Common Features of the Pericentromere and Nucleolus
Abstract
1. Introduction
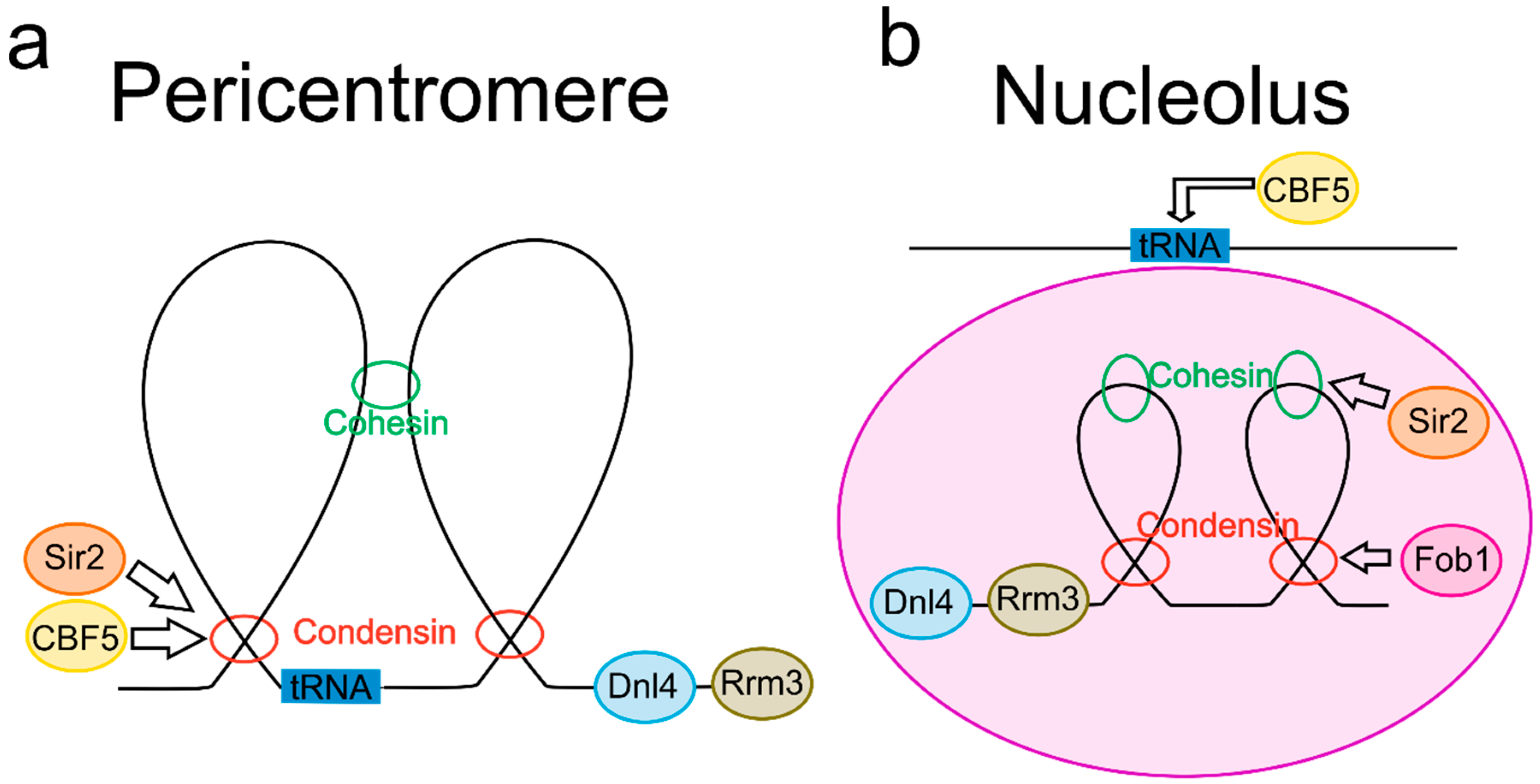
2. Common Features in Both the Pericentromere and the Nucleolus
2.1. DNA Loops Are Enriched in the Pericentromere and Nucleolus
2.2. SMC Proteins in the Pericentromere and Nucleolus Display Common DNA Regulatory Roles
2.3. tRNA Genes Are Localized to Both the Pericentromere and the Nucleolus
2.4. Replication Fork Stalling in Pericentromere and rDNA
2.5. Recombination Control in the Pericentromere and rDNA
2.6. Phase Separation in the Nucleolus and Pericentromere
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Verdaasdonk, J.S.; Bloom, K. Centromeres: Unique chromatin structures that drive chromosome segregation. Nat. Rev. Mol. Cell Biol. 2011, 12, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, D.W.; Mao, Y.; Sullivan, K.F. Centromeres and kinetochores: From epigenetics to mitotic checkpoint signaling. Cell 2003, 112, 407–421. [Google Scholar] [CrossRef]
- Blat, Y.; Kleckner, N. Cohesins bind to preferential sites along yeast chromosome III, with differential regulation along arms versus the centric region. Cell 1999, 98, 249–259. [Google Scholar] [CrossRef]
- Megee, P.C.; Mistrot, C.; Guacci, V.; Koshland, D. The centromeric sister chromatid cohesion site directs Mcd1p binding to adjacent sequences. Mol. Cell 1999, 4, 445–450. [Google Scholar] [CrossRef]
- D’Ambrosio, C.; Schmidt, C.K.; Katou, Y.; Kelly, G.; Itoh, T.; Shirahige, K.; Uhlmann, F. Identification of cis-acting sites for condensin loading onto budding yeast chromosomes. Genes Dev. 2008, 22, 2215–2227. [Google Scholar] [CrossRef]
- Lawrimore, J.; Bloom, K. The regulation of chromosome segregation via centromere loops. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 352–370. [Google Scholar] [CrossRef]
- Kobayashi, T. Ribosomal RNA gene repeats, their stability and cellular senescence. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2014, 90, 119–129. [Google Scholar] [CrossRef]
- Hertwig, O. Lehrbuch der Entwicklungsgeschichte des Menschen und der Wirbeltiere (Textbook of developmental History of Humans and Vertebrates); Nabu Press: Charleston, SC, USA, 1906; p. 548. [Google Scholar]
- Hofmann, A.; Heermann, D.W. The role of loops on the order of eukaryotes and prokaryotes. FEBS Lett. 2015, 589, 2958–2965. [Google Scholar] [CrossRef]
- Filipski, J.; Mucha, M. Structure, function and DNA composition of Saccharomyces cerevisiae chromatin loops. Gene 2002, 300, 63–68. [Google Scholar] [CrossRef]
- Yeh, E.; Haase, J.; Paliulis, L.V.; Joglekar, A.; Bond, L.; Bouck, D.; Salmon, E.D.; Bloom, K.S. Pericentric chromatin is organized into an intramolecular loop in mitosis. Curr. Biol. 2008, 18, 81–90. [Google Scholar] [CrossRef]
- Stephens, A.D.; Haase, J.; Vicci, L.; Taylor, R.M., 2nd; Bloom, K. Cohesin, condensin, and the intramolecular centromere loop together generate the mitotic chromatin spring. J. Cell Biol. 2011, 193, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Aze, A.; Sannino, V.; Soffientini, P.; Bachi, A.; Costanzo, V. Centromeric DNA replication reconstitution reveals DNA loops and ATR checkpoint suppression. Nat. Cell Biol. 2016, 18, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.A.; Vagnarelli, P.; Dong, Y.; Hori, T.; McEwen, B.F.; Fukagawa, T.; Flors, C.; Earnshaw, W.C. A super-resolution map of the vertebrate kinetochore. Proc. Natl. Acad. Sci. USA 2010, 107, 10484–10489. [Google Scholar] [CrossRef] [PubMed]
- Lawrimore, J.; Vasquez, P.A.; Falvo, M.R.; Taylor, R.M., 2nd; Vicci, L.; Yeh, E.; Forest, M.G.; Bloom, K. DNA loops generate intracentromere tension in mitosis. J. Cell Biol. 2015, 210, 553–564. [Google Scholar] [CrossRef]
- Lawrimore, J.; Aicher, J.K.; Hahn, P.; Fulp, A.; Kompa, B.; Vicci, L.; Falvo, M.; Taylor, R.M., 2nd; Bloom, K. ChromoShake: A chromosome dynamics simulator reveals that chromatin loops stiffen centromeric chromatin. Mol. Biol. Cell 2016, 27, 153–166. [Google Scholar] [CrossRef]
- Stephens, A.D.; Quammen, C.W.; Chang, B.; Haase, J.; Taylor, R.M., 2nd; Bloom, K. The spatial segregation of pericentric cohesin and condensin in the mitotic spindle. Mol. Biol. Cell 2013, 24, 3909–3919. [Google Scholar] [CrossRef]
- Lawrimore, J.; Doshi, A.; Friedman, B.; Yeh, E.; Bloom, K. Geometric partitioning of cohesin and condensin is a consequence of chromatin loops. Mol. Biol. Cell 2018, 29, 2737–2750. [Google Scholar] [CrossRef]
- Vas, A.C.; Andrews, C.A.; Kirkland Matesky, K.; Clarke, D.J. In vivo analysis of chromosome condensation in Saccharomyces cerevisiae. Mol. Biol. Cell 2007, 18, 557–568. [Google Scholar] [CrossRef]
- Hirano, T. Condensins: Universal organizers of chromosomes with diverse functions. Genes Dev. 2012, 26, 1659–1678. [Google Scholar] [CrossRef]
- McFarlane, R.J.; Humphrey, T.C. A role for recombination in centromere function. Trends Genet. 2010, 26, 209–213. [Google Scholar] [CrossRef]
- Guacci, V.; Hogan, E.; Koshland, D. Chromosome condensation and sister chromatid pairing in budding yeast. J. Cell Biol. 1994, 125, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Harris, B.; Bose, T.; Lee, K.K.; Wang, F.; Lu, S.; Ross, R.T.; Zhang, Y.; French, S.L.; Beyer, A.L.; Slaughter, B.D.; et al. Cohesion promotes nucleolar structure and function. Mol. Biol. Cell 2014, 25, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Mayan, M.; Aragon, L. Cis-interactions between non-coding ribosomal spacers dependent on RNAP-II separate RNAP-I and RNAP-III transcription domains. Cell Cycle 2010, 9, 4328–4337. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xue, Y.; Acar, M. Live-Cell Imaging of Chromatin Condensation Dynamics by CRISPR. iScience 2018, 4, 216–235. [Google Scholar] [CrossRef] [PubMed]
- Lazar-Stefanita, L.; Scolari, V.F.; Mercy, G.; Muller, H.; Guerin, T.M.; Thierry, A.; Mozziconacci, J.; Koszul, R. Cohesins and condensins orchestrate the 4D dynamics of yeast chromosomes during the cell cycle. EMBO J. 2017, 36, 2684–2697. [Google Scholar] [CrossRef] [PubMed]
- Merz, K.; Hondele, M.; Goetze, H.; Gmelch, K.; Stoeckl, U.; Griesenbeck, J. Actively transcribed rRNA genes in S. cerevisiae are organized in a specialized chromatin associated with the high-mobility group protein Hmo1 and are largely devoid of histone molecules. Genes Dev. 2008, 22, 1190–1204. [Google Scholar] [CrossRef]
- O’Sullivan, A.C.; Sullivan, G.J.; McStay, B. UBF binding in vivo is not restricted to regulatory sequences within the vertebrate ribosomal DNA repeat. Mol. Cell Biol. 2002, 22, 657–668. [Google Scholar] [CrossRef]
- Gadal, O.; Labarre, S.; Boschiero, C.; Thuriaux, P. Hmo1, an HMG-box protein, belongs to the yeast ribosomal DNA transcription system. EMBO J. 2002, 21, 5498–5507. [Google Scholar] [CrossRef]
- Stefanovsky, V.Y.; Pelletier, G.; Bazett-Jones, D.P.; Crane-Robinson, C.; Moss, T. DNA looping in the RNA polymerase I enhancesome is the result of non-cooperative in-phase bending by two UBF molecules. Nucleic Acids Res. 2001, 29, 3241–3247. [Google Scholar] [CrossRef]
- Stefanovsky, V.Y.; Bazett-Jones, D.P.; Pelletier, G.; Moss, T. The DNA supercoiling architecture induced by the transcription factor xUBF requires three of its five HMG-boxes. Nucleic Acids Res. 1996, 24, 3208–3215. [Google Scholar] [CrossRef]
- Putnam, C.D.; Copenhaver, G.P.; Denton, M.L.; Pikaard, C.S. The RNA polymerase I transactivator upstream binding factor requires its dimerization domain and high-mobility-group (HMG) box 1 to bend, wrap, and positively supercoil enhancer DNA. Mol. Cell Biol. 1994, 14, 6476–6488. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.; Wang, Z.; Roeder, R.G.; Gall, J.G. RNA polymerase III in Cajal bodies and lampbrush chromosomes of the Xenopus oocyte nucleus. Mol. Biol. Cell 2002, 13, 3466–3476. [Google Scholar] [CrossRef] [PubMed]
- Ganji, M.; Shaltiel, I.A.; Bisht, S.; Kim, E.; Kalichava, A.; Haering, C.H.; Dekker, C. Real-time imaging of DNA loop extrusion by condensin. Science 2018, 360, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Snider, C.E.; Stephens, A.D.; Kirkland, J.G.; Hamdani, O.; Kamakaka, R.T.; Bloom, K. Dyskerin, tRNA genes, and condensin tether pericentric chromatin to the spindle axis in mitosis. J. Cell Biol. 2014, 207, 189–199. [Google Scholar] [CrossRef]
- Azvolinsky, A.; Dunaway, S.; Torres, J.Z.; Bessler, J.B.; Zakian, V.A. The S. cerevisiae Rrm3p DNA helicase moves with the replication fork and affects replication of all yeast chromosomes. Genes Dev. 2006, 20, 3104–3116. [Google Scholar] [CrossRef]
- Fritsch, O.; Burkhalter, M.D.; Kais, S.; Sogo, J.M.; Schar, P. DNA ligase 4 stabilizes the ribosomal DNA array upon fork collapse at the replication fork barrier. DNA Repair (Amst) 2010, 9, 879–888. [Google Scholar] [CrossRef]
- Hanlon, S.L.; Li, J.J. Re-replication of a centromere induces chromosomal instability and aneuploidy. PLoS Genet. 2015, 11, e1005039. [Google Scholar] [CrossRef]
- Iwasaki, O.; Tanaka, A.; Tanizawa, H.; Grewal, S.I.; Noma, K. Centromeric localization of dispersed Pol III genes in fission yeast. Mol. Biol. Cell 2010, 21, 254–265. [Google Scholar] [CrossRef]
- Thompson, M.; Haeusler, R.A.; Good, P.D.; Engelke, D.R. Nucleolar clustering of dispersed tRNA genes. Science 2003, 302, 1399–1401. [Google Scholar] [CrossRef]
- Haeusler, R.A.; Pratt-Hyatt, M.; Good, P.D.; Gipson, T.A.; Engelke, D.R. Clustering of yeast tRNA genes is mediated by specific association of condensin with tRNA gene transcription complexes. Genes Dev. 2008, 22, 2204–2214. [Google Scholar] [CrossRef]
- Glynn, E.F.; Megee, P.C.; Yu, H.G.; Mistrot, C.; Unal, E.; Koshland, D.E.; DeRisi, J.L.; Gerton, J.L. Genome-wide mapping of the cohesin complex in the yeast Saccharomyces cerevisiae. PLoS Biol. 2004, 2, E259. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, B.D.; Tuffo, K.M.; Oh, S.; Koshland, D.; Holm, C. Mitotic chromosome condensation requires Brn1p, the yeast homologue of Barren. Mol. Biol. Cell 2000, 11, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, B.D.; Hogan, E.; Koshland, D. In vivo requirements for rDNA chromosome condensation reveal two cell-cycle-regulated pathways for mitotic chromosome folding. Genes Dev. 2004, 18, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, B.D.; Hogan, E.; Koshland, D. In vivo dissection of the chromosome condensation machinery: Reversibility of condensation distinguishes contributions of condensin and cohesin. J. Cell Biol. 2002, 156, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Matos-Perdomo, E.; Machin, F. The ribosomal DNA metaphase loop of Saccharomyces cerevisiae gets condensed upon heat stress in a Cdc14-independent TORC1-dependent manner. Cell Cycle 2018, 17, 200–215. [Google Scholar] [CrossRef]
- Johzuka, K.; Terasawa, M.; Ogawa, H.; Ogawa, T.; Horiuchi, T. Condensin loaded onto the replication fork barrier site in the rRNA gene repeats during S phase in a FOB1-dependent fashion to prevent contraction of a long repetitive array in Saccharomyces cerevisiae. Mol. Cell Biol. 2006, 26, 2226–2236. [Google Scholar] [CrossRef]
- Kobayashi, T.; Horiuchi, T.; Tongaonkar, P.; Vu, L.; Nomura, M. SIR2 regulates recombination between different rDNA repeats, but not recombination within individual rRNA genes in yeast. Cell 2004, 117, 441–453. [Google Scholar] [CrossRef]
- Kendall, A.; Hull, M.W.; Bertrand, E.; Good, P.D.; Singer, R.H.; Engelke, D.R. A CBF5 mutation that disrupts nucleolar localization of early tRNA biosynthesis in yeast also suppresses tRNA gene-mediated transcriptional silencing. Proc. Natl. Acad. Sci. USA 2000, 97, 13108–13113. [Google Scholar] [CrossRef]
- Fukagawa, T.; Nogami, M.; Yoshikawa, M.; Ikeno, M.; Okazaki, T.; Takami, Y.; Nakayama, T.; Oshimura, M. Dicer is essential for formation of the heterochromatin structure in vertebrate cells. Nat. Cell Biol. 2004, 6, 784–791. [Google Scholar] [CrossRef]
- Eckert, C.A.; Gravdahl, D.J.; Megee, P.C. The enhancement of pericentromeric cohesin association by conserved kinetochore components promotes high-fidelity chromosome segregation and is sensitive to microtubule-based tension. Genes Dev. 2007, 21, 278–291. [Google Scholar] [CrossRef]
- Freeman, L.; Aragon-Alcaide, L.; Strunnikov, A. The condensin complex governs chromosome condensation and mitotic transmission of rDNA. J. Cell Biol. 2000, 149, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, N.; Nakamura, T.; Kokubu, A.; Ebe, M.; Nagao, K.; Yanagida, M. Dissection of the essential steps for condensin accumulation at kinetochores and rDNAs during fission yeast mitosis. J. Cell Biol. 2008, 180, 1115–1131. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. At the heart of the chromosome: SMC proteins in action. Nat. Rev. Mol. Cell Biol. 2006, 7, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Strunnikov, A.V.; Hogan, E.; Koshland, D. SMC2, a Saccharomyces cerevisiae gene essential for chromosome segregation and condensation, defines a subgroup within the SMC family. Genes Dev. 1995, 9, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Terakawa, T.; Bisht, S.; Eeftens, J.M.; Dekker, C.; Haering, C.H.; Greene, E.C. The condensin complex is a mechanochemical motor that translocates along DNA. Science 2017, 358, 672–676. [Google Scholar] [CrossRef]
- Kimura, K.; Rybenkov, V.V.; Crisona, N.J.; Hirano, T.; Cozzarelli, N.R. 13S condensin actively reconfigures DNA by introducing global positive writhe: Implications for chromosome condensation. Cell 1999, 98, 239–248. [Google Scholar] [CrossRef]
- Baxter, J.; Sen, N.; Martinez, V.L.; De Carandini, M.E.; Schvartzman, J.B.; Diffley, J.F.; Aragon, L. Positive supercoiling of mitotic DNA drives decatenation by topoisomerase II in eukaryotes. Science 2011, 331, 1328–1332. [Google Scholar] [CrossRef]
- Strick, T.R.; Bensimon, D.; Croquette, V. Micro-mechanical measurement of the torsional modulus of DNA. Genetica 1999, 106, 57–62. [Google Scholar] [CrossRef]
- Haering, C.H.; Lowe, J.; Hochwagen, A.; Nasmyth, K. Molecular architecture of SMC proteins and the yeast cohesin complex. Mol. Cell 2002, 9, 773–788. [Google Scholar] [CrossRef]
- Gruber, S.; Haering, C.H.; Nasmyth, K. Chromosomal cohesin forms a ring. Cell 2003, 112, 765–777. [Google Scholar] [CrossRef]
- Skibbens, R.V. Condensins and cohesins—One of these things is not like the other! J. Cell Sci. 2019, 132. [Google Scholar] [CrossRef] [PubMed]
- Burmann, F.; Lee, B.G.; Than, T.; Sinn, L.; O’Reilly, F.J.; Yatskevich, S.; Rappsilber, J.; Hu, B.; Nasmyth, K.; Lowe, J. A folded conformation of MukBEF and cohesin. Nat. Struct. Mol. Biol. 2019, 26, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Stigler, J.; Camdere, G.O.; Koshland, D.E.; Greene, E.C. Single-Molecule Imaging Reveals a Collapsed Conformational State for DNA-Bound Cohesin. Cell Rep. 2016, 15, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, C.; Ciosk, R.; Nasmyth, K. Cohesins: Chromosomal proteins that prevent premature separation of sister chromatids. Cell 1997, 91, 35–45. [Google Scholar] [CrossRef]
- Nasmyth, K.; Haering, C.H. Cohesin: Its roles and mechanisms. Annu. Rev. Genet. 2009, 43, 525–558. [Google Scholar] [CrossRef]
- Heidinger-Pauli, J.M.; Mert, O.; Davenport, C.; Guacci, V.; Koshland, D. Systematic reduction of cohesin differentially affects chromosome segregation, condensation, and DNA repair. Curr. Biol. 2010, 20, 957–963. [Google Scholar] [CrossRef]
- Davidson, I.F.; Bauer, B.; Goetz, D.; Tang, W.; Wutz, G.; Peters, J.M. DNA loop extrusion by human cohesin. Science 2019. [Google Scholar] [CrossRef]
- Rao, S.S.; Huntley, M.H.; Durand, N.C.; Stamenova, E.K.; Bochkov, I.D.; Robinson, J.T.; Sanborn, A.L.; Machol, I.; Omer, A.D.; Lander, E.S.; et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 2014, 159, 1665–1680. [Google Scholar] [CrossRef]
- Gassler, J.; Brandão, H.B.; Imakaev, M.; Flyamer, I.M.; Ladstätter, S.; Bickmore, W.A.; Peters, J.M.; Mirny, L.A.; Tachibana, K. A mechanism of cohesin-dependent loop extrusion organizes zygotic genome architecture. EMBO J. 2017, 36, 3600–3618. [Google Scholar] [CrossRef]
- Rao, S.S.P.; Huang, S.C.; Glenn St Hilaire, B.; Engreitz, J.M.; Perez, E.M.; Kieffer-Kwon, K.R.; Sanborn, A.L.; Johnstone, S.E.; Bascom, G.D.; Bochkov, I.D.; et al. Cohesin Loss Eliminates All Loop Domains. Cell 2017, 171, 305–320.e24. [Google Scholar] [CrossRef]
- Nuebler, J.; Fudenberg, G.; Imakaev, M.; Abdennur, N.; Mirny, L.A. Chromatin organization by an interplay of loop extrusion and compartmental segregation. Proc. Natl. Acad. Sci. USA 2018, 115, E6697–E6706. [Google Scholar] [CrossRef] [PubMed]
- Wutz, G.; Varnai, C.; Nagasaka, K.; Cisneros, D.A.; Stocsits, R.R.; Tang, W.; Schoenfelder, S.; Jessberger, G.; Muhar, M.; Hossain, M.J.; et al. Topologically associating domains and chromatin loops depend on cohesin and are regulated by CTCF, WAPL, and PDS5 proteins. EMBO J. 2017, 36, 3573–3599. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Valsakumar, V.; Poorey, K.; Bekiranov, S.; Smith, J.S. Genome-wide analysis of functional sirtuin chromatin targets in yeast. Genome Biol. 2013, 14, R48. [Google Scholar] [CrossRef] [PubMed]
- Guacci, V.; Koshland, D.; Strunnikov, A. A direct link between sister chromatid cohesion and chromosome condensation revealed through the analysis of MCD1 in S. cerevisiae. Cell 1997, 91, 47–57. [Google Scholar] [CrossRef]
- Dauban, L.; Kamgoue, A.; Wang, R.; Leger-Silvestre, I.; Beckouet, F.; Cantaloube, S.; Gadal, O. Quantification of the dynamic behaviour of ribosomal DNA genes and nucleolus during yeast Saccharomyces cerevisiae cell cycle. J. Struct. Biol. 2019, 208, 152–164. [Google Scholar] [CrossRef]
- Bose, T.; Lee, K.K.; Lu, S.; Xu, B.; Harris, B.; Slaughter, B.; Unruh, J.; Garrett, A.; McDowell, W.; Box, A.; et al. Cohesin proteins promote ribosomal RNA production and protein translation in yeast and human cells. PLoS Genet. 2012, 8, e1002749. [Google Scholar] [CrossRef]
- Takahashi, Y.; Yong-Gonzalez, V.; Kikuchi, Y.; Strunnikov, A. SIZ1/SIZ2 control of chromosome transmission fidelity is mediated by the sumoylation of topoisomerase II. Genetics 2006, 172, 783–794. [Google Scholar] [CrossRef]
- Stephens, A.D.; Snider, C.E.; Bloom, K. The SUMO deconjugating peptidase Smt4 contributes to the mechanism required for transition from sister chromatid arm cohesion to sister chromatid pericentromere separation. Cell Cycle 2015, 14, 2206–2218. [Google Scholar] [CrossRef]
- Strunnikov, A.V.; Aravind, L.; Koonin, E.V. Saccharomyces cerevisiae SMT4 encodes an evolutionarily conserved protease with a role in chromosome condensation regulation. Genetics 2001, 158, 95–107. [Google Scholar]
- Bachant, J.; Alcasabas, A.; Blat, Y.; Kleckner, N.; Elledge, S.J. The SUMO-1 isopeptidase Smt4 is linked to centromeric cohesion through SUMO-1 modification of DNA topoisomerase II. Mol. Cell 2002, 9, 1169–1182. [Google Scholar] [CrossRef]
- Porter, A.C.; Farr, C.J. Topoisomerase II: Untangling its contribution at the centromere. Chromosome Res. Int. J. Mol. Supramol. Evol. Asp. Chromosome Biol. 2004, 12, 569–583. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, C.; Kelly, G.; Shirahige, K.; Uhlmann, F. Condensin-dependent rDNA decatenation introduces a temporal pattern to chromosome segregation. Curr. Biol. 2008, 18, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Uuskula-Reimand, L.; Hou, H.; Samavarchi-Tehrani, P.; Rudan, M.V.; Liang, M.; Medina-Rivera, A.; Mohammed, H.; Schmidt, D.; Schwalie, P.; Young, E.J.; et al. Topoisomerase II beta interacts with cohesin and CTCF at topological domain borders. Genome Biol. 2016, 17, 182. [Google Scholar] [CrossRef] [PubMed]
- Warsi, T.H.; Navarro, M.S.; Bachant, J. DNA topoisomerase II is a determinant of the tensile properties of yeast centromeric chromatin and the tension checkpoint. Mol. Biol. Cell 2008, 19, 4421–4433. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.A.; Queiroz-Machado, J.; Sunkel, C.E. Condensin-dependent localisation of topoisomerase II to an axial chromosomal structure is required for sister chromatid resolution during mitosis. J. Cell Sci. 2003, 116, 4763–4776. [Google Scholar] [CrossRef]
- Dewar, H.; Tanaka, K.; Nasmyth, K.; Tanaka, T.U. Tension between two kinetochores suffices for their bi-orientation on the mitotic spindle. Nature 2004, 428, 93–97. [Google Scholar] [CrossRef]
- Vagnarelli, P.; Morrison, C.; Dodson, H.; Sonoda, E.; Takeda, S.; Earnshaw, W.C. Analysis of Scc1-deficient cells defines a key metaphase role of vertebrate cohesin in linking sister kinetochores. EMBO Rep. 2004, 5, 167–171. [Google Scholar] [CrossRef]
- De Wit, E.; Vos, E.S.; Holwerda, S.J.; Valdes-Quezada, C.; Verstegen, M.J.; Teunissen, H.; Splinter, E.; Wijchers, P.J.; Krijger, P.H.; de Laat, W. CTCF Binding Polarity Determines Chromatin Looping. Mol. Cell 2015, 60, 676–684. [Google Scholar] [CrossRef]
- Splinter, E.; Heath, H.; Kooren, J.; Palstra, R.J.; Klous, P.; Grosveld, F.; Galjart, N.; de Laat, W. CTCF mediates long-range chromatin looping and local histone modification in the beta-globin locus. Genes Dev. 2006, 20, 2349–2354. [Google Scholar] [CrossRef]
- Parelho, V.; Hadjur, S.; Spivakov, M.; Leleu, M.; Sauer, S.; Gregson, H.C.; Jarmuz, A.; Canzonetta, C.; Webster, Z.; Nesterova, T.; et al. Cohesins functionally associate with CTCF on mammalian chromosome arms. Cell 2008, 132, 422–433. [Google Scholar] [CrossRef]
- Wendt, K.S.; Yoshida, K.; Itoh, T.; Bando, M.; Koch, B.; Schirghuber, E.; Tsutsumi, S.; Nagae, G.; Ishihara, K.; Mishiro, T.; et al. Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature 2008, 451, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Herold, M.; Bartkuhn, M.; Renkawitz, R. CTCF: Insights into insulator function during development. Development 2012, 139, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Heger, P.; Marin, B.; Schierenberg, E. Loss of the insulator protein CTCF during nematode evolution. BMC Mol. Biol. 2009, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Defossez, P.A.; Gilson, E. The vertebrate protein CTCF functions as an insulator in Saccharomyces cerevisiae. Nucleic Acids Res. 2002, 30, 5136–5141. [Google Scholar] [CrossRef] [PubMed]
- Fernius, J.; Marston, A.L. Establishment of cohesion at the pericentromere by the Ctf19 kinetochore subcomplex and the replication fork-associated factor, Csm3. PLoS Genet. 2009, 5, e1000629. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.M.; Waples, W.G.; Lavoie, B.D.; Biggins, S. Pericentromeric sister chromatid cohesion promotes kinetochore biorientation. Mol. Biol. Cell 2009, 20, 3818–3827. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.M.; Bennett, M.; Friedman, B.; Lawrimore, J.; Yeh, E.; Bloom, K. Fork pausing allows centromere DNA loop formation and kinetochore assembly. Proc. Natl. Acad. Sci. USA 2018, 115, 11784–11789. [Google Scholar] [CrossRef]
- Fudenberg, G.; Imakaev, M.; Lu, C.; Goloborodko, A.; Abdennur, N.; Mirny, L.A. Formation of Chromosomal Domains by Loop Extrusion. Cell Rep. 2016, 15, 2038–2049. [Google Scholar] [CrossRef]
- Oler, A.J.; Alla, R.K.; Roberts, D.N.; Wong, A.; Hollenhorst, P.C.; Chandler, K.J.; Cassiday, P.A.; Nelson, C.A.; Hagedorn, C.H.; Graves, B.J.; et al. Human RNA polymerase III transcriptomes and relationships to Pol II promoter chromatin and enhancer-binding factors. Nat. Struct. Mol. Biol. 2010, 17, 620–628. [Google Scholar] [CrossRef]
- Zentner, G.E.; Saiakhova, A.; Manaenkov, P.; Adams, M.D.; Scacheri, P.C. Integrative genomic analysis of human ribosomal DNA. Nucleic Acids Res. 2011, 39, 4949–4960. [Google Scholar] [CrossRef]
- Van de Nobelen, S.; Rosa-Garrido, M.; Leers, J.; Heath, H.; Soochit, W.; Joosen, L.; Jonkers, I.; Demmers, J.; van der Reijden, M.; Torrano, V.; et al. CTCF regulates the local epigenetic state of ribosomal DNA repeats. Epigenetics Chromatin 2010, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Torrano, V.; Navascues, J.; Docquier, F.; Zhang, R.; Burke, L.J.; Chernukhin, I.; Farrar, D.; Leon, J.; Berciano, M.T.; Renkawitz, R.; et al. Targeting of CTCF to the nucleolus inhibits nucleolar transcription through a poly(ADP-ribosyl)ation-dependent mechanism. J. Cell Sci. 2006, 119, 1746–1759. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, P.A.; Maggert, K.A. The CCCTC-binding factor (CTCF) of Drosophila contributes to the regulation of the ribosomal DNA and nucleolar stability. PLoS ONE 2011, 6, e16401. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Jia, J.; Wu, C.; Yao, M.; Li, M.; Jin, J.; Jiang, C.; Cai, Y.; Pei, D.; Pan, G.; et al. Ribosomal RNA gene transcription mediated by the master genome regulator protein CCCTC-binding factor (CTCF) is negatively regulated by the condensin complex. J. Biol. Chem. 2013, 288, 26067–26077. [Google Scholar] [CrossRef]
- Dieci, G.; Fiorino, G.; Castelnuovo, M.; Teichmann, M.; Pagano, A. The expanding RNA polymerase III transcriptome. Trends Genet 2007, 23, 614–622. [Google Scholar] [CrossRef]
- Kuhn, R.M.; Clarke, L.; Carbon, J. Clustered tRNA genes in Schizosaccharomyces pombe centromeric DNA sequence repeats. Proc. Natl. Acad. Sci. USA 1991, 88, 1306–1310. [Google Scholar] [CrossRef]
- Wang, L.; Haeusler, R.A.; Good, P.D.; Thompson, M.; Nagar, S.; Engelke, D.R. Silencing near tRNA genes requires nucleolar localization. J. Biol. Chem. 2005, 280, 8637–8639. [Google Scholar] [CrossRef]
- Bertrand, E.; Houser-Scott, F.; Kendall, A.; Singer, R.H.; Engelke, D.R. Nucleolar localization of early tRNA processing. Genes Dev. 1998, 12, 2463–2468. [Google Scholar] [CrossRef]
- Belagal, P.; Normand, C.; Shukla, A.; Wang, R.; Leger-Silvestre, I.; Dez, C.; Bhargava, P.; Gadal, O. Decoding the principles underlying the frequency of association with nucleoli for RNA polymerase III-transcribed genes in budding yeast. Mol. Biol. Cell 2016, 27, 3164–3177. [Google Scholar] [CrossRef]
- Jin, Q.W.; Fuchs, J.; Loidl, J. Centromere clustering is a major determinant of yeast interphase nuclear organization. J. Cell Sci. 2000, 113, 1903–1912. [Google Scholar]
- Hamdani, O.; Dhillon, N.; Hsieh, T.S.; Fujita, T.; Ocampo, J.; Kirkland, J.G.; Lawrimore, J.; Kobayashi, T.J.; Friedman, B.; Fulton, D.; et al. tRNA Genes Affect Chromosome Structure and Function via Local Effects. Mol. Cell Biol. 2019, 39. [Google Scholar] [CrossRef] [PubMed]
- Gard, S.; Light, W.; Xiong, B.; Bose, T.; McNairn, A.J.; Harris, B.; Fleharty, B.; Seidel, C.; Brickner, J.H.; Gerton, J.L. Cohesinopathy mutations disrupt the subnuclear organization of chromatin. J. Cell Biol. 2009, 187, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.L.; Elgin, S.C. Putting boundaries on silence. Cell 1999, 99, 459–462. [Google Scholar] [CrossRef]
- Scott, K.C.; Merrett, S.L.; Willard, H.F. A heterochromatin barrier partitions the fission yeast centromere into discrete chromatin domains. Curr. Biol. 2006, 16, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.C.; White, C.V.; Willard, H.F. An RNA polymerase III-dependent heterochromatin barrier at fission yeast centromere 1. PLoS ONE 2007, 2, e1099. [Google Scholar] [CrossRef]
- Raab, J.R.; Chiu, J.; Zhu, J.; Katzman, S.; Kurukuti, S.; Wade, P.A.; Haussler, D.; Kamakaka, R.T. Human tRNA genes function as chromatin insulators. EMBO J. 2012, 31, 330–350. [Google Scholar] [CrossRef]
- Deshpande, A.M.; Newlon, C.S. DNA replication fork pause sites dependent on transcription. Science 1996, 272, 1030–1033. [Google Scholar] [CrossRef]
- Pryce, D.W.; Ramayah, S.; Jaendling, A.; McFarlane, R.J. Recombination at DNA replication fork barriers is not universal and is differentially regulated by Swi1. Proc. Natl. Acad. Sci. USA 2009, 106, 4770–4775. [Google Scholar] [CrossRef]
- Hodgson, B.; Calzada, A.; Labib, K. Mrc1 and Tof1 regulate DNA replication forks in different ways during normal S phase. Mol. Biol. Cell 2007, 18, 3894–3902. [Google Scholar] [CrossRef]
- Voineagu, I.; Narayanan, V.; Lobachev, K.S.; Mirkin, S.M. Replication stalling at unstable inverted repeats: Interplay between DNA hairpins and fork stabilizing proteins. Proc. Natl. Acad. Sci. USA 2008, 105, 9936–9941. [Google Scholar] [CrossRef]
- Voineagu, I.; Surka, C.F.; Shishkin, A.A.; Krasilnikova, M.M.; Mirkin, S.M. Replisome stalling and stabilization at CGG repeats, which are responsible for chromosomal fragility. Nat. Struct. Mol. Biol. 2009, 16, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Lambert, S.; Baldacci, G.; Murray, J.M.; Carr, A.M. Nearby inverted repeats fuse to generate acentric and dicentric palindromic chromosomes by a replication template exchange mechanism. Genes Dev. 2009, 23, 2876–2886. [Google Scholar] [CrossRef] [PubMed]
- Greenfeder, S.A.; Newlon, C.S. Replication forks pause at yeast centromeres. Mol. Cell Biol. 1992, 12, 4056–4066. [Google Scholar] [CrossRef] [PubMed]
- Katou, Y.; Kanoh, Y.; Bando, M.; Noguchi, H.; Tanaka, H.; Ashikari, T.; Sugimoto, K.; Shirahige, K. S-phase checkpoint proteins Tof1 and Mrc1 form a stable replication-pausing complex. Nature 2003, 424, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.L.; Pot, I.; Chang, M.; Xu, H.; Aneliunas, V.; Kwok, T.; Newitt, R.; Aebersold, R.; Boone, C.; Brown, G.W.; et al. Identification of protein complexes required for efficient sister chromatid cohesion. Mol. Biol. Cell 2004, 15, 1736–1745. [Google Scholar] [CrossRef] [PubMed]
- Calzada, A.; Hodgson, B.; Kanemaki, M.; Bueno, A.; Labib, K. Molecular anatomy and regulation of a stable replisome at a paused eukaryotic DNA replication fork. Genes Dev. 2005, 19, 1905–1919. [Google Scholar] [CrossRef]
- Ivessa, A.S.; Zhou, J.Q.; Schulz, V.P.; Monson, E.K.; Zakian, V.A. Saccharomyces Rrm3p, a 5’ to 3’ DNA helicase that promotes replication fork progression through telomeric and subtelomeric DNA. Genes Dev. 2002, 16, 1383–1396. [Google Scholar] [CrossRef]
- Mohanty, B.K.; Bairwa, N.K.; Bastia, D. The Tof1p-Csm3p protein complex counteracts the Rrm3p helicase to control replication termination of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2006, 103, 897–902. [Google Scholar] [CrossRef]
- Biggins, S. The composition, functions, and regulation of the budding yeast kinetochore. Genetics 2013, 194, 817–846. [Google Scholar] [CrossRef]
- Mythreye, K.; Bloom, K.S. Differential kinetochore protein requirements for establishment versus propagation of centromere activity in Saccharomyces cerevisiae. J. Cell Biol. 2003, 160, 833–843. [Google Scholar] [CrossRef]
- Mirkin, E.V.; Mirkin, S.M. Replication fork stalling at natural impediments. Microbiol. Mol. Biol. Rev. 2007, 71, 13–35. [Google Scholar] [CrossRef] [PubMed]
- Brewer, B.J.; Fangman, W.L. A replication fork barrier at the 3’ end of yeast ribosomal RNA genes. Cell 1988, 55, 637–643. [Google Scholar] [CrossRef]
- Little, R.D.; Platt, T.H.; Schildkraut, C.L. Initiation and termination of DNA replication in human rRNA genes. Mol. Cell Biol. 1993, 13, 6600–6613. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Horiuchi, T. A yeast gene product, Fob1 protein, required for both replication fork blocking and recombinational hotspot activities. Genes Cells 1996, 1, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T. The replication fork barrier site forms a unique structure with Fob1p and inhibits the replication fork. Mol. Cell Biol. 2003, 23, 9178–9188. [Google Scholar] [CrossRef] [PubMed]
- Greenfeder, S.A.; Newlon, C.S. A replication map of a 61-kb circular derivative of Saccharomyces cerevisiae chromosome III. Mol. Biol. Cell 1992, 3, 999–1013. [Google Scholar] [CrossRef]
- Fachinetti, D.; Bermejo, R.; Cocito, A.; Minardi, S.; Katou, Y.; Kanoh, Y.; Shirahige, K.; Azvolinsky, A.; Zakian, V.A.; Foiani, M. Replication termination at eukaryotic chromosomes is mediated by Top2 and occurs at genomic loci containing pausing elements. Mol. Cell 2010, 39, 595–605. [Google Scholar] [CrossRef]
- Mundbjerg, K.; Jorgensen, S.W.; Fredsoe, J.; Nielsen, I.; Pedersen, J.M.; Bentsen, I.B.; Lisby, M.; Bjergbaek, L.; Andersen, A.H. Top2 and Sgs1-Top3 Act Redundantly to Ensure rDNA Replication Termination. PLoS Genet. 2015, 11, e1005697. [Google Scholar] [CrossRef]
- Wilson, T.E.; Grawunder, U.; Lieber, M.R. Yeast DNA ligase IV mediates non-homologous DNA end joining. Nature 1997, 388, 495–498. [Google Scholar] [CrossRef]
- Termolino, P.; Cremona, G.; Consiglio, M.F.; Conicella, C. Insights into epigenetic landscape of recombination-free regions. Chromosoma 2016, 125, 301–308. [Google Scholar] [CrossRef]
- Liebman, S.W.; Symington, L.S.; Petes, T.D. Mitotic recombination within the centromere of a yeast chromosome. Science 1988, 241, 1074–1077. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.S.; Greenwell, P.W.; Dominska, M.; Gawel, M.; Hamilton, M.; Petes, T.D. A fine-structure map of spontaneous mitotic crossovers in the yeast Saccharomyces cerevisiae. PLoS Genet. 2009, 5, e1000410. [Google Scholar] [CrossRef] [PubMed]
- Jaco, I.; Canela, A.; Vera, E.; Blasco, M.A. Centromere mitotic recombination in mammalian cells. J. Cell Biol. 2008, 181, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, R.; Michel, B.; Gangloff, S. Replication fork pausing and recombination or “gimme a break”. Genes Dev. 2000, 14, 1–10. [Google Scholar]
- Mehta, A.; Haber, J.E. Sources of DNA double-strand breaks and models of recombinational DNA repair. Cold Spring Harb. Perspect. Biol. 2014, 6, a016428. [Google Scholar] [CrossRef]
- Syeda, A.H.; Hawkins, M.; McGlynn, P. Recombination and replication. Cold Spring Harb. Perspect. Biol. 2014, 6, a016550. [Google Scholar] [CrossRef]
- Johzuka, K.; Horiuchi, T. Replication fork block protein, Fob1, acts as an rDNA region specific recombinator in S. cerevisiae. Genes Cells 2002, 7, 99–113. [Google Scholar] [CrossRef]
- Kobayashi, T.; Heck, D.J.; Nomura, M.; Horiuchi, T. Expansion and contraction of ribosomal DNA repeats in Saccharomyces cerevisiae: Requirement of replication fork blocking (Fob1) protein and the role of RNA polymerase I. Genes Dev. 1998, 12, 3821–3830. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Horiuchi, T.; Kobayashi, T. Transcription-dependent recombination and the role of fork collision in yeast rDNA. Genes Dev. 2003, 17, 1497–1506. [Google Scholar] [CrossRef]
- Keil, R.L.; Roeder, G.S. Cis-acting, recombination-stimulating activity in a fragment of the ribosomal DNA of S. cerevisiae. Cell 1984, 39, 377–386. [Google Scholar] [CrossRef]
- Gottlieb, S.; Esposito, R.E. A new role for a yeast transcriptional silencer gene, SIR2, in regulation of recombination in ribosomal DNA. Cell 1989, 56, 771–776. [Google Scholar] [CrossRef]
- Nambiar, M.; Smith, G.R. Pericentromere-Specific Cohesin Complex Prevents Meiotic Pericentric DNA Double-Strand Breaks and Lethal Crossovers. Mol. Cell 2018, 71, 540–553.e4. [Google Scholar] [CrossRef] [PubMed]
- Christman, M.F.; Dietrich, F.S.; Fink, G.R. Mitotic recombination in the rDNA of S. cerevisiae is suppressed by the combined action of DNA topoisomerases I and II. Cell 1988, 55, 413–425. [Google Scholar] [CrossRef]
- Canela, A.; Maman, Y.; Jung, S.; Wong, N.; Callen, E.; Day, A.; Kieffer-Kwon, K.R.; Pekowska, A.; Zhang, H.; Rao, S.S.P.; et al. Genome Organization Drives Chromosome Fragility. Cell 2017, 170, 507–521.e18. [Google Scholar] [CrossRef]
- Gothe, H.J.; Bouwman, B.A.M.; Gusmao, E.G.; Piccinno, R.; Petrosino, G.; Sayols, S.; Drechsel, O.; Minneker, V.; Josipovic, N.; Mizi, A.; et al. Spatial Chromosome Folding and Active Transcription Drive DNA Fragility and Formation of Oncogenic MLL Translocations. Mol. Cell 2019, 75, 267–283.e12. [Google Scholar] [CrossRef]
- Correll, C.C.; Bartek, J.; Dundr, M. The Nucleolus: A Multiphase Condensate Balancing Ribosome Synthesis and Translational Capacity in Health, Aging and Ribosomopathies. Cells 2019, 8, 869. [Google Scholar] [CrossRef]
- Brangwynne, C.P.; Mitchison, T.J.; Hyman, A.A. Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 4334–4339. [Google Scholar] [CrossRef]
- Feric, M.; Vaidya, N.; Harmon, T.S.; Mitrea, D.M.; Zhu, L.; Richardson, T.M.; Kriwacki, R.W.; Pappu, R.V.; Brangwynne, C.P. Coexisting Liquid Phases Underlie Nucleolar Subcompartments. Cell 2016, 165, 1686–1697. [Google Scholar] [CrossRef]
- Weber, S.C.; Brangwynne, C.P. Inverse size scaling of the nucleolus by a concentration-dependent phase transition. Curr. Biol. 2015, 25, 641–646. [Google Scholar] [CrossRef]
- Girke, P.; Seufert, W. Compositional reorganization of the nucleolus in budding yeast mitosis. Mol. Biol. Cell 2019, 30, 591–606. [Google Scholar] [CrossRef]
- Sawyer, I.A.; Sturgill, D.; Dundr, M. Membraneless nuclear organelles and the search for phases within phases. Wiley Interdiscip. Rev. RNA 2019, 10, e1514. [Google Scholar] [CrossRef] [PubMed]
- Mitrea, D.M.; Cika, J.A.; Guy, C.S.; Ban, D.; Banerjee, P.R.; Stanley, C.B.; Nourse, A.; Deniz, A.A.; Kriwacki, R.W. Nucleophosmin integrates within the nucleolus via multi-modal interactions with proteins displaying R-rich linear motifs and rRNA. Elife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, F.M.; van Koningsbruggen, S.; Navascues, J.; Lamond, A.I. The multifunctional nucleolus. Nat. Rev. Mol. Cell Biol. 2007, 8, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Frottin, F.; Schueder, F.; Tiwary, S.; Gupta, R.; Korner, R.; Schlichthaerle, T.; Cox, J.; Jungmann, R.; Hartl, F.U.; Hipp, M.S. The nucleolus functions as a phase-separated protein quality control compartment. Science 2019, 365, 342–347. [Google Scholar] [CrossRef]
- Verdaasdonk, J.S.; Vasquez, P.A.; Barry, R.M.; Barry, T.; Goodwin, S.; Forest, M.G.; Bloom, K. Centromere tethering confines chromosome domains. Mol. Cell 2013, 52, 819–831. [Google Scholar] [CrossRef]
- Tjong, H.; Gong, K.; Chen, L.; Alber, F. Physical tethering and volume exclusion determine higher-order genome organization in budding yeast. Genome Res. 2012, 22, 1295–1305. [Google Scholar] [CrossRef]
- Wang, R.; Mozziconacci, J.; Bancaud, A.; Gadal, O. Principles of chromatin organization in yeast: Relevance of polymer models to describe nuclear organization and dynamics. Curr. Opin. Cell Biol. 2015, 34, 54–60. [Google Scholar] [CrossRef]
- Vasquez, P.A.; Hult, C.; Adalsteinsson, D.; Lawrimore, J.; Forest, M.G.; Bloom, K. Entropy gives rise to topologically associating domains. Nucleic Acids Res. 2016, 44, 5540–5549. [Google Scholar] [CrossRef]
- Wong, H.; Marie-Nelly, H.; Herbert, S.; Carrivain, P.; Blanc, H.; Koszul, R.; Fabre, E.; Zimmer, C. A predictive computational model of the dynamic 3D interphase yeast nucleus. Curr. Biol. 2012, 22, 1881–1890. [Google Scholar] [CrossRef]
- Hult, C.; Adalsteinsson, D.; Vasquez, P.A.; Lawrimore, J.; Bennett, M.; York, A.; Cook, D.; Yeh, E.; Forest, M.G.; Bloom, K. Enrichment of dynamic chromosomal crosslinks drive phase separation of the nucleolus. Nucleic Acids Res. 2017, 45, 11159–11173. [Google Scholar] [CrossRef]
- Lampson, M.A.; Cheeseman, I.M. Sensing centromere tension: Aurora B and the regulation of kinetochore function. Trends Cell Biol. 2011, 21, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Palomba, F.; Niedzialkowska, E.; Digman, M.A.; Gratton, E.; Stukenberg, P.T. The inner centromere is a biomolecular condensate scaffolded by the chromosomal passenger complex. Nat. Cell Biol. 2019, 21, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Fang, Y.; Spector, D.L.; Hirano, T. Spatial and temporal regulation of Condensins I and II in mitotic chromosome assembly in human cells. Mol. Biol. Cell 2004, 15, 3296–3308. [Google Scholar] [CrossRef] [PubMed]
- Ochs, R.L.; Press, R.I. Centromere autoantigens are associated with the nucleolus. Exp. Cell Res. 1992, 200, 339–350. [Google Scholar] [CrossRef]
- Carvalho, C.; Pereira, H.M.; Ferreira, J.; Pina, C.; Mendonca, D.; Rosa, A.C.; Carmo-Fonseca, M. Chromosomal G-dark bands determine the spatial organization of centromeric heterochromatin in the nucleus. Mol. Biol. Cell 2001, 12, 3563–3572. [Google Scholar] [CrossRef] [PubMed]
- Padeken, J.; Heun, P. Centromeres in nuclear architecture. Cell Cycle 2013, 12, 3455–3456. [Google Scholar] [CrossRef][Green Version]
- Wong, L.H.; Brettingham-Moore, K.H.; Chan, L.; Quach, J.M.; Anderson, M.A.; Northrop, E.L.; Hannan, R.; Saffery, R.; Shaw, M.L.; Williams, E.; et al. Centromere RNA is a key component for the assembly of nucleoproteins at the nucleolus and centromere. Genome Res. 2007, 17, 1146–1160. [Google Scholar] [CrossRef]
- Yi, Q.; Chen, Q.; Liang, C.; Yan, H.; Zhang, Z.; Xiang, X.; Zhang, M.; Qi, F.; Zhou, L.; Wang, F. HP1 links centromeric heterochromatin to centromere cohesion in mammals. EMBO Rep. 2018, 19. [Google Scholar] [CrossRef]
- Strom, A.R.; Emelyanov, A.V.; Mir, M.; Fyodorov, D.V.; Darzacq, X.; Karpen, G.H. Phase separation drives heterochromatin domain formation. Nature 2017, 547, 241. [Google Scholar] [CrossRef]
- Larson, A.G.; Elnatan, D.; Keenen, M.M.; Trnka, M.J.; Johnston, J.B.; Burlingame, A.L.; Agard, D.A.; Redding, S.; Narlikar, G.J. Liquid droplet formation by HP1alpha suggests a role for phase separation in heterochromatin. Nature 2017, 547, 236. [Google Scholar] [CrossRef]
- Hickman, M.A.; Froyd, C.A.; Rusche, L.N. Reinventing heterochromatin in budding yeasts: Sir2 and the origin recognition complex take center stage. Eukaryot. Cell 2011, 10, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.J. Potential Role of Phase Separation of Repetitive DNA in Chromosomal Organization. Genes (Basel) 2017, 8, 279. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawrimore, C.J.; Bloom, K. Common Features of the Pericentromere and Nucleolus. Genes 2019, 10, 1029. https://doi.org/10.3390/genes10121029
Lawrimore CJ, Bloom K. Common Features of the Pericentromere and Nucleolus. Genes. 2019; 10(12):1029. https://doi.org/10.3390/genes10121029
Chicago/Turabian StyleLawrimore, Colleen J., and Kerry Bloom. 2019. "Common Features of the Pericentromere and Nucleolus" Genes 10, no. 12: 1029. https://doi.org/10.3390/genes10121029
APA StyleLawrimore, C. J., & Bloom, K. (2019). Common Features of the Pericentromere and Nucleolus. Genes, 10(12), 1029. https://doi.org/10.3390/genes10121029




