SWEET Transporters for the Nourishment of Embryonic Tissues during Maize Germination
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Silico Analysis of the SWEET Family in Maize
2.2. Plant Material
2.3. Determination of the Phases of Germination by Evaluating the Water and Oxygen Uptake
2.4. RNA Extraction
2.5. Determination of ZmSWEET and α-Amylase mRNA Levels by RT-PCR
2.6. Analysis by qPCR of mRNA of ZmSWEET, Malate Synthase, Sucrose Phosphate Synthase, and Sucrose Transporter 1 in Scutellum and Embryo Axis
2.7. Determination of Soluble Sugars, Starch and Lipid Content
2.8. Determination of Cell Wall and Vacuolar Invertase Activity
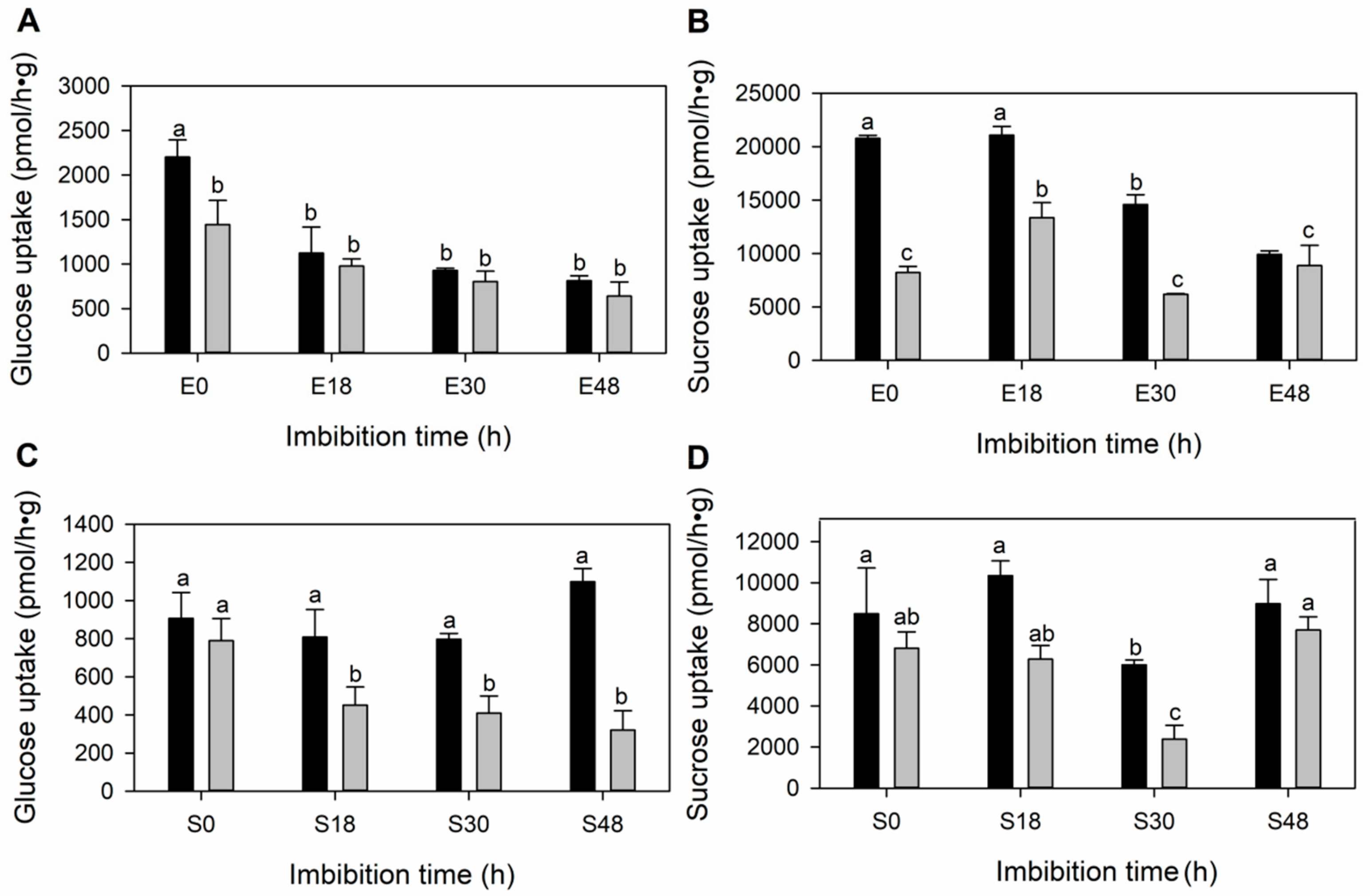
2.9. Sugar Uptake in Embryonic Tissues
3. Results
3.1. Maize SWEET Family
3.2. Transcripts for Several SWEETs Were Found at the Embryo Axis and the Scutellum During the Germination
3.3. Metabolic Activity during Germination Reveals High Suc Content in Maize Embryonic Tissues
3.4. The Sugar Transport along the Germination in the Embryonic Tissues Is Mainly Diffusional
3.5. The Abundance of Sugars Affects the Embryo Axis ZmSWEET mRNA Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Julius, B.T.; Leach, K.A.; Tran, T.M.; Mertz, R.A.; Braun, D.M. Sugar Transporters in Plants: New Insights and Discoveries. Plant Cell Physiol. 2017, 58, 1442–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rottmann, T.; Klebl, F.; Schneider, S.; Kischka, D.; Rüscher, D.; Sauer, N.; Stadler, R. Sugar Transporter STP7 Specificity for l-Arabinose and d-Xylose Contrasts with the Typical Hexose Transporters STP8 and STP12. Plant Physiol. 2018, 176, 2330–2350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.Q.; Hou, B.H.; Lalonde, S.; Takanaga, H.; Hartung, M.L.; Qu, X.Q.; Guo, W.J.; Kim, J.G.; Underwood, W.; Chaudhuri, B.; et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 2010, 468, 527–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.Q.; Qu, X.Q.; Hou, B.H.; Sosso, D.; Osorio, S.; Fernie, A.R.; Frommer, W.B. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 2012, 335, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Bezrutczyk, M.; Hartwig, T.; Horschman, M.; Char, S.N.; Yang, J.; Yang, B.; Frommer, W.B.; Sosso, D. Impaired phloem loading in zmsweet13a,b,c sucrose transporter triple knock-out mutants in Zea mays. New Phytol. 2018, 218, 594–603. [Google Scholar] [CrossRef]
- Lin, I.W.; Sosso, D.; Chen, L.Q.; Gase, K.; Kim, S.G.; Kessler, D.; Klinkenberg, P.M.; Gorder, M.K.; Hou, B.H.; Qu, X.Q.; et al. Nectar secretion requires sucrose phosphate synthases and the sugar transporter SWEET9. Nature 2014, 508, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, H.; Zhu, Y.; Fang, L.; An, X.; Wan, K.; Whalen, C.C.; Yang, X.; Lauzardo, M.; Cao, J.; et al. Structure of a eukaryotic SWEET transporter in a homo-trimeric complex. HHS Public Access. 2016, 527, 148–155. [Google Scholar]
- Zhang, Z.; Zou, L.; Ren, C.; Ren, F.; Wang, Y.; Fan, P.; Li, S.; Liang, Z. VvSWEET10 Mediates Sugar Accumulation in Grapes. Genes 2019, 10, 255. [Google Scholar] [CrossRef]
- Chen, H.Y.; Huh, J.H.; Yu, Y.C.; Ho, L.H.; Chen, L.Q.; Tholl, D.; Frommer, W.B.; Guo, W.J. The Arabidopsis vacuolar sugar transporter SWEET2 limits carbon sequestration from roots and restricts Pythium infection. Plant J. 2015, 83, 1046–1058. [Google Scholar] [CrossRef]
- Sosso, D.; Luo, D.; Li, Q.B.; Sasse, J.; Yang, J.; Gendrot, G.; Suzuki, M.; Koch, K.E.; McCarty, D.R.; Chourey, P.S.; et al. Seed filling in domesticated maize and rice depends on SWEET-mediated hexose transport. Nat. Genet. 2015, 47, 1489–1493. [Google Scholar] [CrossRef]
- Rosental, L.; Nonogaki, H.; Fait, A. Activation and regulation of primary metabolism during seed germination. Seed Sci. Res. 2014, 24, 1–15. [Google Scholar] [CrossRef]
- Ma, Z.; Marsolais, F.; Bernards, M.A.; Sumarah, M.W.; Bykova, N.V.; Igamberdiev, A.U. Glyoxylate cycle and metabolism of organic acids in the scutellum of barley seeds during germination. Plant Sci. 2016, 248, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Corona-Carrillo, J.; Flores-Ponce, M.; Chávez-Nájera, G.; Díaz-Pontones, D. Peroxidase activity in scutella of maize in association with anatomical changes during germination and grain storage. Springerplus 2014, 3, 399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafon-Placette, C.; Köhler, C. Embryo and endosperm, partners in seed development. Curr. Opin. Plant Biol. 2014, 17, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Enríquez-Arredondo, C.; Sánchez-Nieto, S.; Rendón-Huerta, E.; González-Halphen, D.; Gavilanes-Ruíz, M.; Díaz-Pontones, D. The plasma membrane H+-ATPase of maize embryos localizes in regions that are critical during the onset of germination. Plant Sci. 2005, 169, 11–19. [Google Scholar] [CrossRef]
- Sánchez-Linares, L.; Gavilanes-Ruíz, M.; Díaz-Pontones, D.; Guzmán-Chávez, F.; Calzada-Alejo, V.; Zurita-Villegas, V.; Luna-Loaiza, V.; Moreno-Sánchez, R.; Bernal-Lugo, I.; Sánchez-Nieto, S. Early carbon mobilization and radicle protrusion in maize germination. J. Exp. Bot. 2012, 63, 4513–4526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bick, J.A.; Neelam, A.; Smith, E.; Nelson, S.J.; Hall, J.L.; Williams, L.E. Expression analysis of a sucrose carrier in the germinating seedling of Ricinus communis. Plant Mol. Biol. 1998, 38, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Matsukura, C.; Saitoh, T.; Hirose, T.; Ohsugi, R.; Perata, P.; Yamaguchi, J. Sugar uptake and transport in rice embryo. Expression of companion cell-specific sucrose transporter (OsSUT1) induced by sugar and light. Plant Physiol. 2000, 124, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Aoki, N.; Scofield, G.N.; Wang, X.-D.; Offler, C.E.; Patrick, J.W.; Furbank, R.T. Pathway of Sugar Transport in Germinating Wheat Seeds. Plant Physiol. 2006, 141, 1255–1263. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.-Y.; Chang, Y.-M.; Chen, S.C.-C.; Lu, C.-H.; Wu, Y.-H.; Lu, M.-Y.J.; Chen, D.-R.; Shih, A.C.-C.; Sheue, C.-R.; Huang, H.-C.; et al. Anatomical and transcriptional dynamics of maize embryonic leaves during seed germination. Proc. Natl. Acad. Sci. USA 2013, 110, 3979–3984. [Google Scholar] [CrossRef] [Green Version]
- Van Bel, M.; Diels, T.; Vancaester, E.; Kreft, L.; Botzki, A.; Van de Peer, Y.; Coppens, F.; Vandepoele, K. PLAZA 4.0: An integrative resource for functional, evolutionary and comparative plant genomics. Nucleic Acids Res. 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Schwacke, R.; Schneider, A.; van der Graaff, E.; Fischer, K.; Catoni, E.; Desimone, M.; Frommer, W.B.; Flügge, U.-I.; Kunze, R. ARAMEMNON, a novel database for Arabidopsis integral membrane proteins. Plant Physiol. 2003, 131, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef] [PubMed]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Koetsier, G.; Cantor, E.; Biolabs, E. A Practical Guide to Analyzing Nucleic Acid Concentration and Purity with Microvolume Spectrophotometers; New England BioLabs, Inc.: Ipswich, MA, USA, 2019. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar]
- Kersey, P.J.; Allen, J.E.; Allot, A.; Barba, M.; Boddu, S.; Bolt, B.J.; Carvalho-Silva, D.; Christensen, M.; Davis, P.; Grabmueller, C.; et al. Ensembl Genomes 2018: An integrated omics infrastructure for non-vertebrate species. Nucleic Acids Res. 2018, 46, D802–D808. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3--new capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 2012, 13, 134. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 45. [Google Scholar] [CrossRef]
- Ramírez-Pimentel, G.J.; Covarrubias-Prieto, J.; Chablé-Moreno, F.; Chávez-Muñoz, A.; García-Rodríguez, J.; Aguirre-Mancilla, L.C.; Raya-Pérez, J.C. Extraction and comparison of carbohydrates, proteins and lipids content in corn. Ciencia y Tecnologia Agropecuaria 2015, 3, 20–29. [Google Scholar]
- Pelleschi, S.; Rocher, J.-P.; Prioul, J.-L. Effect of water restriction on carbohydrate metabolism and photosynthesis in mature maize leaves. Plant Cell Environ. 1997, 20, 493–503. [Google Scholar] [CrossRef]
- Bergmeyer, H.-U.; Bemt, S. Sucrose. In Methods of Enzymatic Analysis vol 3; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1974; pp. 1176–1179. [Google Scholar]
- Lichtner, F.T.; Spanswick, R.M. Electrogenic sucrose transport in developing soybean cotyledons. Plant Physiol. 1981, 67, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Eom, J.S.; Chen, L.Q.; Sosso, D.; Julius, B.T.; Lin, I.W.; Qu, X.Q.; Braun, D.M.; Frommer, W.B. SWEETs, transporters for intracellular and intercellular sugar translocation. Curr. Opin. Plant Biol. 2015, 25, 53–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Si, W.; Qin, Q.; Wu, H.; Jiang, H. Deciphering evolutionary dynamics of SWEET genes in diverse plant lineages. Sci. Rep. 2018, 8, 13440. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Piron, M.C.; Meyer, S.; Merdinoglu, D.; Bertsch, C.; Mestre, P. The SWEET family of sugar transporters in grapevine: VvSWEET4 is involved in the interaction with Botrytis cinerea. J. Exp. Bot. 2014, 65, 6589–6601. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Frommer, W.B. Structure and function of SemiSWEET and SWEET sugar transporters. Trends Biochem. Sci. 2015, 40, 480–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nonogaki, H. Seed Germination and Reserve Mobilization. Encycl. Life Sci. 2008, 1–9. [Google Scholar] [CrossRef]
- Feenstra, A.D.; Alexander, L.E.; Song, Z.; Korte, A.R.; Yandeau-Nelson, M.D.; Nikolau, B.J.; Lee, Y.J. Spatial Mapping and Profiling of Metabolite Distributions during Germination. Plant Physiol. 2017, 174, 2532–2548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Xu, A.; Zhang, L.; Yin, Z.; Wei, C. Spatiotemporal accumulation and characteristics of starch in developing maize caryopses. Plant Physiol. Biochem. 2018, 130, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013; Volume 9781461446, ISBN 9781461446934. [Google Scholar]
- Turgeon, R.; Medville, R. The absence of phloem loading in willow leaves. Plant Biol. 1998, 95, 12055–12060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slewinski, T.L. Diverse functional roles of monosaccharide transporters and their homologs in vascular plants: A physiological perspective. Mol. Plant 2011, 4, 641–662. [Google Scholar] [CrossRef] [PubMed]
- Sherson, S.M.; Alford, H.L.; Forbes, S.M.; Wallace, G.; Smith, S.M. Roles of cell-wall invertases and monosaccharide transporters in the growth and development of Arabidopsis. J. Exp. Bot. 2003, 54, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, T.E. Sucrose efflux and export from the maize scutellum. Plant Cell Environ. 1987, 10, 259–266. [Google Scholar]
- Yan, D.; Duermeyer, L.; Leoveanu, C.; Nambara, E. The Functions of the Endosperm During Seed Germination. Plant Cell Physiol. 2014, 55, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- Shannon, J.C. Movement of 14C-Labeled Assimilates into Kernels of Zea mays L: I. Pattern and Rate of Sugar Movement. Plant Physiol. 1972, 49, 198–202. [Google Scholar] [CrossRef]
- Ma, Z.; Bykova, N.V.; Igamberdiev, A.U. Cell signaling mechanisms and metabolic regulation of germination and dormancy in barley seeds. Crop J. 2017, 5, 459–477. [Google Scholar] [CrossRef]
- Siao, W.; Chen, J.Y.; Hsiao, H.H.; Chung, P.; Wang, S.J. Characterization of OsSUT2 expression and regulation in germinating embryos of rice seeds. Rice 2011, 4, 39–49. [Google Scholar] [CrossRef]
- Chandran, D.; Reinders, A.; Ward, J.M. Substrate specificity of the Arabidopsis thaliana sucrose transporter AtSUC2. J. Biol. Chem. 2003, 278, 44320–44325. [Google Scholar] [CrossRef]
- Carpaneto, A.; Geiger, D.; Bamberg, E.; Sauer, N.; Fromm, J.; Hedrich, R. Phloem-localized, proton-coupled sucrose carrier ZmSUT1 mediates sucrose efflux under the control of the sucrose gradient and the proton motive force. J. Biol. Chem. 2005, 280, 21437–21443. [Google Scholar] [CrossRef]
- Aguilera-Alvarado, G.P.; Guevara-García, Á.A.; Estrada-Antolín, S.A.; Sánchez-Nieto, S. Biochemical properties and subcellular localization of six members of the HXK family in maize and its metabolic contribution to embryo germination. BMC Plant Biol. 2019, 19, 27. [Google Scholar] [CrossRef]
- Sekhon, R.S.; Briskine, R.; Hirsch, C.N.; Myers, C.L.; Springer, N.M.; Buell, C.R.; de Leon, N.; Kaeppler, S.M. Maize Gene Atlas Developed by RNA Sequencing and Comparative Evaluation of Transcriptomes Based on RNA Sequencing and Microarrays. PLoS ONE 2013, 8, e61005. [Google Scholar] [CrossRef] [PubMed]
- Frank Baker, R.; Leach, K.A.; Braun, D.M. SWEET as sugar: New sucrose effluxers in plants. Mol. Plant 2012, 5, 766–768. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Yang, C.; Tian, Z.; Li, J. AtSWEET4, a hexose facilitator, mediates sugar transport to axial sinks and affects plant development. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Coria, M.; Sánchez-Sánchez, T.; Martínez-Marcelo, V.H.; Aguilera-Alvarado, G.P.; Flores-Barrera, M.; King-Díaz, B.; Sánchez-Nieto, S. SWEET Transporters for the Nourishment of Embryonic Tissues during Maize Germination. Genes 2019, 10, 780. https://doi.org/10.3390/genes10100780
López-Coria M, Sánchez-Sánchez T, Martínez-Marcelo VH, Aguilera-Alvarado GP, Flores-Barrera M, King-Díaz B, Sánchez-Nieto S. SWEET Transporters for the Nourishment of Embryonic Tissues during Maize Germination. Genes. 2019; 10(10):780. https://doi.org/10.3390/genes10100780
Chicago/Turabian StyleLópez-Coria, Montserrat, Tomás Sánchez-Sánchez, Víctor Hugo Martínez-Marcelo, G. Paulina Aguilera-Alvarado, Mireya Flores-Barrera, Beatriz King-Díaz, and Sobeida Sánchez-Nieto. 2019. "SWEET Transporters for the Nourishment of Embryonic Tissues during Maize Germination" Genes 10, no. 10: 780. https://doi.org/10.3390/genes10100780




