Radiotherapy Scheme Effect on PD-L1 Expression for Locally Advanced Rectal Cancer
Abstract
1. Introduction
2. Material and Methods
2.1. Patients
2.2. Evaluation of PD-L1 Expression by Immunohistochemistry
2.3. TILs Assessment
2.4. Assessment of Response to Neoadjuvant Radiotherapy
2.5. Statistical Analyses
3. Results
3.1. Patients, Tumor, and Treatment Characteristics
3.2. Effect of Short-Course versus Long-Course RT on PD-L1+ Cells
3.3. Relation between CD8 TILs Infiltration and PD-L1+ Cells
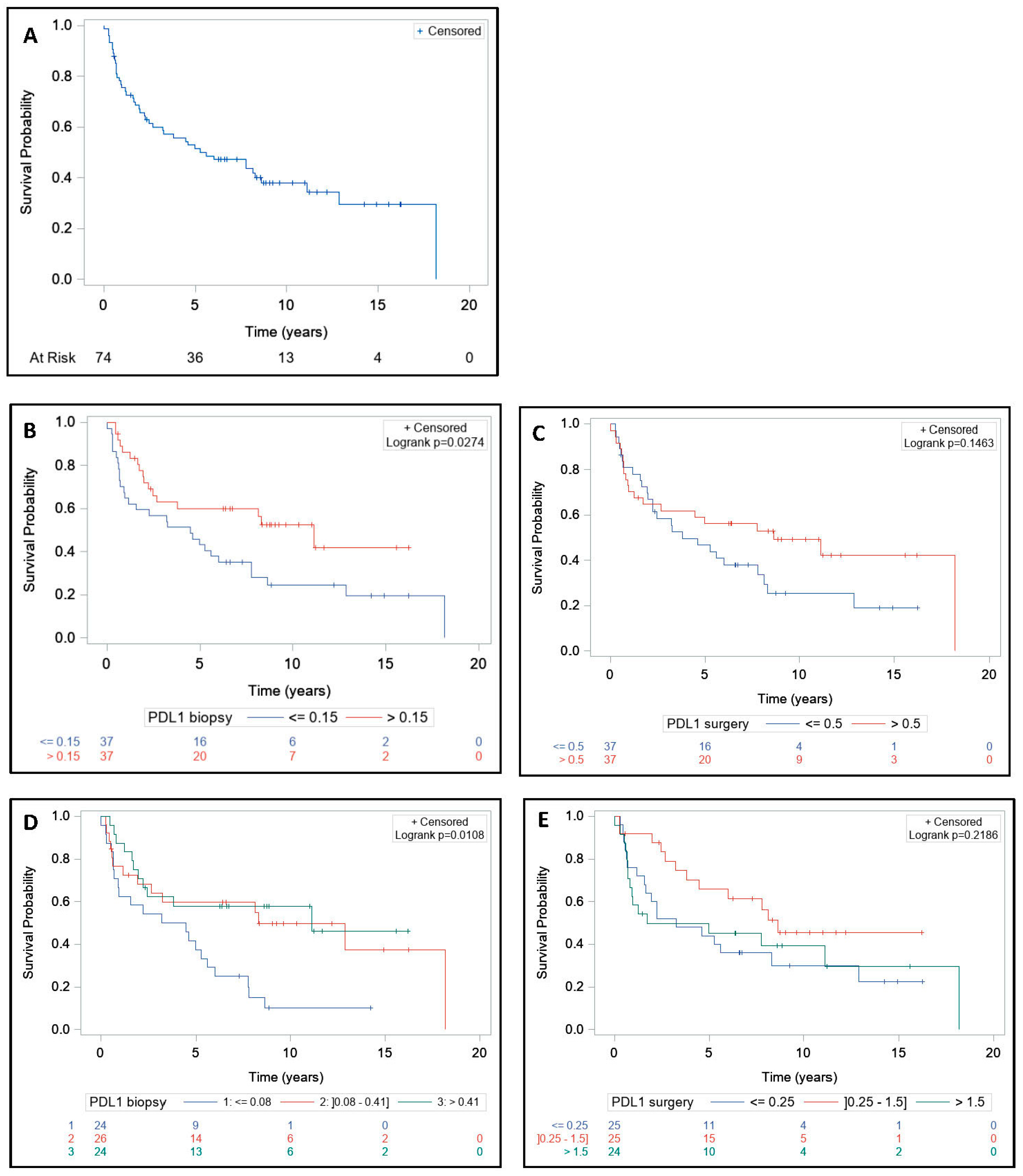
3.4. Survival and Tumor Regression Grade Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D.; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. 4), iv263. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Cederquist, L.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.A.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 874–901. [Google Scholar] [CrossRef] [PubMed]
- Bosset, J.-F.; Calais, G.; Mineur, L.; Maingon, P.; Stojanovic-Rundic, S.; Bensadoun, R.-J.; Bardet, E.; Beny, A.; Ollier, J.-C.; Bolla, M.; et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: Long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014, 15, 184–190. [Google Scholar] [CrossRef]
- Sauer, R.; Liersch, T.; Merkel, S.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Raab, H.-R.; Villanueva, M.-T.; Witzigmann, H.; et al. Preoperative Versus Postoperative Chemoradiotherapy for Locally Advanced Rectal Cancer: Results of the German CAO/ARO/AIO-94 Randomized Phase III Trial After a Median Follow-Up of 11 Years. J. Clin. Oncol. 2012, 30, 1926–1933. [Google Scholar] [CrossRef]
- Erlandsson, J.; Holm, T.; Pettersson, D.; Berglund, Å.; Cedermark, B.; Radu, C.; Johansson, H.; Machado, M.; Hjern, F.; Hallböök, O.; et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): A multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017, 18, 336–346. [Google Scholar] [CrossRef]
- Gerard, J.-P.; Conroy, T.; Bonnetain, F.; Bouché, O.; Chapet, O.; Closon-Dejardin, M.-T.; Untereiner, M.; LeDuc, B.; Francois, É.; Maurel, J.; et al. Preoperative Radiotherapy with or Without Concurrent Fluorouracil and Leucovorin in T3-4 Rectal Cancers: Results of FFCD. J. Clin. Oncol. 2006, 24, 4620–4625. [Google Scholar] [CrossRef]
- Wang, X.-J.; Zheng, Z.-R.; Chi, P.; Lin, H.-M.; Lu, X.-R.; Huang, Y. Effect of Interval between Neoadjuvant Chemoradiotherapy and Surgery on Oncological Outcome for Rectal Cancer: A Systematic Review and Meta-Analysis. Gastroenterol. Res. Pract. 2016, 2016, 1–13. [Google Scholar] [CrossRef][Green Version]
- Formenti, S.C.; DeMaria, S. Systemic effects of local radiotherapy. Lancet Oncol. 2009, 10, 718–726. [Google Scholar] [CrossRef]
- Galon, J.; Costes, A.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Zinzindohoué, F.; Bruneval, P.; Cugnenc, P.-H.; et al. Type, Density, and Location of Immune Cells Within Human Colorectal Tumors Predict Clinical Outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef]
- Tosolini, M.; Kirilovsky, A.; Mlecnik, B.; Fredriksen, T.; Mauger, S.; Bindea, G.; Berger, A.; Bruneval, P.; Fridman, W.H.; Pagès, F.; et al. Clinical Impact of Different Classes of Infiltrating T Cytotoxic and Helper Cells (Th1, Th2, Treg, Th17) in Patients with Colorectal Cancer. Cancer Res. 2011, 71, 1263–1271. [Google Scholar] [CrossRef]
- Mei, Z.; Liu, Y.; Liu, C.; Cui, A.; Liang, Z.; Wang, G.; Peng, H.; Cui, L.; Li, C. Tumour-infiltrating inflammation and prognosis in colorectal cancer: Systematic review and meta-analysis. Br. J. Cancer 2014, 110, 1595–1605. [Google Scholar] [CrossRef]
- Fridman, W.H.; Pagès, F.; Sautès-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Mirjolet, C.; Charon-Barra, C.; Ladoire, S.; Arbez-Gindre, F.; Bertaut, A.; Ghiringhelli, F.; Leroux, A.; Peiffert, D.; Borg, C.; Bosset, J.F.; et al. Tumor lymphocyte immune response to preoperative radiotherapy in locally advanced rectal cancer: The LYMPHOREC study. OncoImmunology 2017, 7, e1396402. [Google Scholar] [CrossRef] [PubMed]
- Dovedi, S.J.; Adlard, A.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.J.; Stratford, I.J.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired Resistance to Fractionated Radiotherapy Can Be Overcome by Concurrent PD-L1 Blockade. Cancer Res. 2014, 74, 5458–5468. [Google Scholar] [CrossRef] [PubMed]
- Grapin, M.; Richard, C.; Limagne, E.; Boidot, R.; Morgand, V.; Bertaut, A.; Derangere, V.; Laurent, P.-A.; Thibaudin, M.; Fumet, J.D.; et al. Optimized fractionated radiotherapy with anti-PD-L1 and anti-TIGIT: A promising new combination. J. Immunother. Cancer 2019, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Hong, M.; Ahn, S.; Choi, Y.-L.; Kim, K.-M.; Oh, D.; Ahn, Y.; Jung, S.-H.; Ahn, M.-J.; Park, K.; et al. Changes in tumour expression of programmed death-ligand 1 after neoadjuvant concurrent chemoradiotherapy in patients with squamous oesophageal cancer. Eur. J. Cancer 2016, 52, 1–9. [Google Scholar] [CrossRef]
- Sheng, J.; Fang, W.; Yu, J.; Chen, N.; Zhan, J.; Ma, Y.; Yang, Y.; Huang, Y.; Zhao, H.; Zhang, L. Expression of programmed death ligand-1 on tumor cells varies pre and post chemotherapy in non-small cell lung cancer. Sci. Rep. 2016, 6, 20090. [Google Scholar] [CrossRef]
- Wimberly, H.; Brown, J.R.; Schalper, K.; Haack, H.; Silver, M.R.; Nixon, C.; Bossuyt, V.; Pusztai, L.; Lannin, N.R.; Rimm, D.L. PD-L1 Expression Correlates with Tumor-Infiltrating Lymphocytes and Response to Neoadjuvant Chemotherapy in Breast Cancer. Cancer Immunol. Res. 2014, 3, 326–332. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Reichling, C.; Taieb, J.; Derangere, V.; Klopfenstein, Q.; Le Malicot, K.; Gornet, J.-M.; Becheur, H.; Fein, F.; Cojocarasu, O.; Kaminsky, M.C.; et al. Artificial intelligence-guided tissue analysis combined with immune infiltrate assessment predicts stage III colon cancer outcomes in PETACC08 study. Gut 2019, 69, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Loibl, S.; Noske, A.; Roller, M.; Müller, B.M.; Komor, M.; Budczies, J.; Niesporek, S.; Kronenwett, R.; Hanusch, C.; et al. Tumor-Associated Lymphocytes as an Independent Predictor of Response to Neoadjuvant Chemotherapy in Breast Cancer. J. Clin. Oncol. 2010, 28, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Mandard, A.M.; Dalibard, F.; Mandard, J.C.; Marnay, J.; Henry-Amar, M.; Petiot, J.F.; Roussel, A.; Jacob, J.H. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994, 73, 2680–2686. [Google Scholar] [CrossRef]
- Hecht, M.; Büttner-Herold, M.; Erlenbach-Wünsch, K.; Haderlein, M.; Croner, R.; Grützmann, R.; Hartmann, A.; Fietkau, R.; Distel, L.V. PD-L1 is upregulated by radiochemotherapy in rectal adenocarcinoma patients and associated with a favourable prognosis. Eur. J. Cancer 2016, 65, 52–60. [Google Scholar] [CrossRef]
- Lim, Y.J.; Koh, J.; Kim, S.; Jeon, S.-R.; Chie, E.K.; Kim, K.; Kang, G.H.; Han, S.-W.; Kim, T.-Y.; Jeong, S.-Y.; et al. Chemoradiation-Induced Alteration of Programmed Death-Ligand 1 and CD8+ Tumor-Infiltrating Lymphocytes Identified Patients with Poor Prognosis in Rectal Cancer: A Matched Comparison Analysis. Int. J. Radiat. Oncol. 2017, 99, 1216–1224. [Google Scholar] [CrossRef]
- Berntsson, J.; Eberhard, J.; Nodin, B.; Leandersson, K.; Larsson, A.H.; Jirstrom, K. Expression of programmed cell death protein 1 (PD-1) and its ligand PD-L1 in colorectal cancer: Relationship with sidedness and prognosis. OncoImmunology 2018, 7, e1465165. [Google Scholar] [CrossRef]
- Bosset, J.F.; Collette, L.; Calais, G.; Mineur, L.; Maingon, P.; Radošević-Jelić, L.; Daban, A.; Bardet, E.; Beny, A.; Ollier, J.-C. Chemotherapy with Preoperative Radiotherapy in Rectal Cancer. N. Engl. J. Med. 2006, 355, 1114–1123. [Google Scholar] [CrossRef]
- Chen, T.-W.; Huang, K.C.-Y.; Chiang, S.-F.; Chen, W.T.-L.; Ke, T.-W.; Chao, K.C. Prognostic relevance of programmed cell death-ligand 1 expression and CD8+ TILs in rectal cancer patients before and after neoadjuvant chemoradiotherapy. J. Cancer Res. Clin. Oncol. 2019, 145, 1043–1053. [Google Scholar] [CrossRef]
- Droeser, R.A.; Hirt, C.; Viehl, C.T.; Frey, D.M.; Nebiker, C.A.; Huber, X.; Zlobec, I.; Eppenberger-Castori, S.; Tzankov, A.; Rosso, R.; et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur. J. Cancer 2013, 49, 2233–2242. [Google Scholar] [CrossRef]
- Taube, J.M.; Anders, R.A.; Young, G.D.; Xu, H.; Sharma, R.; McMiller, T.L.; Chen, S.; Klein, A.P.; Pardoll, E.M.; Topalian, S.L.; et al. Colocalization of Inflammatory Response with B7-H1 Expression in Human Melanocytic Lesions Supports an Adaptive Resistance Mechanism of Immune Escape. Sci. Transl. Med. 2012, 4, 127ra37. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Fox, B.A.; Bifulco, C.B.; Masucci, G.; Rau, T.T.; Botti, G.; Marincola, F.M.; Ciliberto, G.; Pagès, F.; Ascierto, P.A.; et al. Immunoscore and Immunoprofiling in cancer: An update from the melanoma and immunotherapy bridge. J. Trans. Med. 2016, 14, 273. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Ogura, A.; Akiyoshi, T.; Yamamoto, N.; Kawachi, H.; Ishikawa, Y.; Mori, S.; Oba, K.; Nagino, M.; Fukunaga, Y.; Ueno, M. Pattern of programmed cell death-ligand 1 expression and CD8-positive T-cell infiltration before and after chemoradiotherapy in rectal cancer. Eur. J. Cancer 2018, 91, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Peng, Q.; Du, K.; He, J.-Y.; Dong, Y.; Lin, X.; Li, J.; Wu, J. Tumor cell PD-L1 predicts poor local control for rectal cancer patients following neoadjuvant radiotherapy. Cancer Manag. Res. 2017, 9, 249–258. [Google Scholar] [CrossRef]



| Cohort | Pre-Treatment | Post-Treatment | |||||
|---|---|---|---|---|---|---|---|
| PDL1 ≤ 0.15 | PDL1 > 0.15 | p Value | PDL1 ≤ 0.5 | PDL1 > 0.5 | p Value 4 | ||
| Age (Years) | 0.14 | 0.39 | |||||
| Mean (SD 1) | 68.1 (10.0) | 66.2 (10.7) | 69.9 (9.0) | 67.0 (11.0) | 69.1 (9.0) | ||
| Median [range] | 69.0 [29.0–85.0] | 68.0 [29.0–81.0] | 70.0 [48.0–85.0] | 69.0 [29.0–84.0] | 70.0 [43.0–85.0] | ||
| Gender | 0.09 | 0.22 | |||||
| Male | 47 (63.5%) | 27 (73.0%) | 20 (54.1%) | 26 (70.3%) | 21 (56.8%) | ||
| Female | 27 (36.5%) | 10 (27.0%) | 17 (45.9%) | 11 (29.7%) | 16 (43.2%) | ||
| Tumor Distance from Anal Verge (mm) | 0.08 | 0.15 | |||||
| N | 72 | 36 | 36 | 37 | 35 | ||
| Mean (SD) | 6.2 (3.8) | 6.9 (3.8) | 5.4 (3.8) | 6.5 (3.5) | 5.7 (4.1) | ||
| Median [range] | 6.0 [0.0–18.0] | 6.5 [0.0–18.0] | 4.5 [0.0–15.0] | 7.0 [0.0–15.0] | 6.0 [0.0–18.0] | ||
| Tumor Distance from Anal Verge (mm) | 0.01 | 0.36 | |||||
| <5 cm | 25 (34.7%) | 7 (19.4%) | 18 (50.0%) | 10 (27.0%) | 15 (42.9%) | ||
| [5–10] cm | 33 (45.8%) | 22 (61.1%) | 11 (30.6%) | 19 (51.4%) | 14 (40.0%) | ||
| ≥10 cm | 14 (19.4%) | 7 (19.4%) | 7 (19.4%) | 8 (21.6%) | 6 (17.1%) | ||
| Missing | 2 | 1 | 1 | 0 | 2 | ||
| Histopathological Subtype | 0.21 | 0.74 | |||||
| Poorly differentiated adenocarcinoma | 2 (2.9%) | 2 (5.6%) | 0 (0.0%) | 1 (2.9%) | 1 (2.8%) | ||
| Moderately differentiated adenocarcinoma | 36 (51.4%) | 21 (58.3%) | 15 (44.1%) | 20 (58.8%) | 16 (44.4%) | ||
| Highly differentiated adenocarcinoma | 30 (42.9%) | 12 (33.3%) | 18 (52.9%) | 12 (35.3%) | 18 (50.0%) | ||
| Other | 2 (2.9%) | 1 (2.8%) | 1 (2.9%) | 1 (2.9%) | 1 (2.8%) | ||
| Missing | 4 | 1 | 3 | 3 | 1 | ||
| cT Stage | 0.59 | 0.70 | |||||
| T2 | 2 (3.0%) | 0 (0.0%) | 2 (6.1%) | 1 (3.2%) | 1 (2.9%) | ||
| T3 | 58 (87.9%) | 30 (90.9%) | 28 (84.8%) | 26 (83.9%) | 32 (91.4%) | ||
| T4 | 6 (9.1%) | 3 (9.1%) | 3 (9.1%) | 4 (12.9%) | 2 (5.7%) | ||
| Missing | 8 | 4 | 4 | 6 | 2 | ||
| cN Stage | 0.88 | 0.37 | |||||
| N0 | 34 (55.7%) | 17 (56.7%) | 17 (54.8%) | 19 (61.3%) | 15 (50.0%) | ||
| N+ | 27 (44.3%) | 13 (43.3%) | 14 (45.2%) | 12 (38.7%) | 15 (50.0%) | ||
| Missing | 13 | 7 | 6 | 6 | 7 | ||
| cM Stage | 1.00 | 1.00 | |||||
| M0 | 66 (95.7%) | 33 (97.1%) | 33 (94.3%) | 34 (94.4%) | 32 (97.0%) | ||
| M+ | 3 (4.3%) | 1 (2.9%) | 2 (5.7%) | 2 (5.6%) | 1 (3.0%) | ||
| Missing | 5 | 3 | 2 | 1 | 4 | ||
| Radiation Dose Per Fraction (Gy) | 0.46 | 0.22 | |||||
| ≤2 | 47 (63.5%) | 25 (67.6%) | 22 (59.5%) | 26 (70.3%) | 21 (56.8%) | ||
| >2 | 27 (36.5%) | 12 (32.4%) | 15 (40.5%) | 11 (29.7%) | 16 (43.2%) | ||
| Chemotherapy | 0.11 | 0.88 | |||||
| No | 44 (60.3%) | 19 (51.4%) | 25 (69.4%) | 22 (59.5%) | 22 (61.1%) | ||
| Yes | 29 (39.7%) | 18 (48.6%) | 11 (30.6%) | 15 (40.5%) | 14 (38.9%) | ||
| Missing | 1 | 0 | 1 | 0 | 1 | ||
| Neoadjuvant Chemotherapy | |||||||
| No | 68 (100.0%) | 34 (100.0%) | 34 (100.0%) | 33 (100.0%) | 35 (100.0%) | ||
| Missing | 6 | 3 | 3 | 4 | 2 | ||
| Concurrent Chemotherapy | 0.73 | 0.46 | |||||
| No | 52 (73.2%) | 25 (71.4%) | 27 (75.0%) | 25 (69.4%) | 27 (77.1%) | ||
| Yes | 19 (26.8%) | 10 (28.6%) | 9 (25.0%) | 11 (30.6%) | 8 (22.9%) | ||
| Missing | 3 | 2 | 1 | 1 | 2 | ||
| Type of Concurrent Chemotherapy | 0.82 | 0.92 | |||||
| 5FU 2 | 1 (5.3%) | 0 (0.0%) | 1 (11.1%) | 1 (9.1%) | 0 (0.0%) | ||
| Xeloda | 3 (15.8%) | 2 (20.0%) | 1 (11.1%) | 2 (18.2%) | 1 (12.5%) | ||
| Folinic acid + 5FU | 12 (63.2%) | 7 (70.0%) | 5 (55.6%) | 7 (63.6%) | 5 (62.5%) | ||
| Folfox | 1 (5.3%) | 0 (0.0%) | 1 (11.1%) | 0 (0.0%) | 1 (12.5%) | ||
| Cisplatine + 5FU | 2 (10.5%) | 1 (10.0%) | 1 (11.1%) | 1 (9.1%) | 1 (12.5%) | ||
| Adjuvant Chemotherapy | 0.20 | 0.78 | |||||
| No | 55 (76.4%) | 26 (70.3%) | 29 (82.9%) | 27 (75.0%) | 28 (77.8%) | ||
| Yes | 17 (23.6%) | 11 (29.7%) | 6 (17.1%) | 9 (25.0%) | 8 (22.2%) | ||
| Missing | 2 | 0 | 2 | 1 | 1 | ||
| Type of Adjuvant Chemotherapy | 1.00 | 0.56 | |||||
| Folinic acid + 5 FU | 9 (52.9%) | 6 (54.5%) | 3 (50.0%) | 5 (55.6%) | 4 (50.0%) | ||
| Folfox | 4 (23.5%) | 2 (18.2%) | 2 (33.3%) | 1 (11.1%) | 3 (37.5%) | ||
| Other | 4 (23.5%) | 3 (27.3%) | 1 (16.7%) | 3 (33.3%) | 1 (12.5%) | ||
| Time between End of Radiotherapy and Surgery (Days) | 0.92 | 0.11 | |||||
| N | 74 | 37 | 37 | 37 | 37 | ||
| Mean (SD) | 43.4 (35.3) | 46.6 (48.8) | 40.2 (11.0) | 42.9 (13.2) | 43.9 (48.5) | ||
| Median [Range] | 40.0 [4.0–319.0] | 42.0 [4.0–319.0] | 40.0 [19.0–65.0] | 42.0 [15.0–77.0] | 39.0 [4.0–319.0] | ||
| Conservative Surgery | 0.07 | 0.18 | |||||
| No | 32 (43.8%) | 12 (33.3%) | 20 (54.1%) | 13 (36.1%) | 19 (51.4%) | ||
| Yes | 41 (56.2%) | 24 (66.7%) | 17 (45.9%) | 23 (63.9%) | 18 (48.6%) | ||
| Missing | 1 | 1 | 0 | 1 | 0 | ||
| Margins | 1.00 | 0.35 | |||||
| R0 | 69 (94.5%) | 34 (94.4%) | 35 (94.6%) | 36 (97.3%) | 33 (91.7%) | ||
| R1 | 4 (5.5%) | 2 (5.6%) | 2 (5.4%) | 1 (2.7%) | 3 (8.3%) | ||
| Missing | 1 | 1 | 0 | 0 | 1 | ||
| pN+ | 0.11 | 0.67 | |||||
| No | 49 (67.1%) | 21 (58.3%) | 28 (75.7%) | 24 (64.9%) | 25 (69.4%) | ||
| Yes | 24 (32.9%) | 15 (41.7%) | 9 (24.3%) | 13 (35.1%) | 11 (30.6%) | ||
| Missing | 1 | 1 | 0 | 0 | 1 | ||
| ypT Stage | 0.69 | 0.40 | |||||
| 0 | 4 (5.4%) | 2 (5.4%) | 2 (5.4%) | 3 (8.1%) | 1 (2.7%) | ||
| 1 | 3 (4.1%) | 1 (2.7%) | 2 (5.4%) | 1 (2.7%) | 2 (5.4%) | ||
| 2 | 16 (21.6%) | 7 (18.9%) | 9 (24.3%) | 5 (13.5%) | 11 (29.7%) | ||
| 3 | 46 (62.2%) | 23 (62.2%) | 23 (62.2%) | 25 (67.6%) | 21 (56.8%) | ||
| 4 | 5 (6.8%) | 4 (10.8%) | 1 (2.7%) | 3 (8.1%) | 2 (5.4%) | ||
| ypN Stage | 0.29 | 0.43 | |||||
| 0 | 49 (68.1%) | 22 (61.1%) | 27 (75.0%) | 23 (63.9%) | 26 (72.2%) | ||
| 1 | 15 (20.8%) | 8 (22.2%) | 7 (19.4%) | 7 (19.4%) | 8 (22.2%) | ||
| 2 | 8 (11.1%) | 6 (16.7%) | 2 (5.6%) | 6 (16.7%) | 2 (5.6%) | ||
| Missing | 2 | 1 | 1 | 1 | 1 | ||
| TRG 3 | 0.58 | 0.21 | |||||
| 1 | 4 (5.5%) | 2 (5.6%) | 2 (5.4%) | 3 (8.1%) | 1 (2.8%) | ||
| 2 | 18 (24.7%) | 9 (25.0%) | 9 (24.3%) | 11 (29.7%) | 7 (19.4%) | ||
| 3 | 24 (32.9%) | 9 (25.0%) | 15 (40.5%) | 11 (29.7%) | 13 (36.1%) | ||
| 4 | 23 (31.5%) | 13 (36.1%) | 10 (27.0%) | 12 (32.4%) | 11 (30.6%) | ||
| 5 | 4 (5.5%) | 3 (8.3%) | 1 (2.7%) | 0 (0.0%) | 4 (11.1%) | ||
| Missing | 1 | 1 | 0 | 0 | 1 | ||
| ≤2 Gy | >2 Gy | p Value 4 | |
|---|---|---|---|
| PD-L1+ Cells Rate on Biopsy | 0.56 | ||
| n1 | 47 | 27 | |
| Mean (SD 2) | 1.1 (2.9) | 1.1 (1.5) | |
| Median [range] | 0.1 [0.0–17.0] | 0.2 [0.0–4.8] | |
| Median PD-L1+ Cells Rate on Biopsy | 0.46 | ||
| ≤0.15 | 25 (53.2%) | 12 (44.4%) | |
| >0.15 | 22 (46.8%) | 15 (55.6%) | |
| PD-L1+ Cells Rate on Surgical Specimen | 0.33 | ||
| n | 47 | 27 | |
| Mean (SD) | 1.8 (4.3) | 1.4 (1.6) | |
| Median [range] | 0.5 [0.0–27.5] | 0.6 [0.0–5.9] | |
| Median PD-L1+ Cells Rate on Surgical Specimen | 0.22 | ||
| ≤0.5 | 26 (55.3%) | 11 (40.7%) | |
| >0.5 | 21 (44.7%) | 16 (59.3%) | |
| Delta PD-L1 3 | 0.52 | ||
| N | 47 | 27 | |
| Mean (SD) | 0.7 (4.9) | 0.4 (1.8) | |
| Median [range] | 0.2 [-17.0–25.9] | 0.1 [-4.1–3.1] |
| PD-L1 Biopsy ≤ 0.15 (n = 27) | PD-L1 Biopsy > 0.15 (n = 26) | p Value 3 | |
| CD8 TILs 1 on Biopsy | 0.65 | ||
| Mean (SD 2) | 47.4 (32.3) | 68.8 (70.4) | |
| Median [range] | 44.5 [1.3–113.0] | 42.8 [0.3–290.7] | |
| Intraepithelial CD8 TILs on Biopsy | 0.62 | ||
| Mean (SD) | 10.5 (13.4) | 14.8 (19.4) | |
| Median [range] | 6.0 [0.0–50.9] | 6.0 [0.0–87.2] | |
| Stromal CD8 TILs on Biopsy | 0.69 | ||
| Mean (SD) | 36.9 (23.9) | 54.1 (54.3) | |
| Median [range] | 37.8 [1.3–87.3] | 34.2 [0.3–203.5] | |
| PD-L1 Surgery ≤ 0.5 (n = 37) | PD-L1 Surgery > 0.5 (n = 36) | p Value | |
| CD8 TILs on Surgical Specimen | 0.99 | ||
| Mean (SD) | 63.6 (49.2) | 71.0 (71.6) | |
| Median [range] | 55.8 [1.7–273.7] | 51.5 [4.0–392.8] | |
| Intraepithelial CD8 TILs on Surgical Specimen | 0.17 | ||
| Mean (SD) | 6.6 (10.9) | 11.2 (15.6) | |
| Median [range] | 1.6 [0.0–49.2] | 4.4 [0.0–65.9] | |
| Intraepithelial CD8 TILs on Surgical Specimen | 0.82 | ||
| Mean (SD) | 57.0 (44.5) | 59.8 (60.9) | |
| Median [range] | 49.2 [1.7–251.8] | 46.9 [2.8–353.6] |
| Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| HR 3 | 95% CI 4 | p Value 5 | HR | 95% CI | p Value 5 | ||
| Gender | n = 74 | 0.93 | |||||
| Female vs Male | 1.025 | [0.542–1.940] | |||||
| Age (Years) | n = 74 | 0.97 | |||||
| >69 vs ≤69 | 1.012 | [0.543–1.884] | |||||
| Tumor Distance from Anal Verge (cm) | n = 72 | 0.37 | |||||
| ≥10 cm vs <5 cm | 1.398 | [0.570–3.427] | |||||
| [5,6,7,8,9,10] cm vs <5 cm | 1.667 | [0.811–3.425] | |||||
| Histological Subtype | n = 70 | 0.79 | |||||
| Highly differentiated vs Poorly or Moderately differentiated adenocarcinoma | 0.827 | [0.428–1.597] | |||||
| Other vs Poorly or Moderately differentiated adenocarcinoma | 1.211 | [0.284–5.170] | |||||
| cN Stage | n = 61 | 0.39 | |||||
| N+ vs N0 | 1.346 | [0.678–2.674] | |||||
| Radiation Dose Per Fraction (Gy) | n = 74 | 0.10 | |||||
| >2 vs ≤2 | 1.686 | [0.899–3.163] | |||||
| Chemotherapy | n = 73 | 0.82 | |||||
| Yes vs No | 1.074 | [0.577–1.996] | |||||
| Concurrent Chemotherapy | n = 71 | 0.88 | |||||
| Yes vs No | 0.949 | [0.470–1.915] | |||||
| Adjuvant Chemotherapy | n = 72 | 0.24 | |||||
| Yes vs No | 1.499 | [0.763–2.945] | |||||
| Time between End of Radiotherapy and Surgery (Days) | n = 74 | 0.24 | |||||
| >40 vs ≤40 | 1.437 | [0.775–2.663] | |||||
| Conservative Surgery | n = 73 | 0.61 | |||||
| Yes vs No | 1.175 | [0.626–2.205] | |||||
| pN+ | n = 73 | 0.14 | |||||
| Yes vs No | 1.613 | [0.845–3.080] | |||||
| ypT Stage | n = 74 | 0.0045 | 2.64 | [1.160–6.031] | 0.02 | ||
| 3–4 vs 0–2 | 3.27 | [1.444–7.404] | |||||
| ypN Stage | n = 72 | 0.29 | |||||
| 1–2 vs 0 | 1.419 | [0.740–2.719] | |||||
| TRG 1 | n = 73 | 0.21 | |||||
| 3–5 vs 1–2 | 1.579 | [0.771–3.235] | |||||
| Median PD-L1 Biopsy | n = 74 | 0.10 | |||||
| >0.15 vs ≤0.15 | 0.596 | [0.317–1.119] | |||||
| Median PD-L1 Surgery | n = 74 | 0.05 | |||||
| >0.5 vs ≤0.5 | 0.542 | [0.287–1.022] | |||||
| Delta PD-L1 2 | n = 74 | 0.90 | |||||
| >0.2 vs ≤0.2 | 0.962 | [0.521–1.777] | |||||
| Evolution of PD-L1+ Cells Rate | n = 74 | 0.07 | |||||
| High-to-high vs low-to-low | 0.329 | [0.127–0.856] | |||||
| High-to-low vs low-to-low | 1.154 | [0.513–2.594] | |||||
| Low-to-high vs low-to-low | 1.007 | [0.450–2.251] | |||||
| High-to-high vs other | 0.317 | [0.132–0.758] | 0.0098 | 0.33 | [0.136–0.811] | 0.01 | |
| PD-L1 Biopsy | n = 74 | 0.0065 | |||||
| >0.08 vs ≤0.08 | 0.422 | [0.227–0.786] | |||||
| Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Variable | HR 3 | 95% CI 4 | p Value 5 | HR | 95% CI | p Value 5 | |
| Gender | n = 74 | 0.83 | |||||
| Female vs Male | 0.937 | [0.508–1.728] | |||||
| Age (Years) | n = 74 | 0.85 | |||||
| >69 vs ≤69 | 0.945 | [0.522–1.710] | |||||
| Tumor Distance from Anal Verge (cm) | n = 72 | 0.21 | |||||
| ≥10 cm vs <5 cm | 1.628 | [0.684–3.874] | |||||
| [5,6,7,8,9,10] cm vs <5 cm | 1.878 | [0.929–3.798] | |||||
| Histological Subtype | n = 70 | 0.81 | |||||
| Highly differentiated vs Poorly or Moderately differentiated adenocarcinoma | 0.824 | [0.442–1.538] | |||||
| Other vs Poorly or Moderately differentiated adenocarcinoma | 1.056 | [0.249–4.484] | |||||
| cN Stage | n = 61 | 0.17 | |||||
| N+ vs N0 | 1.571 | [0.815–3.029] | |||||
| Radiation Dose Per Fraction (Gy) | n = 74 | 0.30 | |||||
| >2 vs ≤2 | 1.373 | [0.752–2.507] | |||||
| Chemotherapy | n = 73 | 0.71 | |||||
| Yes vs No | 1.119 | [0.617–2.030] | |||||
| Concurrent Chemotherapy | n = 71 | 0.89 | |||||
| Yes vs No | 1.048 | [0.536–2.051] | |||||
| Adjuvant Chemotherapy | n = 72 | 0.12 | |||||
| Yes vs No | 1.658 | [0.869–3.163] | |||||
| Time between End of Radiotherapy and Surgery (Days) | n = 74 | 0.34 | |||||
| >40 vs ≤40 | 1.329 | [0.739–2.392] | |||||
| Conservative Surgery | n = 73 | 0.63 | |||||
| Yes vs No | 1.159 | [0.633–2.123] | |||||
| pN+ | n = 73 | 0.13 | |||||
| Yes vs No | 1.611 | [0.866–2.995] | |||||
| ypT Stage | n = 74 | 0.0092 | |||||
| 3–4 vs 0–2 | 2.656 | [1.274–5.537] | 2.223 | [1.055–4.684] | 0.03 | ||
| ypN Stage | n = 72 | 0.36 | |||||
| 1–2 vs 0 | 1.336 | [0.715–2.494] | |||||
| TRG 1 | n = 73 | 0.16 | |||||
| 3–5 vs 1–2 | 1.63 | [0.823–3.229] | |||||
| Median PD-L1 Biopsy | n = 74 | 0.03 | |||||
| >0.15 vs ≤0.15 | 0.512 | [0.279–0.938] | |||||
| Median PD-L1 Surgery | n = 74 | 0.14 | |||||
| >0.5 vs ≤0.5 | 0.645 | [0.355–1.171] | |||||
| Delta PD-L1 2 | n = 74 | 0.84 | |||||
| >0.2 vs ≤0.2 | 1.062 | [0.591–1.907] | |||||
| Evolution of PD-L1+ Cells Rate | n = 74 | 0.06 | |||||
| High-to-high vs low-to-low | 0.344 | [0.141–0.842] | |||||
| High-to-low vs low-to-low | 0.914 | [0.411–2.032] | |||||
| Low-to-high vs low-to-low | 1.158 | [0.546–2.456] | |||||
| High-to-high vs other | 0.338 | [0.150–0.760] | 0.0087 | 0.402 | [0.177–0.916] | 0.03 | |
| PD-L1 Biopsy | n = 4 | 0.0037 | |||||
| >0.08 vs ≤0.08 | 0.414 | [0.229–0.751] | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boustani, J.; Derangère, V.; Bertaut, A.; Adotevi, O.; Morgand, V.; Charon-Barra, C.; Ghiringhelli, F.; Mirjolet, C. Radiotherapy Scheme Effect on PD-L1 Expression for Locally Advanced Rectal Cancer. Cells 2020, 9, 2071. https://doi.org/10.3390/cells9092071
Boustani J, Derangère V, Bertaut A, Adotevi O, Morgand V, Charon-Barra C, Ghiringhelli F, Mirjolet C. Radiotherapy Scheme Effect on PD-L1 Expression for Locally Advanced Rectal Cancer. Cells. 2020; 9(9):2071. https://doi.org/10.3390/cells9092071
Chicago/Turabian StyleBoustani, Jihane, Valentin Derangère, Aurélie Bertaut, Olivier Adotevi, Véronique Morgand, Céline Charon-Barra, François Ghiringhelli, and Céline Mirjolet. 2020. "Radiotherapy Scheme Effect on PD-L1 Expression for Locally Advanced Rectal Cancer" Cells 9, no. 9: 2071. https://doi.org/10.3390/cells9092071
APA StyleBoustani, J., Derangère, V., Bertaut, A., Adotevi, O., Morgand, V., Charon-Barra, C., Ghiringhelli, F., & Mirjolet, C. (2020). Radiotherapy Scheme Effect on PD-L1 Expression for Locally Advanced Rectal Cancer. Cells, 9(9), 2071. https://doi.org/10.3390/cells9092071







