Sexual Dimorphism in Osteoclasts
Abstract
1. Introduction
2. Sexual Dimorphism in the Innate Immune System
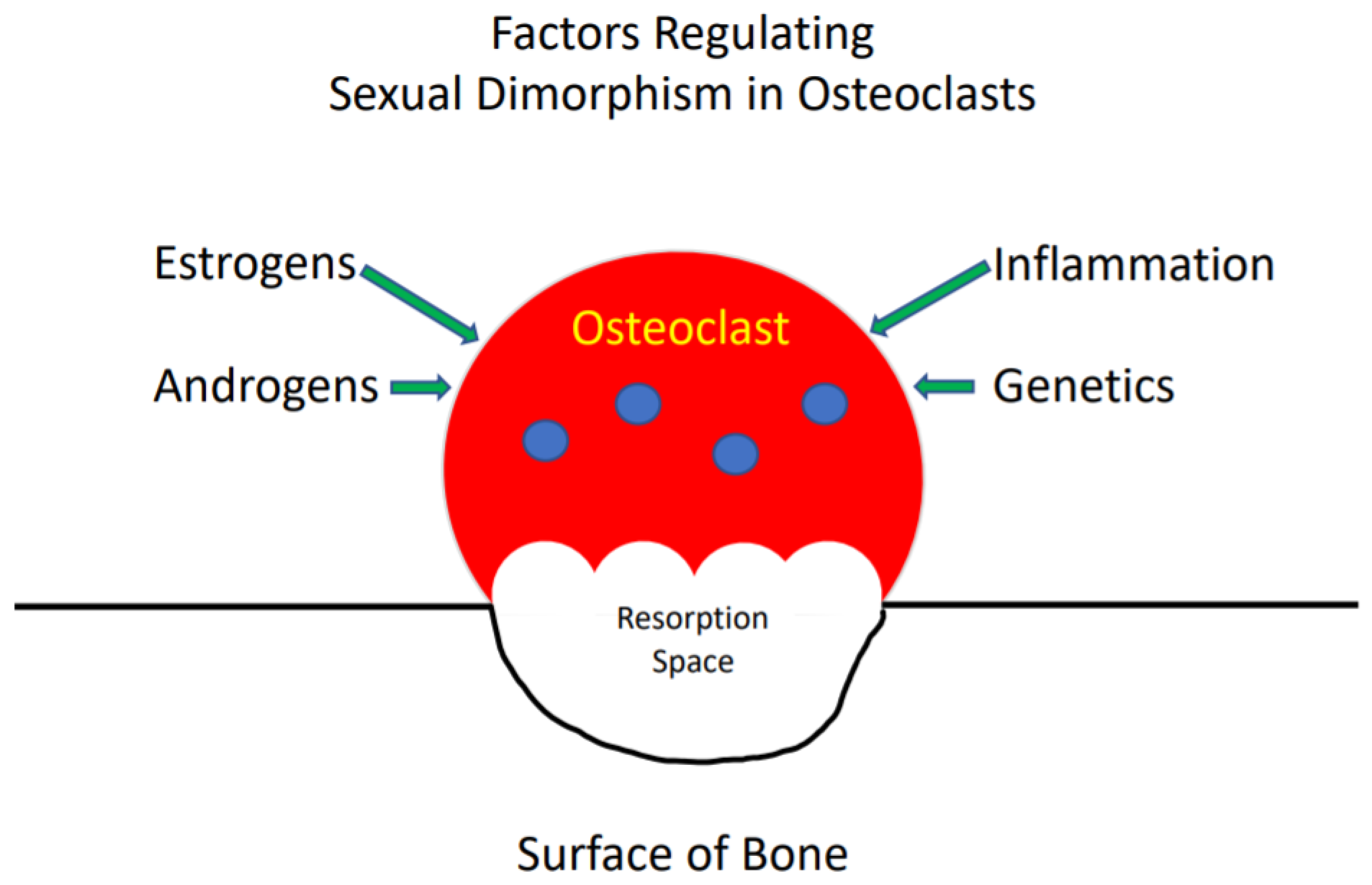
3. Osteoclast Sexual Dimorphism
4. Effects of Sex Steroids on Osteoclasts
Estrogens
5. Androgens
6. Inflammation
7. Genetics
8. Summary
Funding
Conflicts of Interest
References
- Lorenzo, J.; Horowitz, M.; Choi, Y. Osteoimmunology: Interactions of the Bone and Immune System. Endocr. Rev. 2008, 29, 403–440. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef]
- Jacome-Galarza, C.E.; Lee, S.K.; Lorenzo, J.A.; Aguila, H.L. Identification, characterization, and isolation of a common progenitor for osteoclasts, macrophages, and dendritic cells from murine bone marrow and periphery. J. Bone Min. Res. 2013, 28, 1203–1213. [Google Scholar] [CrossRef]
- Madel, M.B.; Ibáñez, L.; Wakkach, A.; de Vries, T.J.; Teti, A.; Apparailly, F.; Blin-Wakkach, C. Immune Function and Diversity of Osteoclasts in Normal and Pathological Conditions. Front. Immunol. 2019, 10, 1408. [Google Scholar] [CrossRef]
- Lorenzo, J.A.; Canalis, E.; Raisz, L.G. Metabolic Bone Disease. In Williams Text Book of Endocrinology; Kronenberg, H., Melmed, S., Polonsky, K.S., Larsen, P.R., Eds.; Saunders-Elsevier: Philadelphia, PA, USA, 2008; Volume 11, pp. 1269–1310. [Google Scholar]
- Yan, Y.; Wang, L.; Ge, L.; Pathak, J.L. Osteocyte-Mediated Translation of Mechanical Stimuli to Cellular Signaling and Its Role in Bone and Non-bone-Related Clinical Complications. Curr. Osteoporos. Rep. 2020, 18, 67–80. [Google Scholar] [CrossRef]
- Pietschmann, P.; Rauner, M.; Sipos, W.; Kerschan-Schindl, K. Osteoporosis: An age-related and gender-specific disease--a mini-review. Gerontology 2009, 55, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Pisitkun, P.; Deane, J.A.; Difilippantonio, M.J.; Tarasenko, T.; Satterthwaite, A.B.; Bolland, S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science 2006, 312, 1669–1672. [Google Scholar] [CrossRef] [PubMed]
- Berghöfer, B.; Frommer, T.; Haley, G.; Fink, L.; Bein, G.; Hackstein, H. TLR7 ligands induce higher IFN-alpha production in females. J. Immunol. 2006, 177, 2088–2096. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Reviews. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Marriott, I.; Bost, K.L.; Huet-Hudson, Y.M. Sexual dimorphism in expression of receptors for bacterial lipopolysaccharides in murine macrophages: A possible mechanism for gender-based differences in endotoxic shock susceptibility. J. Reprod. Immunol. 2006, 71, 12–27. [Google Scholar] [CrossRef]
- Paglia, D.N.; Yang, X.; Kalinowski, J.; Jastrzebski, S.; Drissi, H.; Lorenzo, J. Runx1 Regulates Myeloid Precursor Differentiation Into Osteoclasts Without Affecting Differentiation Into Antigen Presenting or Phagocytic Cells in Both Males and Females. Endocrinology 2016, 157, 3058–3069. [Google Scholar] [CrossRef] [PubMed]
- Jacquin, C.; Gran, D.E.; Lee, S.K.; Lorenzo, J.A.; Aguila, H.L. Identification of multiple osteoclast precursor populations in murine bone marrow. J. Bone Miner. Res. 2006, 21, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.; Jastrzebski, S.; Kalinowski, J.; Zeng, S.; Bae, S.; Giannoppulou, E.; Kahn, N.M.; Drissi, H.; Shin, B.; Lee, S.K.; et al. Sexual dimorphism in early osteoclasts demonstrates enhanced inflammatory pathway activation in female cells. Abstract: Annual Meeting of the American Society for Bone and Mineral Research, 2020. Available online: https://www.asbmr.org/ItineraryBuilder/PresentationDetail.aspx?pid=17c07bb3-47ef-4d3e-8486-585d7b00216e&ptag=AuthorDetail&aid=00000000-0000-0000-0000-000000000000 (accessed on 12 September 2020).
- Valerio, M.S.; Basilakos, D.S.; Kirkpatrick, J.E.; Chavez, M.; Hathaway-Schrader, J.; Herbert, B.A.; Kirkwood, K.L. Sex-based differential regulation of bacterial-induced bone resorption. J. Periodontal Res. 2017, 52, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Zarei, A.; Yang, C.; Gibbs, J.; Davis, J.L.; Ballard, A.; Zeng, R.; Cox, L.; Veis, D.J. Manipulation of the Alternative NF-κB Pathway in Mice Has Sexually Dimorphic Effects on Bone. JBMR Plus 2019, 3, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Oursler, M.J.; Osdoby, P.; Pyfferoen, J.; Riggs, B.L.; Spelsberg, T.C. Avian osteoclasts as estrogen target cells. Proc. Natl. Acad. Sci. USA 1991, 88, 6613–6617. [Google Scholar] [CrossRef]
- Martin-Millan, M.; Almeida, M.; Ambrogini, E.; Han, L.; Zhao, H.; Weinstein, R.S.; Jilka, R.L.; O’Brien, C.A.; Manolagas, S.C. The Estrogen Receptor-{alpha} in Osteoclasts Mediates the Protective Effects of Estrogens on Cancellous But Not Cortical Bone. Mol. Endocrinol. 2010, 6, 6. [Google Scholar]
- Kameda, T.; Mano, H.; Yuasa, T.; Mori, Y.; Miyazawa, K.; Shiokawa, M.; Nakamaru, Y.; Hiroi, E.; Hiura, K.; Kameda, A.; et al. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J. Exp. Med. 1997, 186, 489–495. [Google Scholar] [CrossRef]
- Nakamura, T.; Imai, Y.; Matsumoto, T.; Sato, S.; Takeuchi, K.; Igarashi, K.; Harada, Y.; Azuma, Y.; Krust, A.; Yamamoto, Y.; et al. Estrogen Prevents Bone Loss via Estrogen Receptor alpha and Induction of Fas Ligand in Osteoclasts. Cell 2007, 130, 811–823. [Google Scholar] [CrossRef]
- Krum, S.A.; Miranda-Carboni, G.A.; Hauschka, P.V.; Carroll, J.S.; Lane, T.F.; Freedman, L.P.; Brown, M. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J. 2008, 27, 535–545. [Google Scholar] [CrossRef]
- Kovacic, N.; Grcevic, D.; Katavic, V.; Lukic, I.K.; Grubisic, V.; Mihovilovic, K.; Cvija, H.; Croucher, P.I.; Marusic, A. Fas receptor is required for estrogen deficiency-induced bone loss in mice. Lab. Investig. 2010, 18, 18. [Google Scholar] [CrossRef]
- Hughes, D.E.; Dai, A.; Tiffee, J.C.; Li, H.H.; Mundy, G.R.; Boyce, B.F. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-beta. Nat. Med. 1996, 2, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.A.; Riggs, B.L.; Spelsberg, T.C.; Oursler, M.J. Osteoclasts and transforming growth factor-beta: Estrogen-mediated isoform-specific regulation of production. Endocrinology 1996, 137, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Ponte, F.; Nookaew, I.; Ucer Ozgurel, S.; Marques-Carvalho, A.; Iyer, S.; Warren, A.; Aykin-Burns, N.; Krager, K.; Sardao, V.A.; et al. Estrogens decrease osteoclast number by attenuating mitochondria oxidative phosphorylation and ATP production in early osteoclast precursors. Sci. Rep. 2020, 10, 11933. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.J.; Yaroslavskiy, B.B.; Griswold, R.D.; Zadorozny, E.V.; Guo, L.; Tourkova, I.L.; Blair, H.C. Estrogen inhibits RANKL-stimulated osteoclastic differentiation of human monocytes through estrogen and RANKL-regulated interaction of estrogen receptor-a with BCAR1 and Traf6. Exp. Cell Res. 2009, 315, 1287–1301. [Google Scholar] [CrossRef]
- Gavali, S.; Gupta, M.K.; Daswani, B.; Wani, M.R.; Sirdeshmukh, R.; Khatkhatay, M.I. LYN, a key mediator in estrogen-dependent suppression of osteoclast differentiation, survival, and function. Biochim Biophys Acta Mol. Basis Dis. 2019, 1865, 547–557. [Google Scholar] [CrossRef]
- Crusodé de Souza, M.; Sasso-Cerri, E.; Cerri, P.S. Immunohistochemical detection of estrogen receptor beta in alveolar bone cells of estradiol-treated female rats: Possible direct action of estrogen on osteoclast life span. J. Anat. 2009, 215, 673–681. [Google Scholar] [CrossRef]
- Li, J.Y.; Chassaing, B.; Tyagi, A.M.; Vaccaro, C.; Luo, T.; Adams, J.; Darby, T.M.; Weitzmann, M.N.; Mulle, J.G.; Gewirtz, A.T.; et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Investig. 2016, 126, 2049–2063. [Google Scholar] [CrossRef]
- Huber, D.M.; Bendixen, A.C.; Pathrose, P.; Srivastava, S.; Dienger, K.M.; Shevde, N.K.; Pike, J.W. Androgens suppress osteoclast formation induced by rankl and macrophage-colony stimulating factor. Endocrinology 2001, 142, 3800–3808. [Google Scholar] [CrossRef]
- Steffens, J.P.; Coimbra, L.S.; Rossa, C., Jr.; Kantarci, A.; Van Dyke, T.E.; Spolidorio, L.C. Androgen receptors and experimental bone loss—An in vivo and in vitro study. Bone 2015, 81, 683–690. [Google Scholar] [CrossRef][Green Version]
- Michael, H.; Harkonen, P.L.; Vaananen, H.K.; Hentunen, T.A. Estrogen and testosterone use different cellular pathways to inhibit osteoclastogenesis and bone resorption. J. Bone Miner. Res. 2005, 20, 2224–2232. [Google Scholar] [CrossRef]
- Sinnesael, M.; Jardi, F.; Deboel, L.; Laurent, M.R.; Dubois, V.; Zajac, J.D.; Davey, R.A.; Carmeliet, G.; Claessens, F.; Vanderschueren, D. The androgen receptor has no direct antiresorptive actions in mouse osteoclasts. Mol. Cell Endocrinol. 2015, 411, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Ucer, S.; Iyer, S.; Bartell, S.M.; Martin-Millan, M.; Han, L.; Kim, H.N.; Weinstein, R.S.; Jilka, R.L.; O’Brien, C.A.; Almeida, M.; et al. The Effects of Androgens on Murine Cortical Bone Do Not Require AR or ERalpha Signaling in Osteoblasts and Osteoclasts. J. Bone Min. Res. 2015, 30, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Valerio, M.S.; Kirkwood, K.L. Sexual Dimorphism in Immunity to Oral Bacterial Diseases: Intersection of Neutrophil and Osteoclast Pathobiology. J. Dent. Res. 2018, 97, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Kirk, S.G.; Samavati, L.; Liu, Y. MAP kinase phosphatase-1, a gatekeeper of the acute innate immune response. Life Sci. 2020, 241, 117157. [Google Scholar] [CrossRef] [PubMed]
- Novak, S.; Roeder, E.; Kalinowski, J.; Jastrzebski, S.; Aguila, H.L.; Lee, S.-K.; Kalajzic, I.; Lorenzo, J.A. Osteoclasts Derive Predominantly from Bone Marrow-Resident CX(3)CR1(+) Precursor Cells in Homeostasis, whereas Circulating CX(3)CR1(+) Cells Contribute to Osteoclast Development during Fracture Repair. J. Immunol. 2020, 204, 868–878. [Google Scholar] [CrossRef]
- Madel, M.B.; Ibáñez, L.; Ciucci, T.; Halper, J.; Rouleau, M.; Boutin, A.; Hue, C.; Duroux-Richard, I.; Apparailly, F.; Garchon, H.J.; et al. Dissecting the phenotypic and functional heterogeneity of mouse inflammatory osteoclasts by the expression of Cx3cr1. Elife 2020, 9. [Google Scholar] [CrossRef]
- Abe, K.; Aoki, Y. Sex differences in bone resorption in the mouse femur. A light- and scanning electron-microscopic study. Cell Tissue Res. 1989, 255, 15–21. [Google Scholar] [CrossRef]
- Wang, J.; Stern, P.H. Sex-specific effects of estrogen and androgen on gene expression in human monocyte-derived osteoclasts. J. Cell Biochem. 2011, 3, 23297. [Google Scholar] [CrossRef]
- Alsofi, L.; Daley, E.; Hornstra, I.; Morgan, E.F.; Mason, Z.D.; Acevedo, J.F.; Word, R.A.; Gerstenfeld, L.C.; Trackman, P.C. Sex-Linked Skeletal Phenotype of Lysyl Oxidase Like-1 Mutant Mice. Calcif Tissue Int. 2016, 98, 172–185. [Google Scholar] [CrossRef]
- van der Eerden, B.C.; Oei, L.; Roschger, P.; Fratzl-Zelman, N.; Hoenderop, J.G.; van Schoor, N.M.; Pettersson-Kymmer, U.; Schreuders-Koedam, M.; Uitterlinden, A.G.; Hofman, A.; et al. TRPV4 deficiency causes sexual dimorphism in bone metabolism and osteoporotic fracture risk. Bone 2013, 57, 443–454. [Google Scholar] [CrossRef]
- Lee, Y.D.; Yoon, S.H.; Park, C.K.; Lee, J.; Lee, Z.H.; Kim, H.H. Caveolin-1 regulates osteoclastogenesis and bone metabolism in a sex-dependent manner. J. Biol. Chem. 2015, 290, 6522–6530. [Google Scholar] [CrossRef]
- Bloom, A.C.; Collins, F.L.; Van’t Hof, R.J.; Ryan, E.S.; Jones, E.; Hughes, T.R.; Morgan, B.P.; Erlandsson, M.; Bokarewa, M.; Aeschlimann, D.; et al. Deletion of the membrane complement inhibitor CD59a drives age and gender-dependent alterations to bone phenotype in mice. Bone 2016, 84, 253–261. [Google Scholar] [CrossRef]
- Sabag, E.; Halperin, E.; Liron, T.; Hiram-Bab, S.; Frenkel, B.; Gabet, Y. Hormone-Independent Sexual Dimorphism in the Regulation of Bone Resorption by Krox20. J. Bone Min. Res. 2019, 34, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.; Kupferman, J.; Schmidt, E.; Polleux, F.; Delany, A.M.; Lee, S.K. Rac1 Inhibition Via Srgap2 Restrains Inflammatory Osteoclastogenesis and Limits the Clastokine, SLIT3. J. Bone Min. Res. 2020, 35, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; He, T.; Wu, D.; Zhang, L.; Chen, R.; Liu, B.; Yuan, J.; Tickner, J.; Qin, A.; Xu, J.; et al. Conditional Knockout of PKC-δ in Osteoclasts Favors Bone Mass Accrual in Males Due to Decreased Osteoclast Function. Front. Cell Dev. Biol. 2020, 8, 450. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzo, J. Sexual Dimorphism in Osteoclasts. Cells 2020, 9, 2086. https://doi.org/10.3390/cells9092086
Lorenzo J. Sexual Dimorphism in Osteoclasts. Cells. 2020; 9(9):2086. https://doi.org/10.3390/cells9092086
Chicago/Turabian StyleLorenzo, Joseph. 2020. "Sexual Dimorphism in Osteoclasts" Cells 9, no. 9: 2086. https://doi.org/10.3390/cells9092086
APA StyleLorenzo, J. (2020). Sexual Dimorphism in Osteoclasts. Cells, 9(9), 2086. https://doi.org/10.3390/cells9092086



