BV-2 Microglial Cells Respond to Rotenone Toxic Insult by Modifying Pregnenolone, 5α-Dihydroprogesterone and Pregnanolone Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture
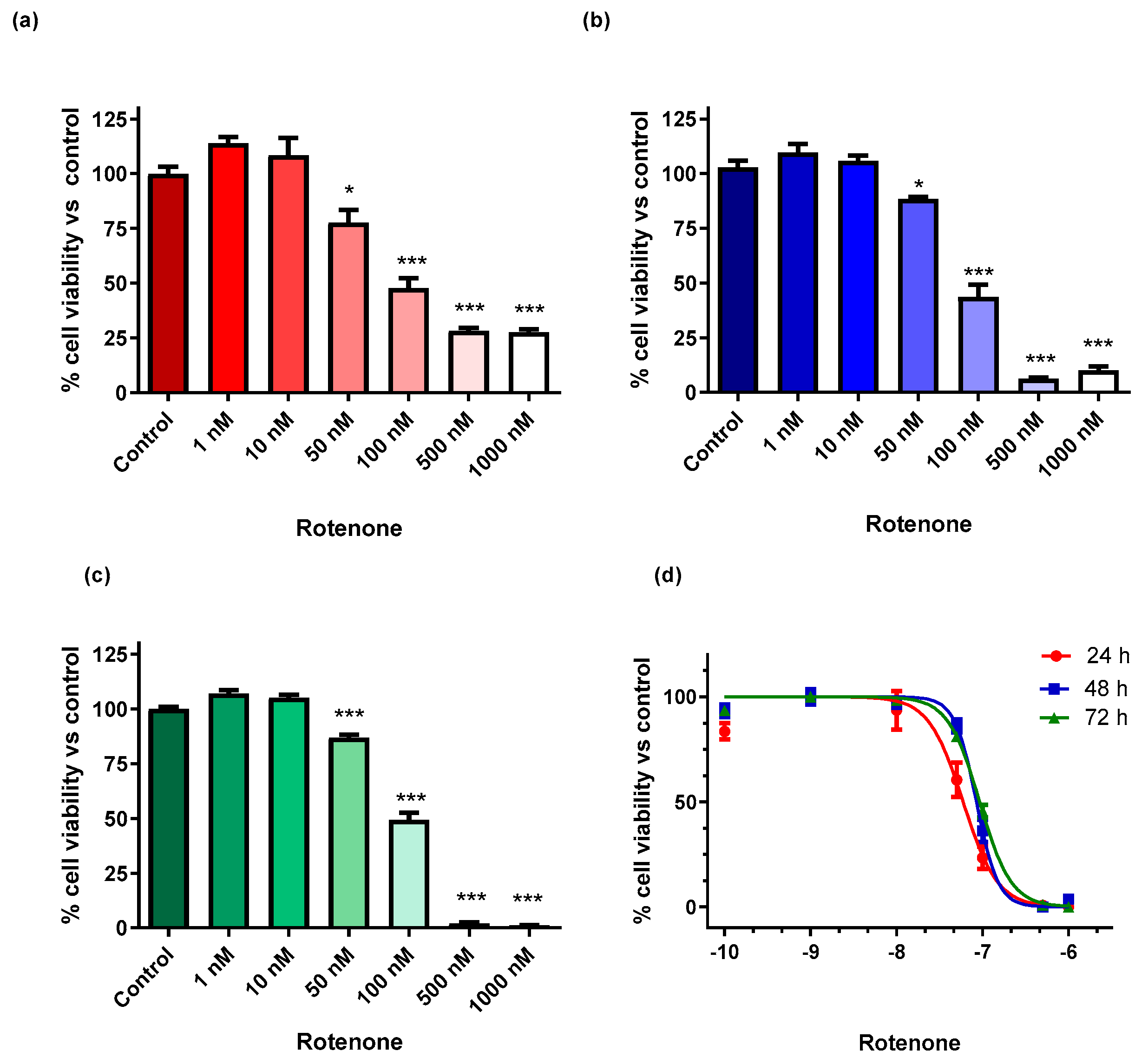
2.3. Viability of BV-2 Microglial Cells Exposed to Rotenone
2.4. Viability Evaluation of BV-2 Cells for Neurosteroids Dosage Normalization
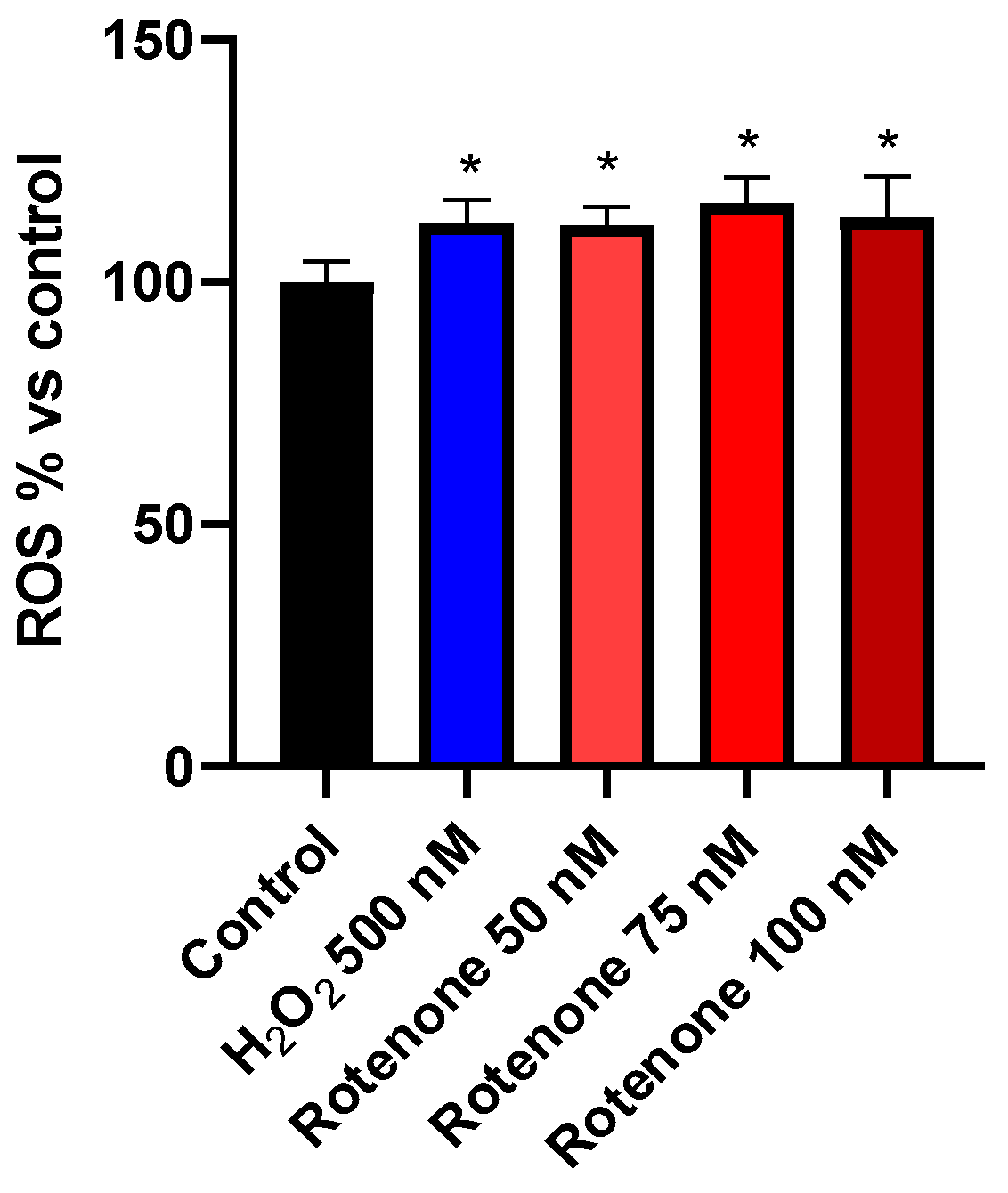
2.5. Determination of ROS
2.6. Sample Processing
2.7. Working Solutions and Calibrators
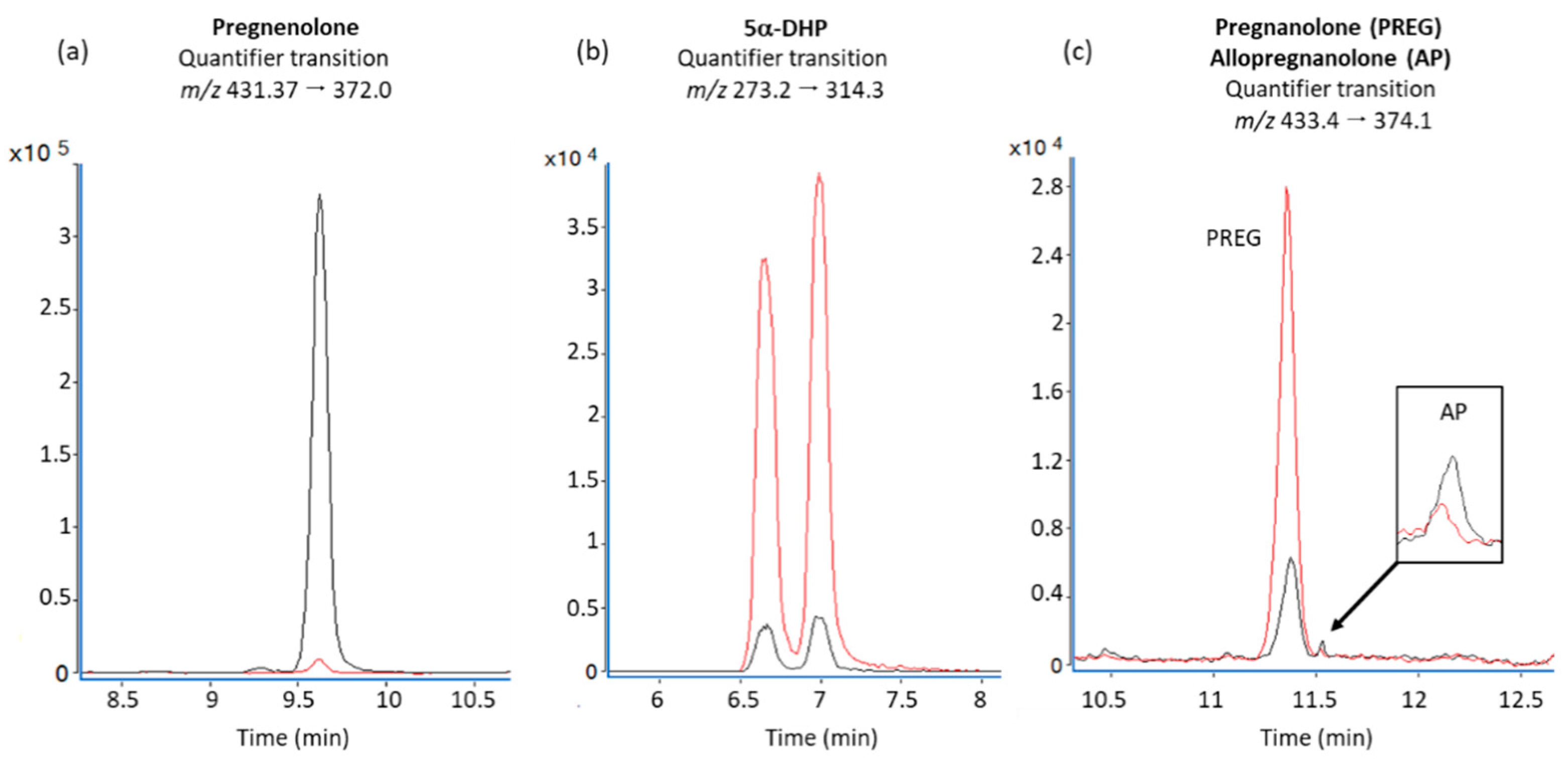
2.8. LC-MS/MS Conditions
2.9. Calibration Curves and Method Validation
2.10. Statistical Analysis
3. Results
3.1. Viability of BV-2 Cells Exposed to Rotenone
3.2. Determination of ROS
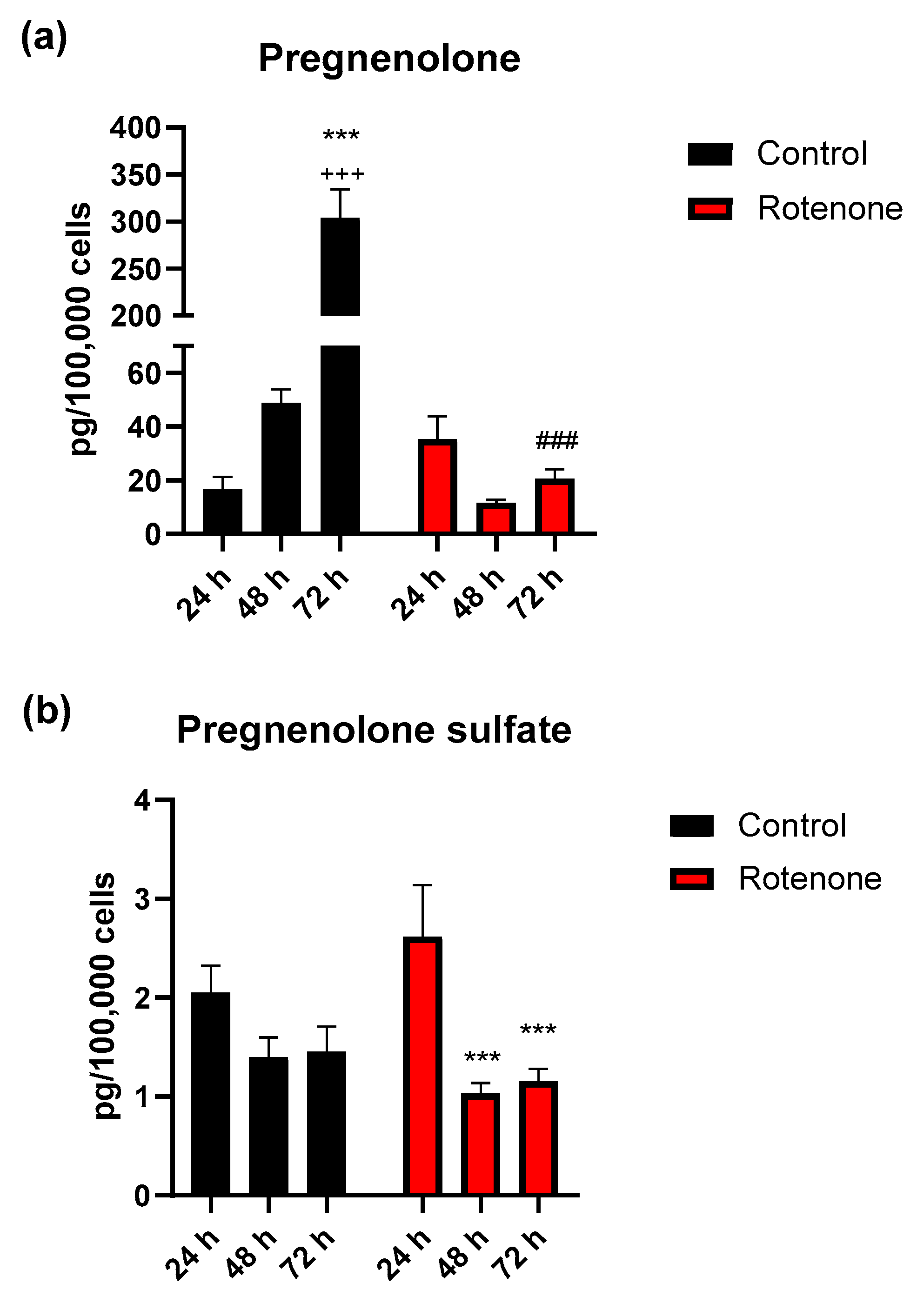
3.3. Effect of Rotenone Exposure on Neurosteroid Levels in BV-2 Cell Medium
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2017, 18, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Troncoso-Escudero, P.; Parra, A.; Nassif, M.; Vidal, R.L. Outside in: Unraveling the Role of Neuroinflammation in the Progression of Parkinson’s Disease. Front. Neurol. 2018, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms Underlying Inflammation in Neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Janssen, B.; Vugts, D.J.; Windhorst, A.D.; Mach, R.H. PET Imaging of Microglial Activation—Beyond Targeting TSPO. Molecules 2018, 23, 607. [Google Scholar] [CrossRef]
- Arcuri, C.; Mecca, C.; Bianchi, R.; Giambanco, I.; Donato, R. The Pathophysiological Role of Microglia in Dynamic Surveillance, Phagocytosis and Structural Remodeling of the Developing CNS. Front. Mol. Neurosci. 2017, 10, 10. [Google Scholar] [CrossRef]
- Sominsky, L.; De Luca, S.N.; Spencer, S.J. Microglia: Key players in neurodevelopment and neuronal plasticity. Int. J. Biochem. Cell Boil. 2018, 94, 56–60. [Google Scholar] [CrossRef]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017, 169, 1276–1290.e17. [Google Scholar] [CrossRef]
- Friedman, B.; Srinivasan, K.; Ayalon, G.; Meilandt, W.J.; Lin, H.; Huntley, M.A.; Cao, Y.; Lee, S.-H.; Haddick, P.C.; Ngu, H.; et al. Diverse Brain Myeloid Expression Profiles Reveal Distinct Microglial Activation States and Aspects of Alzheimer’s Disease Not Evident in Mouse Models. Cell Rep. 2018, 22, 832–847. [Google Scholar] [CrossRef]
- Krasemann, S.; Madore, C.; Cialic, R.; Baufeld, C.; Calcagno, N.; El Fatimy, R.; Beckers, L.; O’Loughlin, E.; Xu, Y.; Fanek, Z.; et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunology 2017, 47, 566–581.e9. [Google Scholar] [CrossRef] [PubMed]
- Butovsky, O.; Weiner, H.L. Microglial signatures and their role in health and disease. Nat. Rev. Neurosci. 2018, 19, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Joers, V.; Tansey, M.G.; Mulas, G.; Carta, A.R. Microglial phenotypes in Parkinson’s disease and animal models of the disease. Prog. Neurobiol. 2016, 155, 57–75. [Google Scholar] [CrossRef] [PubMed]
- Dani, M.; Wood, M.; Mizoguchi, R.; Fan, Z.; Walker, Z.; Morgan, R.; Hinz, R.; Biju, M.; Kuruvilla, T.; Brooks, D.J.; et al. Microglial activation correlates in vivo with both tau and amyloid in Alzheimer’s disease. Brain 2018. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell Boil. 2017, 217, 459–472. [Google Scholar] [CrossRef]
- Bogie, J.F.J.; Stinissen, P.; Hendriks, J.J.A. Macrophage subsets and microglia in multiple sclerosis. Acta Neuropathol. 2014, 128, 191–213. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2015, 53, 1181–1194. [Google Scholar] [CrossRef]
- Di Michele, F.; Longone, P.; Romeo, E.; Lucchetti, S.; Brusa, L.; Pierantozzi, M.; Bassi, A.; Bernardi, G.; Stanzione, P. Decreased plasma and cerebrospinal fluid content of neuroactive steroids in Parkinson’s disease. Neurol. Sci. 2003, 24, 172–173. [Google Scholar] [CrossRef]
- Irwin, R.W.; Solinsky, C.M.; Brinton, R.D. Frontiers in therapeutic development of allopregnanolone for Alzheimer’s disease and other neurological disorders. Front. Cell. Neurosci. 2014, 8, 203. [Google Scholar] [CrossRef]
- Orefice, N.; Carotenuto, A.; Mangone, G.; Bues, B.; Rehm, R.; Cerillo, I.; Saccà, F.; Calignano, A.; Orefice, G.; Orefice, N. Assessment of neuroactive steroids in cerebrospinal fluid comparing acute relapse and stable disease in relapsing-remitting multiple sclerosis. J. Steroid Biochem. Mol. Boil. 2016, 159, 1–7. [Google Scholar] [CrossRef]
- Rone, M.B.; Fan, J.; Papadopoulos, V. Cholesterol transport in steroid biosynthesis: Role of protein–protein interactions and implications in disease states. Biochim. et Biophys. Acta (BBA) - Mol. Cell Boil. Lipids 2009, 1791, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Giatti, S.; Boraso, M.; Melcangi, R.; Viviani, B. Neuroactive steroids, their metabolites, and neuroinflammation. J. Mol. Endocrinol. 2012, 49, R125–R134. [Google Scholar] [CrossRef] [PubMed]
- Gottfried-Blackmore, A.; Sierra, A.; Jellinck, P.H.; McEwen, B.S.; Bulloch, K. Brain microglia express steroid-converting enzymes in the mouse. J. Steroid Biochem. Mol. Boil. 2008, 109, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Zwain, I.; Yen, S.S.C. Neurosteroidogenesis in Astrocytes, Oligodendrocytes, and Neurons of Cerebral Cortex of Rat Brain. Endocrinology 1999, 140, 3843–3852. [Google Scholar] [CrossRef]
- Compagnone, N.A.; Mellon, S.H. Neurosteroids: Biosynthesis and Function of These Novel Neuromodulators. Front. Neuroendocr. 2000, 21, 1–56. [Google Scholar] [CrossRef]
- Pelletier, G. Steroidogenic Enzymes in the Brain: Morphological Aspects. New Perspect. Early Soc.-Cogn. Dev. 2010, 181, 193–207. [Google Scholar] [CrossRef]
- Yilmaz, C.; Karali, K.; Fodelianaki, G.; Gravanis, A.; Chavakis, T.; Charalampopoulos, I.; Alexaki, V.I. Neurosteroids as regulators of neuroinflammation. Front. Neuroendocr. 2019, 55, 100788. [Google Scholar] [CrossRef]
- Bader, S.; Wolf, L.; Milenkovic, V.M.; Gruber, M.; Nothdurfter, C.; Rupprecht, R.; Wetzel, C.H. Differential effects of TSPO ligands on mitochondrial function in mouse microglia cells. Psychoneuroendocrinology 2019, 106, 65–76. [Google Scholar] [CrossRef]
- Blasi, E.; Barluzzi, R.; Bocchini, V.; Mazzolla, R.; Bistoni, F. Immortalization of murine microglial cells by a v-raf / v-myc carrying retrovirus. J. Neuroimmunol. 1990, 27, 229–237. [Google Scholar] [CrossRef]
- Wolf, L.; Bauer, A.; Melchner, D.; Hallof-Buestrich, H.; Stoertebecker, P.; Haen, E.; Kreutz, M.; Sarubin, N.; Milenkovic, V.; Wetzel, C.H.; et al. Enhancing Neurosteroid Synthesis – Relationship to the Pharmacology of Translocator Protein (18 kDa) (TSPO) Ligands and Benzodiazepines. Pharmacopsychiatry 2015, 48, 72–77. [Google Scholar] [CrossRef]
- Henn, A. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX 2009, 26, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, K.; Fiddler, J.; Soh, T.; Clarke, S. BV-2 Microglial Cells Used in a Model of Neuroinflammation. FASEB J. 2015, 29 (Suppl. 1), 608.2. [Google Scholar] [CrossRef]
- Corsi, L.; Dongmo, B.M.; Avallone, R. Supplementation of omega 3 fatty acids improves oxidative stress in activated BV2 microglial cell line. Int. J. Food Sci. Nutr. 2015, 66, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Stansley, B.; Post, J.; Hensley, K. A comparative review of cell culture systems for the study of microglial biology in Alzheimer’s disease. J. Neuroinflamm. 2012, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- ICH Harmonised Guideline: Bioanalytical Method Validation M10. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-ich-guideline-m10-bioanalytical-method-validation-step-2b_en.pdf (accessed on 21 August 2020).
- Rustichelli, C.; Pinetti, D.; Lucchi, C.; Ravazzini, F.; Puia, G. Simultaneous determination of pregnenolone sulphate, dehydroepiandrosterone and allopregnanolone in rat brain areas by liquid chromatography–electrospray tandem mass spectrometry. J. Chromatogr. B 2013, 930, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Meletti, S.; Lucchi, C.; Monti, G.; Giovannini, G.; Bedin, R.; Trenti, T.; Rustichelli, C.; Biagini, G. Decreased allopregnanolone levels in cerebrospinal fluid obtained during status epilepticus. Epilepsia 2016, 58, e16–e20. [Google Scholar] [CrossRef]
- Biagini, G.; Longo, D.; Baldelli, E.; Zoli, M.; Rogawski, M.A.; Bertazzoni, G.; Avoli, M. Neurosteroids and epileptogenesis in the pilocarpine model: Evidence for a relationship between P450scc induction and length of the latent period. Epilepsia 2009, 50, 53–58. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.-S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Ghadery, C.; Koshimori, Y.; Coakeley, S.; Harris, M.; Rusjan, P.; Kim, J.; Houle, S.; Strafella, A.P. Microglial activation in Parkinson’s disease using [18F]-FEPPA. J. Neuroinflamm. 2017, 14, 8. [Google Scholar] [CrossRef]
- Guilarte, T.R. TSPO in diverse CNS pathologies and psychiatric disease: A critical review and a way forward. Pharmacol. Ther. 2019, 194, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Beckers, L.; Ory, D.; Geric, I.; Declercq, L.; Koole, M.; Kassiou, M.; Bormans, G.; Baes, M. Increased Expression of Translocator Protein (TSPO) Marks Pro-inflammatory Microglia but Does Not Predict Neurodegeneration. Mol. Imaging Boil. 2017, 20, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Gavish, M.; Veenman, L. Regulation of Mitochondrial, Cellular, and Organismal Functions by TSPO. Sci. Technol. Catal. 2006 2018, 82, 103–136. [Google Scholar] [CrossRef]
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapere, J.-J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.-R.; et al. Translocator protein (18 kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef]
- Papadopoulos, V.; Fan, J.; Zirkin, B. Translocator protein (18 kDa): An update on its function in steroidogenesis. J. Neuroendocr. 2018, 30, e12500. [Google Scholar] [CrossRef] [PubMed]
- Di Michele, F.; Luchetti, S.; Bernardi, G.; Romeo, E.; Longone, P. Neurosteroid and neurotransmitter alterations in Parkinson’s disease. Front. Neuroendocr. 2013, 34, 132–142. [Google Scholar] [CrossRef]
- Luchetti, S.; Huitinga, I.; Swaab, D. Neurosteroid and GABA-A receptor alterations in Alzheimer’s disease, Parkinson’s disease and multiple sclerosis. Neuroscience 2011, 191, 6–21. [Google Scholar] [CrossRef]
- Noorbakhsh, F.; Baker, G.B.; Power, C. Allopregnanolone and neuroinflammation: A focus on multiple sclerosis. Front. Cell. Neurosci. 2014, 8, 134. [Google Scholar] [CrossRef]
- Biagini, G.; Rustichelli, C.; Curia, G.; Vinet, J.; Lucchi, C.; Pugnaghi, M.; Meletti, S. Neurosteroids and Epileptogenesis. J. Neuroendocr. 2013, 25, 980–990. [Google Scholar] [CrossRef]
- Guennoun, R.; Labombarda, F.; Deniselle, M.G.; Liere, P.; De Nicola, A.; Schumacher, M. Progesterone and allopregnanolone in the central nervous system: Response to injury and implication for neuroprotection. J. Steroid Biochem. Mol. Boil. 2015, 146, 48–61. [Google Scholar] [CrossRef]
- Liang, J.J.; Rasmusson, A.M. Overview of the Molecular Steps in Steroidogenesis of the GABAergic Neurosteroids Allopregnanolone and Pregnanolone. Chronic Stress 2018, 2, 2470547018818555. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Su, C.; Ng, S. Non-genomic mechanisms of progesterone action in the brain. Front. Mol. Neurosci. 2013, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.J.; Belelli, D.; Peden, D.R.; Vardy, A.W.; Peters, J.A. Neurosteroid modulation of GABAA receptors. Prog. Neurobiol. 2003, 71, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Schwab, C.; McGeer, P.L. Astrocytes are GABAergic cells that modulate microglial activity. Glia 2010, 59, 152–165. [Google Scholar] [CrossRef]
- Tsutsui, K.; Haraguchi, S. Neuroprotective actions of cerebellar and pineal allopregnanolone on Purkinje cells. FASEB BioAdv. 2020, 2, 149–159. [Google Scholar] [CrossRef]
- Balan, I.; Beattie, M.C.; O’Buckley, T.K.; Aurelian, L.; Morrow, A.L. Endogenous Neurosteroid (3α,5α)3-Hydroxypregnan-20-one Inhibits Toll-like-4 Receptor Activation and Pro-inflammatory Signaling in Macrophages and Brain. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Orihuela, R.; A McPherson, C.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2015, 173, 649–665. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avallone, R.; Lucchi, C.; Puja, G.; Codeluppi, A.; Filaferro, M.; Vitale, G.; Rustichelli, C.; Biagini, G. BV-2 Microglial Cells Respond to Rotenone Toxic Insult by Modifying Pregnenolone, 5α-Dihydroprogesterone and Pregnanolone Levels. Cells 2020, 9, 2091. https://doi.org/10.3390/cells9092091
Avallone R, Lucchi C, Puja G, Codeluppi A, Filaferro M, Vitale G, Rustichelli C, Biagini G. BV-2 Microglial Cells Respond to Rotenone Toxic Insult by Modifying Pregnenolone, 5α-Dihydroprogesterone and Pregnanolone Levels. Cells. 2020; 9(9):2091. https://doi.org/10.3390/cells9092091
Chicago/Turabian StyleAvallone, Rossella, Chiara Lucchi, Giulia Puja, Alessandro Codeluppi, Monica Filaferro, Giovanni Vitale, Cecilia Rustichelli, and Giuseppe Biagini. 2020. "BV-2 Microglial Cells Respond to Rotenone Toxic Insult by Modifying Pregnenolone, 5α-Dihydroprogesterone and Pregnanolone Levels" Cells 9, no. 9: 2091. https://doi.org/10.3390/cells9092091
APA StyleAvallone, R., Lucchi, C., Puja, G., Codeluppi, A., Filaferro, M., Vitale, G., Rustichelli, C., & Biagini, G. (2020). BV-2 Microglial Cells Respond to Rotenone Toxic Insult by Modifying Pregnenolone, 5α-Dihydroprogesterone and Pregnanolone Levels. Cells, 9(9), 2091. https://doi.org/10.3390/cells9092091






