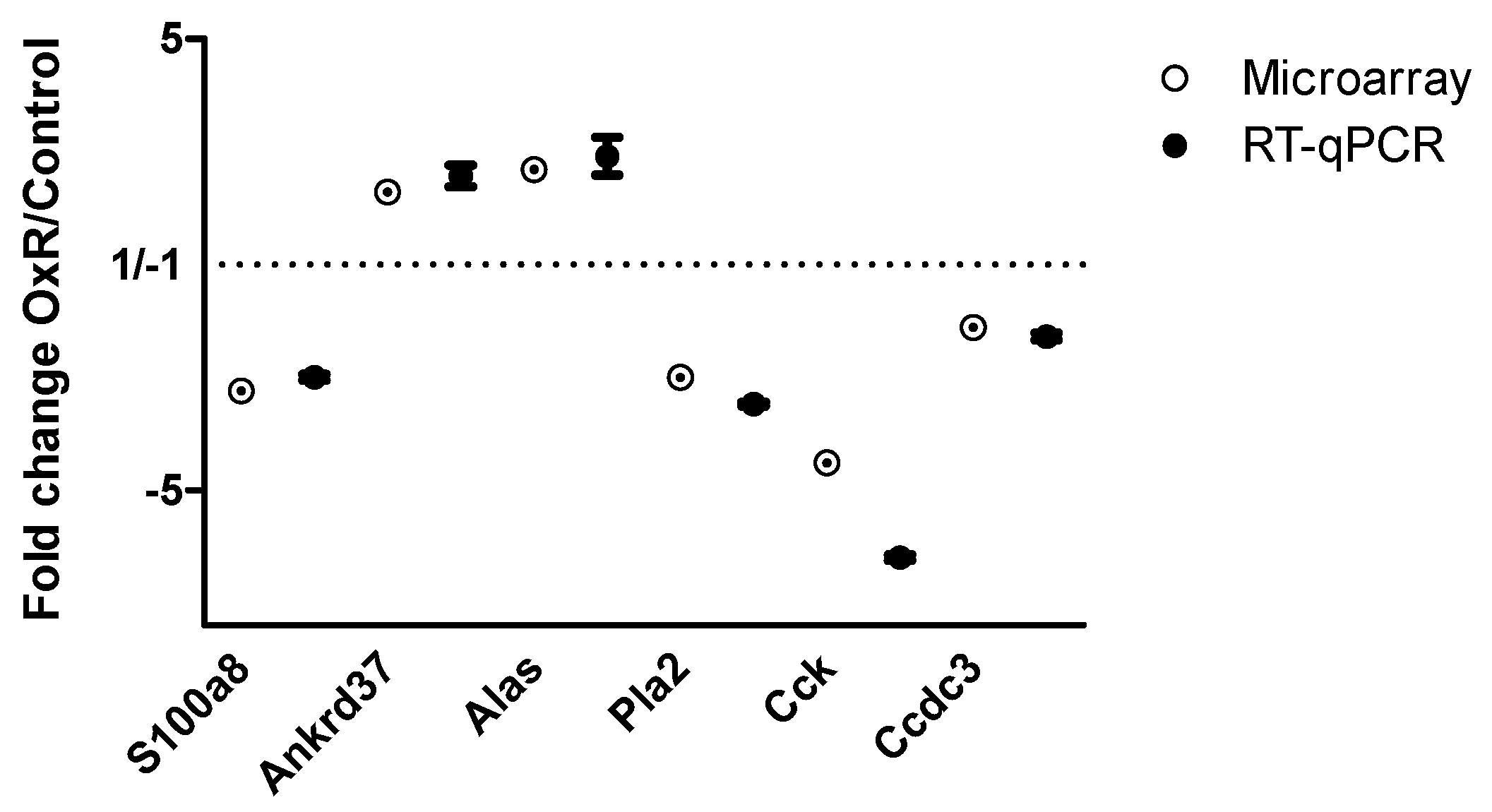
3.3. Gene Expression in Epididymal White Adipose Tissue (eWAT) was Altered Between Oxygen Restriction (OxR) and Control Mice
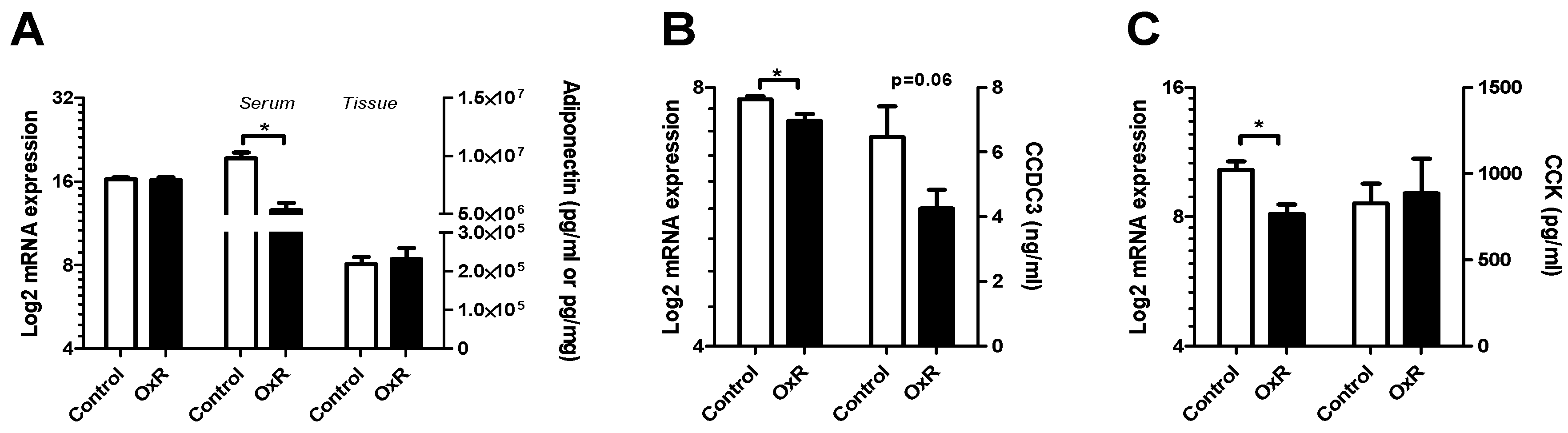
3.4. Metabolic Serum Adipokines Affected by Oxygen Restriction (OxR)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Unger, R.H.; Scherer, P.E. Gluttony, sloth and the metabolic syndrome: A roadmap to lipotoxicity. Trends Endocrinol. Metab. 2010, 21, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. AMS 2013, 9, 191–200. [Google Scholar] [CrossRef]
- Wang, P.; Mariman, E.; Renes, J.; Keijer, J. The secretory function of adipocytes in the physiology of white adipose tissue. J. Cell. Physiol. 2008, 216, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-α: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Bolus, W.R.; Hasty, A.H. Contributions of innate type 2 inflammation to adipose function. J. Lipid Res. 2018. [Google Scholar] [CrossRef]
- Stefan, N.; Kantartzis, K.; Machann, J.; Schick, F.; Thamer, C.; Rittig, K.; Balletshofer, B.; Machicao, F.; Fritsche, A.; Haring, H.U. Identification and characterization of metabolically benign obesity in humans. Arch. Intern. Med. 2008, 168, 1609–1616. [Google Scholar] [CrossRef]
- Primeau, V.; Coderre, L.; Karelis, A.D.; Brochu, M.; Lavoie, M.E.; Messier, V.; Sladek, R.; Rabasa-Lhoret, R. Characterizing the profile of obese patients who are metabolically healthy. Int. J. Obes. 2011, 35, 971–981. [Google Scholar] [CrossRef]
- Engin, A. Adipose tissue hypoxia in obesity and its impact on preadipocytes and macrophages: Hypoxia hypothesis. Adv. Exp. Med. Biol. 2017, 960, 305–326. [Google Scholar] [CrossRef] [PubMed]
- Melo, L.C.; Silva, M.A.; Calles, A.C. Obesity and lung function: A systematic review. Einstein 2014, 12, 120–125. [Google Scholar] [CrossRef]
- Pasarica, M.; Sereda, O.R.; Redman, L.M.; Albarado, D.C.; Hymel, D.T.; Roan, L.E.; Rood, J.C.; Burk, D.H.; Smith, S.R. Reduced adipose tissue oxygenation in human obesity: Evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 2009, 58, 718–725. [Google Scholar] [CrossRef]
- Bolinder, J.; Kerckhoffs, D.A.; Moberg, E.; Hagstrom-Toft, E.; Arner, P. Rates of skeletal muscle and adipose tissue glycerol release in nonobese and obese subjects. Diabetes 2000, 49, 797–802. [Google Scholar] [CrossRef]
- Ye, J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int. J. Obes. 2009, 33, 54–66. [Google Scholar] [CrossRef]
- Hosogai, N.; Fukuhara, A.; Oshima, K.; Miyata, Y.; Tanaka, S.; Segawa, K.; Furukawa, S.; Tochino, Y.; Komuro, R.; Matsuda, M.; et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 2007, 56, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Rausch, M.E.; Weisberg, S.; Vardhana, P.; Tortoriello, D.V. Obesity in c57bl/6j mice is characterized by adipose tissue hypoxia and cytotoxic t-cell infiltration. Int. J. Obes. 2008, 32, 451–463. [Google Scholar] [CrossRef]
- Ye, J.; Gao, Z.; Yin, J.; He, Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1118–E1128. [Google Scholar] [CrossRef]
- Heilbronn, L.K.; Campbell, L.V. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr. Pharm. Des. 2008, 14, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Goossens, G.H.; Bizzarri, A.; Venteclef, N.; Essers, Y.; Cleutjens, J.P.; Konings, E.; Jocken, J.W.; Cajlakovic, M.; Ribitsch, V.; Clement, K.; et al. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation 2011, 124, 67–76. [Google Scholar] [CrossRef]
- Lecoultre, V.; Peterson, C.M.; Covington, J.D.; Ebenezer, P.J.; Frost, E.A.; Schwarz, J.M.; Ravussin, E. Ten nights of moderate hypoxia improves insulin sensitivity in obese humans. Diabetes Care 2013, 36, e197–e198. [Google Scholar] [CrossRef]
- Duivenvoorde, L.P.; van Schothorst, E.M.; Bunschoten, A.; Keijer, J. Dietary restriction of mice on a high-fat diet induces substrate efficiency and improves metabolic health. J. Mol. Endocrinol. 2011, 47, 81–97. [Google Scholar] [CrossRef]
- Hoevenaars, F.P.; van Schothorst, E.M.; Horakova, O.; Voigt, A.; Rossmeisl, M.; Pico, C.; Caimari, A.; Kopecky, J.; Klaus, S.; Keijer, J. Bioclaims standard diet (biosd): A reference diet for nutritional physiology. Genes Nutr. 2012, 7, 399–404. [Google Scholar] [CrossRef]
- Hoevenaars, F.P.; Bekkenkamp-Grovenstein, M.; Janssen, R.J.; Heil, S.G.; Bunschoten, A.; Hoek-van den Hil, E.F.; Snaas-Alders, S.; Teerds, K.; van Schothorst, E.M.; Keijer, J. Thermoneutrality results in prominent diet-induced body weight differences in c57bl/6j mice, not paralleled by diet-induced metabolic differences. Mol. Nutr. Food Res. 2014, 58, 799–807. [Google Scholar] [CrossRef]
- van der Stelt, I.; Hoevenaars, F.; Siroka, J.; de Ronde, L.; Friedecky, D.; Keijer, J.; van Schothorst, E. Metabolic response of visceral white adipose tissue of obese mice exposed for 5 days to human room temperature compared to mouse thermoneutrality. Front. Physiol. 2017, 8, 179. [Google Scholar] [CrossRef]
- Duivenvoorde, L.P.; van Schothorst, E.M.; Swarts, H.J.; Keijer, J. Assessment of metabolic flexibility of old and adult mice using three noninvasive, indirect calorimetry-based treatments. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 282–293. [Google Scholar] [CrossRef]
- Dillard, T.A.; Moores, L.K.; Bilello, K.L.; Phillips, Y.Y. The preflight evaluation. A comparison of the hypoxia inhalation test with hypobaric exposure. Chest 1995, 107, 352–357. [Google Scholar] [CrossRef]
- Tamisier, R.; Gilmartin, G.S.; Launois, S.H.; Pepin, J.L.; Nespoulet, H.; Thomas, R.; Levy, P.; Weiss, J.W. A new model of chronic intermittent hypoxia in humans: Effect on ventilation, sleep, and blood pressure. J. Appl. Physiol. 2009, 107, 17–24. [Google Scholar] [CrossRef]
- Hoevenaars, F.P.; Keijer, J.; Swarts, H.J.; Snaas-Alders, S.; Bekkenkamp-Grovenstein, M.; van Schothorst, E.M. Effects of dietary history on energy metabolism and physiological parameters in c57bl/6j mice. Exp. Physiol. 2013, 98, 1053–1062. [Google Scholar] [CrossRef]
- Van Schothorst, E.M.; Pagmantidis, V.; de Boer, V.C.; Hesketh, J.; Keijer, J. Assessment of reducing RNA input for agilent oligo microarrays. Anal. Biochem. 2007, 363, 315–317. [Google Scholar] [CrossRef]
- Van Helden, Y.G.; Godschalk, R.W.; Swarts, H.J.; Hollman, P.C.; van Schooten, F.J.; Keijer, J. β-carotene affects gene expression in lungs of male and female bcmo1-/- mice in opposite directions. Cell. Mol. Life Sci. CMLS 2011, 68, 489–504. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoek-van den Hil, E.F.; Keijer, J.; Bunschoten, A.; Vervoort, J.J.; Stankova, B.; Bekkenkamp, M.; Herreman, L.; Venema, D.; Hollman, P.C.; Tvrzicka, E.; et al. Quercetin induces hepatic lipid omega-oxidation and lowers serum lipid levels in mice. PloS ONE 2013, 8, e51588. [Google Scholar] [CrossRef]
- Benita, Y.; Kikuchi, H.; Smith, A.D.; Zhang, M.Q.; Chung, D.C.; Xavier, R.J. An integrative genomics approach identifies hypoxia inducible factor-1 (Hif-1)-target genes that form the core response to hypoxia. Nucl. Acids Res. 2009, 37, 4587–4602. [Google Scholar] [CrossRef] [PubMed]
- Hofer, T.; Wenger, R.H.; Kramer, M.F.; Ferreira, G.C.; Gassmann, M. Hypoxic up-regulation of erythroid 5-aminolevulinate synthase. Blood 2003, 101, 348–350. [Google Scholar] [CrossRef]
- Van den Borst, B.; Schols, A.M.; de Theije, C.; Boots, A.W.; Kohler, S.E.; Goossens, G.H.; Gosker, H.R. Characterization of the inflammatory and metabolic profile of adipose tissue in a mouse model of chronic hypoxia. J. Appl. Physiol. 2013, 114, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Famulla, S.; Horrighs, A.; Cramer, A.; Sell, H.; Eckel, J. Hypoxia reduces the response of human adipocytes towards tnfα resulting in reduced nf-κb signaling and mcp-1 secretion. Int. J. Obes. 2012, 36, 986–992. [Google Scholar] [CrossRef]
- Poitou, C.; Viguerie, N.; Cancello, R.; De Matteis, R.; Cinti, S.; Stich, V.; Coussieu, C.; Gauthier, E.; Courtine, M.; Zucker, J.D.; et al. Serum amyloid a: Production by human white adipocyte and regulation by obesity and nutrition. Diabetologia 2005, 48, 519–528. [Google Scholar] [CrossRef]
- Jernas, M.; Palming, J.; Sjoholm, K.; Jennische, E.; Svensson, P.A.; Gabrielsson, B.G.; Levin, M.; Sjogren, A.; Rudemo, M.; Lystig, T.C.; et al. Separation of human adipocytes by size: Hypertrophic fat cells display distinct gene expression. FASEB J. 2006, 20, 1540–1542. [Google Scholar] [CrossRef]
- Salans, L.B.; Knittle, J.L.; Hirsch, J. The role of adipose cell size and adipose tissue insulin sensitivity in the carbohydrate intolerance of human obesity. J. Clin. Invest. 1968, 47, 153–165. [Google Scholar] [CrossRef]
- Han, C.Y.; Subramanian, S.; Chan, C.K.; Omer, M.; Chiba, T.; Wight, T.N.; Chait, A. Adipocyte-derived serum amyloid a3 and hyaluronan play a role in monocyte recruitment and adhesion. Diabetes 2007, 56, 2260–2273. [Google Scholar] [CrossRef]
- Gencer, S.; van der Vorst, E.P.C.; Aslani, M.; Weber, C.; Doring, Y.; Duchene, J. Atypical chemokine receptors in cardiovascular disease. Thromb. Haemost. 2019, 119, 534–541. [Google Scholar] [CrossRef]
- Kwon, B. Is cd137 ligand (cd137l) signaling a fine tuner of immune responses? Immune Netw. 2015, 15, 121–124. [Google Scholar] [CrossRef]
- Forsberg, M.H.; Ciecko, A.E.; Bednar, K.J.; Itoh, A.; Kachapati, K.; Ridgway, W.M.; Chen, Y.G. Cd137 plays both pathogenic and protective roles in type 1 diabetes development in nod mice. J. Immunol. 2017, 198, 3857–3868. [Google Scholar] [CrossRef]
- Zhou, S.N.; Ran, R.Z.; Tan, L.L.; Guo, H. Current perspectives of sa-4-1bbl in immune modulation during cancer. Exp. Ther. Med. 2018, 15, 2699–2702. [Google Scholar] [CrossRef]
- Lushchak, O.V.; Piroddi, M.; Galli, F.; Lushchak, V.I. Aconitase post-translational modification as a key in linkage between krebs cycle, iron homeostasis, redox signaling, and metabolism of reactive oxygen species. Redox Rep. 2014, 19, 8–15. [Google Scholar] [CrossRef]
- Janero, D.R.; Hreniuk, D. Suppression of TCA cycle activity in the cardiac muscle cell by hydroperoxide-induced oxidant stress. Am. J. Physiol. 1996, 270, C1735–C1742. [Google Scholar] [CrossRef]
- Drapier, J.C.; Hibbs, J.B.J. Aconitases: A class of metalloproteins highly sensitive to nitric oxide synthesis. Methods Enzymol. 1996, 269, 26–36. [Google Scholar] [CrossRef]
- Tretter, L.; Ambrus, A. Measurement of ROS homeostasis in isolated mitochondria. Methods Enzymol. 2014, 547, 199–223. [Google Scholar] [CrossRef]
- Ishimoto, Y.; Tanaka, T.; Yoshida, Y.; Inagi, R. Physiological and pathophysiological role of reactive oxygen species and reactive nitrogen species in the kidney. Clin. Exp. Pharmacol. Physiol. 2018, 45, 1097–1105. [Google Scholar] [CrossRef]
- Poyton, R.O.; Ball, K.A.; Castello, P.R. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol. Metab. 2009, 20, 332–340. [Google Scholar] [CrossRef]
- Smith, S.J.; Saggerson, E.D. Regulation of pyruvate dehydrogenase activity in white adipocyte mitochondria by palmitoyl carnitine and citrate. Int. J. Biochem. 1979, 10, 785–790. [Google Scholar] [CrossRef]
- Taylor, W.M.; Halperin, M.L. Regulation of pyruvate dehydrogenase in muscle. Inhibition by citrate. J. Biol. Chem. 1973, 248, 6080–6083. [Google Scholar] [PubMed]
- Lam, K.S.; Xu, A. Adiponectin: Protection of the endothelium. Curr. Diabetes Rep. 2005, 5, 254–259. [Google Scholar] [CrossRef]
- Trujillo, M.E.; Scherer, P.E. Adiponectin-journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J. Intern. Med. 2005, 257, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Lam, K.S.; Wang, Y.; Wu, D.; Lam, M.C.; Shen, J.; Wong, L.; Hoo, R.L.; Zhang, J.; Xu, A. Hypoxia dysregulates the production of adiponectin and plasminogen activator inhibitor-1 independent of reactive oxygen species in adipocytes. Biochem. Biophys. Res. Commun. 2006, 341, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Mazzatti, D.; Lim, F.L.; O’Hara, A.; Wood, I.S.; Trayhurn, P. A microarray analysis of the hypoxia-induced modulation of gene expression in human adipocytes. Arch. Physiol. Biochem. 2012, 118, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Song, H.D.; Li, R.Y.; Zhou, L.B.; Zhu, Z.D.; Hu, R.M.; Han, Z.G.; Chen, J.L. The gene expression profiling of human visceral adipose tissue and its secretory functions. Biochem. Biophys. Res. Commun. 2003, 300, 839–846. [Google Scholar] [CrossRef]
- Koob, G.F.; Annau, Z.; Rubin, R.J.; Montgomery, M.R. Effect of hypoxic hypoxia and carbon monoxide on food intake, water intake, and body weight in two strains of rats. Life Sci. 1974, 14, 1511–1520. [Google Scholar] [CrossRef]
- Butterfield, G.E.; Gates, J.; Fleming, S.; Brooks, G.A.; Sutton, J.R.; Reeves, J.T. Increased energy intake minimizes weight loss in men at high altitude. J. Appl. Physiol. 1992, 72, 1741–1748. [Google Scholar] [CrossRef]
- Helwig, M.; Archer, Z.A.; Heldmaier, G.; Tups, A.; Mercer, J.G.; Klingenspor, M. Photoperiodic regulation of satiety mediating neuropeptides in the brainstem of the seasonal siberian hamster (Phodopus sungorus). J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2009, 195, 631–642. [Google Scholar] [CrossRef]
- Kobayashi, S.; Fukuhara, A.; Taguchi, T.; Matsuda, M.; Tochino, Y.; Otsuki, M.; Shimomura, I. Identification of a new secretory factor, ccdc3/favine, in adipocytes and endothelial cells. Biochem. Biophys. Res. Commun. 2010, 392, 29–35. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoevenaars, F.P.M.; Keijer, J.; van der Stelt, I.; Duivenvoorde, L.P.M.; Herreman, L.; van Nes, R.; Friedecký, D.; Hegeman, M.A.; van Schothorst, E.M. White Adipose Tissue Response of Obese Mice to Ambient Oxygen Restriction at Thermoneutrality: Response Markers Identified, but no WAT Inflammation. Genes 2019, 10, 359. https://doi.org/10.3390/genes10050359
Hoevenaars FPM, Keijer J, van der Stelt I, Duivenvoorde LPM, Herreman L, van Nes R, Friedecký D, Hegeman MA, van Schothorst EM. White Adipose Tissue Response of Obese Mice to Ambient Oxygen Restriction at Thermoneutrality: Response Markers Identified, but no WAT Inflammation. Genes. 2019; 10(5):359. https://doi.org/10.3390/genes10050359
Chicago/Turabian StyleHoevenaars, Femke P. M., Jaap Keijer, Inge van der Stelt, Loes P. M. Duivenvoorde, Laure Herreman, Robin van Nes, David Friedecký, Maria A. Hegeman, and Evert M. van Schothorst. 2019. "White Adipose Tissue Response of Obese Mice to Ambient Oxygen Restriction at Thermoneutrality: Response Markers Identified, but no WAT Inflammation" Genes 10, no. 5: 359. https://doi.org/10.3390/genes10050359
APA StyleHoevenaars, F. P. M., Keijer, J., van der Stelt, I., Duivenvoorde, L. P. M., Herreman, L., van Nes, R., Friedecký, D., Hegeman, M. A., & van Schothorst, E. M. (2019). White Adipose Tissue Response of Obese Mice to Ambient Oxygen Restriction at Thermoneutrality: Response Markers Identified, but no WAT Inflammation. Genes, 10(5), 359. https://doi.org/10.3390/genes10050359





