Transcriptomic Signatures of Experimental Alkaloid Consumption in a Poison Frog
Abstract
:1. Introduction
| Glossary | |
| Alkaloid absorption | Up-take of alkaloids from dietary items. |
| Biotransformation | Structural change of a compound. For example, the conversion of pumiliotoxin (+)-251D into allopumiliotoxin (+)-267A known from Dendrobates and Adelphobates [39]. |
| Alkaloid degradation | Breakdown of alkaloids or alkaloid derivatives. |
| Alkaloid metabolism | Biotransformation, excretion or degradation of alkaloids. |
| Bioaccumulation | Accumulation of alkaloids because they are not metabolized at the same rate in which they are absorbed. |
| Alkaloid sequestering | Ability to absorb alkaloids, and internalize and store them in selected tissues or cells. |
| Alkaloid resistance | Avoidance of alkaloid toxicity. It can be achieved through several mechanisms, including mutations in target ion-channels and receptors, sequestering or metabolism. |
| Alkaloid processing | Absorption, metabolism and sequestering of alkaloids. |
2. Methods
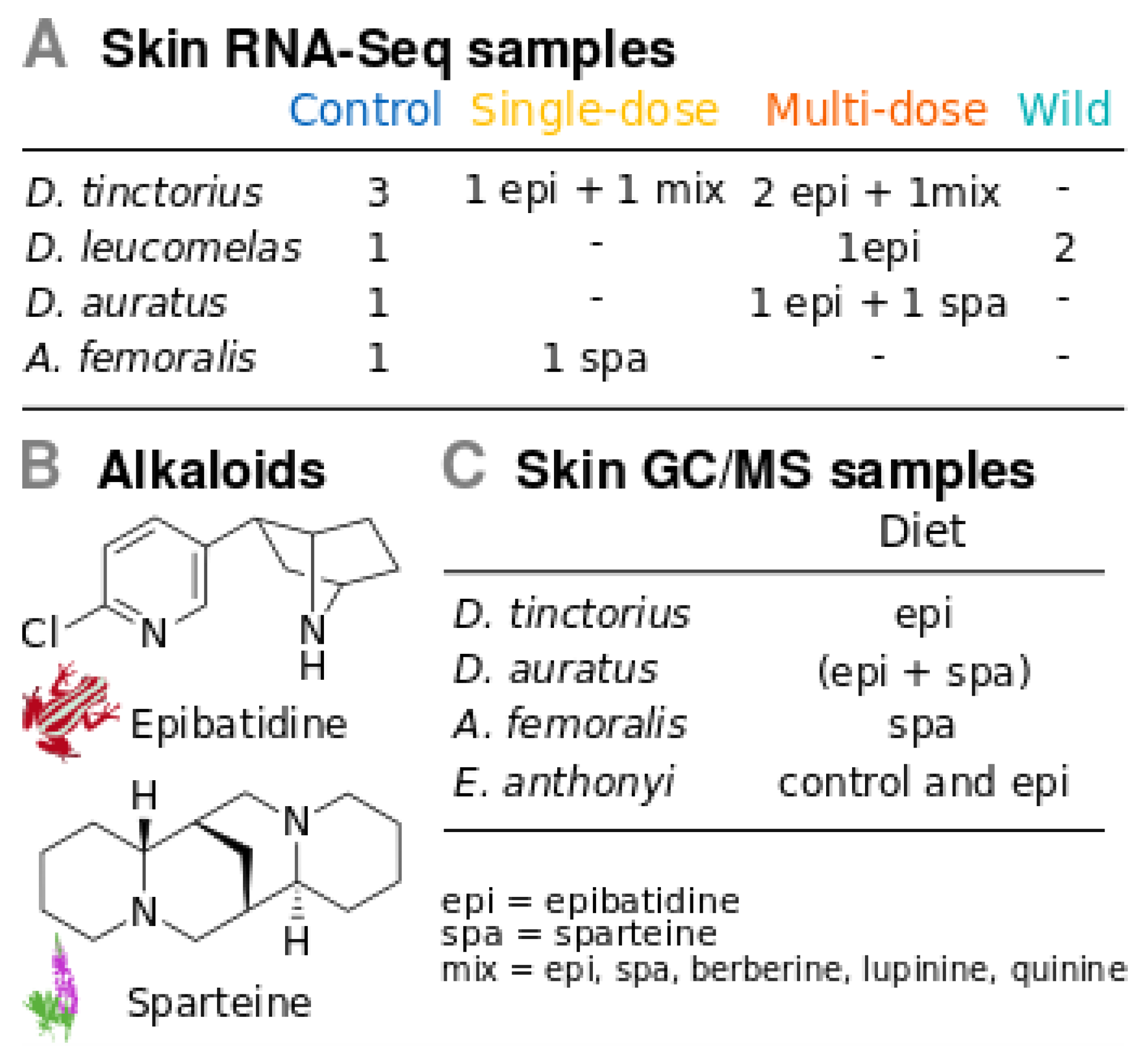
2.1. Poison Frogs
2.2. Experimental Design for Gene Expression
2.3. RNA-Seq, Transcriptome Assemblies and Annotation
2.4. Gene Expression in Dendrobates Tinctorius
2.5. Multi-Species Comparison
2.6. Alkaloid Sequestering Confirmation Tests
3. Results
3.1. Transcriptomes
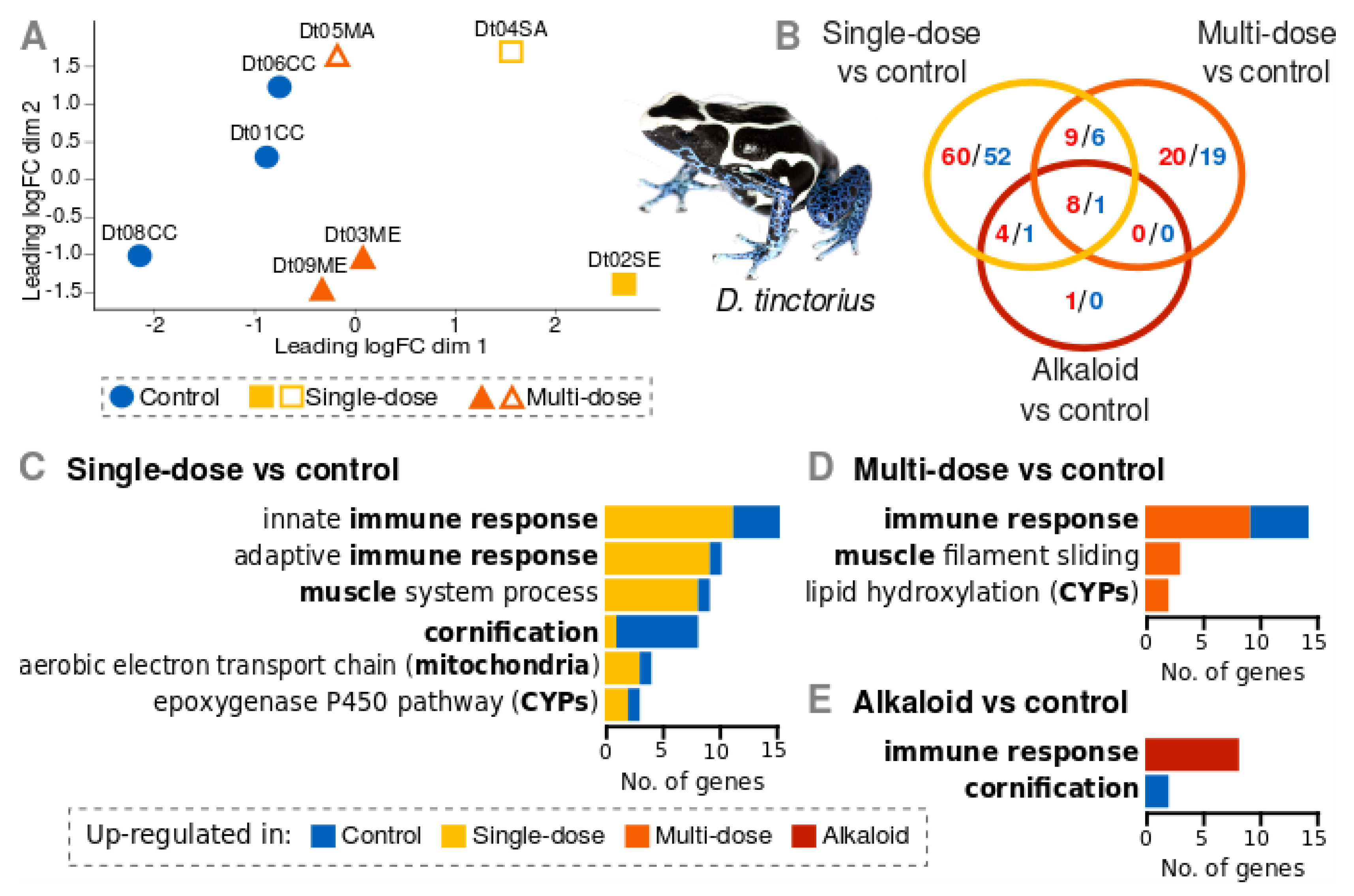
3.2. Gene Expression of D. Tinctorius
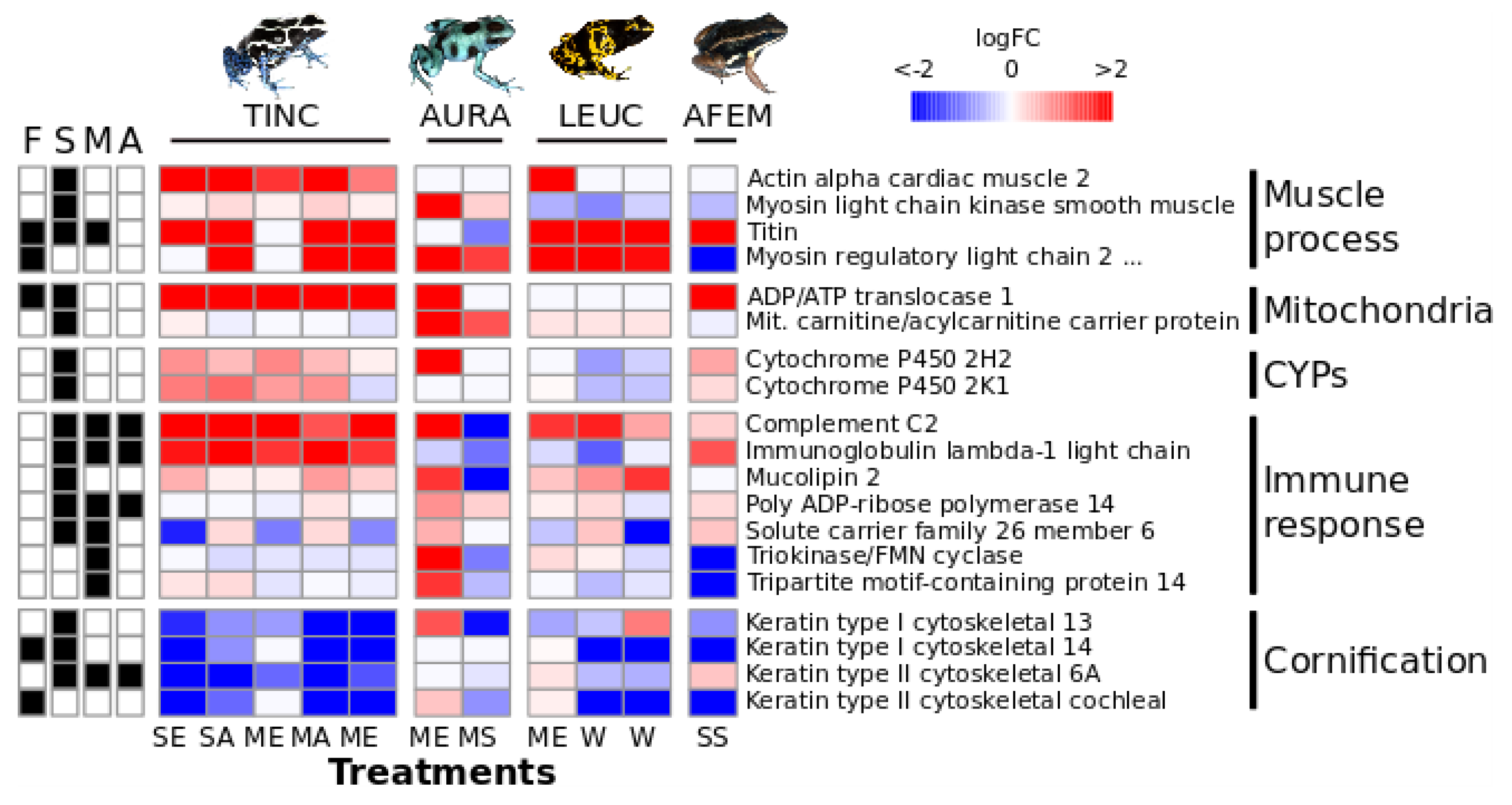
3.3. Multi-Species Comparison
3.4. Alkaloid Sequestering
4. Discussion
4.1. Gene Expression Patterns
4.2. Potential Biological Significance
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Data Availability
References
- Daly, J.W.; Garraffo, H.M.; Spande, T.F.; Jaramillo, C.; Rand, A.S. Dietary source for skin alkaloids of poison frogs (Dendrobatidae)? J. Chem. Ecol. 1994, 20, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Daly, J.W.; Gusovsky, F.; Myers, C.W.; Yotsu-Yamashita, M.; Yasumoto, T. First occurrence of tetrodotoxin in a dendrobatid frog (Colostethus inguinalis), with further reports for the bufonid genus Atelopus. Toxicon 1994, 32, 279–285. [Google Scholar] [CrossRef]
- Hantak, M.M.; Grant, T.; Reinsch, S.; Mcginnity, D.; Loring, M.; Toyooka, N.; Saporito, R.A. Dietary alkaloid sequestration in a poison frog: An experimental test of alkaloid uptake in Melanophryniscus stelzneri (Bufonidae). J. Chem. Ecol. 2013, 39, 1400–1406. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Poth, D.; Schulz, S.; Vences, M. Discovery of skin alkaloids in a miniaturized eleutherodactylid frog from Cuba. Biol. Let. 2011, 7, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Saporito, R.A.; Donnelly, M.A.; Spande, T.F.; Garrafo, H.M. A review of chemical ecology in poison frogs. Chemoecology 2012, 22, 159–168. [Google Scholar] [CrossRef]
- Santos, J.; Coloma, L.; Cannatella, D. Multiple, recurring origins of aposematism and diet specialization in poison frogs. PNAS 2003, 100, 12792–12797. [Google Scholar] [CrossRef] [Green Version]
- Vences, M.; Kosuch, J.; Boistel, R.; Haddad, C.F.; La Marca, E.; Lötters, S.; Veith, M. Convergent evolution of aposematic coloration in Neotropical poison frogs: a molecular phylogenetic perspective. Org. Divers. Evol. 2003, 3, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.C.; Cannatella, D.C. Phenotypic integration emerges from aposematism and scale in poison frogs. PNAS 2011, 108, 6175–6180. [Google Scholar] [CrossRef] [Green Version]
- Tarvin, R.; Borghese, C.M.; Sachs, W.; Santos, J.C.; Lu, Y.; O’Connell, L.A.; Cannatella, D.C.; Harris, R.A.; Zakon, H.H. Interacting amino acid replacements allow poison frogs to evolve epibatidine resistance. Science 2017, 357, 1261–1266. [Google Scholar] [CrossRef] [Green Version]
- Hagman, M.; Forsman, A. Correlated evolution of conspicuous coloration and body size in poison frogs (Dendrobatidae). Evolution 2003, 57, 2904–2910. [Google Scholar] [CrossRef]
- Vences, M.; Glaw, F.; Böhme, W. Evolutionary correlates of microphagy in alkaloid-containing frogs (Amphibia: Anura). Zool. Anz. 1998, 236, 217–230. [Google Scholar]
- Pröhl, H.; Ostrowski, T. 2011. Behavioural elements reflect phenotypic colour divergence in a poison frog. Evol. Ecol. 2011, 25, 993–1015. [Google Scholar] [CrossRef]
- Rudh, A.; Rogell, B.; Håstad, O.; Qvarnström, A. Rapid population divergence linked with co-variation between coloration and sexual display in strawberry poison frogs. Evolution 2011, 65, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.C. Fast molecular evolution associated with high active metabolic rates in poison frogs. Mol. Biol. Evol. 2012, 29, 2001–2018. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.C.; Baquero, M.; Barrio-Amorós, C.L.; Coloma, L.A.; Erdtmann, L.K.; Lima, A.P.; Cannatella, D.C. Aposematism increases acoustic diversification and speciation in poison frogs. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dugas, M.B.; Halbrook, S.R.; Killius, A.M.; del Sol, J.F.; Richards-Zawacki, C.L. Colour and escape behaviour in polymorphic populations of an aposematic poison frog. Ethology 2015, 121, 813–822. [Google Scholar] [CrossRef]
- Tarvin, R.; Powell, E.A.; Santos, J.C.; Ron, S.R.; Cannatella, D.C. The birth of aposematism: High phenotypic divergence and low genetic diversity in a young clade of poison frogs. Mol. Phyl. Evol. 2017, 109, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, A.; Becza, N.; Saporito, R.A. Escape behaviour of aposematic (Oophaga pumilio) and cryptic (Craugastor sp.) frogs in response to simulated predator approach. J. Trop. Ecol. 2017, 33, 165–169. [Google Scholar] [CrossRef]
- Gibson, G.G.; Skett, P. Introduction to Drug Metabolism, 3rd ed.; Nelson Thornes Ltd.: Hampshire, UK, 2001; p. 256. [Google Scholar]
- Santos, J.C.; Tarvin, R.D.; O’Connell, L.A. A review of chemical defense in poison frogs (Dendrobatidae): Ecology, pharmacokinetics, and autoresistance. In Chemical Signals in Vertebrates 13; Schulte, B.A., Goodwin, T.E., Ferkin, M.H., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 305–337. [Google Scholar]
- Caty, S.N.; Alvarez-Buylla, A.; Byrd, G.D.; Vidoudez, C.; Roland, A.B.; Tapia, E.E.; Budnik, B.; Trauger, S.A.; Coloma, L.A.; O’Connell, L.A. Molecular physiology of chemical defenses in a poison frog. J. Exp. Biol. 2019, 222, jeb204149. [Google Scholar] [CrossRef]
- Grant, T.; Colombo, P.; Verrastro, L.; Saporito, R.A. The occurence of defensive alkaloids in non-integumentary tissue of the Brazilian red-belly toad Melanophryniscus simplex (Bufonidae). Chemoecology 2012, 22, 169–178. [Google Scholar] [CrossRef]
- Neuwirth, M.; Daly, J.; Myers, C.; Tice, L. Morphology of the granular secretory glands in skin of poison-dart frogs (Dendrobatidae). Tissue Cell 1979, 11, 755–771. [Google Scholar] [CrossRef]
- Delfino, G.; Giachi, F.; Nosi, D.; Malentacchi, C. Serous cutaneous glands in Phyllobates bicolor (Anura: Dendrobatidae): An ontogenetic, ultrastructural study on secretory product biosynthesis and maturation. Copeia 2010, 1, 27–37. [Google Scholar] [CrossRef]
- Prates, I.; Antoniazzi, M.M.; Sciani, J.M.; Pimenta, D.C.; Toledo, L.F.; Haddad, C.F.; Jared, C. Skin glands, poison and mimicry in dendrobatid and leptodactylid amphibians. J. Morph. 2012, 273, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Wang, G.K. Batrachotoxin-resistant Na+ channels derived from point mutations in transmembrane segment D4-S6. Biophys. J. 1999, 76, 3141–3149. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Mitchell, J.; Tikhonov, D.B.; Zhorov, B.S.; Wang, G.K. How batrachotoxin modifies the sodium channel permeation pathway: Computer modeling and site-directed mutagenesis. Mol. Pharmacol. 2006, 69, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Hanifin, C.T.; Gilly, W.F. Evolutionary history of a complex adaptation: Tetrodotoxin resistance in salamanders. Evolution 2015, 69, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Tarvin, R.D.; Santos, J.C.; O’connell, L.A.; Zakon, H.H.; Cannatella, D.C. Convergent substitutions in a sodium channel suggest multiple origins of toxin resistance in poison frogs. Mol. Biol. Evol. 2016, 33, 1068–1081. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Wang, G.K. Single rat muscle Na+ channel mutation confers batrachotoxin autoresistance found in poison-dart frog Phyllobates terribilis. PNAS 2017, 114, 10491–10496. [Google Scholar] [CrossRef]
- Márquez, R.; Ramírez-Castañeda, V.; Amézquita, A. Does batrachotoxin autoresistance co-evolve with toxicity in Phyllobates poison-dart frogs? Evolution 2018, 73, 390–400. [Google Scholar] [CrossRef]
- Spande, T.F.; Garraffo, H.M.; Edwards, M.W.; Yeh, H.J.; Pannell, L.; Daly, J.W. Epibatidine: A novel (chloropyridyl) azabicycloheptane with potent analgesic activity from an Ecuadoran poison frog. J. Am. Chem. Soc. 1992, 114, 3475–3478. [Google Scholar] [CrossRef]
- Badio, B.; Daly, J.W. Epibatidine, a potent analgetic and nicotinic antagonist. Mol. Pharmacol. 1994, 45, 563–569. [Google Scholar] [PubMed]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [CrossRef] [PubMed]
- Lembeck, F. Epibatidine: High potency and broad spectrum activity on neuronal and neuromuscular nicotinic acetylcholine receptors. Naunyn Schmiedebergs Arch. Pharmacol. 1999, 359, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.C.; Coloma, L.A.; Summers, K.; Caldwell, J.P.; Ree, R.; Cannatella, D.C. Amazonian amphibian diversity is primarily derived from late Miocene Andean lineages. PLoS Biol. 2009, 7, e56. [Google Scholar] [CrossRef] [PubMed]
- Toft, C.A. Evolution of diet specialization in poison-dart frogs (Dendrobatidae). Herpetologica 1995, 51, 202–216. [Google Scholar]
- Caldwell, J.P. The evolution of myrmecophagy and its correlates in poison frogs (Family Dendrobatidae). J. Zool. 1996, 240, 75–101. [Google Scholar] [CrossRef]
- Daly, J.W.; Garraffo, H.M.; Spande, T.F.; Clark, V.C.; Ma, J.; Ziffer, H.; Cover, J.F. Evidence for an enantioselective pumiliotoxin 7-hydroxylase in dendrobatid poison frogs of the genus Dendrobates. PNAS 2003, 100, 11092–11097. [Google Scholar] [CrossRef]
- AmphibiaWeb. Available online: https://amphibiaweb.org (accessed on 10 March 2019).
- Pyron, R.A.; Wiens, J.J. A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Mol. Phyl. Evol. 2011, 61, 543–583. [Google Scholar] [CrossRef]
- Grant, T.; Rada, M.; Anganoy-Criollo, M.; Batista, A.; Dias, P.H.; Jeckel, A.M.; Machado, D.J.; Rueda-Almonacid, J.V. Phylogenetic systematics of dart-poison frogs and their relatives revisited (Anura: Dendrobatoidea). S. Am. J. Herpetol. 2017, 12, s1. [Google Scholar] [CrossRef]
- Singh, S.; Avor, K.S.; Pouw, B.; Seale, T.W.; Basmadjian, G.P. Design and synthesis of isoxazole containing bioisosteres of epibatidine as potent nicotinic acetylcholine receptor agonists. Chem. Pharm. Bull. 1999, 47, 1501–1505. [Google Scholar] [CrossRef]
- Grant, T.; Frost, D.R.; Caldwell, J.P.; Gagliardo, R.O.N.; Haddad, C.F.; Kok, P.J.; Means, D.B.; Noonan, B.P.; Schargel, W.E.; Wheeler, W.C. Phylogenetic systematics of dart-poison frogs and their relatives (Amphibia: Athesphatanura: Dendrobatidae). Bull. Am. Mus. Nat. Hist. 2006, 299, 1–262. [Google Scholar] [CrossRef]
- Amézquita, A.; Ramos, Ó.; González, M.C.; Rodríguez, C.; Medina, I.; Simões, P.I.; Pimentel Lima, A. Conspicuousness, color resemblance, and toxicity in geographically diverging mimicry: The pan-Amazonian frog Allobates femoralis. Evolution 2017, 71, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Saporito, R.A.; Grant, T. Comment on Amézquita et al. (2018) "Conspicuousness, color resemblance, and toxicity in geographically diverging mimicry: The pan-Amazonian frog Allobates femoralis". Evolution 2018, 72, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Yovo, K. Les Alcaloïdes Quinolizidiniques des Graines des Lupins: Contribution à Une Étude Pharmacologique et Toxicologique Comparée de la Spartéine et de la Lupanine. Ph.D. Thesis, Université François-Rabelais de Tours, Tours, France, 1982. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature Biotechnol. 2011, 29, 644. [Google Scholar] [CrossRef] [PubMed]
- Cabau, C.; Escudié, F.; Djari, A.; Guiguen, Y.; Bobe, J.; Klopp, C. Compacting and correcting Trinity and Oases RNA-Seq de novo assemblies. PeerJ 2017, 5, e2988. [Google Scholar] [CrossRef] [PubMed]
- Smith-Unna, R.; Boursnell, C.; Patro, R.; Hibberd, J.M.; Kelly, S. TransRate: Reference-free quality assessment of de novo transcriptome assemblies. Genome Res. 2016, 26, 1134–1144. [Google Scholar] [CrossRef]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef]
- Musacchia, F.; Basu, S.; Petrosino, G.; Salvemini, M.; Sanges, R. Annocript: A flexible pipeline for the annotation of transcriptomes able to identify putative long noncoding RNAs. Bioinformatics 2015, 31, 2199–2201. [Google Scholar] [CrossRef]
- The UniProt Consortium. UniProt: The universal protein knowledge base. Nucleic Acids Res. 2017, 45, D158–D169. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R package version 2.5-2. 2018. Available online: https://CRAN.R-project.org/package=vegan (accessed on 10 March 2019).
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Soneson, C.; Love, M.I.; Robinson, M.D. Differential analyses for RNA-seq: Transcript-level estimates improve gene-level inferences. F1000Research 2015, 4, 1521. [Google Scholar] [CrossRef] [PubMed]
- Mortari, M.R.; Schwartz, E.N.; Schwartz, C.A.; Pires, O.R., Jr.; Santos, M.M.; Bloch, C., Jr.; Sebben, A. Main alkaloids from the Brazilian dendrobatidae frog Epipedobates flavopictus: Pumiliotoxin 251D, histrionicotoxin and decahydroquinolines. Toxicon 2004, 43, 303–310. [Google Scholar] [CrossRef]
- Mukhtar, H.; Khan, W.A. Cutaneous Cytochrome P-450. Drug Metab. Rev. 1989, 20, 657–673. [Google Scholar] [CrossRef]
- Guengerich, F.P. Cytochrome P-450 3A4: Regulation and role in drug metabolism. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 1–7. [Google Scholar] [CrossRef]
- Boelsterli, U.A. Mechanistic Toxicology: The Molecular Basis of How Chemicals Disrupt Biological Targets, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; p. 416. [Google Scholar]
- Klein, J.; Figueroa, F. Evolution of the major histocompatibility complex. Crit. Rev. Immunol. 1986, 6, 295–386. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK10757/ (accessed on 10 March 2019).
- Leslie, G.A.; Clem, L.W. Production of anti-hapten antibodies by several classes of lower vertebrates. J. Immunol. 1969, 103, 613–617. [Google Scholar] [PubMed]
- Wang, C.W.; Hung, S.I. Immunology of cutaneous adverse drug reactions. In Advances in Diagnosis and Management of Cutaneous Adverse Drug Reactions; Shear, N., Dodiuk-Gad, R., Eds.; Adis: Singapore, 2019; pp. 23–37. [Google Scholar]
- Stuckert, A.M.; Moore, E.; Coyle, K.P.; Davison, I.; MacManes, M.D.; Roberts, R.; Summers, K. Variation in pigmentation gene expression is associated with distinct aposematic color morphs in the poison frog Dendrobates auratus. BMC Evol. Biol. 2019, 19, 85. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, E.; Rodríguez, A.; Grau, J.H.; Lötters, S.; Künzel, S.; Saporito, R.A.; Ringler, E.; Schulz, S.; Wollenberg Valero, K.C.; Vences, M. Transcriptomic Signatures of Experimental Alkaloid Consumption in a Poison Frog. Genes 2019, 10, 733. https://doi.org/10.3390/genes10100733
Sanchez E, Rodríguez A, Grau JH, Lötters S, Künzel S, Saporito RA, Ringler E, Schulz S, Wollenberg Valero KC, Vences M. Transcriptomic Signatures of Experimental Alkaloid Consumption in a Poison Frog. Genes. 2019; 10(10):733. https://doi.org/10.3390/genes10100733
Chicago/Turabian StyleSanchez, Eugenia, Ariel Rodríguez, Jose H. Grau, Stefan Lötters, Sven Künzel, Ralph A. Saporito, Eva Ringler, Stefan Schulz, Katharina C. Wollenberg Valero, and Miguel Vences. 2019. "Transcriptomic Signatures of Experimental Alkaloid Consumption in a Poison Frog" Genes 10, no. 10: 733. https://doi.org/10.3390/genes10100733
APA StyleSanchez, E., Rodríguez, A., Grau, J. H., Lötters, S., Künzel, S., Saporito, R. A., Ringler, E., Schulz, S., Wollenberg Valero, K. C., & Vences, M. (2019). Transcriptomic Signatures of Experimental Alkaloid Consumption in a Poison Frog. Genes, 10(10), 733. https://doi.org/10.3390/genes10100733




