NIR-Triggered Hyperthermal Effect of Polythiophene Nanoparticles Synthesized by Surfactant-Free Oxidative Polymerization Method on Colorectal Carcinoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polythiophene Nanoparticles
2.3. Physico-Chemical Characterization
2.4. Photothermal Properties of Polythiophene Nanocomposites
2.5. Cell Culture
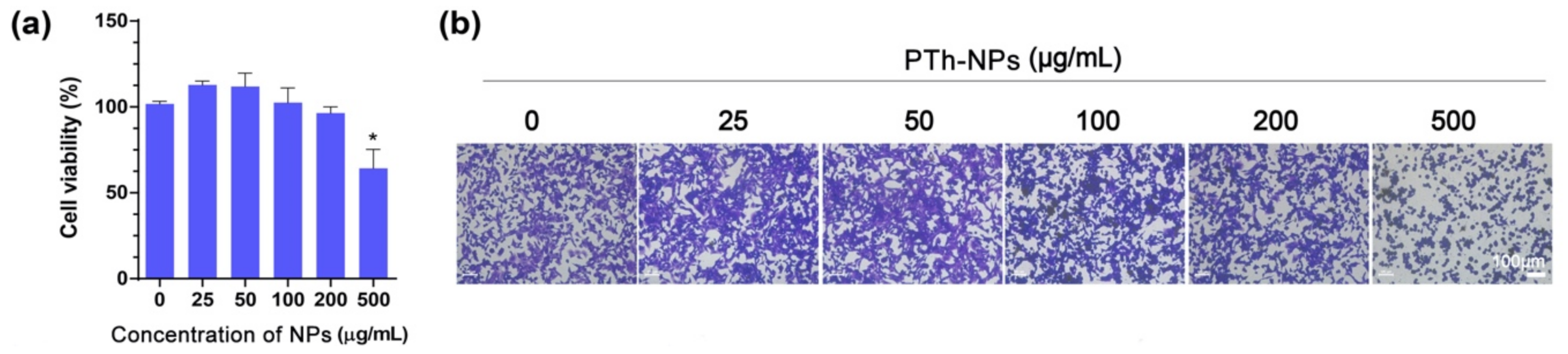
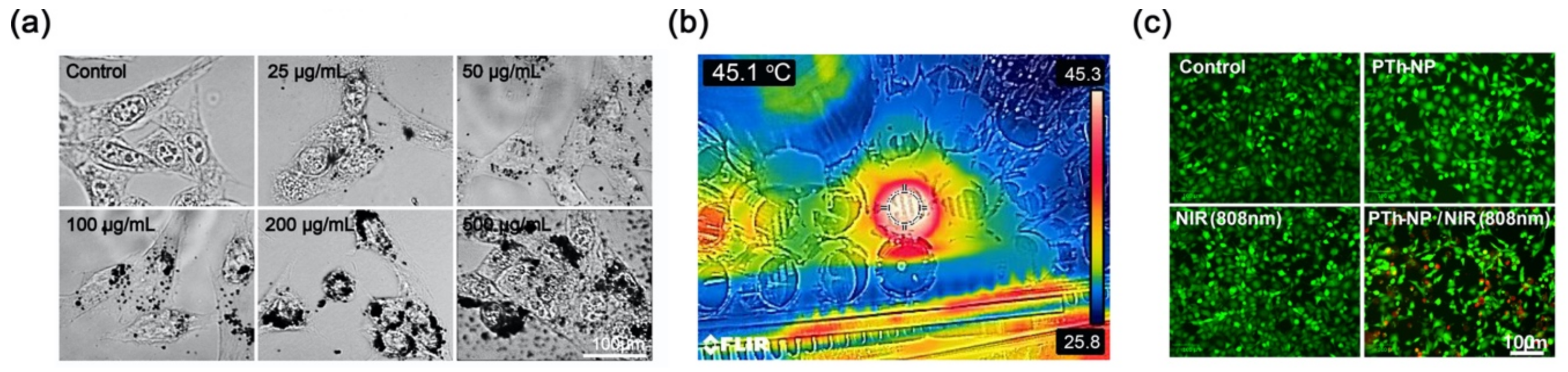
2.6. Cytotoxicity
2.7. Cellular Uptake Assay
2.8. Hyperthermia Effects of PTh–NPs
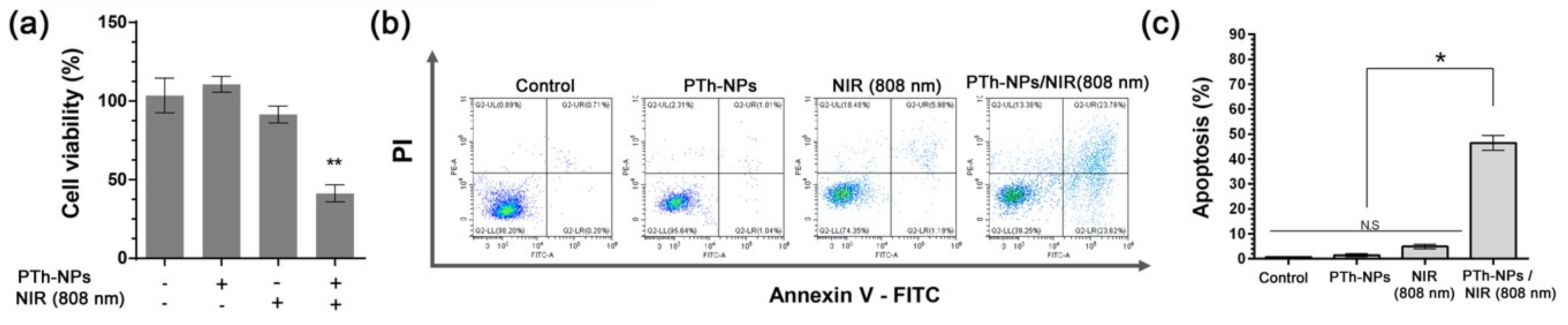
2.9. In Vitro Cell Apoptosis Analysis
2.10. Statistical Analysis
3. Results and Discussion
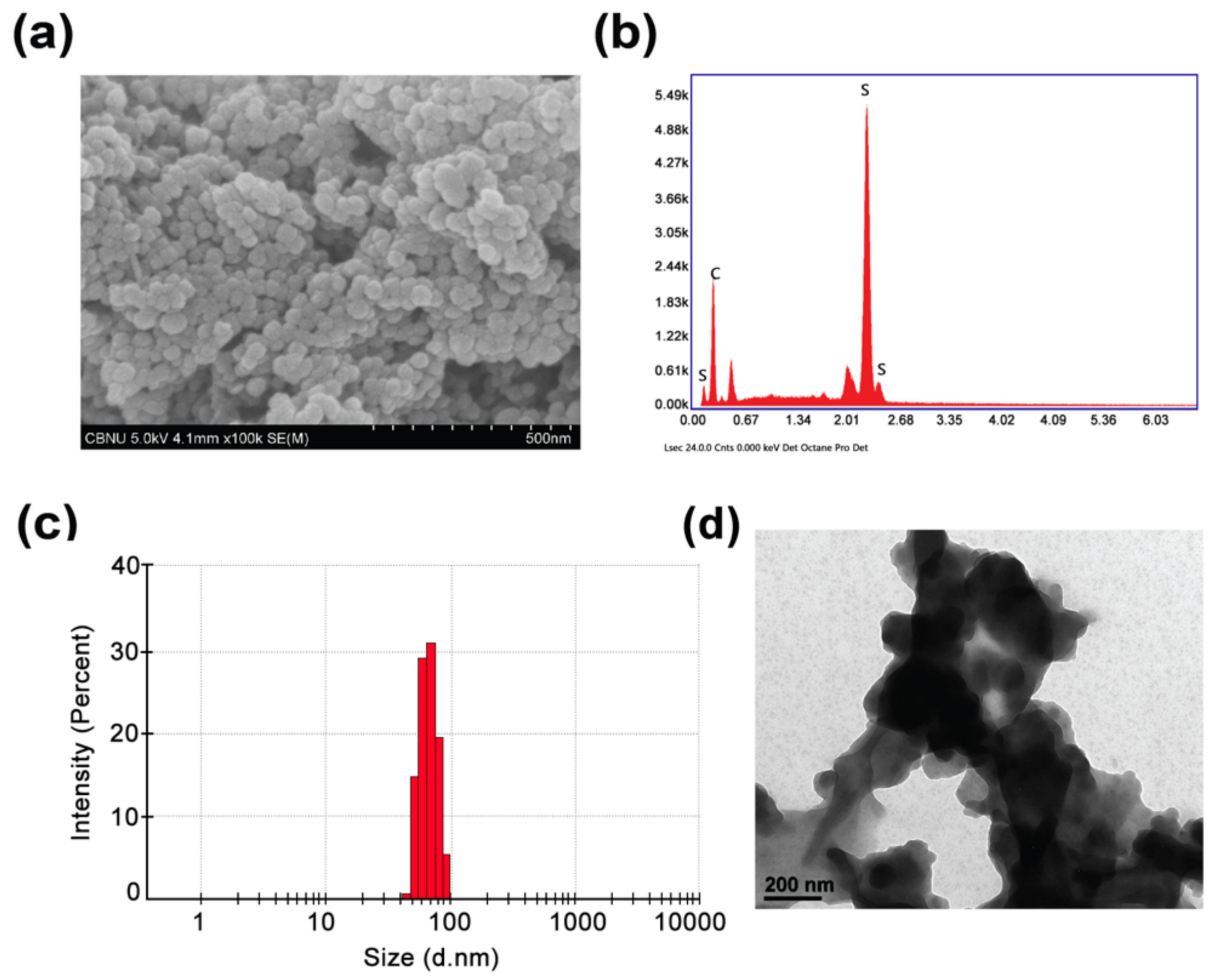
3.1. Physicochemical Characterization
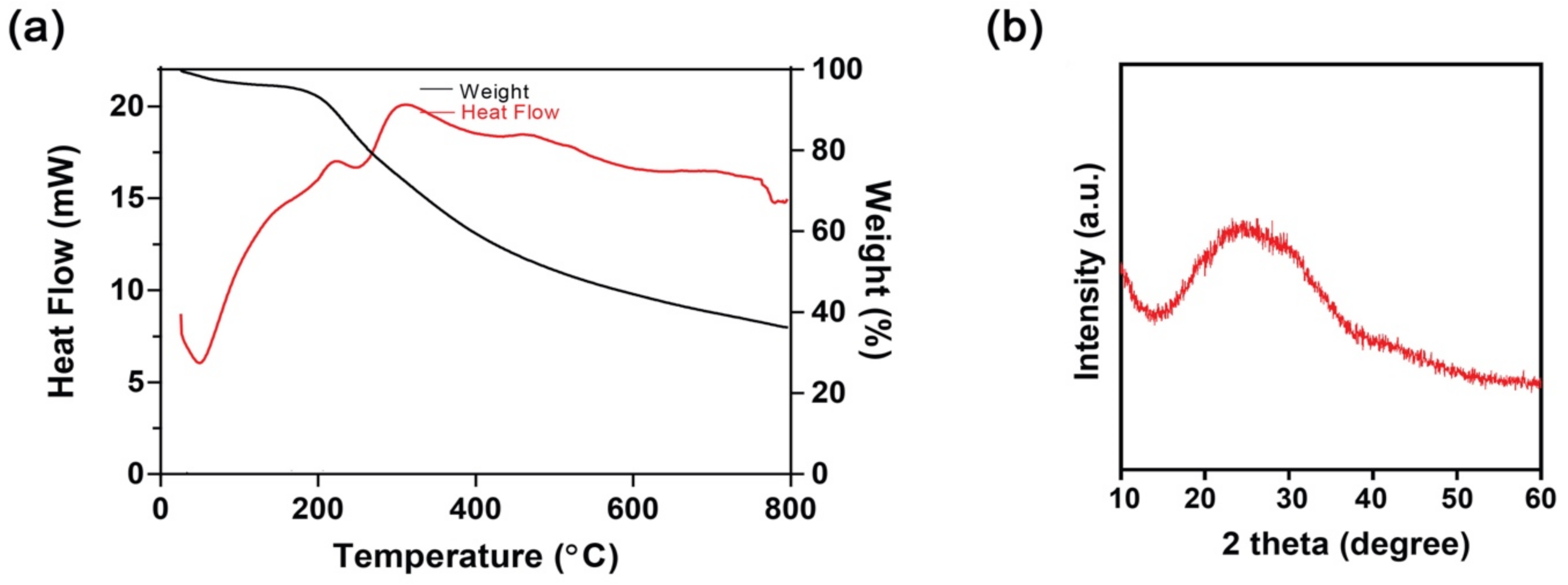
3.2. Thermogravimetric (TGA) and Differential Scanning Calorimetry (DSC) Study
3.3. Photothermal Analysis and Photothermal Stability of PTh–NPs
3.4. In Vitro Cell Ablation Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pal, I.; Rajesh, Y.; Banik, P.; Dey, G.; Dey, K.K.; Bharti, R.; Naskar, D.; Chakraborty, S.; Ghosh, S.K.; Das, S.K.; et al. Prevention of epithelial to mesenchymal transition in colorectal carcinoma by regulation of the E-cadherin-β-catenin-vinculin axis. Cancer Lett. 2019, 452, 254–263. [Google Scholar] [CrossRef]
- Mármol, I.; Sánchez-De-Diego, C.; Pradilla-Dieste, A.; Cerrada, E.; Rodríguez-Yoldi, M. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Diaz, L.A. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H.; Ryan, D.P. Cytoreductive surgery plus hyperthermic perioperative chemotherapy to treat peritoneal metastases from colorectal cancer: standard of care or an experimental approach? Lancet Oncol. 2012, 13, e362–e369. [Google Scholar] [CrossRef]
- Ciardiello, D.; Vitiello, P.P.; Cardone, C.; Martini, G.; Troiani, T.; Martinelli, E.; Ciardiello, F. Immunotherapy of colorectal cancer: Challenges for therapeutic efficacy. Cancer Treat. Rev. 2019, 76, 22–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Deng, W.; Li, N.; Neri, S.; Sharma, A.; Jiang, W.; Lin, S.H. Combining Immunotherapy and Radiotherapy for Cancer Treatment: Current Challenges and Future Directions. Front. Pharmacol. 2018, 9, 185. [Google Scholar] [CrossRef] [Green Version]
- Gasiulė, S.; Dreize, N.; Kaupinis, A.; Ražanskas, R.; Čiupas, L.; Stankevicius, V.; Lesauskaitė, V.; Laurinavicius, A.; Valius, M.; Vilkaitis, G. Molecular Insights into miRNA-Driven Resistance to 5-Fluorouracil and Oxaliplatin Chemotherapy: miR-23b Modulates the Epithelial–Mesenchymal Transition of Colorectal Cancer Cells. J. Clin. Med. 2019, 8, 2115. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Chen, P.; Zhang, Y.; Lu, W.; Ding, W.; Luo, Y.; Wen, S.; Xu, R.; Liu, P.-P.; Huang, P. Synergy between Auranofin and Celecoxib against Colon Cancer In Vitro and In Vivo through a Novel Redox-Mediated Mechanism. Cancers 2019, 11, 931. [Google Scholar] [CrossRef] [Green Version]
- Bhattarai, D.P.; Tiwari, A.P.; Maharjan, B.; Tumurbaatar, B.; Park, C.H.; Kim, C.S. Sacrificial template-based synthetic approach of polypyrrole hollow fibers for photothermal therapy. J. Colloid Interface Sci. 2019, 534, 447–458. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Bhattarai, D.P.; Maharjan, B.; Ko, S.W.; Kim, H.Y.; Park, C.H.; Kim, C.S. Polydopamine-based Implantable Multifunctional Nanocarpet for Highly Efficient Photothermal-chemo Therapy. Sci. Rep. 2019, 9, 2943. [Google Scholar] [CrossRef]
- Vogl, T.J.; Zegelman, A.; Bechstein, W.O.; Zeuzem, S.; Zangos, S. Treatment of liver metastases of colorectal carcinoma: overview of hyperthermal ablation methods. Dtsch. Med. Wochenschr. (1946) 2013, 138, 792–798. [Google Scholar]
- Zhang, X.; Wu, J.; Williams, G.R.; Yang, Y.; Niu, S.; Qian, Q.; Zhu, L.-M. Dual-responsive molybdenum disulfide/copper sulfide-based delivery systems for enhanced chemo-photothermal therapy. J. Colloid Interface Sci. 2019, 539, 433–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirrahimi, M.; Abed, Z.; Beik, J.; Shiri, I.; Dezfuli, A.S.; Mahabadi, V.P.; Kamrava, S.K.; Ghaznavi, H.; Shakeri-Zadeh, A. A thermo-responsive alginate nanogel platform co-loaded with gold nanoparticles and cisplatin for combined cancer chemo-photothermal therapy. Pharmacol. Res. 2019, 143, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, D.P.; Shrestha, S.; Shrestha, B.K.; Park, C.H.; Kim, C.S. A controlled surface geometry of polyaniline doped titania nanotubes biointerface for accelerating MC3T3-E1 cells growth in bone tissue engineering. Chem. Eng. J. 2018, 350, 57–68. [Google Scholar] [CrossRef]
- Bhattarai, D.P.; Hwang, T.I.; Kim, J.I.; Lee, J.H.; Chun, S.; Kim, B.-S.; Park, C.H.; Kim, C.S. Synthesis of polypyrrole nanorods via sacrificial removal of aluminum oxide nanopore template: A study on cell viability, electrical stimulation and neuronal differentiation of PC12 cells. Mater. Sci. Eng. C 2020, 107, 110325. [Google Scholar] [CrossRef]
- Rezk, A.I.; Bhattarai, D.P.; Park, J.; Park, C.H.; Park, C.H. Polyaniline-coated titanium oxide nanoparticles and simvastatin-loaded poly(ε-caprolactone) composite nanofibers scaffold for bone tissue regeneration application. Colloids Surf. B 2020, 192, 111007. [Google Scholar] [CrossRef]
- German, N.; Popov, A.; Ramanaviciene, A.; Ramanavicius, A. Enzymatic Formation of Polyaniline, Polypyrrole, and Polythiophene Nanoparticles with Embedded Glucose Oxidase. Nanomaterials 2019, 9, 806. [Google Scholar] [CrossRef] [Green Version]
- Osawa, S.; Ito, M.; Tanaka, K.; Kuwano, J. Electrochemical polymerization of thiophene under ultrasonic field. Synth. Met. 1987, 18, 145–150. [Google Scholar] [CrossRef]
- Wei, Y.; Chan, C.C.; Tian, J.; Jang, G.W.; Hsueh, K.F. Electrochemical polymerization of thiophenes in the presence of bithiophene or terthiophene: kinetics and mechanism of the polymerization. Chem. Mater. 1991, 3, 888–897. [Google Scholar] [CrossRef]
- Wang, J.; Neoh, K.G.; Kang, E.-T. Comparative study of chemically synthesized and plasma polymerized pyrrole and thiophene thin films. Thin Solid Films 2004, 446, 205–217. [Google Scholar] [CrossRef]
- Gnanakan, S.R.P.; Rajasekhar, M.; Subramania, A. Synthesis of polythiophene nanoparticles by surfactant-assisted dilute polymerization method for high performance redox supercapacitors. Int. J. Electrochem. Sci. 2009, 4, 1289–1301. [Google Scholar]
- Li, X.-G.; Li, J.; Huang, M.-R. Facile Optimal Synthesis of Inherently Electroconductive Polythiophene Nanoparticles. Chem. A Eur. J. 2009, 15, 6446–6455. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, J.M.; Cheong, I.W.; Lee, H.; Kim, J.H. A facile route of polythiophene nanoparticles via Fe3+-catalyzed oxidative polymerization in aqueous medium. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 2097–2107. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Xu, D.; Kong, E.S.-W.; Zhang, Y. Facile synthesis of dispersible spherical polythiophene nanoparticles by copper(II) catalyzed oxidative polymerization in aqueous medium. Synth. Met. 2010, 160, 921–926. [Google Scholar] [CrossRef]
- Inagaki, C.S.; Oliveira, M.M.; Zarbin, A.J. Direct and one-step synthesis of polythiophene/gold nanoparticles thin films through liquid/liquid interfacial polymerization. J. Colloid Interface Sci. 2018, 516, 498–510. [Google Scholar] [CrossRef]
- Inagaki, C.S.; Oliveira, M.M.; Bergamini, M.F.; Marcolino-Junior, L.H.; Zarbin, A.J. Facile synthesis and dopamine sensing application of three component nanocomposite thin films based on polythiophene, gold nanoparticles and carbon nanotubes. J. Electroanal. Chem. 2019, 840, 208–217. [Google Scholar] [CrossRef]
- Bhattarai, D.P.; Awasthi, G.P.; Maharjan, B.; Lee, J.; Kim, B.-S.; Park, C.H.; Kim, C.S. Synthesis of polythiophene nanoparticles by surfactant-free chemical oxidative polymerization method: Characterization, in vitro biomineralization, and cytotoxicity evaluation. J. Ind. Eng. Chem. 2019, 77, 243–252. [Google Scholar] [CrossRef]
- Kim, B.-S.; Yang, S.-S.; Kim, C.S. Incorporation of BMP-2 nanoparticles on the surface of a 3D-printed hydroxyapatite scaffold using an ε-polycaprolactone polymer emulsion coating method for bone tissue engineering. Colloids Surf. B 2018, 170, 421–429. [Google Scholar] [CrossRef]
- Strohbehn, J.W.; Douple, E.B. Hyperthermia and Cancer Therapy: A Review of Biomedical Engineering Contributions and Challenges. IEEE Trans. Biomed. Eng. 1984, 31, 779–787. [Google Scholar] [CrossRef]
- Bao, Z.; Liu, X.; Liu, Y.; Liu, H.; Zhao, K. Near-infrared light-responsive inorganic nanomaterials for photothermal therapy. Asian J. Pharm. Sci. 2016, 11, 349–364. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, L.; Fan, Y.; Feng, Q.; Cui, F.-Z. Biocompatibility and Toxicity of Nanoparticles and Nanotubes. J. Nanomater. 2012, 2012, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Isidoro, C.; Maneerat, E.; Giovia, A.; Carlo, F.; Caputo, G.; Ekkapongpisit, M.; Follo, C. Biocompatibility, endocytosis, and intracellular trafficking of mesoporous silica and polystyrene nanoparticles in ovarian cancer cells: effects of size and surface charge groups. Int. J. Nanomed. 2012, 7, 4147–4158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowley, L.C.; Marfell, B.J.; Scott, A.P.; Waterhouse, N.J. Quantitation of Apoptosis and Necrosis by Annexin V Binding, Propidium Iodide Uptake, and Flow Cytometry. Cold Spring Harb. Protoc. 2016, 2016, 087288. [Google Scholar] [CrossRef] [PubMed]
- Shellman, Y.G.; Howe, W.R.; Miller, L.A.; Goldstein, N.B.; Pacheco, T.R.; Mahajan, R.L.; LaRue, S.M.; Norris, D.A. Hyperthermia Induces Endoplasmic Reticulum-Mediated Apoptosis in Melanoma and Non-Melanoma Skin Cancer Cells. J. Investig. Dermatol. 2008, 128, 949–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, C.-H.; Lin, F.-L.; Hou, S.-M.; Liu, J.-F. Hyperthermia Induces Apoptosis through Endoplasmic Reticulum and Reactive Oxygen Species in Human Osteosarcoma Cells. Int. J. Mol. Sci. 2014, 15, 17380–17395. [Google Scholar] [CrossRef] [Green Version]
- Slimen, I.B.; Najar, T.; Ghram, A.; Debbabi, H.; Ben Mrad, M.; Abdrabbah, M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperth. 2014, 30, 513–523. [Google Scholar] [CrossRef]
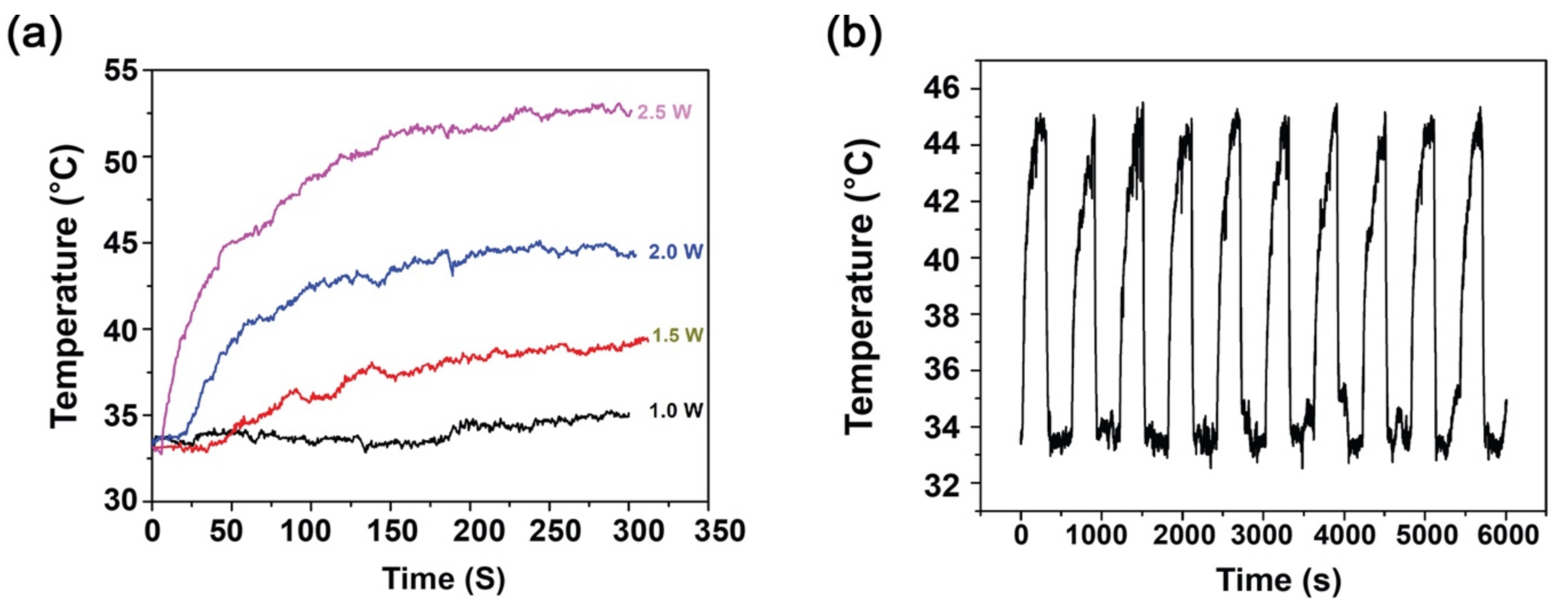
| Concentration of PTh–NPs (µg/mL) | Maximum Temperature (°C) Recorded under Different Power Supply of NIR 808 nm for Different Concentration of Substrate | |||
|---|---|---|---|---|
| 1.0 W/cm2 | 1.5 W/cm2 | 2.0 W/cm2 | 2.5 W/cm2 | |
| 100 | 35.0 | 39.2 | 45.1 | 53.5 |
| 200 | 36.0 | 42.5 | 49.4 | 57.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattarai, D.P.; Kim, B.S. NIR-Triggered Hyperthermal Effect of Polythiophene Nanoparticles Synthesized by Surfactant-Free Oxidative Polymerization Method on Colorectal Carcinoma Cells. Cells 2020, 9, 2122. https://doi.org/10.3390/cells9092122
Bhattarai DP, Kim BS. NIR-Triggered Hyperthermal Effect of Polythiophene Nanoparticles Synthesized by Surfactant-Free Oxidative Polymerization Method on Colorectal Carcinoma Cells. Cells. 2020; 9(9):2122. https://doi.org/10.3390/cells9092122
Chicago/Turabian StyleBhattarai, Deval Prasad, and Beom Su Kim. 2020. "NIR-Triggered Hyperthermal Effect of Polythiophene Nanoparticles Synthesized by Surfactant-Free Oxidative Polymerization Method on Colorectal Carcinoma Cells" Cells 9, no. 9: 2122. https://doi.org/10.3390/cells9092122




