Differential Expression of Genes Related to Sexual Determination Can Modify the Reproductive Cycle of Astyanax scabripinnis (Characiformes: Characidae) in B Chromosome Carrier Individuals
Abstract
1. Introduction
2. Materials and Methods
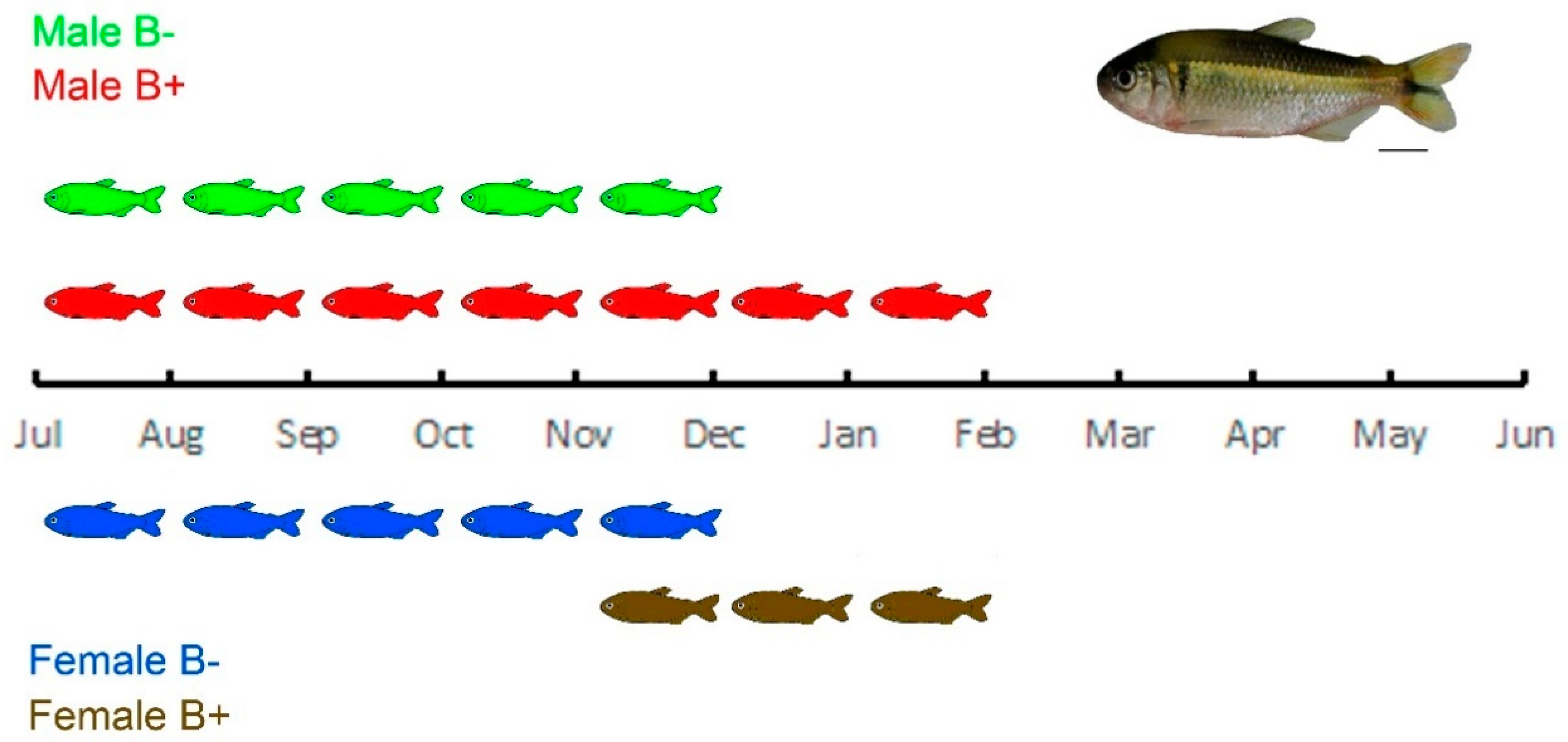
2.1. Characterization of the Study Object
2.2. Cytogenetics
2.3. Histological Analysis
2.4. RNA Extraction and cDNA Synthesis
2.5. Quantitative PCR in Real-Time (qRT-PCR)
2.6. Illumina Sequencing and Coverage Analysis of Sex-Associated Genes
3. Results
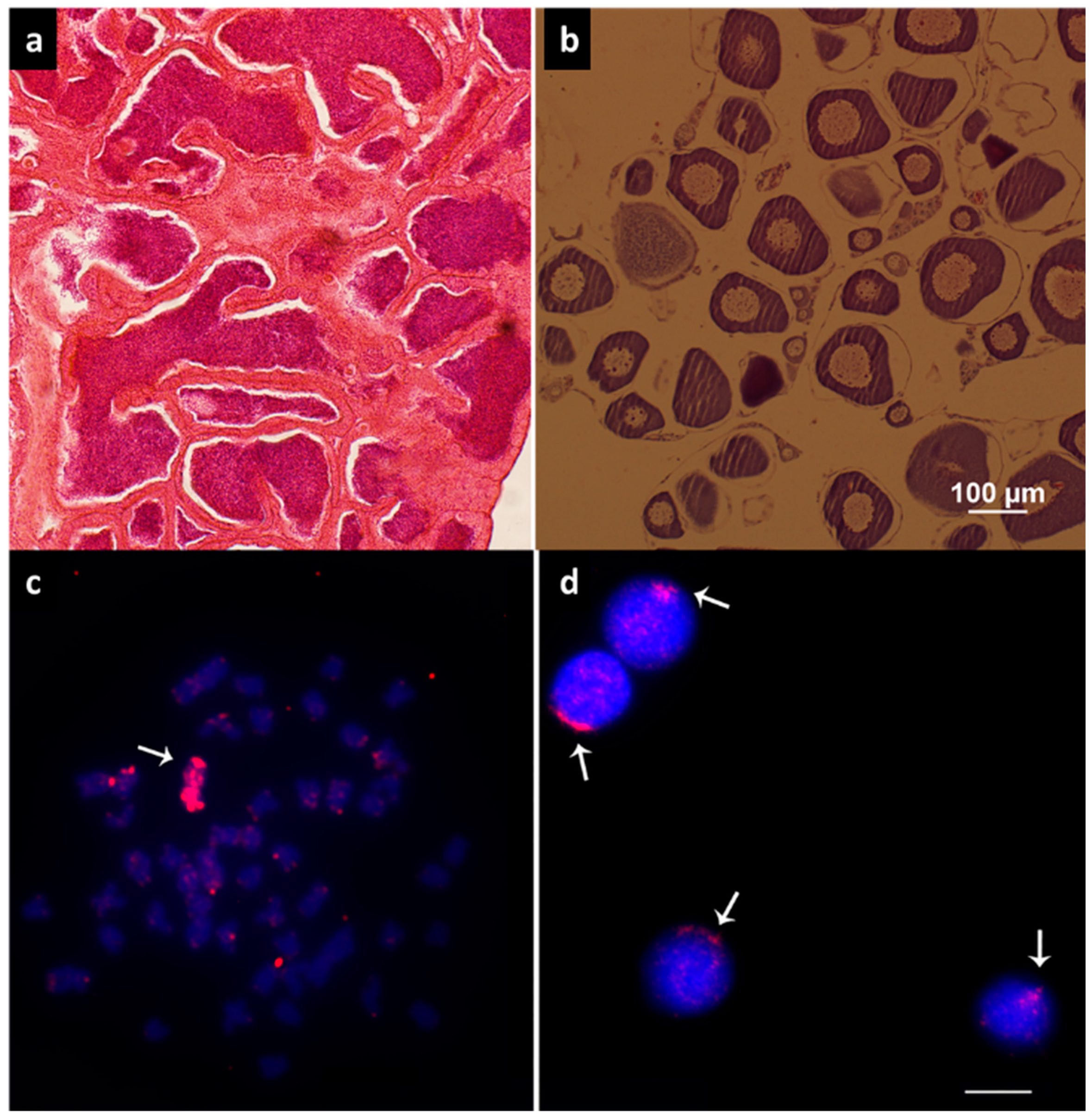
3.1. Histological Analysis and Cytogenetics
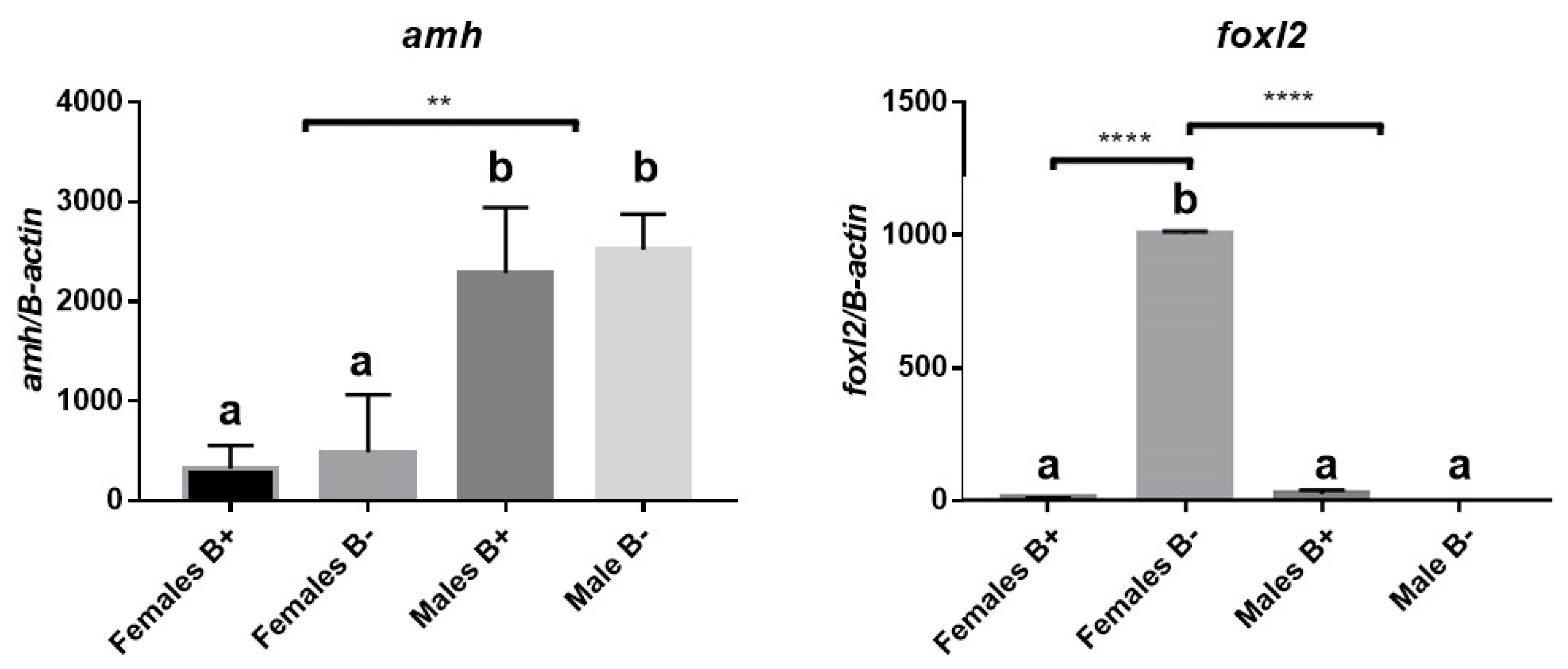
3.2. Analysis of Gene Expression of Amh and Foxl2a
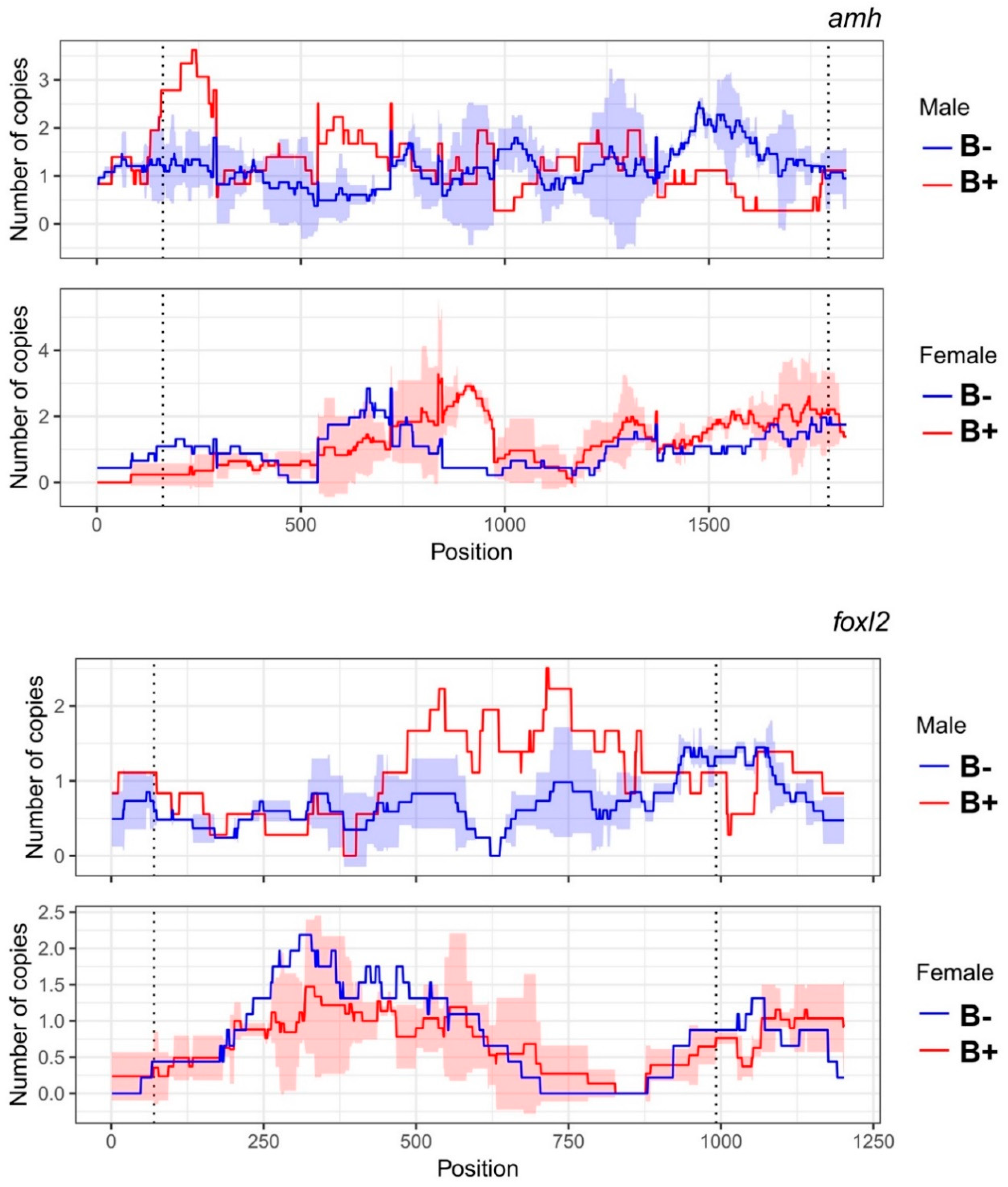
3.3. Number of Gene Copies between Individuals with and without B Chromosomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trukhina, A.V.; Lukina, N.A.; Wackerow-Kouzova, N.D.; Smirnov, A.F. The variety of vertebrate mechanisms of sex determination. BioMed Res. Int. 2013, 2013, 587460. [Google Scholar] [CrossRef] [PubMed]
- Devlin, R.H.; Nagahama, Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences. Aquaculture 2002, 208, 191–364. [Google Scholar] [CrossRef]
- Martínez, P.; Viñas, A.M.; Sánchez, L.; Díaz, N.; Ribas, L.; Piferrer, F. Genetic architecture of sex determination in fish: Applications to sex ratio control in aquaculture. Front. Genet. 2014, 5, 340. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Nagahama, Y.; Shinomiya, A.; Sato, T.; Matsuda, C.; Kobayashi, T.; Morrey, C.E.; Shibata, N.; Asakawa, S.; Shimizu, N.; et al. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature 2002, 417, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Myosho, T.; Otake, H.; Masuyama, H.; Matsuda, M.; Kuroki, Y.; Fujiyama, A.; Naruse, K.; Hamaguchi, S.; Sakaizumi, M. Tracing the emergence of a novel sex-determining gene in medaka, Oryzias luzonensis. Genetics 2012, 191, 163–170. [Google Scholar] [CrossRef]
- Yano, A.; Nicol, B.; Jouanno, E.; Quillet, E.; Fostier, A.; Guyomard, R.; Guiguen, Y. The sexually dimorphic on the Y-chromosome gene (sdY) is a conserved male-specific Y-chromosome sequence in many salmonids. Evol. Appl. 2013, 6, 486–496. [Google Scholar] [CrossRef]
- Hattori, R.S.; Murai, Y.; Oura, M.; Masuda, S.; Majhi, S.K.; Sakamoto, T.; Fernandino, J.I.; Somoza, G.M.; Yokota, M.; Strussmann, C.A. A Y-linked anti-Mullerian hormone duplication takes over a critical role in sex determination. Proc. Natl. Acad. Sci. USA 2012, 109, 2955–2959. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Zhang, Y.; Sarida, M.; Hattori, R.S.; Strüssmann, C.A. Coexistence of Genotypic and Temperature-Dependent Sex Determination in Pejerrey Odontesthes bonariensis. PLoS ONE 2014, 9, e102574. [Google Scholar] [CrossRef]
- Bej, D.K.; Miyoshi, K.; Hattori, R.S.; Strüssmann, C.A.; Yamamoto, Y. A Duplicated, Truncated amh Gene Is Involved in Male Sex Determination in an Old World Silverside. G3 Genes Genomes Genet. 2017, 7, 2489–2495. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Yoshinaga, N.; Yazawa, T.; Gen, K.; Kitano, T. Cortisol Is Involved in Temperature-Dependent Sex Determination in the Japanese Flounder. Endocrinology 2010, 151, 3900–3908. [Google Scholar] [CrossRef]
- Haugen, T.; Almeida, F.F.; Andersson, E.; Bogerd, J.; Male, R.; Skaar, K.S.; Schulz, R.W.; Sørhus, E.; Wijgerde, T.; Taranger, G.L. Sex differentiation in Atlantic cod (Gadus morhua L.): Morphological and gene expression studies. Reprod. Biol. Endocrinol. 2012, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Mankiewicz, J.L.; Godwin, J.; Holler, B.L.; Turner, P.M.; Murashige, R.; Shamey, R.; Daniels, H.V.; Borski, R.J. Masculinizing Effect of Background Color and Cortisol in a Flatfish with Environmental Sex-Determination. Integr. Comp. Biol. 2013, 53, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Siegfried, K.R. In search of determinants: Gene expression during gonadal sex differentiation. J. Fish Biol. 2010, 76, 1879–1902. [Google Scholar] [CrossRef] [PubMed]
- Eshel, O.; Shirak, A.; Dor, L.; Band, M.; Zak, T.; Markovich-Gordon, M.; Chalifa-Caspi, V.; Feldmesser, E.; Weller, J.I.; Seroussi, E.; et al. Identification of male-specific amh duplication, sexually differentially expressed genes and microRNAs at early embryonic development of Nile tilapia (Oreochromis niloticus). BMC Genom. 2014, 15, 774. [Google Scholar] [CrossRef] [PubMed]
- Pannetier, M. FOXL2 activates P450 aromatase gene transcription: Towards a better characterization of the early steps of mammalian ovarian development. J. Mol. Endocrinol. 2006, 36, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Bertho, S.; Pasquier, J.; Pan, Q.; Le Trionnaire, G.; Bobe, J.; Postlethwait, J.H.; Pailhoux, E.; Schartl, M.; Herpin, A.; Guiguen, Y. Foxl2 and Its Relatives Are Evolutionary Conserved Players in Gonadal Sex Differentiation. Sex. Dev. 2016, 10, 111–129. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Ma, H.; Liu, X.; Shi, H.; Li, M.; Wang, D. Mutation of foxl2 or cyp19a1a results in female to male sex reversal in XX Nile tilapia. Endocrinology 2017, 158, 2634–2647. [Google Scholar] [CrossRef]
- Caulier, M.; Brion, F.; Chadili, E.; Turies, C.; Piccini, B.; Porcher, J.M.; Guiguen, Y.; Hinfray, N. Localization of steroidogenic enzymes and Foxl2a in the gonads of mature zebrafish (Danio rerio). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 188, 96–106. [Google Scholar] [CrossRef]
- Reis, R.E.; Albert, J.S.; Di Dario, F.; Mincarone, M.M.; Petry, P.; Rocha, L.A. Fish biodiversity and conservation in South America. J. Fish Biol. 2016, 89, 12–47. [Google Scholar] [CrossRef]
- Gery, J. Characoids of the World; TFH Publications: Neptune, NJ, USA, 1977. [Google Scholar]
- Eschemeyer, W. Catalog of Fishes: Genera, Species, References. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 16 March 2018).
- Tenorio, R.C.C.D.O.; Vitorino, C.D.A.; Souza, I.L.; Oliveira, C.; Venere, P.C. Comparative cytogenetics in Astyanax (Characiformes: Characidae) with focus on the cytotaxonomy of the group. Neotrop. Ichthyol. 2013, 11, 553–564. [Google Scholar] [CrossRef]
- Salvador, L.B.; Moreira-Filho, O. B chromosomes in Astyanax scabripinnis (Pisces, Characidae). Heredity. 1992, 69, 50–56. [Google Scholar] [CrossRef]
- Moreira-Filho, O.; Galetti, P.M.; Bertollo, L.A.C. B chromosomes in the fish Astyanax scabripinnis (Characidae, Tetragonopterinae): An overview in natural populations. Cytogenet. Genome Res. 2004, 106, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Camacho, J.P.M. B chromosomes. In The Evolution of the Genome; Gregory, T.R., Ed.; Elsevier Academic Press: London, UK, 2005; pp. 223–286. ISBN 0123014638. [Google Scholar]
- Banaei-Moghaddam, A.M.; Martis, M.M.; Macas, J.; Gundlach, H.; Himmelbach, A.; Altschmied, L.; Mayer, K.F.X.; Houben, A. Genes on B chromosomes: Old questions revisited with new tools. Biochim. Biophys. Acta 2015, 1849, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Camacho, J.P.M.; Sharbel, T.F.; Beukeboom, L.W. B-chromosome evolution. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2000, 355, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Martins, C. The modern view of b chromosomes under the impact of high scale omics analyses. Cells 2019, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Domínguez, B.; Ruiz-Ruano, F.J.; Cabrero, J.; Corral, J.M.; López-León, M.D.; Sharbel, T.F.; Camacho, J.P.M. Protein-coding genes in B chromosomes of the grasshopper Eyprepocnemis plorans. Sci. Rep. 2017, 7, 45200. [Google Scholar] [CrossRef]
- Makunin, A.I.; Dementyeva, P.V.; Graphodatsky, A.S.; Volobouev, V.T.; Kukekova, A.V.; Trifonov, V.A. Genes on B chromosomes of vertebrates. Mol. Cytogenet. 2014, 7, 99. [Google Scholar] [CrossRef]
- Castro, J.P.; Hattori, R.S.; Yoshinaga, T.T.; Silva, D.M.Z.D.A.; Foresti, F.; Santos, M.H.; Almeida, M.C.; Artoni, R.F. Differential Expression of dmrt1 in Astyanax scabripinnis (Teleostei, Characidade) Is Correlated with B Chromosome Occurrence. Zebrafish 2018, 16, 182–188. [Google Scholar] [CrossRef]
- Vicente, V.E.; Moreira-Filho, O.; Camacho, J.P. Sex-ratio distortion associated with the presence of a B chromosome in Astyanax scabripinnis (Teleostei, Characidae). Cytogenet. Cell Genet. 1996, 74, 70–75. [Google Scholar] [CrossRef]
- Silva, D.M.Z.D.A.; Pansonato-Alves, J.C.; Utsunomia, R.; Araya-Jaime, C.; Ruiz-Ruano, F.J.; Daniel, S.N.; Hashimoto, D.T.; Oliveira, C.; Camacho, J.P.M.; Porto-Foresti, F.; et al. Delimiting the Origin of a B Chromosome by FISH Mapping, Chromosome Painting and DNA Sequence Analysis in Astyanax paranae (Teleostei, Characiformes). PLoS ONE 2014, 9, e94896. [Google Scholar] [CrossRef]
- Porto-Foresti, F.; Oliveira, C.; Maistro, E.L.; Foresti, F. Estimated frequency of B-chromosomes and population density of Astyanax scabripinnis paranae in a small stream. Braz. J. Genet. 1997, 20. [Google Scholar] [CrossRef]
- Cornelio, D.; Castro, J.P.; Santos, M.H.; Vicari, M.R.; de Almeida, M.C.; Moreira-Filho, O.; Camacho, J.P.M.; Artoni, R.F. Hermaphroditism can compensate for the sex ratio in the Astyanax scabripinnis species complex (Teleostei: Characidae): Expanding the B chromosome study model. Rev. Fish Biol. Fish. 2017, 27, 681–689. [Google Scholar] [CrossRef]
- Bertollo, L.A.C.; Takahashi, C.S.; Moreira-Filho, O. Cytotaxonomic considerations on Hoplias lacerdae (Pisces, Erythrinidae). Braz. J. Genet. 1978, 1, 103–120. [Google Scholar]
- Vicari, M.R.; de Pistune Mello, H.F.; Castro, J.P.; de Almeida, M.C.; Bertollo, L.A.C.; Moreira-Filho, O.; Camacho, J.P.M.; Artoni, R.F. New insights on the origin of B chromosomes in Astyanax scabripinnis obtained by chromosome painting and FISH. Genetica 2011, 139, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Vazzoler, A.E.A.M. Biologia da Reprodução de Peixes Teleósteos: Teoria e Prática; EDUEM: Maringá, Brazil, 1996. [Google Scholar]
- Bruford, M.W.; Hanotte, O.; Brookfield, J.F.Y.; Burke, T. Single-locus and multilocus DNA fingerprinting. In Molecular Genetics Analyses of Populations: A Practical Approach; HoelzelL, A.R., Ed.; IRL Press: Oxford, UK, 1992; pp. 225–269. ISBN 0-19-963277-4. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Carvalho, M.L.; Oliveira, C.; Foresti, F. Nuclear DNA content of thirty species of Neotropical fishes. Genet. Mol. Biol. 1998, 21, 47–54. [Google Scholar] [CrossRef]
- Spargo, A.; Ponstingl, H.; Ning, Z. SSAHA2: Sequence Search and Alignment by Hashing Algorithm. Available online: https://www.sanger.ac.uk/science/tools/ssaha2-0 (accessed on 16 March 2018).
- Pfennig, F.; Standke, A.; Gutzeit, H.O. The role of Amh signaling in teleost fish—Multiple functions not restricted to the gonads. Gen. Comp. Endocrinol. 2015, 223, 87–107. [Google Scholar] [CrossRef]
- Poonlaphdecha, S.; Pepey, E.; Canonne, M.; de Verdal, H.; Baroiller, J.F.; D’Cotta, H. Temperature induced-masculinisation in the Nile tilapia causes rapid up-regulation of both dmrt1 and amh expressions. Gen. Comp. Endocrinol. 2013, 193, 234–242. [Google Scholar] [CrossRef]
- Morinaga, C.; Saito, D.; Nakamura, S.; Sasaki, T.; Asakawa, S.; Shimizu, N.; Mitani, H.; Furutani-Seiki, M.; Tanaka, M.; Kondoh, H. The hotei mutation of medaka in the anti-Mullerian hormone receptor causes the dysregulation of germ cell and sexual development. Proc. Natl. Acad. Sci. USA 2007, 104, 9691–9696. [Google Scholar] [CrossRef]
- Li, Y.; Jing, X.A.; Aldrich, J.C.; Clifford, C.; Chen, J.; Akbari, O.S.; Ferree, P.M. Unique sequence organization and small RNA expression of a “selfish” B chromosome. Chromosoma 2017, 126, 753–768. [Google Scholar] [CrossRef]
- Ramos, É.; Cardoso, A.L.; Brown, J.; Marques, D.F.; Fantinatti, B.E.A.; Cabral-de-Mello, D.C.; Oliveira, R.A.; O’Neill, R.J.; Martins, C. The repetitive DNA element BncDNA, enriched in the B chromosome of the cichlid fish Astatotilapia latifasciata, transcribes a potentially noncoding RNA. Chromosoma 2017, 126, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Maistro, E.L.; Foresti, F.; Oliveira, C. New Occurrence of a Macro-B-Chromosome in Astyanax-Scabripinnis-Paranae (Pisces, Characiformes, Characidae). Braz. J. Genet. 1994, 17, 153–156. [Google Scholar]
- Silva, D.M.Z.D.A.; Utsunomia, R.; Ruiz-Ruano, F.J.; Oliveira, C.; Foresti, F. The complete mitochondrial genome sequence of Astyanax paranae (Teleostei: Characiformes). Mitochondrial DNA Part B 2016, 1, 586–587. [Google Scholar] [CrossRef]
- Castro, J.P.; Moura, M.O.; Moreira-Filho, O.; Shibatta, O.A.; Santos, M.H.; Nogaroto, V.; Vicari, M.R.; de Almeida, M.C.; Artoni, R.F. Diversity of the Astyanax scabripinnis species complex (Teleostei: Characidae) in the Atlantic Forest, Brazil: Species limits and evolutionary inferences. Rev. Fish Biol. Fish. 2015, 25, 231–244. [Google Scholar] [CrossRef]
- Castro, J.P.; Moura, M.O.; Moreira-Filho, O.; Shibatta, O.A.; Santos, M.H.; Nogaroto, V.; Vicari, M.R.; de Almeida, M.C.; Artoni, R.F. Evidence of incipient speciation in Astyanax scabripinnis species complex (Teleostei: Characidae). Neotrop. Ichthyol. 2014, 12, 429–438. [Google Scholar] [CrossRef]
- Pandian, T.J. Sexuality in Fishes; Science Publishers Enfield/CRC Press: New Hampshire, UK, 2010; ISBN 9781578086856. [Google Scholar]
- Pandian, T.J. Genetic Sex Differentiation in Fish; Science Publishers Enfield/CRC Press: New Hampshire, UK, 2012; ISBN 9781466517103. [Google Scholar]
| Gene | Primer Sequence (5′–3′) | Use |
|---|---|---|
| ame_amh_Fw1 | CTGGGATGTTGAAGACGA | Conventional PCR |
| ame_amh_Rv1 | GAGGAATTAATCAGCTCCAGAA | Conventional PCR |
| ame_fox2_Fw1 | ACGTTCTTGGGCTCAGAGGA | Conventional PCR |
| ame_foxl2a_Rv1 | AGACTTGCCGGGTTGGAAGTG | Conventional PCR |
| amh_rtf1 | CCTCACTGCTCTTCCTGACG | qRT-PCR |
| amh_rtR1 | AAACACCCAACACAGCTTGC | qRT-PCR |
| foxl2a_rtF1 | ACCTGAGCCTTAACGAGTGC | qRT-PCR |
| foxl2a_rtR1 | ATGTCTTCACACGTCGGGTC | qRT-PCR |
| β-actin F_RT | ATCATGAAGTGCGACGTGGA | qRT-PCR |
| β-actin R_RT | TATTTACGCTCAGGTGGGGC | qRT-PCR |
| Gene | ♀ (B+) | ♀ (B−) | ♂ (B+) | ♂ (B−) | Total |
|---|---|---|---|---|---|
| amh | 3 | 3 | 3 | 3 | 12 |
| foxl2a | 3 | 3 | 3 | 3 | 12 |
| Total | 24 |
| Sex | Samples | Number of B | Number of Reads (mi) | Coverage |
|---|---|---|---|---|
| Male | 2 | 0 | 93.7 | 7.5 |
| 1 | 1 | 52.2 | 4.2 | |
| Female | 1 | 0 | 43.7 | 3.5 |
| 2 | 1 | 96 | 7.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, J.P.; Hattori, R.S.; Yoshinaga, T.T.; Silva, D.M.Z.d.A.; Ruiz-Ruano, F.J.; Foresti, F.; Santos, M.H.; Almeida, M.C.d.; Moreira-Filho, O.; Artoni, R.F. Differential Expression of Genes Related to Sexual Determination Can Modify the Reproductive Cycle of Astyanax scabripinnis (Characiformes: Characidae) in B Chromosome Carrier Individuals. Genes 2019, 10, 909. https://doi.org/10.3390/genes10110909
Castro JP, Hattori RS, Yoshinaga TT, Silva DMZdA, Ruiz-Ruano FJ, Foresti F, Santos MH, Almeida MCd, Moreira-Filho O, Artoni RF. Differential Expression of Genes Related to Sexual Determination Can Modify the Reproductive Cycle of Astyanax scabripinnis (Characiformes: Characidae) in B Chromosome Carrier Individuals. Genes. 2019; 10(11):909. https://doi.org/10.3390/genes10110909
Chicago/Turabian StyleCastro, Jonathan Pena, Ricardo Shohei Hattori, Túlio Teruo Yoshinaga, Duílio Mazzoni Zerbinato de Andrade Silva, Francisco J. Ruiz-Ruano, Fausto Foresti, Mateus Henrique Santos, Mara Cristina de Almeida, Orlando Moreira-Filho, and Roberto Ferreira Artoni. 2019. "Differential Expression of Genes Related to Sexual Determination Can Modify the Reproductive Cycle of Astyanax scabripinnis (Characiformes: Characidae) in B Chromosome Carrier Individuals" Genes 10, no. 11: 909. https://doi.org/10.3390/genes10110909
APA StyleCastro, J. P., Hattori, R. S., Yoshinaga, T. T., Silva, D. M. Z. d. A., Ruiz-Ruano, F. J., Foresti, F., Santos, M. H., Almeida, M. C. d., Moreira-Filho, O., & Artoni, R. F. (2019). Differential Expression of Genes Related to Sexual Determination Can Modify the Reproductive Cycle of Astyanax scabripinnis (Characiformes: Characidae) in B Chromosome Carrier Individuals. Genes, 10(11), 909. https://doi.org/10.3390/genes10110909






