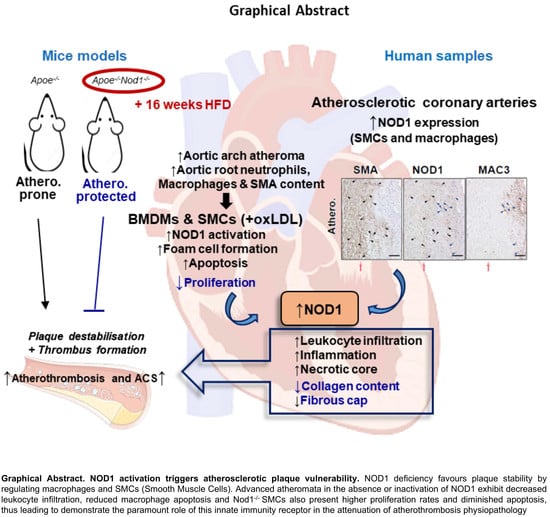
Deletion or Inhibition of NOD1 Favors Plaque Stability and Attenuates Atherothrombosis in Advanced Atherogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Samples
2.2. Animal Procedures
2.3. Cell Procedures
2.4. Histological Analysis and Lesion Quantification
2.5. Immunostaining
2.6. Western Blot Analysis
2.7. qRT-PCR
2.8. Quantification and Statistical Analysis
3. Results
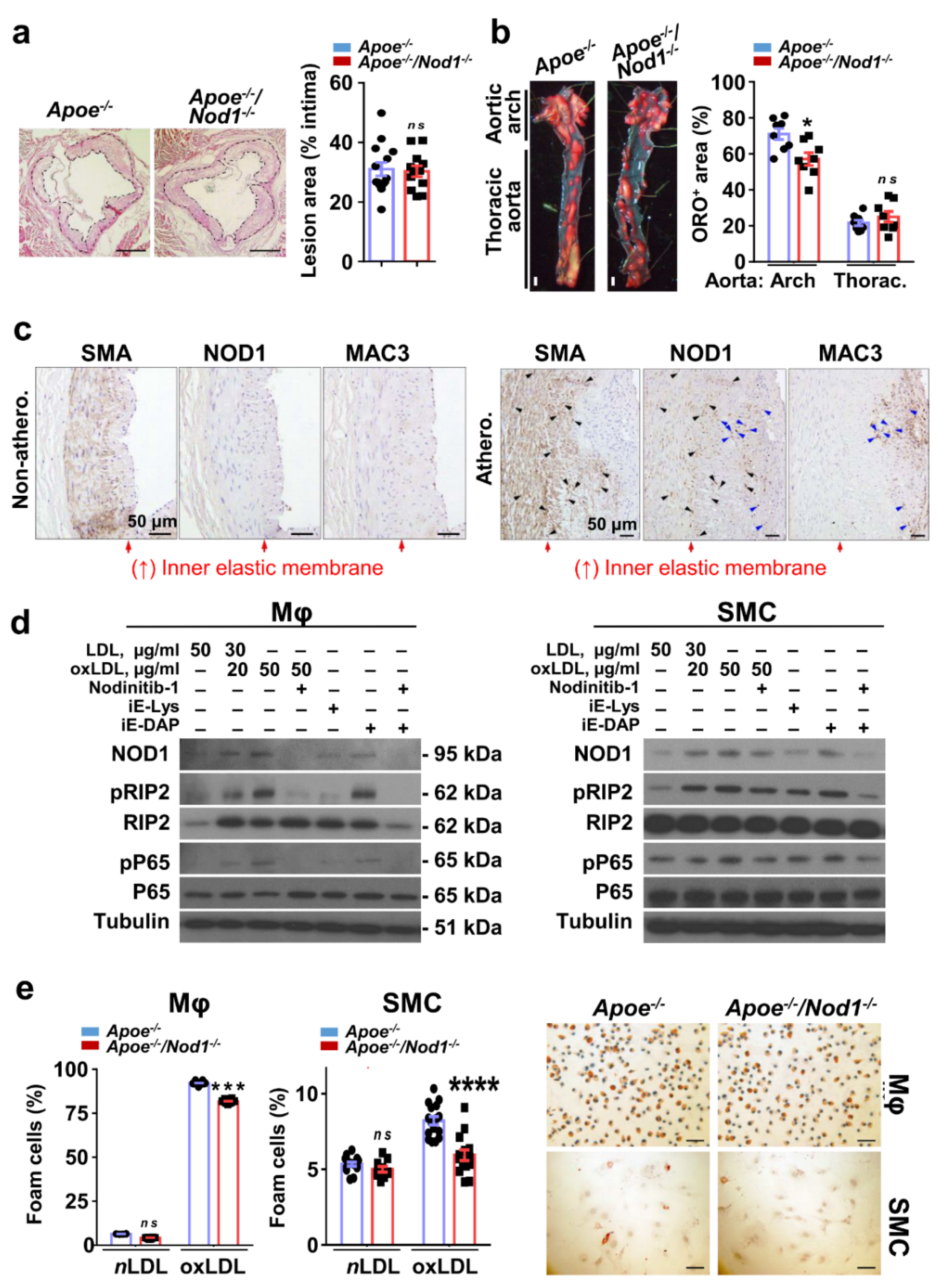
3.1. NOD1 in Vascular Smooth Muscle Cells and Macrophages Plays a Key Role in Murine and Human Atherosclerosis Plaque Formation
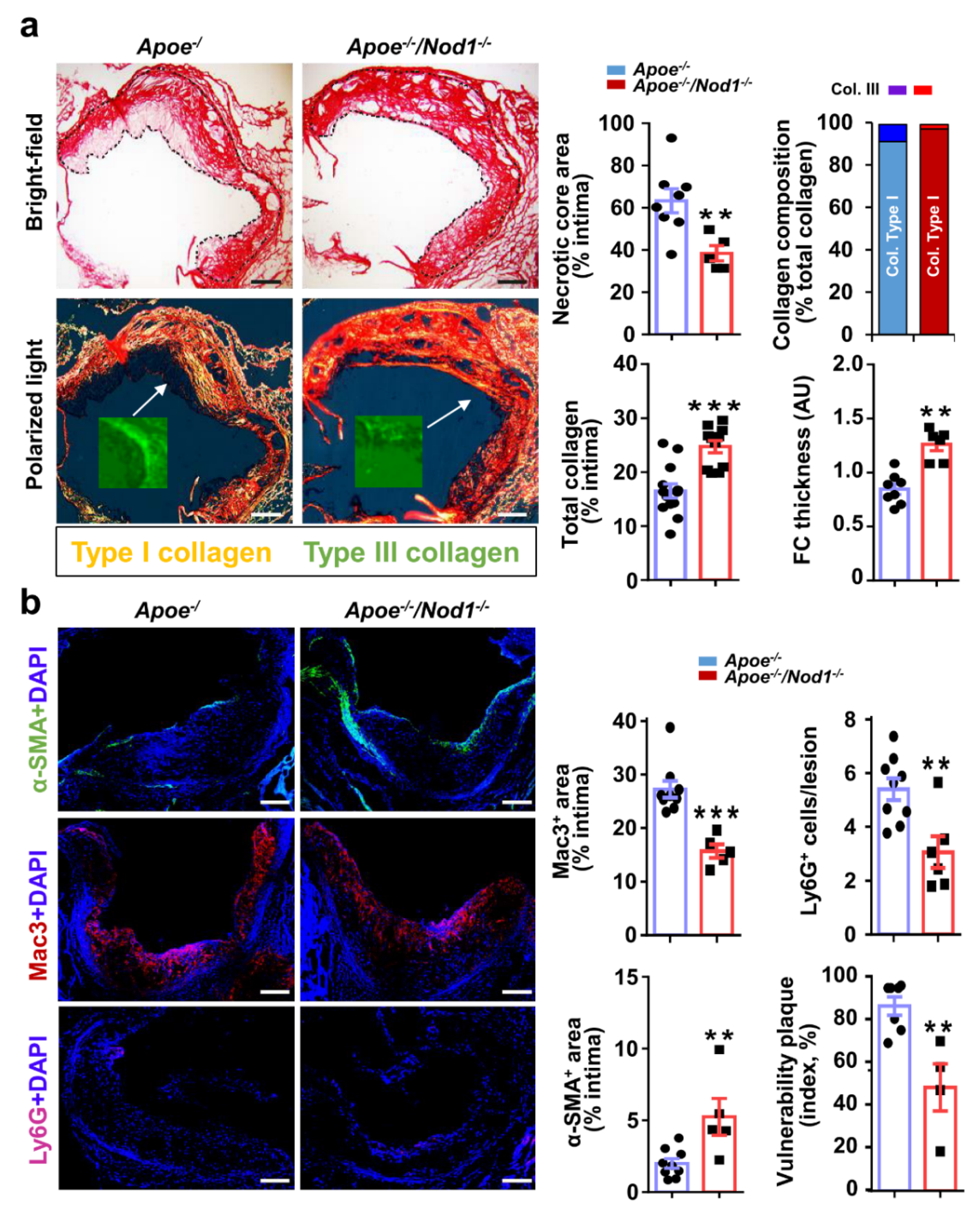
3.2. NOD1 Deficiency Modulates Structural and Compositional Features of Vulnerable Plaques
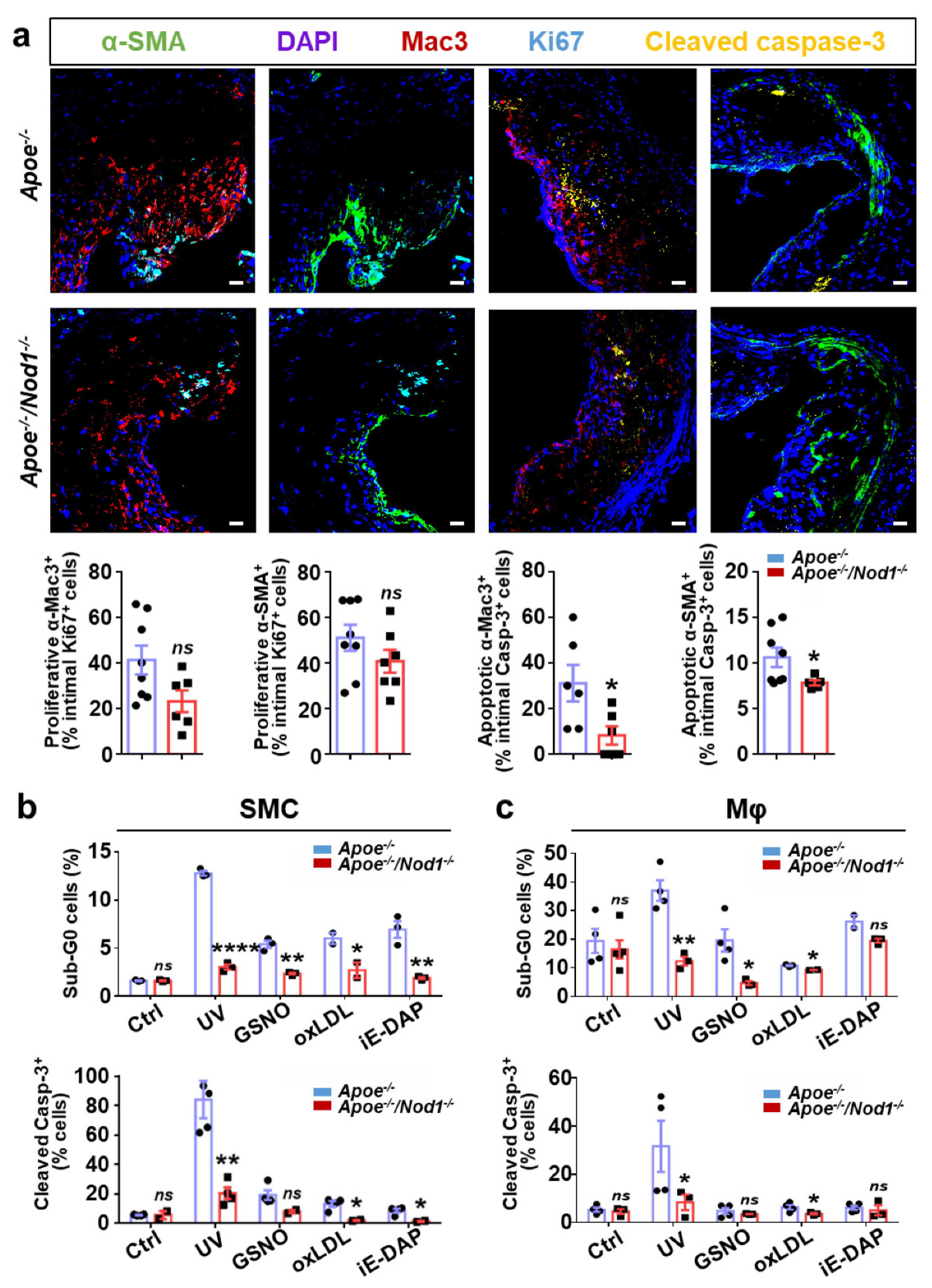
3.3. Nod1 Inactivation Increases SMC Proliferation and Reduces Macrophage Apoptosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviations | Acronyms |
| ACS | Acute coronary syndrome |
| BMDM | Bone-marrow derived macrophage |
| FC | Fibrous cap |
| HE | Haematoxylin-eosin |
| HFD | High-fat diet |
| iE-DAP | γ-D-glutamyl-meso-diaminopimelic acid |
| NC | Necrotic core |
| ORO | Oil Red O |
| oxLDL | Oxidized low-density lipoprotein |
| SMC | Smooth muscle cell |
References
- Falk, E.; Nakano, M.; Bentzon, J.F.; Finn, A.V.; Virmani, R. Update on acute coronary syndromes: The pathologists’ view. Eur. Heart J. 2013, 34, 719–728. [Google Scholar] [CrossRef]
- Libby, P. Mechanisms of acute coronary syndromes and their implications for therapy. N. Engl. J. Med. 2013, 368, 2004–2013. [Google Scholar] [CrossRef]
- Viles-Gonzalez, J.F.; Fuster, V.; Badimon, J.J. Atherothrombosis: A widespread disease with unpredictable and life-threatening consequences. Eur. Heart J. 2004, 25, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Huff, M.W.; Pickering, J.G. Can a vascular smooth muscle-derived foam-cell really change its spots? Arter. Thromb. Vasc. Biol. 2015, 35, 492–495. [Google Scholar] [CrossRef][Green Version]
- Fuster, J.J.; Gonzalez-Navarro, H.; Vinue, A.; Molina-Sanchez, P.; Andres-Manzano, M.J.; Nakayama, K.I.; Nakayama, K.; Diez-Juan, A.; Bernad, A.; Rodriguez, C.; et al. Deficient p27 phosphorylation at serine 10 increases macrophage foam cell formation and aggravates atherosclerosis through a proliferation-independent mechanism. Arter. Thromb. Vasc. Biol. 2011, 31, 2455–2463. [Google Scholar] [CrossRef]
- Naghavi, M.; Libby, P.; Falk, E.; Casscells, S.W.; Litovsky, S.; Rumberger, J.; Badimon, J.J.; Stefanadis, C.; Moreno, P.; Pasterkamp, G.; et al. From vulnerable plaque to vulnerable patient: A call for new definitions and risk assessment strategies: Part I. Circulation 2003, 108, 1664–1672. [Google Scholar] [CrossRef]
- Silvestre-Roig, C.; de Winther, M.P.; Weber, C.; Daemen, M.J.; Lutgens, E.; Soehnlein, O. Atherosclerotic plaque destabilization: Mechanisms, models, and therapeutic strategies. Circ. Res. 2014, 114, 214–226. [Google Scholar] [CrossRef]
- Schwartz, S.M.; Galis, Z.S.; Rosenfeld, M.E.; Falk, E. Plaque rupture in humans and mice. Arter. Thromb. Vasc. Biol. 2007, 27, 705–713. [Google Scholar] [CrossRef]
- Gonzalez-Ramos, S.; Paz-Garcia, M.; Rius, C.; Del Monte-Monge, A.; Rodriguez, C.; Fernandez-Garcia, V.; Andres, V.; Martinez-Gonzalez, J.; Lasuncion, M.A.; Martin-Sanz, P.; et al. Endothelial NOD1 directs myeloid cell recruitment in atherosclerosis through VCAM-1. FASEB J. 2019, 33, 3912–3921. [Google Scholar] [CrossRef]
- Hansson, G.K.; Libby, P.; Tabas, I. Inflammation and plaque vulnerability. J. Intern. Med. 2015, 278, 483–493. [Google Scholar] [CrossRef]
- Hasegawa, M.; Fujimoto, Y.; Lucas, P.C.; Nakano, H.; Fukase, K.; Nunez, G.; Inohara, N. A critical role of RICK/RIP2 polyubiquitination in Nod-induced NF-kappaB activation. Embo J. 2008, 27, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Nishio, H.; Kanno, S.; Onoyama, S.; Ikeda, K.; Tanaka, T.; Kusuhara, K.; Fujimoto, Y.; Fukase, K.; Sueishi, K.; Hara, T. Nod1 ligands induce site-specific vascular inflammation. Arter. Thromb. Vasc. Biol. 2011, 31, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Prieto, P.; Vallejo-Cremades, M.T.; Benito, G.; González-Peramato, P.; Francés, D.; Agra, N.; Terrón, V.; Gónzalez-Ramos, S.; Delgado, C.; Ruiz-Gayo, M.; et al. NOD1 receptor is up-regulated in diabetic human and murine myocardium. Clin. Sci. 2014, 127. [Google Scholar] [CrossRef] [PubMed]
- Val-Blasco, A.; Piedras, M.J.G.M.; Ruiz-Hurtado, G.; Suarez, N.; Prieto, P.; Gonzalez-Ramos, S.; Gómez-Hurtado, N.; Delgado, C.; Pereira, L.; Benito, G.; et al. Role of NOD1 in heart failure progression via regulation of Ca2+ handling. J. Am. Coll. Cardiol. 2017, 69. [Google Scholar] [CrossRef]
- Yang, H.; Li, N.; Song, L.N.; Wang, L.; Tian, C.; Tang, C.S.; Du, J.; Li, H.H.; Yu, X.H.; Wang, H.X. Activation of NOD1 by DAP contributes to myocardial ischemia/reperfusion injury via multiple signaling pathways. Apoptosis 2015, 20, 512–522. [Google Scholar] [CrossRef]
- Gonzalez-Navarro, H.; Vinue, A.; Vila-Caballer, M.; Fortuno, A.; Beloqui, O.; Zalba, G.; Burks, D.; Diez, J.; Andres, V. Molecular mechanisms of atherosclerosis in metabolic syndrome: Role of reduced IRS2-dependent signaling. Arter. Thromb. Vasc. Biol. 2008, 28, 2187–2194. [Google Scholar] [CrossRef]
- Centa, M.; Ketelhuth, D.F.J.; Malin, S.; Gisterå, A. Quantification of Atherosclerosis in Mice. JoVE 2019, e59828. [Google Scholar] [CrossRef]
- Fuster, J.J.; Fernandez, P.; Gonzalez-Navarro, H.; Silvestre, C.; Nabah, Y.N.; Andres, V. Control of cell proliferation in atherosclerosis: Insights from animal models and human studies. Cardiovasc. Res. 2010, 86, 254–264. [Google Scholar] [CrossRef]
- Johnson, J.L. Emerging regulators of vascular smooth muscle cell function in the development and progression of atherosclerosis. Cardiovasc. Res. 2014, 103, 452–460. [Google Scholar] [CrossRef]
- Allahverdian, S.; Pannu, P.S.; Francis, G.A. Contribution of monocyte-derived macrophages and smooth muscle cells to arterial foam cell formation. Cardiovasc. Res. 2012, 95, 165–172. [Google Scholar] [CrossRef]
- Chaabane, C.; Coen, M.; Bochaton-Piallat, M.L. Smooth muscle cell phenotypic switch: Implications for foam cell formation. Curr. Opin. Lipidol. 2014, 25, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Manning-Tobin, J.J.; Moore, K.J.; Seimon, T.A.; Bell, S.A.; Sharuk, M.; Alvarez-Leite, J.I.; de Winther, M.P.; Tabas, I.; Freeman, M.W. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arter. Thromb. Vasc. Biol. 2009, 29, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Stefanadis, C.; Antoniou, C.K.; Tsiachris, D.; Pietri, P. Coronary atherosclerotic vulnerable plaque: Current perspectives. J. Am. Heart Assoc. 2017, 6, e005543. [Google Scholar] [CrossRef]
- Hartwig, H.; Silvestre-Roig, C.; Hendrikse, J.; Beckers, L.; Paulin, N.; Van der Heiden, K.; Braster, Q.; Drechsler, M.; Daemen, M.J.; Lutgens, E.; et al. Atherosclerotic plaque destabilization in mice: A comparative study. PLoS ONE 2015, 10, e0141019. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakashima, Y.; Plump, A.S.; Raines, E.W.; Breslow, J.L.; Ross, R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arter. Thromb. 1994, 14, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Durand, E.; Mallat, Z.; Addad, F.; Vilde, F.; Desnos, M.; Guerot, C.; Tedgui, A.; Lafont, A. Time courses of apoptosis and cell proliferation and their relationship to arterial remodeling and restenosis after angioplasty in an atherosclerotic rabbit model. J. Am. Coll. Cardiol. 2002, 39, 1680–1685. [Google Scholar] [CrossRef][Green Version]
- Kanno, S.; Nishio, H.; Tanaka, T.; Motomura, Y.; Murata, K.; Ihara, K.; Onimaru, M.; Yamasaki, S.; Kono, H.; Sueishi, K.; et al. Activation of an innate immune receptor, Nod1, accelerates atherogenesis in Apoe-/- mice. J. Immunol. 2015, 194, 773–780. [Google Scholar] [CrossRef]
- Vlacil, A.-K.; Schuett, J.; Ruppert, V.; Soufi, M.; Oberoi, R.; Shahin, K.; Wächter, C.; Tschernig, T.; Lei, Y.; Liu, F.; et al. Deficiency of Nucleotide-binding oligomerization domain-containing proteins (NOD) 1 and 2 reduces atherosclerosis. Basic Res. Cardiol. 2020, 115, 47. [Google Scholar] [CrossRef]
- Geng, Y.J.; Libby, P. Evidence for apoptosis in advanced human atheroma. Colocalization with interleukin-1 beta-converting enzyme. Am. J. Pathol. 1995, 147, 251–266. [Google Scholar]
- da Silva Correia, J.; Miranda, Y.; Leonard, N.; Hsu, J.; Ulevitch, R.J. Regulation of Nod1-mediated signaling pathways. Cell Death Differ. 2007, 14, 830–839. [Google Scholar] [CrossRef]
- Coussens, N.P.; Mowers, J.C.; McDonald, C.; Nunez, G.; Ramaswamy, S. Crystal structure of the Nod1 caspase activation and recruitment domain. Biochem. Biophys. Res. Commun. 2007, 353, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Komori, R.; Taniguchi, M.; Ichikawa, Y.; Uemura, A.; Oku, M.; Wakabayashi, S.; Higuchi, K.; Yoshida, H. Ultraviolet a induces endoplasmic reticulum stress response in human dermal fibroblasts. Cell Struct. Funct. 2012, 37, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Nakato, R.; Ohkubo, Y.; Konishi, A.; Shibata, M.; Kaneko, Y.; Iwawaki, T.; Nakamura, T.; Lipton, S.A.; Uehara, T. Regulation of the unfolded protein response via S-nitrosylation of sensors of endoplasmic reticulum stress. Sci. Rep. 2015, 5, 14812. [Google Scholar] [CrossRef] [PubMed]
- Seimon, T.; Tabas, I. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J. Lipid Res. 2009, 50, S382–S387. [Google Scholar] [CrossRef]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef]
- Penz, S.; Reininger, A.J.; Brandl, R.; Goyal, P.; Rabie, T.; Bernlochner, I.; Rother, E.; Goetz, C.; Engelmann, B.; Smethurst, P.A.; et al. Human atheromatous plaques stimulate thrombus formation by activating platelet glycoprotein VI. FASEB J. 2005, 19, 898–909. [Google Scholar] [CrossRef]
- Rekhter, M.D. Collagen synthesis in atherosclerosis: Too much and not enough. Cardiovasc. Res. 1999, 41, 376–384. [Google Scholar] [CrossRef]
- Fatema, K.; Hirono, O.; Masakane, I.; Nitobe, J.; Kaneko, K.; Zhang, X.; Takeishi, Y.; Kubota, I. Dynamic assessment of myocardial involvement in patients with end-stage renal disease by ultrasonic tissue characterization and serum markers of collagen metabolism. Clin. Cardiol. 2004, 27, 228–234. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Ramos, S.; Fernández-García, V.; Recalde, M.; Rodríguez, C.; Martínez-González, J.; Andrés, V.; Martín-Sanz, P.; Boscá, L. Deletion or Inhibition of NOD1 Favors Plaque Stability and Attenuates Atherothrombosis in Advanced Atherogenesis. Cells 2020, 9, 2067. https://doi.org/10.3390/cells9092067
González-Ramos S, Fernández-García V, Recalde M, Rodríguez C, Martínez-González J, Andrés V, Martín-Sanz P, Boscá L. Deletion or Inhibition of NOD1 Favors Plaque Stability and Attenuates Atherothrombosis in Advanced Atherogenesis. Cells. 2020; 9(9):2067. https://doi.org/10.3390/cells9092067
Chicago/Turabian StyleGonzález-Ramos, Silvia, Victoria Fernández-García, Miriam Recalde, Cristina Rodríguez, José Martínez-González, Vicente Andrés, Paloma Martín-Sanz, and Lisardo Boscá. 2020. "Deletion or Inhibition of NOD1 Favors Plaque Stability and Attenuates Atherothrombosis in Advanced Atherogenesis" Cells 9, no. 9: 2067. https://doi.org/10.3390/cells9092067
APA StyleGonzález-Ramos, S., Fernández-García, V., Recalde, M., Rodríguez, C., Martínez-González, J., Andrés, V., Martín-Sanz, P., & Boscá, L. (2020). Deletion or Inhibition of NOD1 Favors Plaque Stability and Attenuates Atherothrombosis in Advanced Atherogenesis. Cells, 9(9), 2067. https://doi.org/10.3390/cells9092067