The Amazonian Red Side-Necked Turtle Rhinemys rufipes (Spix, 1824) (Testudines, Chelidae) Has a GSD Sex-Determining Mechanism with an Ancient XY Sex Microchromosome System
Abstract
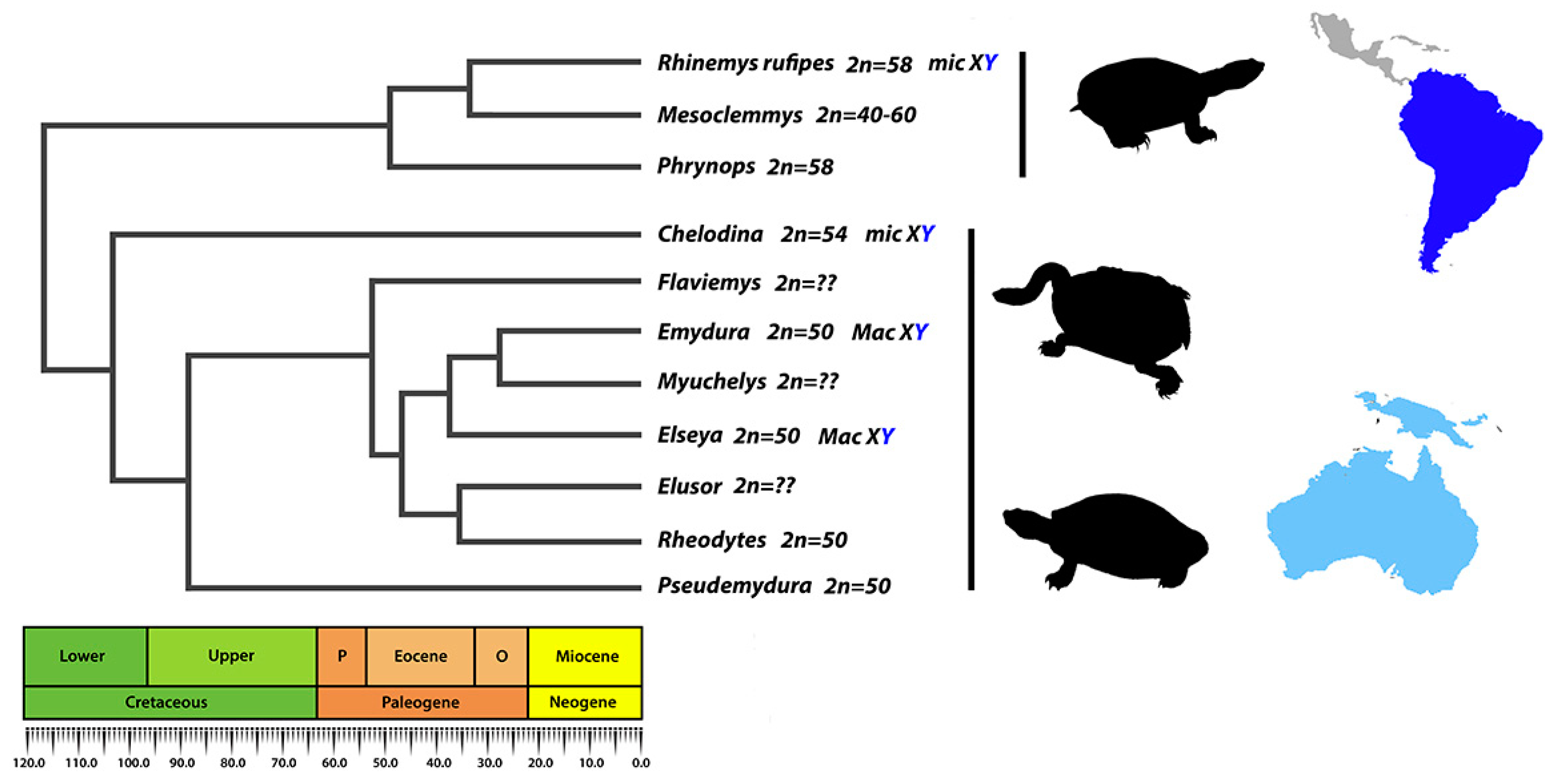
1. Introduction
2. Material and Methods
2.1. Sampling, Mitotic Chromosomes Preparation, and C-Banding
2.2. Probes for Chromosome Hybridization
2.3. Fluorescence In Situ Hybridization (FISH) for Repetitive DNA Mapping
2.4. Preparation of Probes for Comparative Genomic Hybridization (CGH)
2.5. Comparative Genomic Hybridization (CGH)
2.6. Microscopic Analyses
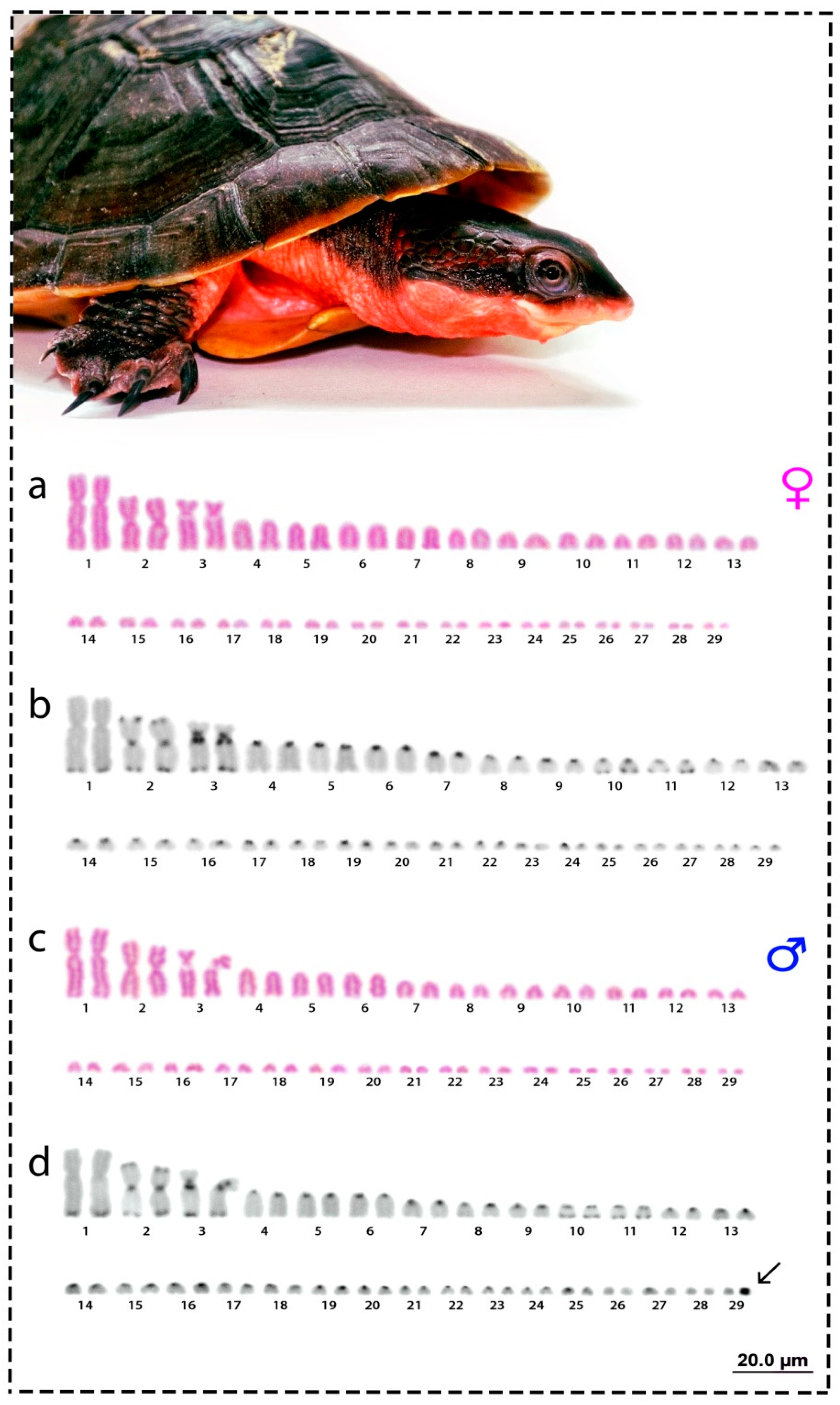
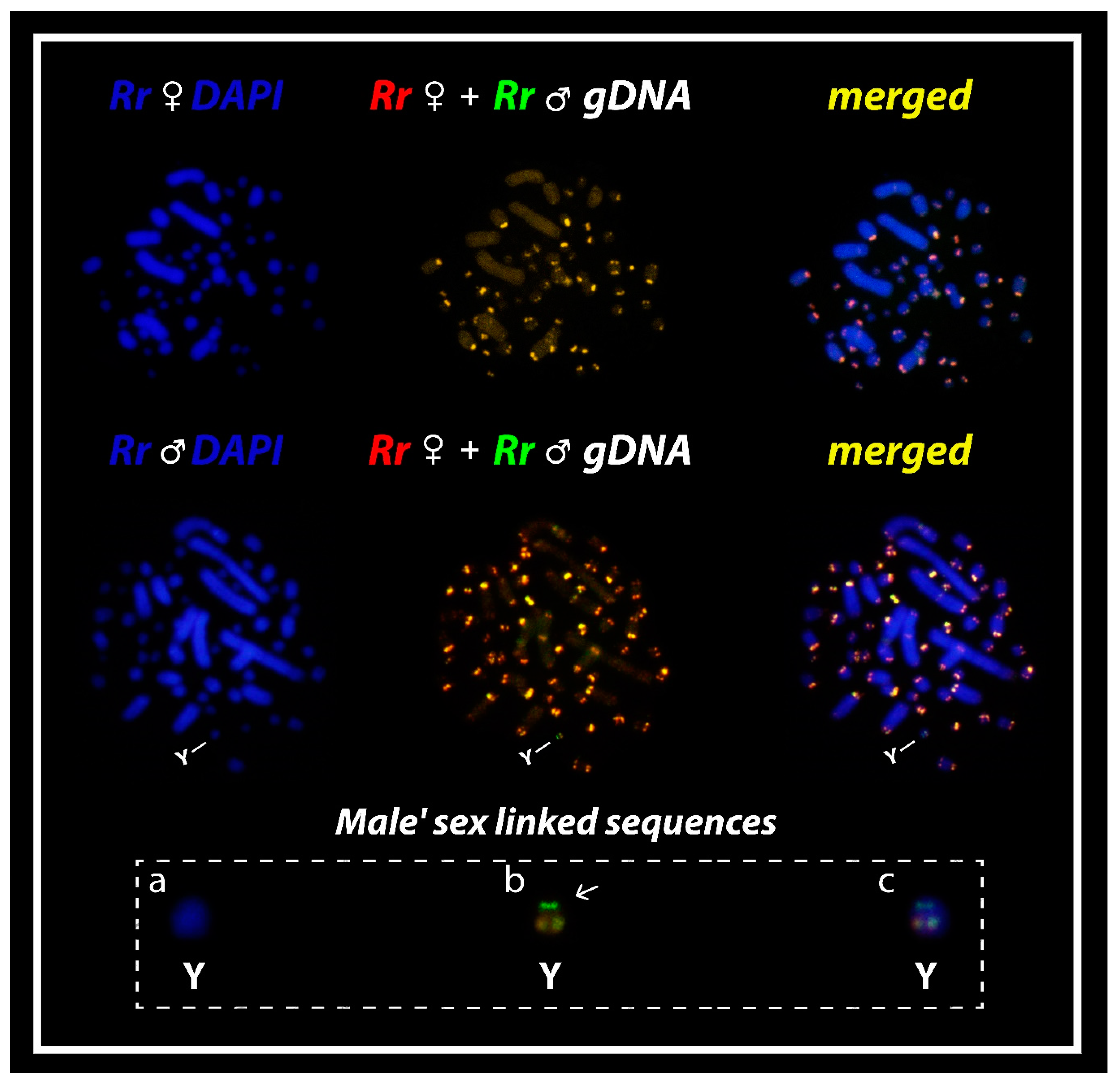
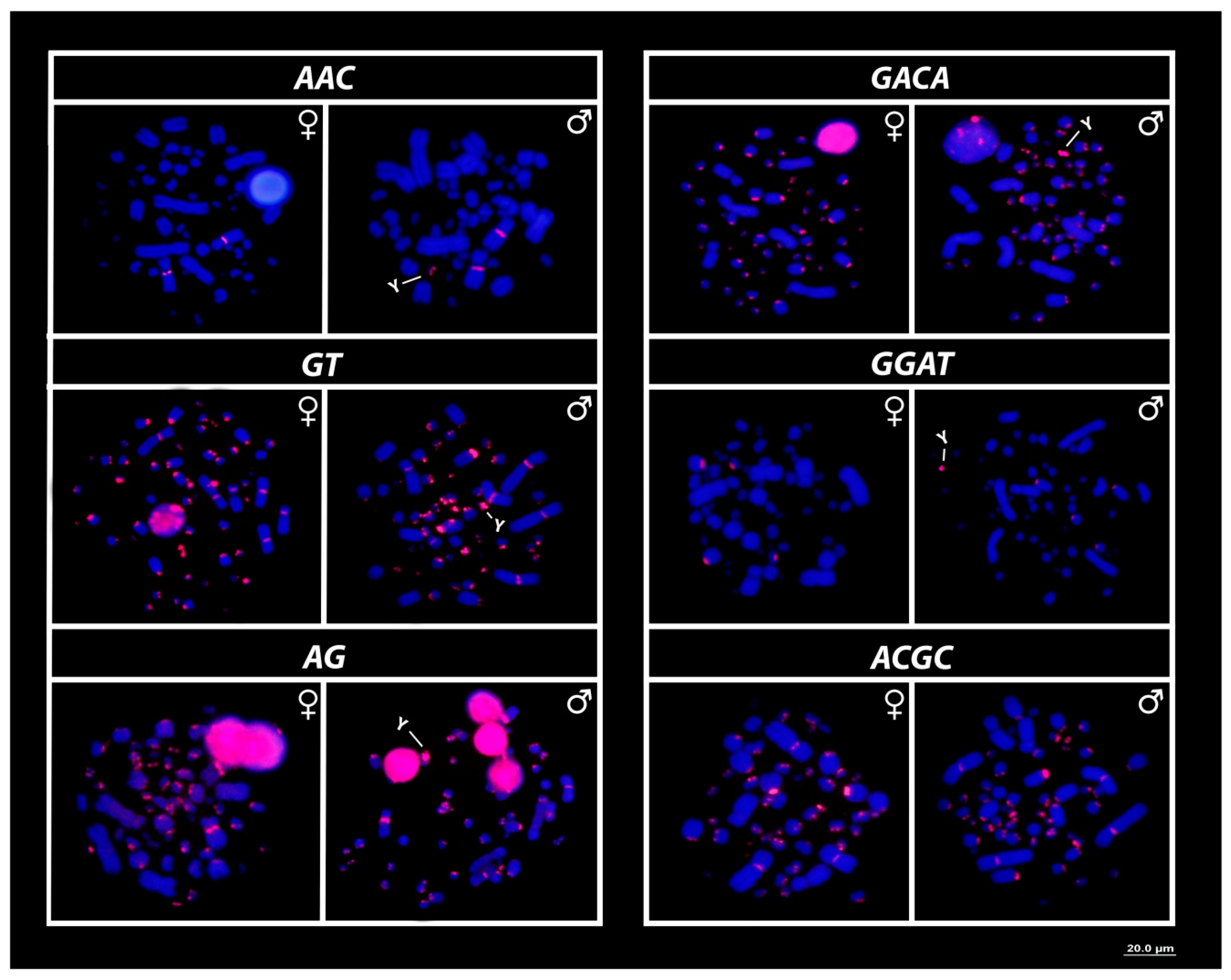
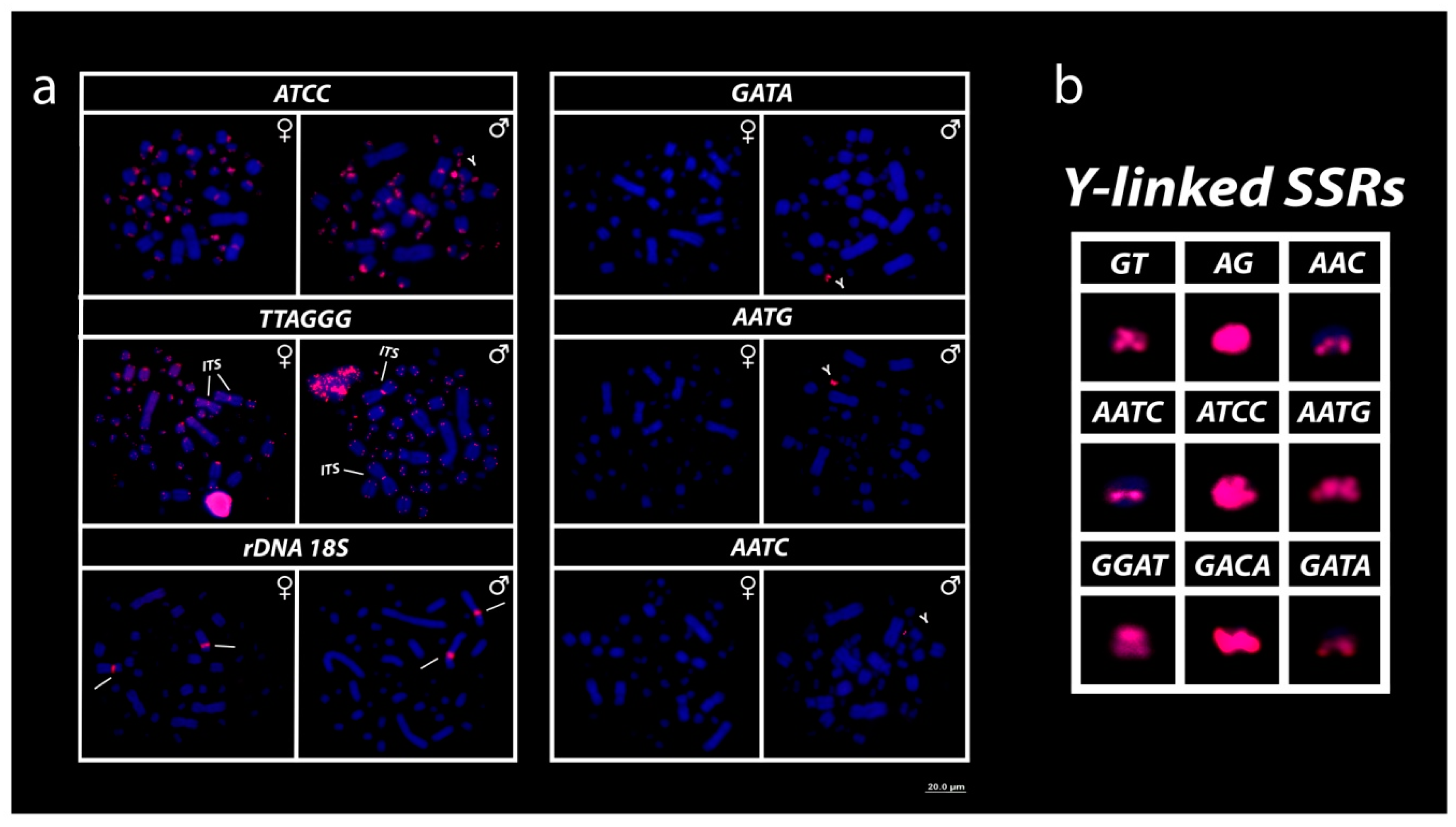
3. Results
3.1. Karyotype and C-Positive Heterochromatin
3.2. Comparative Genomic Hybridization (CGH)
3.3. Mapping of 18S rDNA, Telomeric Repeats and SSRs Motifs
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ernst, C.H.E.; Barbour, R.W. Turtles of the World, 1st ed.; Smithsonian Institution Press: Washington, DC, USA, 1989; p. 313. [Google Scholar]
- Ferreira, G.S.; Bronzati, M.; Langer, M.C.; Sterli, J. Phylogeny, biogeography and diversification patterns of side-necked turtles (Testudines: Pleurodira). R. Soc. Open Sci. 2018, 5, 171773. [Google Scholar] [CrossRef] [PubMed]
- The Reptile Database. Available online: http://www.reptile-database.org (accessed on 22 May 2020).
- Bull, J.J.; Legler, J.M. Karyotypes of side-necked turtles (Testudines: Pleurodira). Can. J. Zool. 2018, 58, 828–841. [Google Scholar] [CrossRef]
- McBee, K.; Bickham, J.W.; Rhodin, A.G.J.; Mittermeier, R.A. Karyotipic variation in the genus Platemys (Testudines: Pleurodira). Copeia 1985, 2, 445–449. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Augstenová, B.; Clemente, L.; Auer, M.; Fritz, U.; Praschag, P.; Protiva, T.; Velenský, P.; Kratochvíl, L.; Rovatsos, M. Sex is determined by XX/XY sex chromosomes in Australasian side-necked turtles (Testudines: Chelidae). Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Viana, P.F.; Ribeiro, L.B.; Lima, T.; de Carvalho, V.T.; Vogt, R.C.; Gross, M.C.; Feldberg, E. An optimized protocol for obtaining mitotic chromosomes from cultured reptilian lymphocytes. Nucleus 2016, 59, 191–195. [Google Scholar] [CrossRef]
- Cavalcante, M.G.; Bastos, C.E.M.C.; Nagamachi, C.Y.; Pieczarka, J.C.; Vicari, M.R.; Noronha, R.C.R. Physical mapping of repetitive DNA suggests 2n reduction in Amazon turtles Podocnemis (Testudines: Podocnemididae). PLoS ONE 2018, 13, e0197536. [Google Scholar] [CrossRef]
- Ezaz, T.; Valenzuela, N.; Grützner, F.; Miura, I.; Georges, A.; Burke, R.L.; Graves, J.A.M. An XX/XY sex microchromosome system in a freshwater turtle, Chelodina longicollis (Testudines: Chelidae) with genetic sex determination. Chromosome Res. 2006, 14, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Martinez, P.A.; Ezaz, T.; Valenzuela, N.; Georges, A.; Graves, J.A.M. An XX/XY heteromorphic sex chromosome system in the Australian chelid turtle Emydura macquarii: A new piece in the puzzle of sex chromosome evolution in turtles. Chromosome Res. 2008, 16, 815–825. [Google Scholar] [CrossRef]
- Viana, P.F. The puzzle of karyotype evolution in Neotropical chelids. Status (manuscript in preparation).
- Magnusson, W.E.; Lima, A.C.; Costa, V.L.; Vogt, R.C. Home range of the turtle, Phrynops rufipes, in an isolated reserve in central Amazônia, Brazil. Chelonian Conserv. Biol. 1997, 2, 494–499. [Google Scholar]
- Magnusson, W.E.; Lima, A.C.; Costa, V.L.; Lima, O.P. Growth of the turtle, Phrynops rufipes, in central Amazônia, Brazil. Chelonian Conserv. Biol. 1997, 2, 576–581. [Google Scholar]
- Lamar, W.W.; Mendem, F. Notes on the chelid turtle Phrynops rufipes in Colombia. Salamandra 1984, 18, 305–321. [Google Scholar]
- Rueda-Almonacid, J.V.; Carr, J.L.; Mittermeier, R.A.; Rodríguez-Mahecha, J.V.; Mast, R.B.; Vogt, R.C.; Rhodin, A.G.J.; De La Ossa-Velásquez, J.; Rueda, J.N.; Mittermeier, C.G. Las Tortugas y los Cocodrilianos de los Países Andinos del Trópico, 1st ed.; Conservación Internacional: Bogotá, Colombia, 2007; p. 538. [Google Scholar]
- Páez, V.P.; Morales-Betancourt, M.A.; Lasso, C.A.; Castaño-Mora, O.V.; Bock, B.C. Biología y Conservación de las Tortugas Continentales de Colombia, 1st ed.; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2012; p. 528. [Google Scholar]
- Alvarenga, C.C.E. Aspectos de Biologia Reprodutiva de Rhinemys rufipes (Spix, 1824) (Chelidae, Testudines) na Reserva Florestal Adolpho Ducke, Amazonas, Brasil. Master’s Thesis, Universidade Federal do Amazonas, Manaus, Brazil, 2006. [Google Scholar]
- Sanchez, D.E.A. Home range of the turtle, Phrynops rufipes, in an isolated reserve in central Amazônia, Brazil. Master’s Thesis, Universidade Federal do Amazonas, Manaus, Brazil, 2008. [Google Scholar]
- Vogt, R.C. Tartarugas da Amazônia, 1st ed.; Instituto Nacional de Pesquisas da Amazônia: Manaus, Brazil, 2008; p. 104. [Google Scholar]
- Ferrara, C.R.; Fagundes, C.K.; Morcatty, T.Q.; Vogt, R.C. Quelônios Amazônicos: Guia de Identificação e Distribuição, 1st ed.; Wildlife Conservation Society: Manaus, Brazil, 2017; p. 182. [Google Scholar]
- Ewert, M.A.; Etcheberger, C.R.; Nelson, C.E. Turtle sex-determination modes and TSD patterns, and some TSD patterns correlates. In Temperature-Dependent Sex Determination in Vertebrates, 1st ed.; Valenzuela, N., Lance, V.A., Eds.; Smithsonian Books: Washington, DC, USA, 2004; Volume 1, pp. 21–32. [Google Scholar]
- Ferreira-Júnior, P.D. Aspectos Ecológicos da Determinação Sexual em Tartarugas. Acta Amaz. 2009, 39, 139–154. [Google Scholar] [CrossRef]
- Vogt, R.C. Ecologia reprodutiva de uma comunidade de quelônios da Amazônia. In Proceedings of the Congresso Latino-Americano de Zoologia, Belém, Brazil, 26–31 July 1992; p. 131. [Google Scholar]
- Cunha, F.A.; Fernandes, T.; Franco, J.; Vogt, R.C. Reproductive Biology and Hatchling Morphology of the Amazon Toad-headed Turtle (Mesoclemmys raniceps) (Testudines: Chelidae), with Notes on Species Morphology and Taxonomy of the Mesoclemmys Group. Chelonian Conserv. Biol. 2019, 18, 195–209. [Google Scholar] [CrossRef]
- Bickham, J.W.; Hanks, B.G. Diploid-triploid mosaicism and tissue ploidy diversity within Platemys platycephala from Suriname. Cytogenet Genome. Res. 2009, 127, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.M.; Sampaio, M.M.; Assis, M.F.; Ayres, M.; Cunha, O.R. General considerations on the karyotypic evolution of Chelonia from the Amazon region of Brazil. Cytologia. 1976, 41, 559–565. [Google Scholar] [CrossRef]
- Bickham, J.W.; Tucker, P.K.; Legler, J.M. Diploid-triploid mosaicism: An unusual phenomenon in side-necked turtles (Platemys platycephala). Science 1985, 227, 1591–1593. [Google Scholar] [CrossRef]
- Sumner, A.T. A simple technique for demonstrating centromeric heterochromatin. Exp. Cell Res. 1972, 75, 204–206. [Google Scholar] [CrossRef]
- Gross, M.C.; Schneider, C.H.; Valente, G.T.; Porto, J.I.R.; Martins, C.; Feldberg, E. Variability of 18S rDNA locus among Symphysodon fishes: Chromosomal rearrangements. J. Fish. Biol. 2009, 76, 1117–1127. [Google Scholar] [CrossRef]
- Ijdo, J.W.; Wells, R.A.; Baldini, A.; Reeders, S.T. Improved telomere detection using a telomere repeat probe (TTAGGG)n generated by PCR. Nucleic Acids Res. 1991, 19, 4780. [Google Scholar] [CrossRef]
- Kubat, Z.; Hobza, R.; Vyskot, B.; Kejnovsky, E. Microsatellite accumulation in the Y chromosome of Silene latifolia. Genome 2008, 51, 350–356. [Google Scholar] [CrossRef]
- Pinkel, D.; Straume, T.; Gray, J.W. Cytogenetic analysis using quantitative, high sensitivity, fluorescence hybridization. Proc. Natl. Acad. Sci. USA 1986, 83, 2934–2938. [Google Scholar] [CrossRef] [PubMed]
- Viana, P.F.; Ribeiro, L.B.; Souza, G.M.; Chalkidis, H.M.; Gross, M.C.; Feldberg, E. Is the Karyotype of Neotropical Boid Snakes Really Conserved? Cytotaxonomy, Chromosomal Rearrangements and Karyotype Organization in the Boidae Family. PLoS ONE 2016, 11, e0160274. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zwick, M.S.; Hanson, R.E.; Mcknight, T.D.; Islam-Faridi, M.H.; Stelly, D.M.; Wing, R.A.; Price, H.J. A rapid procedure for the isolation of C0t-1 DNA from plants. Genome 1997, 40, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Viana, P.F.; Ezaz, T.; Cioffi, M.B.; Almeida, B.J.; Feldberg, E. Evolutionary Insights of the ZW Sex Chromosomes in Snakes: A New Chapter Added by the Amazonian Puffing Snakes of the Genus Spilotes. Genes 2019, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.B.; Ráb, P.; Ezaz, T.; Bertollo, L.A.C.; Lavoué, S.; de Oliveira, E.A.; Sember, A.; Molina, W.F.; Souza, F.H.S.; Majtánová, Z.; et al. Deciphering the Evolutionary History of Arowana Fishes (Teleostei, Osteoglossiformes, Osteoglossidae): Insight from Comparative Cytogenomics. Int. J. Mol. Sci. 2019, 20, 4296. [Google Scholar] [CrossRef]
- Viana, P.F.; Ezaz, T.; Cioffi, M.B.; Liehr, T.; Al-Rikabi, A.; Goll, L.G.; Rocha, A.M.; Feldberg, E. Landscape of snake’ sex chromosomes evolution spanning 85 MYR reveals ancestry of sequences despite distinct evolutionary trajectories. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Sassi, F.D.; Hatanaka, T.; Moraes, R.L.R.D.; Toma, G.A.; Oliveira, E.A.D.; Liehr, T.; Rab, P.; Bertollo, L.A.C.; Viana, P.F.; Feldberg, E.; et al. An Insight into the Chromosomal Evolution of Lebiasinidae (Teleostei, Characiformes). Genes 2020, 11, 365. [Google Scholar] [CrossRef]
- Levan, A.; Fredga, K.; Sandberg, A.A. Nomenclature for centromeric position on chromosomes. Hereditas 1964, 52, 201–220. [Google Scholar] [CrossRef]
- Guillon, J.-M.; Guéry, L.; Hulin, V.; Girondot, M. A large phylogeny of turtles (Testudines) using molecular data. Contrib. Zool. 2012, 81, 147–158. [Google Scholar] [CrossRef]
- Crawford, N.G.; Parham, J.F.; Sellas, A.B.; Faircloth, B.C.; Glenn, T.C.; Papenfuss, T.J.; Henderson, J.B.; Hansen, M.H.; Simison, W.B. A phylogenomic analysis of turtles. Mol. Phylogenet. Evol. 2015, 83, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.G.; Sterli, J.; Moreira, F.R.; Schrago, C.G. Multilocus phylogeny and statistical biogeography clarify the evolutionary history of major lineages of turtles. Mol. Phylogenet. Evol. 2017, 113, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Colston, T.J.; Kulkarni, P.; Jetz, W.; Pyron, R.A. Phylogenetic and Spatial Distribution of Evolutionary Isolation and Threat in Turtles and Crocodilians (Non-Avian Archosauromorphs). BMC Evol. Biol. 2019, 20. [Google Scholar]
- Ayres, M.; Sampaio, M.M.; Barros, R.M.S.; Dias, L.B.; Cunha, O.R. A karyological study of turtles from the Brazilian Amazon region. Cytogenetics 1969, 8, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Killebrew, F.C. Mitotic chromosomes of turtles: I. the Pelomedusidae. J. Herpetol. 1975, 9, 281–285. [Google Scholar] [CrossRef]
- De Smet, W.H. The chromosomes of 11 species of Chelonia. (REPTILIA). Acta Zool. Pathol. Antverp. 1978, 70, 15–34. [Google Scholar]
- Mittermeier, R.A.; Fedullo, L.P. Cytogenetic analysis of the pleurodine turtle Phrynops hogei and its taxonomic implications. Amphibia-Reptilia 1991, 12, 203–212. [Google Scholar]
- Valenzuela, N.; Adams, D.C.; Janzen, F.J. Pattern does not equal process: Exactly when is sex environmentally determined? Am. Nat. 2003, 161, 676–683. [Google Scholar] [CrossRef]
- Valenzuela, N.; Adams, D.C. Chromosome number and sex determination coevolve in turtles. Evolution 2011, 65, 1808–1813. [Google Scholar] [CrossRef]
- Montiel, E.E.; Badenhorst, D.; Tamplin, J.; Burke, R.L.; Valenzuela, N. Discovery of the youngest sex chromosomes reveals first case of convergent co-option of ancestral autosomes in turtles. Chromosoma 2017, 126, 105–113. [Google Scholar] [CrossRef]
- Lee, L.; Montiel, E.E.; Valenzuela, N. Discovery of putative XX/XY male heterogamety in Emydura subglobosa turtles exposes a novel trajectory of sex chromosome evolution in Emydura. Cytogenet Genome Res. 2019, 158, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Bista, B.; Valenzuela, N. Turtle Insights into the Evolution of the Reptilian Karyotype and the Genomic Architecture of Sex Determination. Genes 2020, 11, 416. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Montiel, E.E.; Navarro-Domínguez, B.M.; Valenzuela, N. Chromosomal rearrangements during turtle evolution altered the synteny of genes involved in vertebrate sex determination. Cytogenet Genome Res. 2019, 157, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.S.; Langer, M.C. A pelomedusoid (Testudines, Pleurodira) plastron from the lower Cretaceous of Alagoas, Brazil. Cretac. Res. 2013, 46, 267–271. [Google Scholar] [CrossRef]
- Romano, P.S.; Gallo, V.; Ramos, R.R.; Antonioli, L. Atolchelys lepida, a new side-necked turtle from the Early Cretaceous of Brazil and the age of crown Pleurodira. Biol. Lett. 2014, 10, 20140290. [Google Scholar] [CrossRef]
- de la Fuente, M.S.; Umazano, A.M.; Sterli, J.; Carballido, J.L. New chelid turtles of the lower section of the Cerro Barcino formation (Aptian-Albian?), Patagonia, Argentina. Cretac. Res. 2011, 32, 527–537. [Google Scholar] [CrossRef]
- Joyce, W.G. Origin, Evolution and Biogeographic History of South American Turtles. Ameghiniana 2014, 51, 81. [Google Scholar] [CrossRef]
- Shaffer, H.B.; McCartney-Melstad, E.; Near, T.J.; Mount, G.G.; Spinks, P.Q. Phylogenomic analyses of 539 highly informative loci dates a fully resolved time tree for the major clades of living turtles (Testudines). Mol. Phylogenet. Evol. 2017, 115, 7–15. [Google Scholar] [CrossRef]
- Matsubara, K.; O’Meally, D.; Azad, B.; Georges, A.; Sarre, S.D.; Graves, J.A.M.; Matsuda, Y.; Ezaz, T. Amplification of microsatellite repeat motifs is associated with the evolutionary differentiation and heterochromatinization of sex chromosomes in Sauropsida. Chromosoma 2016, 125, 111–123. [Google Scholar] [CrossRef]
- Richard, G.F.; Pâques, F. Mini- and microsatellite expansions: The recombination connection. EMBO Rep. 2000, 1, 122–126. [Google Scholar] [CrossRef]
- Martin, P.; Makepeace, K.; Hill, S.A.; Hood, D.W.; Moxon, E.R. Microsatellite instability regulates transcription factor binding and gene expression. Proc. Natl. Acad. Sci. USA 2005, 3800–3804. [Google Scholar] [CrossRef] [PubMed]
- Balaresque, P.; King, T.E.; Parkin, E.J.; Heyer, E.; Carvalho-Silva, D.; Kraaijenbrink, T.; de Knijff, P.; Tyler-Smith, C.; Jobling, M.A. Gene conversion violates the stepwise mutation model for microsatellites in Y-chromosomal palindromic repeats. Hum. Mutat. 2014, 35, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Figliuolo, V.S.P.; Goll, L.; Viana, P.F.; Feldberg, E.; Gross, M.C. First Record on Sex Chromosomes in a Species of the Family Cynodontidae: Cynodon gibbus (Agassiz, 1829). Cytogenet Genome Res. 2020, 160, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Alföldi, J.; Di Palma, F.; Grabherr, M.; Williams, C.; Kong, L.; Mauceli, E.; Russell, P.; Lowe, C.B.; Glor, R.E.; Jaffe, J.D.; et al. The genome of the green anole lizard and a comparative analysis with birds and mammals. Nature 2011, 477, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Castoe, T.A.; de Koning, A.J.; Hall, K.T.; Yokoyama, K.D.; Gu, W.; Smith, E.N.; Feschotte, C.; Uetz, P.; Ray, D.A.; Dobry, J.; et al. Sequencing the genome of the Burmese python (Python molurus bivittatus) as a model for studying extreme adaptations in snakes. Genome Biol. 2011, 12, 406. [Google Scholar] [CrossRef] [PubMed]
- Castoe, T.A.; Hall, K.T.; Mboulas, M.L.G.; Gu, W.; de Koning, A.J.; Fox, S.E.; Poole, W.E.; Vemulapalli, V.; Daza, J.M.; Mockler, T.; et al. Discovery of highly divergent repeat landscapes in snake genomes using high-throughput sequencing. Genome Biol. Evol. 2011, 3, 641–653. [Google Scholar] [CrossRef]
- Adams, R.H.; Blackmon, H.; Reyes-Velasco, J.; Schield, D.R.; Card, D.C.; Andrew, A.L.; Waynewood, N.; Castoe, T.A. Microsatellite landscape evolutionary dynamics across 450 million years of vertebrate genome evolution. Genome 2016, 59, 295–310. [Google Scholar] [CrossRef]
- Shedlock, A.M.; Botka, C.W.; Zhao, S.; Shetty, J.; Zhang, T.; Liu, J.S.; Deschavanne, P.J.; Edwards, S.V. Phylogenomics of nonavian reptiles and the structure of the ancestral amniote genome. Proc. Natl. Acad. Sci. USA 2007, 104, 2767–2772. [Google Scholar] [CrossRef]
- Card, D.C.; Schield, D.R.; Reyes-Velasco, J.; Fujita, M.K.; Andrew, A.L.; Oyler-McCance, S.J.; FIke, J.A.; Tomback, D.F.; Ruggiero, R.P.; Castoe, T.A. Two low coverage bird genomes and a comparison of reference-guided versus de novo genome assemblies. PLoS ONE 2014, 9, e106649. [Google Scholar] [CrossRef]
- Joyce, W.G.; Parham, J.F.; Lyson, T.R.; Warnock, R.C.M.; Donoghue, P.C.J. A divergence dating analysis of turtles using fossil calibrations: An example of best practices. J. Paleontol. 2013, 87, 612–634. [Google Scholar] [CrossRef]
- Deakin, J.E.; Ezaz, T. Understanding the Evolution of Reptile Chromosomes through Applications of Combined Cytogenetics and Genomics Approaches. Cytogenet. Genome Res. 2019, 157, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Deakin, J.E.; Potter, S.; O’Neill, R.; Ruiz-Herrera, A.; Cioffi, M.B.; Eldridge, M.D.; Fukui, K.; Marshall Graves, J.A.M.; Griffin, D.; Grutzner, F.; et al. Chromosomics: Bridging the gap between genomes and chromosomes. Genes 2019, 10, 627. [Google Scholar] [CrossRef]
- Payseur, B.A.; Nachman, M.W. Microsatellite variation and recombination rate in the human genome. Genetics 2000, 156, 1285–1298. [Google Scholar] [PubMed]
- Li, Y.C.; Korol, A.B.; Fahima, T.; Beiles, A.; Nevo, E. Microsatellites: Genomic distribution, putative functions and mutational mechanisms: A review. Mol. Ecol. 2002, 11, 2453–2465. [Google Scholar] [CrossRef]
- Ramsay, L.; Macaulay, M.; Cardle, L.; Morgante, M.; Ivanissevich, S.D.; Maestri, E.; Powell, W.; Waugh, R. Intimate association of microsatellite repeats with retrotransposons and other dispersed repetitive elements in barley. Plant. J. 1999, 17, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Prizon, A.C.; Bruschi, D.P.; Gazolla, C.B.; Borin-Carvalho, L.A.; Portela-Castro, A.L.D.B. Chromosome Spreading of the Retrotransposable Rex-3 Element and Microsatellite Repeats in Karyotypes of the Ancistrus Populations. Zebrafish 2018, 15, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.V.; Wolski, M.A.V.; Nogaroto, V.; Almeida, M.C.; Moreira-Filho, O.; Vicari, M.R. Fragile sites, dysfunctional telomere and chromosome fusions: What is 5S rDNA role? Gene 2017, 608, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Aksenova, A.Y.; Mirkin, S.M.; Clemente, L.; Mazzoleni, S.; Pensabene-Bellavia, E.; Augstenová, B.; Auer, M.; Praschag, P.; Protiva, M.; Velenský, P.; et al. At the beginning of the end and in the middle of the beginning: Structure and maintenance of telomeric DNA repeats and interstitial telomeric sequences. Genes 2019, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Clemente, L.; Mazzoleni, S.; Pensabene-Bellavia, E.; Augstenová, B.; Auer, M.; Praschag, P.; Protiva, T.; Velenský, P.; Wagner, P.; Fritz, U.; et al. Interstitial Telomeric Repeats Are Rare in Turtles. Genes 2020, 11, 657. [Google Scholar] [CrossRef]
- Ocalewicz, K. Telomeres in fishes. Cytogenet. Genome Res. 2013, 141, 114–125. [Google Scholar] [CrossRef]
- Montiel, E.E.; Badenhorst, D.; Lee, L.S.; Literman, R.; Trifonov, V.; Valenzuela, N. Cytogenetic insights into the evolution of chromosomes and sex determination reveal striking homology of turtle sex chromosomes to amphibian autosomes. Cytogenet. Genome Res. 2016, 148, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Rovatsos, M.; Kratochvíl, L.; Altmanova, M.; Pokorna, M.J. Interstitial telomeric motifs in squamate reptiles: When the exceptions outnumber the rule. PLoS ONE 2015, 10, e0134985. [Google Scholar] [CrossRef] [PubMed]
- Meyne, J.; Baker, R.J.; Hobart, H.H.; Hsu, T.C.; Ryder, O.A.; Ward, O.G.; Wiley, J.E.; Wurster-Hill, D.H.; Yates, T.L.; Moyzis, R.K. Distribution of non-telomeric sites of the (TTAGGG)n telomeric sequence in vertebrate chromosomes. Chromosoma 1990, 99, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Ramos, M.A.; Herrán, R.; Ruiz-Rejón, C.; Ruiz-Rejón, M. A satellite DNA of the Sparidae family (Pisces, Perciformes) associated with telomeric sequences. Cytogenet. Cell Genet. 1998, 83, 3–9. [Google Scholar] [CrossRef]
- Nergadze, S.; Santagostino, M.; Salzano, A.; Mondello, C.; Giulotto, E. Contribution of telomerase RNA retrotranscription to DNA double-strand break repair during mammalian genome evolution. Genome Biol. 2007, 8, 260. [Google Scholar] [CrossRef]
- Flint, J.; Craddock, C.F.; Villegas, A.; Bentley, D.P.; Williams, H.J.; Galanello, R.; Cao, A.; Wood, W.G.; Ayyub, H.; Higgs, D.R. Healing of broken human chromosomes by the addition of telomeric repeats. Am. J. Hum. Genet. 1994, 55, 505–512. [Google Scholar]
- Azzalin, C.M.; Nergadze, S.G.; Giulotto, E. Human intrachromosomal telomeric-like repeats: Sequence organization and mechanisms of origin. Chromosoma 2001, 110, 75–82. [Google Scholar] [CrossRef]
- Viana, P.F.; Ezaz, T.; Marajó, L.; Ferreira, M.; Zuanon, J.; Cioffi, M.B.; Bertollo, L.; Gross, M.C.; Feldberg, E. Genomic Organization of Repetitive DNAs and Differentiation of an XX/XY Sex Chromosome System in the Amazonian Puffer Fish, Colomesus asellus (Tetraodontiformes). Cytogenet. Genome Res. 2017, 153, 96–104. [Google Scholar] [CrossRef]
- Bachtrog, D.; Mank, J.E.; Peichel, C.L.; Kirkpatrick, M.; Otto, S.P.; Ashman, T.L.; Hahn, M.W.; Kitano, J.; Mayrose, I.; Ming, R.; et al. Tree of Sex Consortium. Sex determination: Why so many ways of doing it? PLoS Biol. 2014, 12, e1001899. [Google Scholar] [CrossRef]
- Tree of Sex Consortium. Tree of Sex: A database of sexual systems. Sci. Data 2014, 24, 140015. [Google Scholar]
- Pennell, M.W.; Mank, J.E.; Peichel, C.L. Transitions in sex determination and sex chromosomes across vertebrate species. Mol. Ecol. 2018, 27, 3950–3963. [Google Scholar] [CrossRef] [PubMed]
- De Smet, W.H.O. Chromosomes of 23 species of snakes. Acta Zool. Pathol. Antverp. 1978, 70, 85–118. [Google Scholar]
- Baker, R.J.; Mengden, G.A.; Bull, J.J. Karyotypic studies of thirty-eight species of North American snakes. Copeia 1972, 257–265. [Google Scholar] [CrossRef]
- Wright, A.E.; Dean, R.; Zimmer, F.; Mank, J.E. How to make a sex chromosome. Nat. Commun. 2016, 7, 12087. [Google Scholar] [CrossRef]
- Darolti, I.; Wright, A.E.; Sandkam, B.A.; Morris, J.; Bloch, N.I.; Farré, M.; Fuller, R.C.; Bourne, G.R.; Larkin, D.M.; Breden, F.; et al. Extreme heterogeneity in sex chromosome differentiation and dosage compensation in livebearers. Proc. Natl. Acad. Sci. USA 2019, 116, 19031–19036. [Google Scholar] [CrossRef]
- Stock, M.; Horn, A.; Grossen, C.; Lindtke, D.; Sermier, R.; Betto-Colliard, C.; Dufresnes, C.; Bonjour, E.; Dumas, Z.; Luquet, E.; et al. Ever-young sex chromosomes in European tree frogs. PLoS Biol. 2011, 9, e1001062. [Google Scholar] [CrossRef] [PubMed]
- Ogata, M.; Lambert, M.; Ezaz, T.; Miura, I. Reconstruction of female heterogamety from admixture of XX-XY and ZZ-ZW sex-chromosome systems within a frog species. Mol. Ecol. 2018, 27, 4078–4089. [Google Scholar] [CrossRef]
- Nielsen, S.V.; Guzmán-Méndez, I.A.; Gamble, T.; Blumer, M.; Pinto, B.J.; Kratochvíl, L.; Rovatsos, M. Escaping the evolutionary trap? Sex chromosome turnover in basilisks and related lizards (Corytophanidae: Squamata). Biol. Lett. 2019, 15, 20190498. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viana, P.F.; Feldberg, E.; Cioffi, M.B.; de Carvalho, V.T.; Menezes, S.; Vogt, R.C.; Liehr, T.; Ezaz, T. The Amazonian Red Side-Necked Turtle Rhinemys rufipes (Spix, 1824) (Testudines, Chelidae) Has a GSD Sex-Determining Mechanism with an Ancient XY Sex Microchromosome System. Cells 2020, 9, 2088. https://doi.org/10.3390/cells9092088
Viana PF, Feldberg E, Cioffi MB, de Carvalho VT, Menezes S, Vogt RC, Liehr T, Ezaz T. The Amazonian Red Side-Necked Turtle Rhinemys rufipes (Spix, 1824) (Testudines, Chelidae) Has a GSD Sex-Determining Mechanism with an Ancient XY Sex Microchromosome System. Cells. 2020; 9(9):2088. https://doi.org/10.3390/cells9092088
Chicago/Turabian StyleViana, Patrik F., Eliana Feldberg, Marcelo B. Cioffi, Vinicius Tadeu de Carvalho, Sabrina Menezes, Richard C. Vogt, Thomas Liehr, and Tariq Ezaz. 2020. "The Amazonian Red Side-Necked Turtle Rhinemys rufipes (Spix, 1824) (Testudines, Chelidae) Has a GSD Sex-Determining Mechanism with an Ancient XY Sex Microchromosome System" Cells 9, no. 9: 2088. https://doi.org/10.3390/cells9092088
APA StyleViana, P. F., Feldberg, E., Cioffi, M. B., de Carvalho, V. T., Menezes, S., Vogt, R. C., Liehr, T., & Ezaz, T. (2020). The Amazonian Red Side-Necked Turtle Rhinemys rufipes (Spix, 1824) (Testudines, Chelidae) Has a GSD Sex-Determining Mechanism with an Ancient XY Sex Microchromosome System. Cells, 9(9), 2088. https://doi.org/10.3390/cells9092088






