Dynamic High-Sensitivity Quantitation of Procollagen-I by Endogenous CRISPR-Cas9 NanoLuciferase Tagging
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Generation of Split GFP Expressing Stable Cells
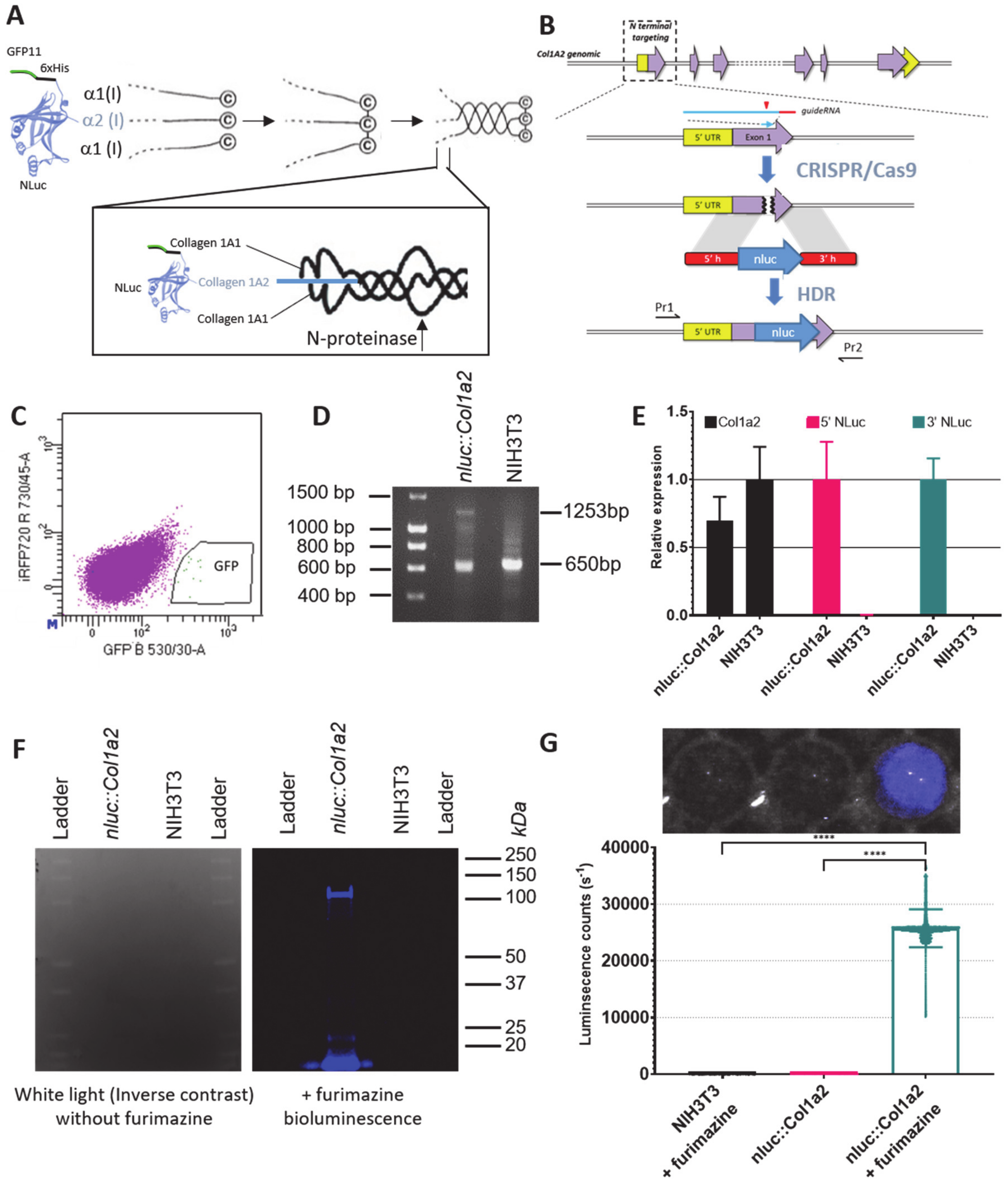
2.3. CRISPR Editing
2.4. DNA and RNA Validation of CRISPR Editing
2.5. In-Gel Detection of NLuc Activity
2.6. Proteomic Validation of CRISPR Editing
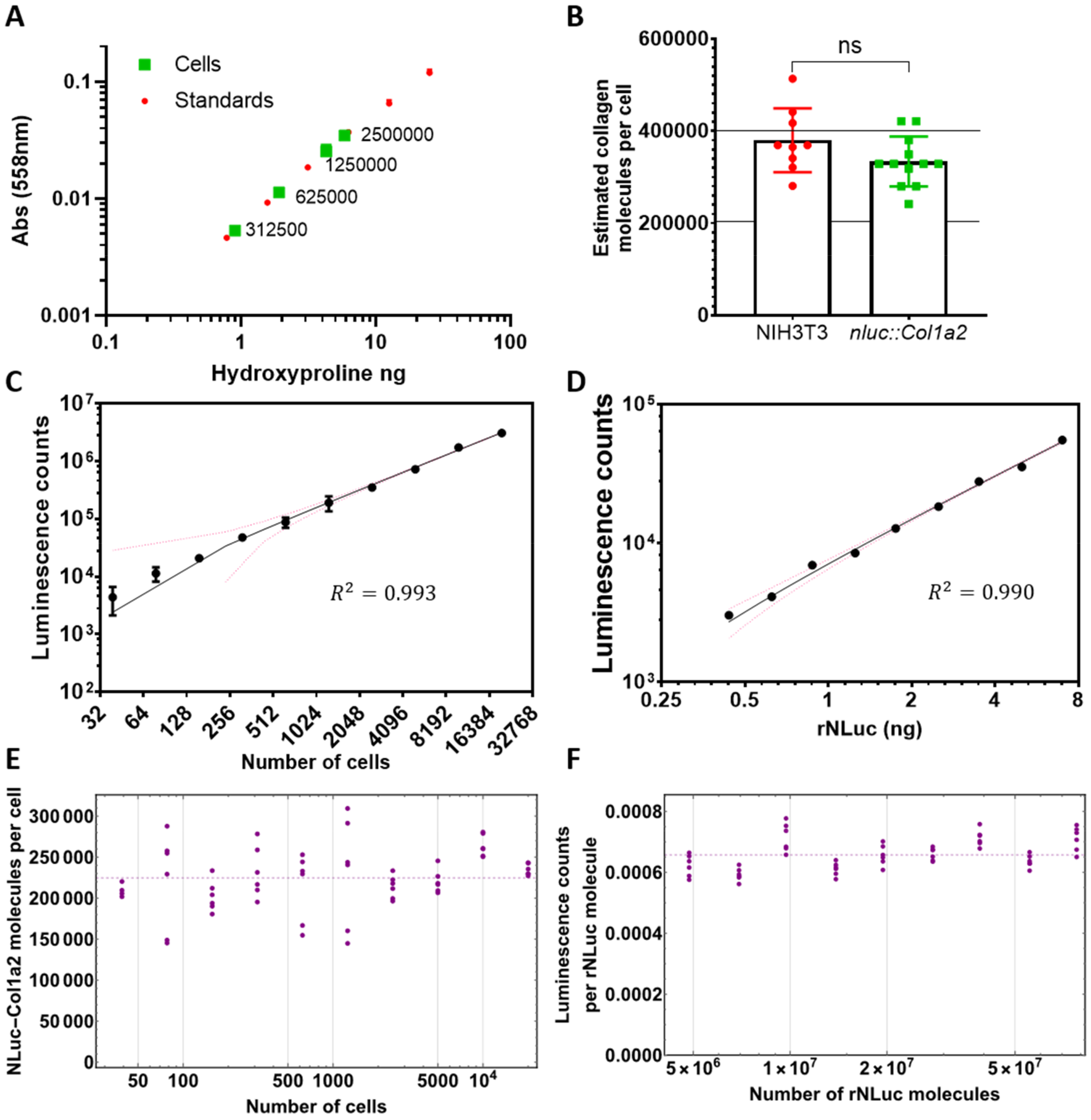
2.7. Quantitation of Absolute Collagen Levels
2.8. Hydroxyproline Assay
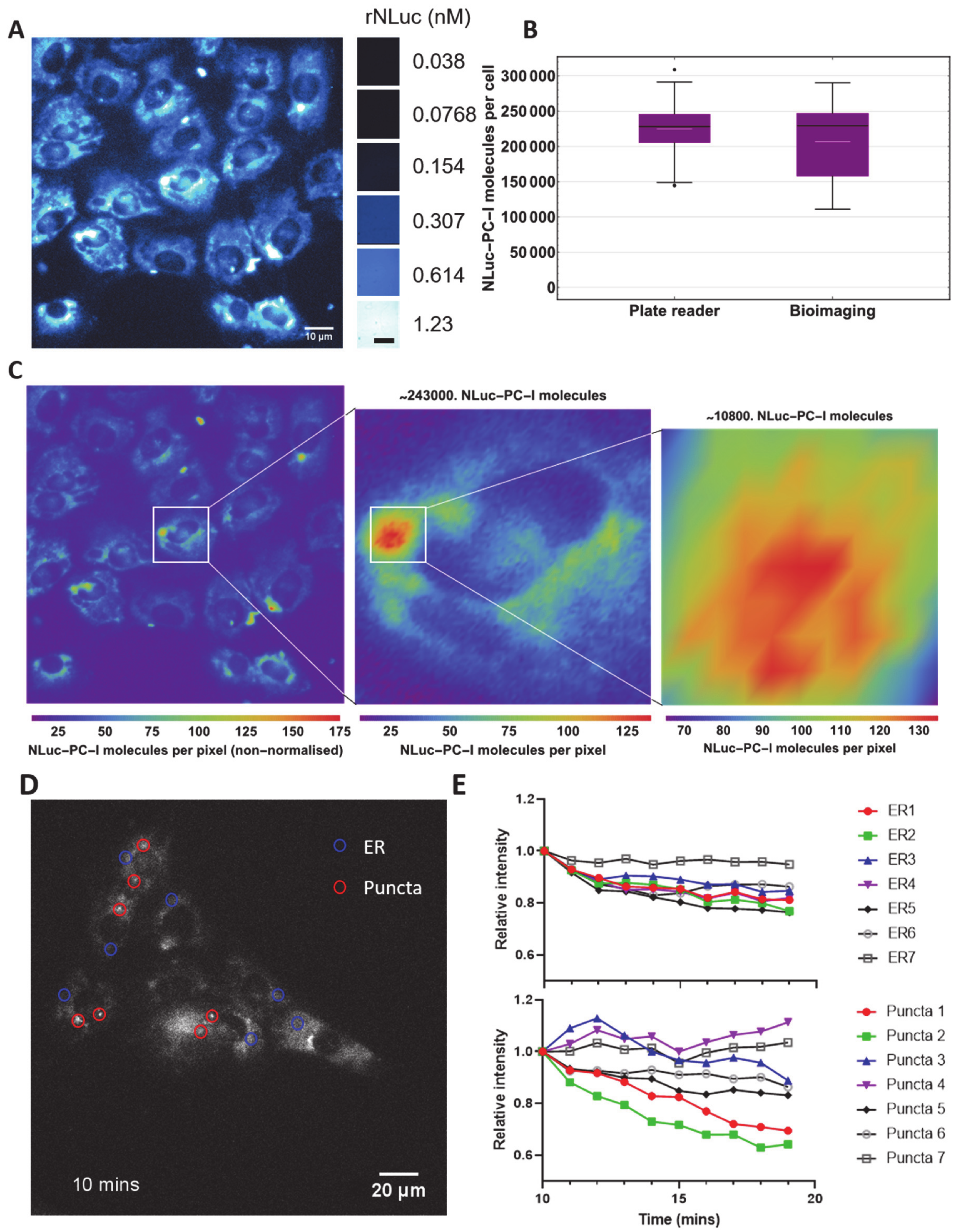
2.9. Bioluminescence Imaging
3. Results
3.1. CRISPR-Cas9 Editing of Col1a2
3.2. Quantitation of NLuc-PC-I
3.3. Direct Imaging and Quantitation of NLuc-PC-I in Cells
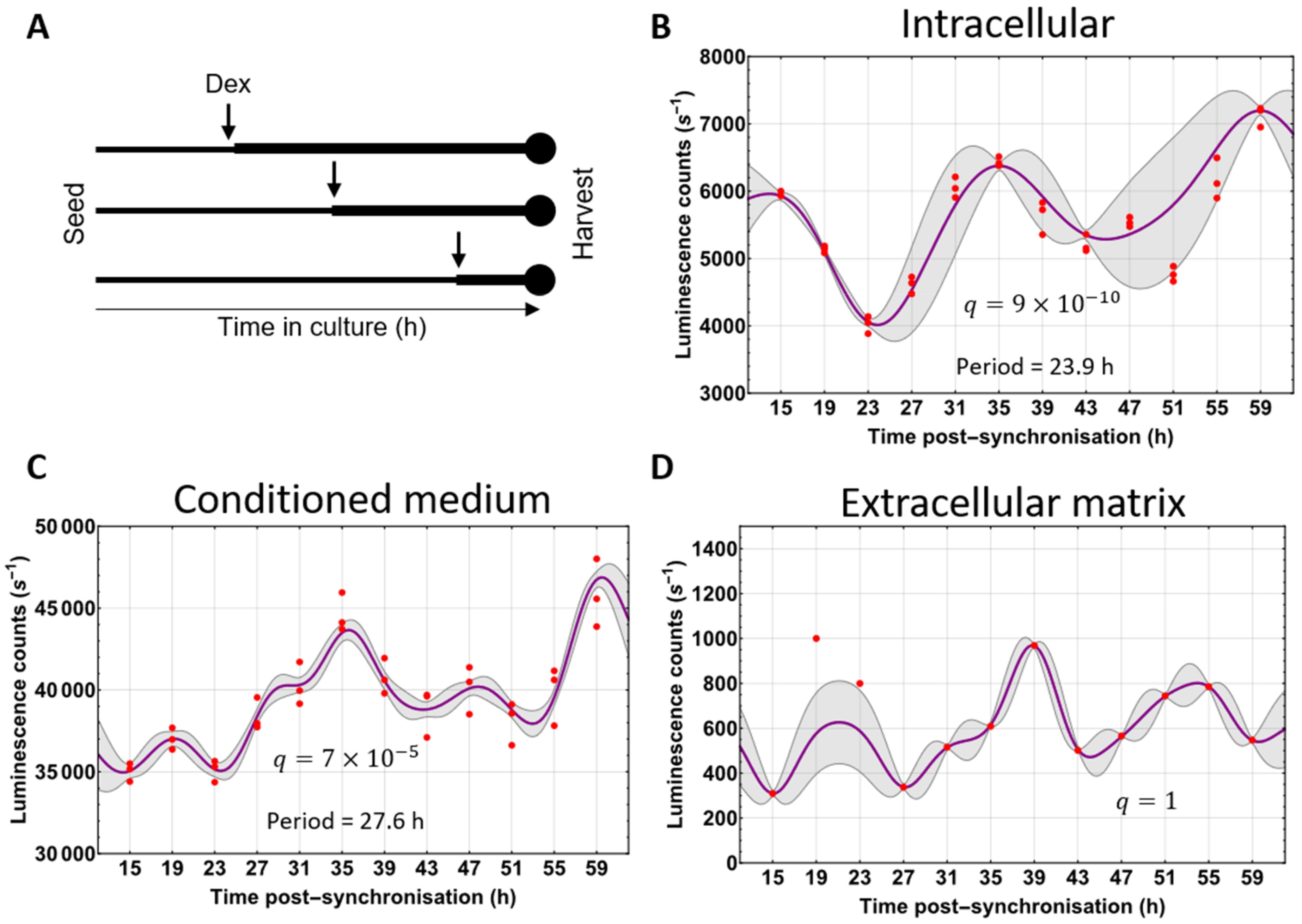
3.4. Circadian Fluctuations of Procollagen-I
3.5. NLuc-PC-I Response to Known Collagen Modulators
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hall, M.P.; Unch, J.; Binkowski, B.F.; Valley, M.P.; Butler, B.L.; Wood, M.G.; Otto, P.; Zimmerman, K.; Vidugiris, G.; Machleidt, T.; et al. Engineered Luciferase Reporter from a Deep Sea Shrimp Utilizing a Novel Imidazopyrazinone Substrate. ACS Chem. Biol. 2012, 7, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Zdzieblik, D.; Oesser, S.; Baumstark, M.W.; Gollhofer, A.; König, D. Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: A randomised controlled trial. Br. J. Nutr. 2015, 114, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Bella, J.; Hulmes, D.J.S. Fibrillar Collagens. Plant-Microbe Interact. 2017, 82, 457–490. [Google Scholar]
- Kalson, N.S.; Lu, Y.; Taylor, S.H.; Starborg, T.; Holmes, D.F.; Kadler, K.E. A structure-based extracellular matrix expansion mechanism of fibrous tissue growth. eLife 2015, 4, e05958. [Google Scholar] [CrossRef]
- Heinemeier, K.M.; Schjerling, P.; Heinemeier, J.; Magnusson, S.P.; Kjaer, M. Lack of tissue renewal in human adult Achilles tendon is revealed by nuclear bomb 14 C. FASEB J. 2013, 27, 2074–2079. [Google Scholar] [CrossRef]
- Chang, J.; Garva, R.; Pickard, A.; Yeung, C.-Y.C.; Mallikarjun, V.; Swift, J.; Holmes, D.F.; Calverley, B.; Lu, Y.; Adamson, A.; et al. Circadian control of the secretory pathway maintains collagen homeostasis. Nat. Cell Biol. 2020, 22, 74–86. [Google Scholar] [CrossRef]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The role of collagen in cancer: from bench to bedside. J. Transl. Med. 2019, 17, 309–322. [Google Scholar] [CrossRef]
- Fang, M.; Yuan, J.; Peng, C.; Li, Y. Collagen as a double-edged sword in tumor progression. Tumor Biol. 2013, 35, 2871–2882. [Google Scholar] [CrossRef]
- Wynn, T.A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Investig. 2007, 117, 524–529. [Google Scholar] [CrossRef]
- Prockop, D.J.; Udenfriend, S. A specific method for the analysis of hydroxyproline in tissues and urine. Anal. Biochem. 1960, 1, 228–239. [Google Scholar] [CrossRef]
- Kadler, K.E.; Baldock, C.; Bella, J.; Boot-Handford, R.P. Collagens at a glance. J. Cell Sci. 2007, 120, 1955–1958. [Google Scholar] [CrossRef]
- Brodsky, B.; Persikov, A.V. Molecular Structure of the Collagen Triple Helix. Cytokines 2005, 70, 301–339. [Google Scholar] [CrossRef]
- Bentley, J.; Hanson, A.N. The hydroxyproline of elastin. Biochim. Biophys. Acta (BBA) Protein Struct. 1969, 175, 339–344. [Google Scholar] [CrossRef]
- Canty, E.G.; Lu, Y.; Meadows, R.S.; Shaw, M.K.; Holmes, D.F.; Kadler, K.E. Coalignment of plasma membrane channels and protrusions (fibripositors) specifies the parallelism of tendon. J. Cell Biol. 2004, 165, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, D.; Sekine, S.; Barsi-Rhyne, B.; Hu, J.; Chen, B.; Gilbert, L.A.; Ishikawa, H.; Leonetti, M.D.; Marshall, W.F.; Weissman, J.S.; et al. Versatile protein tagging in cells with split fluorescent protein. Nat. Commun. 2016, 7, 11046. [Google Scholar] [CrossRef]
- Campeau, E.; Ruhl, V.E.; Rodier, F.; Smith, C.L.; Rahmberg, B.L.; Fuss, J.O.; Campisi, J.; Yaswen, P.; Cooper, P.K.; Kaufman, P.D. A Versatile Viral System for Expression and Depletion of Proteins in Mammalian Cells. PLoS ONE 2009, 4, e6529. [Google Scholar] [CrossRef] [PubMed]
- Pickard, A.; Adamson, A.; Lu, Y.; Chang, J.; Garva, R.; Hodson, N.W.; Kadler, K.E. Collagen assembly and turnover imaged with a CRISPR-Cas9 engineered Dendra2 tag. bioRxiv 2018, 331496. [Google Scholar] [CrossRef]
- Hodgkins, A.; Farne, A.; Perera, S.; Grego, T.; Parry-Smith, D.J.; Skarnes, W.C.; Iyer, V. WGE: A CRISPR database for genome engineering. Bioinformatics 2015, 31, 3078–3080. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.K.; Enwemeka, C.S. A simplified method for the analysis of hydroxyproline in biological tissues. Clin. Biochem. 1996, 29, 225–229. [Google Scholar] [CrossRef]
- Todaro, G.J.; Green, H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell Biol. 1963, 17, 299–313. [Google Scholar] [CrossRef]
- Tanzawa, K.; Berger, J.; Prockop, D.J. Type I procollagen N-proteinase from whole chick embryos. Cleavage of a homotrimer of pro-alpha 1(I) chains and the requirement for procollagen with a triple-helical conformation. J. Biol. Chem. 1985, 260, 1120–1126. [Google Scholar] [PubMed]
- Wu, G.; Anafi, R.C.; Hughes, M.E.; Kornacker, K.; HogenEsch, J.B. MetaCycle: an integrated R package to evaluate periodicity in large scale data. Bioinformatics 2016, 32, 3351–3353. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Pickard, A.; Chang, J.; Alachkar, N.; Calverley, B.; Garva, R.; Arvan, P.; Meng, Q.-J.; Kadler, K.E. Preservation of circadian rhythms by the protein folding chaperone, BiP. FASEB J. 2019, 33, 7479–7489. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.I.; Roth, G.J.; Hilberg, F.; Muller-Quernheim, J.; Prasse, A.; Zissel, G.; Schnapp, A.; Park, J.E. Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. Eur. Respir. J. 2007, 29, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Montes, A.; Ruiz-Corro, L.; López-Reyes, A.; Castrejón-Gómez, E.; Armendariz-Borunda, J. Potent antioxidant role of Pirfenidone in experimental cirrhosis. Eur. J. Pharmacol. 2008, 595, 69–77. [Google Scholar] [CrossRef]
- Veerapathiran, S.; Wohland, T. Fluorescence techniques in developmental biology. J. Biosci. 2018, 43, 541–553. [Google Scholar] [CrossRef]
- Suter, D.M.; Molina, N.; Gatfield, D.; Schneider, K.; Schibler, U.; Naef, F. Mammalian Genes Are Transcribed with Widely Different Bursting Kinetics. Science 2011, 332, 472–474. [Google Scholar] [CrossRef]
- Lees, J.F.; Tasab, M.; Bulleid, N.J. Identification of the molecular recognition sequence which determines the type-specific assembly of procollagen. EMBO J. 1997, 16, 908–916. [Google Scholar] [CrossRef]
- Marini, J.C.; Forlino, A.; Bächinger, H.P.; Bishop, N.J.; Byers, P.H.; De Paepe, A.; Fassier, F.; Fratzl-Zelman, N.; Kozloff, K.M.; Krakow, D.; et al. Osteogenesis imperfecta. Nat. Rev. Dis. Prim. 2017, 3, 17052. [Google Scholar] [CrossRef]
- Morris, J.L.; Cross, S.J.; Lu, Y.; Kadler, K.E.; Lu, Y.; Dallas, S.L.; Martin, P. Live imaging of collagen deposition during skin development and repair in a collagen I - GFP fusion transgenic zebrafish line. Dev. Biol. 2018, 441, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Kadler, K.E.; Hojima, Y.; Prockop, D.J. Assembly of collagen fibrils de novo by cleavage of the type I pC-collagen with procollagen C-proteinase. Assay of critical concentration demonstrates that collagen self-assembly is a classical example of an entropy-driven process. J. Biol. Chem. 1987, 262, 15696–15701. [Google Scholar] [PubMed]
- Hulmes, D.J.; Kadler, K.E.; Mould, A.; Hojima, Y.; Holmes, D.F.; Cummings, C.; Chapman, J.A.; Prockop, D.J. Pleomorphism in type I collagen fibrils produced by persistence of the procollagen N-propeptide. J. Mol. Biol. 1989, 210, 337–345. [Google Scholar] [CrossRef]
- Fleischmajer, R.; Timpl, R.; Tuderman, L.; Raisher, L.; Wiestner, M.; Perlish, J.S.; Graves, P.N. Ultrastructural identification of extension aminopropeptides of type I and III collagens in human skin. Proc. Natl. Acad. Sci. USA 1981, 78, 7360–7364. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, T.; Colige, A.; Syx, D.; Giunta, C.; Lindert, U.; Rohrbach, M.; Aryani, O.; Alanay, Y.; Simsek-Kiper, P.O.; Kroes, H.Y.; et al. Expanding the clinical and mutational spectrum of the Ehlers–Danlos syndrome, dermatosparaxis type. Genet. Med. 2016, 18, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Tolstoshev, P.; Berg, R.A.; Rennard, S.I.; Bradley, K.H.; Trapnell, B.C.; Crystal, R.G. Procollagen production and procollagen messenger RNA levels and activity in human lung fibroblasts during periods of rapid and stationary growth. J. Biol. Chem. 1981, 256, 3135–3140. [Google Scholar]
- Rannikmäe, K.; Davies, G.; Thomson, P.A.; Bevan, S.; Devan, W.J.; Falcone, G.J.; Traylor, M.; Anderson, C.D.; Battey, T.W.; Radmanesh, F.; et al. Common variation in COL4A1/COL4A2 is associated with sporadic cerebral small vessel disease. Neurology 2015, 84, 918–926. [Google Scholar] [CrossRef]
- Gatseva, A.; Sin, Y.Y.; Brezzo, G.; Van Agtmael, T. Basement membrane collagens and disease mechanisms. Essays Biochem. 2019, 63, 297–312. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calverley, B.C.; Kadler, K.E.; Pickard, A. Dynamic High-Sensitivity Quantitation of Procollagen-I by Endogenous CRISPR-Cas9 NanoLuciferase Tagging. Cells 2020, 9, 2070. https://doi.org/10.3390/cells9092070
Calverley BC, Kadler KE, Pickard A. Dynamic High-Sensitivity Quantitation of Procollagen-I by Endogenous CRISPR-Cas9 NanoLuciferase Tagging. Cells. 2020; 9(9):2070. https://doi.org/10.3390/cells9092070
Chicago/Turabian StyleCalverley, Ben C., Karl E. Kadler, and Adam Pickard. 2020. "Dynamic High-Sensitivity Quantitation of Procollagen-I by Endogenous CRISPR-Cas9 NanoLuciferase Tagging" Cells 9, no. 9: 2070. https://doi.org/10.3390/cells9092070
APA StyleCalverley, B. C., Kadler, K. E., & Pickard, A. (2020). Dynamic High-Sensitivity Quantitation of Procollagen-I by Endogenous CRISPR-Cas9 NanoLuciferase Tagging. Cells, 9(9), 2070. https://doi.org/10.3390/cells9092070




