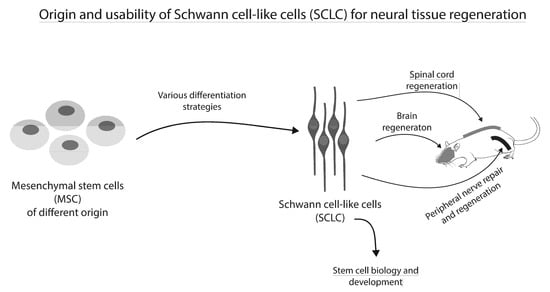
Schwann Cell-Like Cells: Origin and Usability for Repair and Regeneration of the Peripheral and Central Nervous System
Abstract
:1. Introduction
1.1. Schwann Cell Development and Homeostasis
1.2. PNS Injury
1.3. CNS Injury
1.4. SC Transplantation
1.4.1. SC Transplantation in the PNS
1.4.2. SC Transplantation in the CNS
1.5. Biomaterial/Scaffolds
1.6. Immunosuppression Following PNI and SCI
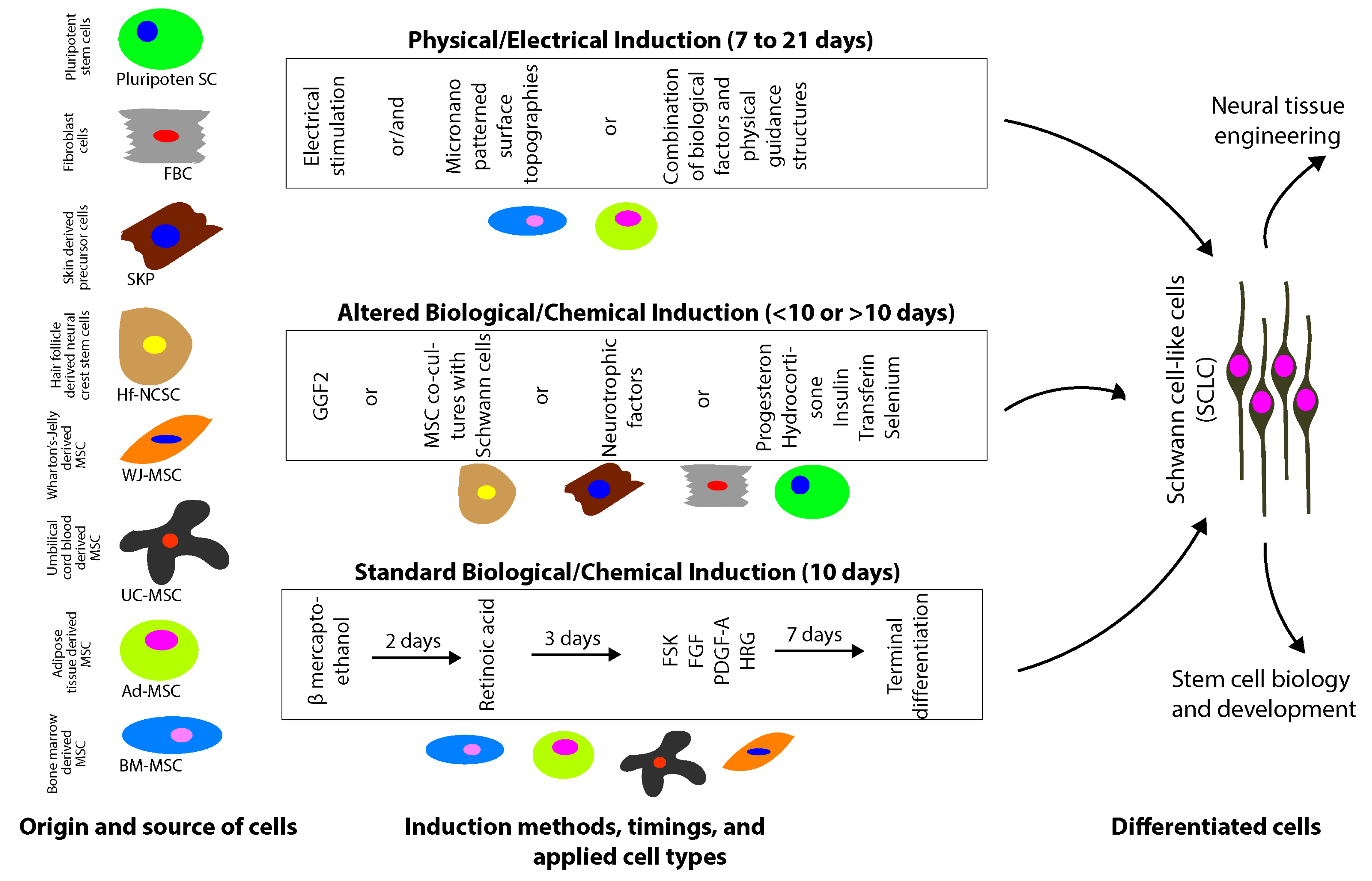
2. Origin and Therapeutic Effects of Schwann Cell-Like Cells (SCLC)
2.1. Mesenchymal Stem/Stromal Cells
2.1.1. Biological/Chemical Induction
2.1.2. Physical-Electrical Induction
2.1.3. Bone Marrow-Derived MSC
In Vitro Characterization
Application in the PNS
Application in the CNS
Limitations
2.1.4. Adipose Tissue-Derived MSC
In Vitro Characterization
Application in the PNS
Application in the CNS
Limitations
2.1.5. Umbilical Cord-Derived MSC
Umbilical Cord Blood-Derived MSC
In Vitro Characterization
In Vivo Application
Limitations
Wharton’s-Jelly-Derived MSC
In Vitro Characterization
Application in the PNS
Application in the CNS
Limitations
2.2. Hair Follicle/Skin-Derived Stem Cells
2.2.1. Neural Crest Stem Cells
In Vitro Characterization
Application in the PNS
Application in the CNS
Limitations
2.2.2. Skin-Derived Precursory Cells
In Vitro Characterization
Application in the PNS
Application in the CNS
Limitations
2.3. Pluripotent Stem Cells
2.3.1. In Vitro Characterization
2.3.2. In Vivo Application
2.3.3. Limitations
2.4. Fibroblasts
| Starting Cell | Induction Factors | Method | Phenotypic Markers | Growth Factor Expression | In Vitro Outcome | In Vivo Outcome | Time (Days) | Subacute/Chronic Injury | Injury | In Vivo Cotreatments | Application in PNS/CNS | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ad-MSC | BME, RA, FSK, bFGF, PDGF, HRG | direct biochemical induction | morphology | BDNF, NGF, GDNF | increased neurites sprouting of NG108-15 neurons, increased neurites length and increased amount of neurites per neuron | increased myelination | 18 days | subacute | rat tibial crush | - | PNS | [134] |
| Ad-MSC | BME, RA, FSK, bFGF, PDGF-AA, HRG | direct biochemical induction | - | BDNF, GDNF, VEGF-A, Angiopoietin-1 | increased neurites length of rat DRG neurons | increased amount and length of axons, increased angiogenesis | 18 days | subacute | 10-mm rat sciatic nerve gap | 14-mm tubular fibrin conduit; Cyclosporine A | PNS | [126] |
| Ad-MSC | BME, RA, FSK, bFGF, PDGF-AA, HRG | direct biochemical induction | morphology | BDNF, GDNF, NGF | withdrawel of differrentiation media cause reversion of the induced SCLC phenotype | - | 18 days | - | - | - | - | [131] |
| Ad-MSC | BME, RA, FSK, bFGF, PDGF, HRG, PROG, Hydrocortisone, Insulin | direct biochemical induction | morphology, GFAP, S100, PMP-22, P0 | BDNF, NGF | - | increased amount of axons, increased myelination, enhanced motor function recovery | 13 days | subacute | 10-mm rat sciatic nerve gap | collagen sponge, cyclosporine A | PNS | [143] |
| BM-MSC | BME, RA, FSK, bFGF, PDGF-AA, GGF-2 | direct biochemical induction | morphology, GFAP, S100, p75, erbB3 | - | increased neurite sprouting, increased neurite length, increase neurite density of rat DRG neuron | - | 18 days | - | - | - | - | [111] |
| BM-MSC | BME, RA, FSK, bFGF, PDGF-AA, HRG | direct biochemical induction | morphology, GFAP, S100, CNPase, p75NTR, P0 | HGF, VEGF | increased number and neurite length of Neuro2A cells | enhanced axonal outgrowth in ex vivo Spinal Cord slices | 12 days | - | - | - | CNS (ex vivo) | [112] |
| BM-MSC | neurosphere induction: bFGF, EGF, B27; SC-like cell induction: FSK, PDGF-AA, bFGF, HRG | two step biochemical induction | morphology, S100, p75 | BDNF, VEGF, HGF, NGF | incresed neurites sprouting, increased neurite length of Neuro2A cells and rat DRG neurons, myelination | functional myelination | 21 days (neurospheres); 14 days (SC-like cells) | Subacute | 5-mm rat sciatic nerve gap | 16-mm chitosan conduit; Cyclosporine A | PNS | [121] |
| BM-MSC | BME, RA, FSK, bFGF, PDGF-AA, HRG | direct biochemical induction | morphology, GFAP, S100, p75, P0 | - | - | increased amount of axons, enhanced motor function outcome | 8–9 days | Subacute | 10-mm rat sciatic nerve gap | 10-mm trans-permeable tubes (Hollow fibers, Amicon, Beverly, MA); tacrolimus | PNS | [116] |
| BM-MSC; Ad-MSC | conditioned SC media | SC co-culture | PMP-22, S100 | - | - | - | 12 days | - | - | - | - | [97] |
| ESC | rosette induction: Stromal feeder cells, BME, SHH, FGF8, BDNF, TGFβ, cAMP, ascorbic acid; SC-like cell induction: HRG, CNTF, cAMP | two step biochemical induction: ESC to neural rosette to SC-like cells | GFAP, S100, MBP | - | - | - | 16 days (rosette); 60 days (SC-like cells) | - | - | - | - | [201] |
| ESC | neurosphere induction: Stromal feeder cell, BME; SC-like cell induction: FSK, bFGF, HRG, ascorbic acid | two step biochemical induction: ESC to neurospheres to SC-like cells | morphology, GFAP, S100, p75, PMP-22, P0, MBP, Krox20 | - | interaction with chicken & rat DRG neurons | - | 14–16 days (neurospheres); 56 days (SC-like cells) | - | - | - | - | [200] |
| ESC/iPSC | NCC induction: stromal feeder cell, B27, FGF2, Rock inhibitor, ascorbic acid; SC-like cell induction: HRG | two step biochemical induction: ESC/iPSC to NCC to SC-like cells | GFAP, S100, p75, erbB3, Sox9, PMP-22, MBP | - | myelination of rat DRG neurons | - | 14 days (neurospheres); 40 days (SC-like cells) | - | - | - | - | [199] |
| ESC/iPSC | rosette induction: CHIR99021, SB431542; SCP induction: NRG1; SC-like cell induction: NRG1, RA, FSK, PDGF-BB | tree step biochemical induction: ESC/iPSC to rosette to SPCs to SC-like cells | morphology, GFAP, S100, PMP-22, PLP | BDNF, GDNF, NGF, CNTF, NT-3, NT-4 | myelination of rat DRG neurons | enhanced myelination, enhanced motor function recovery | 6 days (rosette); 18 days (SPC); 7 days (SC-like cells) | suacute | 6–9 mm mouse sciatic nerve gap | matrigel | PNS | [204] |
| Fibroblasts | SOX10, Krox20 transduction; FSK, bFGF, PDGF, HRG | genetic modification | morphology, GFAP, p75, NG2 | BDNF, GDNF, NGF | increased neurites sprouting of NG108-15 neurons, increased neurites length, increased amount of neurites per neuron, myelination of mice DRG neurons | enhanced myelination, enhanced motor function recovery | 3 days | subacute | 5 mm mouse sciatic nerve gap | 5 mm gelatin hydrogel conduit | PNS | [207] |
| Fibroblasts | SOX10, Krox20 transduction; HRG, FSK | genetic modification | morphology, GFAP, erbB3, MAG, P0, MBP | interaction with murine DRG neurons, increased neurites length | - | 14 days | - | - | - | - | [210] | |
| Hf-NCC | mouse sciatic nerve | In vivo differentiation | GFAP | - | - | enhanced myelination, enhanced electrical signal transduction | Subacute | 2-mm rat sciatic nerve gap | PNS | [174] | ||
| Hf-NCC | GGF-2 | direct biochemical induction | GFAP, S100 | - | - | - | 28 days | - | - | - | [168] | |
| Hf-NCC | BME, RA, FSK, bFGF, PDGF-BB, GGF-2, CHIR99021 (GSK inhibitor, WNT activator), SB431542 (TGFβ1 receptor inhibitor) | direct biochemical induction | morphology, S100, p75, MBP, SOX10, Krox20 | BDNF, FGF2, FGF5, IL6, VEGF | interaction with murine DRG neurons, myelination | - | 4–17 days | - | - | - | [169] | |
| SKP | FSK, HRG | direct biochemical induction | S100, p75, PMP-22, MBP | - | - | integration into CNS white matter in ex vivo spinal cord slices; compact myelin formation in vivo | 10 days | chronic demyelination | shiverer mice brain characterized by extensive demyelination | CNS | [178] | |
| SKP | FSK, HRG | direct biochemical induction | morphology, S100, p75, P0 | - | myleination of rat DRG neurons | alignement with newly formed myelin | 10 days | chronic (implantation 6 days post demyelination) | local demyelination by lysolecthin injection in mice sciatic nerves | PNS | [182] | |
| UCB-MSC | NCC induction: Epidermal Growth Factor, bFGF, B27; SC-like cell induction: RA, FSK, bFGF, PDGF-AA, HRG | two step biochemical induction: UCB-MSC to neurospheres to SC-like cells | morphology, GFAP, S100, Nestin | - | increased neurite sprouting of rat DRG neurons | - | >5 days (neurospheres); 4 days (SC-like cells) | - | - | - | - | [147] |
| UCB-MSC | BME, RA, FSK, bFGF, PDGF-BB, NGF, HRG | direct biochemical induction | morphology, GFAP, S100, p75 | - | - | - | 8 days | - | - | - | - | [148] |
| WJ-MSC | BME, RA, FSK, bFGF, PDGF, HRG | direct biochemical induction | morphology, GFAP, S100, p75, MBP | BDNF, NGF, NT-3 | increased neurite sprouting, increased neurite lenght of rat DRG neurons | - | 12 days | - | - | - | - | [155] |
| WJ-MSC | BME, RA, FSK, bFGF, PDGF, HRG | direct biochemical induction | morphology, GFAP, S100, p75, P0, O4 | - | - | improved amount of axons, myelination, enhanced motor function recovery | 6–7 days | Subacute | 8-mm rat sciatic nerve gap | 8-mm trans-permeable tubes (Hollow fibers, Amicon, Beverly, MA); tacrolimus | PNS | [156] |
2.4.1. In Vitro Characterization
2.4.2. In Vivo Characterization
2.4.3. Limitations
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Johnson, E.O.; Zoubos, A.B.; Soucacos, P.N. Regeneration and repair of peripheral nerves. Injury 2005, 36. [Google Scholar] [CrossRef] [PubMed]
- Hke, A. Mechanisms of Disease: What factors limit the success of peripheral nerve regeneration in humans? Nat. Clin. Pract. Neurol. 2006, 2, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Pfister, B.J.; Gordon, T.; Loverde, J.R.; Kochar, A.S.; Mackinnon, S.E.; Cullen, D.K. Biomedical Engineering Strategies for Peripheral Nerve Repair: Surgical Applications, State of the Art, and Future Challenges. Crit. Rev. Biomed. Eng. 2011, 39, 81–124. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, S.E.; Hudson, A.R. Clinical application of peripheral nerve transplantation. Plast. Reconstr. Surg. 1992, 90, 695–699. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, Y.; Yin, H.-Y.; Guo, Z.-Y.; Xu, F.; Xiao, B.; Jiang, W.-L.; Guo, W.-M.; Meng, H.-Y.; Lu, S.-B.; et al. Differentiation of adipose-derived stem cells into Schwann cell-like cells through intermittent induction: Potential advantage of cellular transient memory function. Stem Cell Res. Ther. 2018, 9, 133. [Google Scholar] [CrossRef] [Green Version]
- Johnson, P.J.; Wood, M.D.; Moore, A.M.; MacKinnon, S.E. Tissue engineered constructs for peripheral nerve surgery. Eur. Sur. 2013, 45, 122–135. [Google Scholar] [CrossRef] [Green Version]
- Gunard, V.; Xu, X.M.; Bunge, M.B. The use of schwann cell transplantation to foster central nervous system repair. Semin. Neurosci. 1993, 5, 401–411. [Google Scholar] [CrossRef]
- Aszmann, O.; Korak, K.; Luegmair, M.; Frey, M. Bridging Critical Nerve Defects through an Acellular Homograft Seeded with Autologous Schwann Cells Obtained from a Regeneration Neuroma of the Proximal Stump. J. Reconstr. Microsurg. 2008, 24, 151–158. [Google Scholar] [CrossRef]
- Hilton, D.A.; Jacob, J.; Househam, L.; Tengah, C. Complications following sural and peroneal nerve biopsies. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1271–1272. [Google Scholar] [CrossRef] [Green Version]
- Stratton, J.A.; Kumar, R.; Sinha, S.; Shah, P.; Stykel, M.; Shapira, Y.; Midha, R.; Biernaskie, J. Purification and Characterization of Schwann Cells from Adult Human Skin and Nerve. eNeuro 2017, 4. [Google Scholar] [CrossRef] [Green Version]
- Rutkowski, J.L.; Kirk, C.J.; Lerner, M.A.; Tennekoon, G.I. Purification and expansion of human Schwann cells in vitro. Nat. Med. 1995, 1, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Andersen, N.D.; Srinivas, S.; Piñero, G.; Monje, P.V. A rapid and versatile method for the isolation, purification and cryogenic storage of Schwann cells from adult rodent nerves. Sci. Rep. 2016, 6, 31781. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, O.A.; Gordon, T. Role of chronic Schwann cell denervation in poor functional recovery after nerve injuries and experimental strategies to combat it. Neurosurgery 2009, 65, A105–A114. [Google Scholar] [CrossRef] [PubMed]
- Cajal, S.R.y. Degeneration and Regeneration of the Nervous System. Nature 1930, 125, 230–231. [Google Scholar] [CrossRef]
- Kidd, G.J.; Ohno, N.; Trapp, B.D. Biology of Schwann cells. Handb. Clin. Neurol. 2013, 115, 55–79. [Google Scholar] [CrossRef]
- Mirsky, R.; Woodhoo, A.; Parkinson, D.B.; Arthur-Farraj, P.; Bhaskaran, A.; Jessen, K.R. Novel signals controlling embryonic Schwann cell development, myelination and dedifferentiation. J. Peripher. Nerv. Syst. 2008, 13, 122–135. [Google Scholar] [CrossRef]
- Murinson, B.B.; Archer, D.R.; Li, Y.; Griffin, J.W. Degeneration of myelinated efferent fibers prompts mitosis in Remak Schwann cells of uninjured C-fiber afferents. J. Neurosci. 2005, 25, 1179–1187. [Google Scholar] [CrossRef] [Green Version]
- Jessen, K.R.; Mirsky, R.; Lloyd, A.C. Schwann Cells: Development and Role in Nerve Repair. Cold Spring Harb. Perspect. Biol. 2015, 7, a020487. [Google Scholar] [CrossRef]
- Webster, H.D. The geometry of peripheral myelin sheaths during their formation and growth in rat sciatic nerves. J. Cell Biol. 1971, 48, 348–367. [Google Scholar] [CrossRef]
- Topilko, P.; Schneider-Maunoury, S.; Levi, G.; Baron-Van Evercooren, A.; Chennoufi, A.B.; Seitanidou, T.; Babinet, C.; Charnay, P. Krox-20 controls myelination in the peripheral nervous system. Nature 1994, 371, 796–799. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The Success and Failure of the Schwann Cell Response to Nerve Injury. Front. Cell. Neurosci. 2019, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cattin, A.L.; Burden, J.J.; Van Emmenis, L.; Mackenzie, F.E.; Hoving, J.J.; Garcia Calavia, N.; Guo, Y.; McLaughlin, M.; Rosenberg, L.H.; Quereda, V.; et al. Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell 2015, 162, 1127–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef] [Green Version]
- Richner, M.; Ulrichsen, M.; Elmegaard, S.L.; Dieu, R.; Pallesen, L.T.; Vaegter, C.B. Peripheral Nerve Injury Modulates Neurotrophin Signaling in the Peripheral and Central Nervous System. Mol. Neurobiol. 2014, 50, 945–970. [Google Scholar] [CrossRef] [PubMed]
- Frostick, S.P.; Yin, Q.; Kemp, G.J. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery 1998, 18, 397–405. [Google Scholar] [CrossRef]
- Terenghi, G. Peripheral nerve regeneration and neurotrophic factors. J. Anat. 1999, 194, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.W.; Keynes, R.J. Peripheral nerve regeneration. Annu. Rev. Neurosci. 1990, 13, 43–60. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. Signals that determine Schwann cell identity. J. Anat. 2002, 200, 367–376. [Google Scholar] [CrossRef]
- Anderson, K.D.; Guest, J.D.; Dietrich, W.D.; Bartlett Bunge, M.; Curiel, R.; Dididze, M.; Green, B.A.; Khan, A.; Pearse, D.D.; Saraf-Lavi, E.; et al. Safety of Autologous Human Schwann Cell Transplantation in Subacute Thoracic Spinal Cord Injury. J. Neurotrauma 2017, 34, 2950–2963. [Google Scholar] [CrossRef]
- Ma, M.S.; Boddeke, E.; Copray, S. Pluripotent Stem Cells for Schwann Cell Engineering. Stem Cell Rev. Rep. 2015, 11, 205–218. [Google Scholar] [CrossRef]
- Rodríguez, F.J.; Verdú, E.; Ceballos, D.; Navarro, X. Nerve guides seeded with autologous schwann cells improve nerve regeneration. Exp. Neurol. 2000, 161, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Fansa, H.; Keilhoff, G. Comparison of different biogenic matrices seeded with cultured Schwann cells for bridging peripheral nerve defects. Neurol. Res. 2004, 26, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Llorens, F.; Gil, V.; del Río, J.A. Emerging functions of myelin-associated proteins during development, neuronal plasticity, and neurodegeneration. FASEB J. 2011, 25, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Zörner, B.; Schwab, M.E. Anti-Nogo on the go: From animal models to a clinical trial. Ann. N. Y. Acad. Sci. 2010, 1198, E22–E34. [Google Scholar] [CrossRef] [PubMed]
- Barres, B.A.; Jacobson, M.D.; Schmid, R.; Sendtner, M.; Raff, M.C. Does oligodendrocyte survival depend on axons? Curr. Biol. 1993, 3, 489–497. [Google Scholar] [CrossRef]
- Ludwin, S.K. Oligodendrocyte survival in Wallerian degeneration. Acta Neuropathol. 1990, 80, 184–191. [Google Scholar] [CrossRef]
- Buffo, A.; Rite, I.; Tripathi, P.; Lepier, A.; Colak, D.; Horn, A.P.; Mori, T.; Götz, M. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc. Natl. Acad. Sci. USA 2008, 105, 3581–3586. [Google Scholar] [CrossRef] [Green Version]
- Pekny, M.; Pekna, M. Astrocyte intermediate filaments in CNS pathologies and regeneration. J. Pathol. 2004, 204, 428–437. [Google Scholar] [CrossRef]
- Pineau, I.; Sun, L.; Bastien, D.; Lacroix, S. Astrocytes initiate inflammation in the injured mouse spinal cord by promoting the entry of neutrophils and inflammatory monocytes in an IL-1 receptor/MyD88-dependent fashion. Brain. Behav. Immun. 2010, 24, 540–553. [Google Scholar] [CrossRef]
- Satake, K.; Matsuyama, Y.; Kamiya, M.; Kawakami, H.; Iwata, H.; Adachi, K.; Kiuchi, K. Nitric oxide via macrophage iNOS induces apoptosis following traumatic spinal cord injury. Brain Res. Mol. Brain Res. 2000, 85, 114–122. [Google Scholar] [CrossRef]
- Antonios, J.P.; Farah, G.J.; Cleary, D.R.; Martin, J.R.; Ciacci, J.D.; Pham, M.H. Immunosuppressive mechanisms for stem cell transplant survival in spinal cord injury. Neurosurg. Focus 2019, 46, E9. [Google Scholar] [CrossRef] [PubMed]
- Lavdas, A.A.; Papastefanaki, F.; Thomaidou, D.; Matsas, R. Schwann cell transplantation for CNS repair. Curr. Med. Chem. 2008, 15, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.J.; Blakemore, W.F. Requirements for Schwann cell migration within CNS environments: A viewpoint. Int. J. Dev. Neurosci. 1993, 11, 641–649. [Google Scholar] [CrossRef]
- Hirano, A.; Zimmerman, H.M.; Levine, S. Electron microscopic observations of peripheral myelin in a central nervous system lesion. Acta Neuropathol. 1969, 12, 348–365. [Google Scholar] [CrossRef]
- Gilmore, S.A.; Sims, T.J. Patterns of Schwann cell myelination of axons within the spinal cord. J. Chem. Neuroanat. 1993, 6, 191–199. [Google Scholar] [CrossRef]
- Black, J.A.; Waxman, S.G.; Smith, K.J. Remyelination of dorsal column axons by endogenous Schwann cells restores the normal pattern of Nav1.6 and Kv1.2 at nodes of Ranvier. Brain 2006, 129, 1319–1329. [Google Scholar] [CrossRef] [Green Version]
- Blakemore, W.F.; Franklin, R.J. Transplantation options for therapeutic central nervous system remyelination. Cell Transplant. 2000, 9, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Zawadzka, M.; Rivers, L.E.; Fancy, S.P.; Zhao, C.; Tripathi, R.; Jamen, F.; Young, K.; Goncharevich, A.; Pohl, H.; Rizzi, M.; et al. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell 2010, 6, 578–590. [Google Scholar] [CrossRef] [Green Version]
- Assinck, P.; Duncan, G.J.; Plemel, J.R.; Lee, M.J.; Stratton, J.A.; Manesh, S.B.; Liu, J.; Ramer, L.M.; Kang, S.H.; Bergles, D.E.; et al. Myelinogenic Plasticity of Oligodendrocyte Precursor Cells following Spinal Cord Contusion Injury. J. Neurosci. 2017, 37, 8635–8654. [Google Scholar] [CrossRef]
- Monteiro de Castro, G.; Deja, N.A.; Ma, D.; Zhao, C.; Franklin, R.J. Astrocyte Activation via Stat3 Signaling Determines the Balance of Oligodendrocyte versus Schwann Cell Remyelination. Am. J. Pathol. 2015, 185, 2431–2440. [Google Scholar] [CrossRef] [Green Version]
- Ulanska-Poutanen, J.; Mieczkowski, J.; Zhao, C.; Konarzewska, K.; Kaza, B.; Pohl, H.B.; Bugajski, L.; Kaminska, B.; Franklin, R.J.; Zawadzka, M. Injury-induced perivascular niche supports alternative differentiation of adult rodent CNS progenitor cells. eLife 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Norenberg, M.D.; Smith, J.; Marcillo, A. The pathology of human spinal cord injury: Defining the problems. J. Neurotrauma 2004, 21, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Bruce, J.H.; Norenberg, M.D.; Kraydieh, S.; Puckett, W.; Marcillo, A.; Dietrich, D. Schwannosis: Role of gliosis and proteoglycan in human spinal cord injury. J. Neurotrauma 2000, 17, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.M.; McGuinness, U.M.; Aguayo, A.J. Axons from CNS neurons regenerate into PNS grafts. Nature 1980, 284, 264–265. [Google Scholar] [CrossRef]
- Harvey, A.R.; Plant, G.W.; Tan, M.M. Schwann cells and the regrowth of axons in the mammalian CNS: A review of transplantation studies in the rat visual system. Clin. Exp. Pharmacol. Physiol. 1995, 22, 569–579. [Google Scholar] [CrossRef]
- Bunge, M.B. Transplantation of purified populations of Schwann cells into lesioned adult rat spinal cord. J. Neurol. 1994, 242, S36–S39. [Google Scholar] [CrossRef]
- Dezawa, M.; Nagano, T. Contacts between regenerating axons and the Schwann cells of sciatic nerve segments grafted to the optic nerve of adult rats. J. Neurocytol. 1993, 22, 1103–1112. [Google Scholar] [CrossRef]
- Blight, A.R.; Young, W. Central axons in injured cat spinal cord recover electrophysiological function following remyelination by Schwann cells. J. Neurol. Sci. 1989, 91, 15–34. [Google Scholar] [CrossRef]
- Bunge, R.P. The role of the Schwann cell in trophic support and regeneration. J. Neurol. 1994, 242, S19–S21. [Google Scholar] [CrossRef]
- Ra, J.C.; Shin, I.S.; Kim, S.H.; Kang, S.K.; Kang, B.C.; Lee, H.Y.; Kim, Y.J.; Jo, J.Y.; Yoon, E.J.; Choi, H.J.; et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011, 20, 1297–1308. [Google Scholar] [CrossRef]
- Halfpenny, C.; Benn, T.; Scolding, N. Cell transplantation, myelin repair, and multiple sclerosis. Lancet Neurol. 2002, 1, 31–40. [Google Scholar] [CrossRef]
- Kocsis, J.D.; Waxman, S.G. Schwann cells and their precursors for repair of central nervous system myelin. Brain 2007, 130, 1978–1980. [Google Scholar] [CrossRef] [Green Version]
- Woodhoo, A.; Sahni, V.; Gilson, J.; Setzu, A.; Franklin, R.J.; Blakemore, W.F.; Mirsky, R.; Jessen, K.R. Schwann cell precursors: A favourable cell for myelin repair in the Central Nervous System. Brain 2007, 130, 2175–2185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavdas, A.A.; Franceschini, I.; Dubois-Dalcq, M.; Matsas, R. Schwann cells genetically engineered to express PSA show enhanced migratory potential without impairment of their myelinating ability in vitro. Glia 2006, 53, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Papastefanaki, F.; Chen, J.; Lavdas, A.A.; Thomaidou, D.; Schachner, M.; Matsas, R. Grafts of Schwann cells engineered to express PSA-NCAM promote functional recovery after spinal cord injury. Brain 2007, 130, 2159–2174. [Google Scholar] [CrossRef] [Green Version]
- Bunge, M.B. Efficacy of Schwann cell transplantation for spinal cord repair is improved with combinatorial strategies. J. Physiol. 2016, 594, 3533–3538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.; Xu, X.M.; Kleitman, N.; Bunge, M.B. Methylprednisolone administration improves axonal regeneration into Schwann cell grafts in transected adult rat thoracic spinal cord. Exp. Neurol. 1996, 138, 261–276. [Google Scholar] [CrossRef]
- Xu, X.M.; Guénard, V.; Kleitman, N.; Aebischer, P.; Bunge, M.B. A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Exp. Neurol. 1995, 134, 261–272. [Google Scholar] [CrossRef]
- Takami, T.; Oudega, M.; Bates, M.L.; Wood, P.M.; Kleitman, N.; Bunge, M.B. Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. J. Neurosci. 2002, 22, 6670–6681. [Google Scholar] [CrossRef] [Green Version]
- Fouad, K.; Schnell, L.; Bunge, M.B.; Schwab, M.E.; Liebscher, T.; Pearse, D.D. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J. Neurosci. 2005, 25, 1169–1178. [Google Scholar] [CrossRef]
- Pearse, D.D.; Pereira, F.C.; Marcillo, A.E.; Bates, M.L.; Berrocal, Y.A.; Filbin, M.T.; Bunge, M.B. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat. Med. 2004, 10, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Menei, P.; Montero-Menei, C.; Whittemore, S.R.; Bunge, R.P.; Bunge, M.B. Schwann cells genetically modified to secrete human BDNF promote enhanced axonal regrowth across transected adult rat spinal cord. Eur. J. Neurosci. 1998, 10, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Golden, K.L.; Pearse, D.D.; Blits, B.; Garg, M.S.; Oudega, M.; Wood, P.M.; Bunge, M.B. Transduced Schwann cells promote axon growth and myelination after spinal cord injury. Exp. Neurol. 2007, 207, 203–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flora, G.; Joseph, G.; Patel, S.; Singh, A.; Bleicher, D.; Barakat, D.J.; Louro, J.; Fenton, S.; Garg, M.; Bunge, M.B.; et al. Combining neurotrophin-transduced schwann cells and rolipram to promote functional recovery from subacute spinal cord injury. Cell Transplant. 2013, 22, 2203–2217. [Google Scholar] [CrossRef] [Green Version]
- Fortun, J.; Hill, C.E.; Bunge, M.B. Combinatorial strategies with Schwann cell transplantation to improve repair of the injured spinal cord. Neurosci. Lett. 2009, 456, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Tetzlaff, W.; Okon, E.B.; Karimi-Abdolrezaee, S.; Hill, C.E.; Sparling, J.S.; Plemel, J.R.; Plunet, W.T.; Tsai, E.C.; Baptiste, D.; Smithson, L.J.; et al. A systematic review of cellular transplantation therapies for spinal cord injury. J. Neurotrauma 2011, 28, 1611–1682. [Google Scholar] [CrossRef]
- Griffin, J.M.; Bradke, F. Therapeutic repair for spinal cord injury: Combinatory approaches to address a multifaceted problem. EMBO Mol. Med. 2020, 12, e11505. [Google Scholar] [CrossRef]
- Terzis, J.; Faibisoff, B.; Williams, B. The nerve gap: Suture under tension vs. graft. Plast. Reconstr. Surg. 1975, 56, 166–170. [Google Scholar] [CrossRef] [Green Version]
- Sarker, M.D.; Naghieh, S.; McInnes, A.D.; Schreyer, D.J.; Chen, X. Regeneration of peripheral nerves by nerve guidance conduits: Influence of design, biopolymers, cells, growth factors, and physical stimuli. Prog. Neurobiol. 2018, 171, 125–150. [Google Scholar] [CrossRef]
- Gillon, R.S.; Cui, Q.; Dunlop, S.A.; Harvey, A.R. Effects of immunosuppression on regrowth of adult rat retinal ganglion cell axons into peripheral nerve allografts. J. Neurosci. Res. 2003, 74, 524–532. [Google Scholar] [CrossRef]
- Fansa, H.; Keilhoff, G.; Horn, T.; Altmann, S.; Wolf, G.; Schneider, W. Stimulation of Schwann cell growth and axon regeneration of peripheral nerves by the immunosuppressive drug FK 506. Handchir. Mikrochir. Plast. Chir. 1999, 31, 323–329; discussion 330–322. [Google Scholar] [CrossRef] [PubMed]
- Sosa, I.; Reyes, O.; Kuffler, D.P. Immunosuppressants: Neuroprotection and promoting neurological recovery following peripheral nerve and spinal cord lesions. Exp. Neurol. 2005, 195, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Ayala-Cuellar, A.P.; Kang, J.H.; Jeung, E.B.; Choi, K.C. Roles of mesenchymal stem cells in tissue regeneration and immunomodulation. Biomol. Ther. 2019, 27, 25–33. [Google Scholar] [CrossRef]
- Soria-Zavala, K.; García-Sánchez, J.; Rodríguez-Barrera, R. Mesenchymal Stem Cells for Clinical Use after Spinal Cord Injury. IntechOpen 2020. [Google Scholar]
- Dezawa, M.; Takahashi, I.; Esaki, M.; Takano, M.; Sawada, H. Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. Eur. J. Neurosci. 2001, 14, 1771–1776. [Google Scholar] [CrossRef]
- Woodbury, D.; Schwarz, E.J.; Prockop, D.J.; Black, I.B. Adult rat and human bone marrow stromal cells differentiate into neurons. J. Neurosci. Res. 2000, 61, 364–370. [Google Scholar] [CrossRef]
- Takahashi, J.; Palmer, T.D.; Gage, F.H. Retinoic acid and neurotrophins collaborate to regulate neurogenesis in adult-derived neural stem cell cultures. J. Neurobiol. 1999, 38, 65–81. [Google Scholar] [CrossRef]
- Shah, N.M.; Groves, A.K.; Anderson, D.J. Alternative neural crest cell fates are instructively promoted by TGFbeta superfamily members. Cell 1996, 85, 331–343. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, L.R.; Avioli, L.V. Activation of extracellular signal-regulated kinases 1 and 2 (ERK1 and ERK2) by FGF-2 and PDGF-BB in normal human osteoblastic and bone marrow stromal cells: Differences in mobility and in-gel renaturation of ERK1 in human, rat, and mouse osteoblastic cells. Biochem. Biophys. Res. Commun. 1997, 238, 134–139. [Google Scholar] [CrossRef]
- Kim, H.A.; Ratner, N.; Roberts, T.M.; Stiles, C.D. Schwann cell proliferative responses to cAMP and Nf1 are mediated by cyclin D1. J. Neurosci. 2001, 21, 1110–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer-Franke, A.; Wilkinson, G.A.; Kruttgen, A.; Hu, M.; Munro, E.; Hanson, M.G., Jr.; Reichardt, L.F.; Barres, B.A. Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron 1998, 21, 681–693. [Google Scholar] [CrossRef] [Green Version]
- Tohill, M.; Mantovani, C.; Wiberg, M.; Terenghi, G. Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neurosci. Lett. 2004, 362, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Caddick, J.; Kingham, P.J.; Gardiner, N.J.; Wiberg, M.; Terenghi, G. Phenotypic and functional characteristics of mesenchymal stem cells differentiated along a Schwann cell lineage. Glia 2006, 54, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Mahanthappa, N.K.; Anton, E.S.; Matthew, W.D. Glial Growth Factor 2, a Soluble Neuregulin, Directly Increases Schwann Cell Motility and Indirectly Promotes Neurite Outgrowth. J. Neurosci. 1996, 16, 4673–4683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, N.M.; Marchionni, M.A.; Isaacs, I.; Stroobant, P.; Anderson, D.J. Glial growth factor restricts mammalian neural crest stem cells to a glial fate. Cell 1994, 77, 349–360. [Google Scholar] [CrossRef]
- Krampera, M.; Marconi, S.; Pasini, A.; Galiè, M. Induction of neural-like differentiation in human mesenchymal stem cells derived from bone marrow, fat, spleen and thymus. Bone 2007. [Google Scholar] [CrossRef] [PubMed]
- Ariza, C.A.; Fleury, A.T.; Tormos, C.J.; Petruk, V.; Chawla, S.; Oh, J.; Sakaguchi, D.S.; Mallapragada, S.K. The Influence of Electric Fields on Hippocampal Neural Progenitor Cells. Stem Cell Rev. Rep. 2010, 6, 585–600. [Google Scholar] [CrossRef]
- Pires, F.; Ferreira, Q.; Rodrigues, C.A.V.; Morgado, J.; Ferreira, F.C. Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: Expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 1158–1168. [Google Scholar] [CrossRef]
- Stewart, E.; Kobayashi, N.R.; Higgins, M.J.; Quigley, A.F.; Jamali, S.; Moulton, S.E.; Kapsa, R.M.I.; Wallace, G.G.; Crook, J.M. Electrical stimulation using conductive polymer polypyrrole promotes differentiation of human neural stem cells: A biocompatible platform for translational neural tissue engineering. Tissue Eng. Part C Methods 2015, 21, 385–393. [Google Scholar] [CrossRef]
- Das, S.R.; Uz, M.; Ding, S.; Lentner, M.T.; Hondred, J.A.; Cargill, A.A.; Sakaguchi, D.S.; Mallapragada, S.; Claussen, J.C. Electrical Differentiation of Mesenchymal Stem Cells into Schwann-Cell-Like Phenotypes Using Inkjet-Printed Graphene Circuits. Adv. Healthc. Mater. 2017, 6, 1601087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thrivikraman, G.; Madras, G.; Basu, B. Intermittent electrical stimuli for guidance of human mesenchymal stem cell lineage commitment towards neural-like cells on electroconductive substrates. Biomaterials 2014, 35, 6219–6235. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Seo, Y.K.; Yoon, H.H.; Kim, C.W.; Park, J.K.; Jeon, S. Electromagnetic fields induce neural differentiation of human bone marrow derived mesenchymal stem cells via ROS mediated EGFR activation. Neurochem. Int. 2013, 62, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Hammerick, K.E.; Longaker, M.T.; Prinz, F.B. In vitro effects of direct current electric fields on adipose-derived stromal cells. Biochem. Biophys. Res. Commun. 2010, 397, 12–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moosazadeh Moghaddam, M.; Bonakdar, S.; Shokrgozar, M.A.; Zaminy, A.; Vali, H.; Faghihi, S. Engineered substrates with imprinted cell-like topographies induce direct differentiation of adipose-derived mesenchymal stem cells into Schwann cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1022–1035. [Google Scholar] [CrossRef]
- Sharma, A.D.; Zbarska, S.; Petersen, E.M.; Marti, M.E.; Mallapragada, S.K.; Sakaguchi, D.S. Oriented growth and transdifferentiation of mesenchymal stem cells towards a Schwann cell fate on micropatterned substrates. J. Biosci. Bioeng. 2016, 121, 325–335. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.; Sokabe, T.; Watabe, T.; Miyazono, K.; Yamashita, J.K.; Obi, S.; Ohura, N.; Matsushita, A.; Kamiya, A.; Ando, J. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1915–H1924. [Google Scholar] [CrossRef] [Green Version]
- Kurpinski, K.; Chu, J.; Hashi, C.; Li, S. Anisotropic mechanosensing by mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 2006, 103, 16095–16100. [Google Scholar] [CrossRef] [Green Version]
- Clause, K.C.; Liu, L.J.; Tobita, K. Directed stem cell differentiation: The role of physical forces. Cell Commun. Adhes. 2010, 17, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Salazar, M.; Gonzalez-Galofre, Z.N.; Casamitjana, J.; Crisan, M.; James, A.W.; Péault, B. Five Decades Later, Are Mesenchymal Stem Cells Still Relevant? Front. Bioeng. Biotechnol. 2020, 8, 148. [Google Scholar] [CrossRef] [Green Version]
- Brohlin, M.; Mahay, D.; Novikov, L.N.; Terenghi, G.; Wiberg, M.; Shawcross, S.G.; Novikova, L.N. Characterisation of human mesenchymal stem cells following differentiation into Schwann cell-like cells. Neurosci. Res. 2009, 64, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Park, H.W.; Lim, M.J.; Jung, H.; Lee, S.P.; Paik, K.S.; Chang, M.S. Human mesenchymal stem cell-derived Schwann cell-like cells exhibit neurotrophic effects, via distinct growth factor production, in a model of spinal cord injury. Glia 2010. [Google Scholar] [CrossRef] [PubMed]
- Saab, A.S.; Nave, K.A. Myelin dynamics: Protecting and shaping neuronal functions. Curr. Opin. Neurobiol. 2017, 47, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Mimura, T.; Dezawa, M.; Kanno, H.; Sawada, H.; Yamamoto, I. Peripheral nerve regeneration by transplantation of bone marrow stromal cell-derived Schwann cells in adult rats. J. Neurosurg. 2004, 101, 806–812. [Google Scholar] [CrossRef]
- Wang, X.; Luo, E.; Li, Y.; Hu, J. Schwann-like mesenchymal stem cells within vein graft facilitate facial nerve regeneration and remyelination. Brain Res. 2011, 1383, 71–80. [Google Scholar] [CrossRef]
- Shimizu, S.; Kitada, M.; Ishikawa, H.; Itokazu, Y.; Wakao, S.; Dezawa, M. Peripheral nerve regeneration by the in vitro differentiated-human bone marrow stromal cells with Schwann cell property. Biochem. Biophys. Res. Commun. 2007, 359, 915–920. [Google Scholar] [CrossRef]
- Zaminy, A.A. Mesenchymal stem cells as an alternative for Schwann cells in rat spinal cord injury. Iran. Biomed. J. 2013, 17, 113–122. [Google Scholar] [CrossRef]
- Galhom, R.A.; Hussein Abd El Raouf, H.H.; Mohammed Ali, M.H. Role of bone marrow derived mesenchymal stromal cells and Schwann-like cells transplantation on spinal cord injury in adult male albino rats. Biomed. Pharmacother. 2018, 108, 1365–1375. [Google Scholar] [CrossRef]
- Biernaskie, J.; Sparling, J.S.; Liu, J.; Shannon, C.P.; Plemel, J.R.; Xie, Y.; Miller, F.D.; Tetzlaff, W. Skin-derived precursors generate myelinating Schwann cells that promote remyelination and functional recovery after contusion spinal cord injury. J. Neurosci. 2007, 27, 9545–9559. [Google Scholar] [CrossRef] [Green Version]
- Shea, G.K.; Tsui, A.Y.; Chan, Y.S.; Shum, D.K. Bone marrow-derived Schwann cells achieve fate commitment—A prerequisite for remyelination therapy. Exp. Neurol. 2010, 224, 448–458. [Google Scholar] [CrossRef]
- Cai, S.; Tsui, Y.P.; Tam, K.W.; Shea, G.K.; Chang, R.S.; Ao, Q.; Shum, D.K.; Chan, Y.S. Directed Differentiation of Human Bone Marrow Stromal Cells to Fate-Committed Schwann Cells. Stem Cell Rep. 2017, 9, 1097–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabatino, M.A.; Santoro, R.; Gueven, S.; Jaquiery, C.; Wendt, D.J.; Martin, I.; Moretti, M.; Barbero, A. Cartilage graft engineering by co-culturing primary human articular chondrocytes with human bone marrow stromal cells. J. Tissue Eng. Regen. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Eichler, H.; Stoeve, J.; Kluter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Ema, H.; Morita, Y.; Yamazaki, S.; Matsubara, A.; Seita, J.; Tadokoro, Y.; Kondo, H.; Takano, H.; Nakauchi, H. Adult mouse hematopoietic stem cells: Purification and single-cell assays. Nat. Protoc. 2006, 1, 2979–2987. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [Green Version]
- Kingham, P.J.; Kalbermatten, D.F.; Mahay, D.; Armstrong, S.J.; Wiberg, M.; Terenghi, G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp. Neurol. 2007, 207, 267–274. [Google Scholar] [CrossRef]
- di Summa, P.G.; Kalbermatten, D.F.; Raffoul, W.; Terenghi, G.; Kingham, P.J. Extracellular matrix molecules enhance the neurotrophic effect of Schwann cell-like differentiated adipose-derived stem cells and increase cell survival under stress conditions. Tissue Eng. Part A 2013, 19, 368–379. [Google Scholar] [CrossRef] [Green Version]
- di Summa, P.G.; Kingham, P.J.; Raffoul, W.; Wiberg, M.; Terenghi, G.; Kalbermatten, D.F. Adipose-derived stem cells enhance peripheral nerve regeneration. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 1544–1552. [Google Scholar] [CrossRef]
- Kaewkhaw, R.; Scutt, A.M.; Haycock, J.W. Anatomical Site Influences the Differentiation of Adipose-Derived Stem Cells for Schwann-Cell Phenotype and Function. Glia 2011, 749, 734–749. [Google Scholar] [CrossRef]
- Faroni, A.; Terenghi, G.; Magnaghi, V. Expression of functional γ-aminobutyric acid type A receptors in schwann-like adult stem cells. J. Mol. Neurosci. 2012, 47, 619–630. [Google Scholar] [CrossRef]
- Faroni, A.; Rothwell, S.W.; Grolla, A.A.a. Differentiation of adipose-derived stem cells into Schwann cell phenotype induces expression of P2X receptors that control cell death. Cell Death Dis. 2013, 4, e743. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Madura, T.; Mantovani, C.; Terenghi, G. Differentiated adipose-derived stem cells promote myelination and enhance functional recovery in a rat model of chronic denervation. J. Neurosci. Res. 2012, 90, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Kingham, P.J.; Kolar, M.K.; Novikova, L.N.; Novikov, L.N.; Wiberg, M. Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells Dev. 2014, 23, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Madura, T.; Sakai, Y.; Yano, K.; Terenghi, G.; Hosokawa, K. Glial differentiation of human adipose-derived stem cells: Implications for cell-based transplantation therapy. Neuroscience 2013, 16, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, M.; Golding, J.P.; Loughlin, A.J.; Kingham, P.J.; Phillips, J.B. Engineered neural tissue with aligned, differentiated adipose-derived stem cells promotes peripheral nerve regeneration across a critical sized defect in rat sciatic nerve. Biomaterials 2015, 37, 242–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- di Summa, P.G.; Kalbermatten, D.F.; Pralong, E.; Raffoul, W.; Kingham, P.J.; Terenghi, G. Long-term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience 2011, 181, 278–291. [Google Scholar] [CrossRef]
- Orbay, H.; Uysal, A.C.; Hyakusoku, H.; Mizuno, H. Differentiated and undifferentiated adipose-derived stem cells improve function in rats with peripheral nerve gaps. J. Plast. Reconstr. Aesthet. Surg. 2012, 65, 657–664. [Google Scholar] [CrossRef]
- Zaminy, A.; Shokrgozar, M.A.; Sadeghi, Y.; Norouzian, M.; Heidari, M.H.; Piryaei, A. Transplantation of schwann cells differentiated from adipose stem cells improves functional recovery in rat spinal cord injury. Arch. Iran. Med. 2013, 16, 533–541. [Google Scholar]
- Yang, L.; Fang, J.; Liao, D.; Wang, W. Schwann cells differentiated from adipose-derived stem cells for the treatment of brain contusion. Mol. Med. Report. 2014, 9, 567–573. [Google Scholar] [CrossRef] [Green Version]
- Faroni, A.; Smith, R.J.P.; Lu, L.; Reid, A.J. Human Schwann-like cells derived from adipose-derived mesenchymal stem cells rapidly de-differentiate in the absence of stimulating medium. Eur. J. Neurosci. 2016, 43, 417–430. [Google Scholar] [CrossRef]
- Topilko, P.; Murphy, P.; Charnay, P. Embryonic development of Schwann cells: Multiple roles for neuregulins along the pathway. Mol. Cell. Neurosci. 1996, 8, 71–75. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Liu, W.; Lu, X.; Liu, Z.; Zhao, X.; Li, G.; Chen, Z. A Modified Approach to Inducing Bone Marrow Stromal Cells to Differentiate into Cells with Mature Schwann Cell Phenotypes. Stem Cells Dev. 2016, 25, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Liu, Y.; Liu, Z.; Ren, S.; Xiong, H.; Chen, J.; Duscher, D.; Machens, H.G.; Liu, W.; Guo, G.; et al. Differentiated human adipose-derived stromal cells exhibit the phenotypic and functional characteristics of mature Schwann cells through a modified approach. Cytotherapy 2019, 21, 987–1003. [Google Scholar] [CrossRef]
- Weiss, M.L.; Troyer, D.L. Stem cells in the umbilical cord. Stem Cell Rev. 2006, 2, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; Choi, J.; Yang, M.S.; Moon, Y.J.; Park, J.S.; Kim, H.C.; Kim, Y.J. Mesenchymal stem cells from cryopreserved human umbilical cord blood. Biochem. Biophys. Res. Commun. 2004. [Google Scholar] [CrossRef] [PubMed]
- Couto, P.S.; Shatirishvili, G.; Bersenev, A.; Verter, F. First decade of clinical trials and published studies with mesenchymal stromal cells from umbilical cord tissue. Regen. Med. 2019, 14, 309–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.T.; Cheng, H.Y.; Zhang, L.; Fan, J.; Chen, Y.Z.; Jiang, X.D.; Xu, R.X. Umbilical cord blood cell-derived neurospheres differentiate into Schwann-like cells. Neuroreport 2009. [Google Scholar] [CrossRef]
- Xiao, Y.Z.; Wang, S. Differentiation of Schwannlike cells from human umbilical cord blood mesenchymal stem cells in vitro. Mol. Med. Report. 2015, 11, 1146–1152. [Google Scholar] [CrossRef]
- Lassing, I.; Mellstrm, K.; Nistr, M. Comparison of PDGF-AA- and PDGF-BB-induced phosphoinositide formation in human and mouse fibroblasts. Exp. Cell Res. 1994, 211, 286–295. [Google Scholar] [CrossRef]
- Benoit, B.O.; Savarese, T.; Joly, M.; Engstrom, C.M.; Pang, L.; Reilly, J.; Recht, L.D.; Ross, A.H.; Quesenberry, P.J. Neurotrophin channeling of neural progenitor cell differentiation. J. Neurobiol. 2001, 46, 265–280. [Google Scholar] [CrossRef]
- Sung, M.A.; Jung, H.J.; Lee, J.W.; Lee, J.Y.; Pang, K.M.; Yoo, S.B.; Alrashdan, M.S.; Kim, S.M.; Jahng, J.W.; Lee, J.H. Human umbilical cord blood-derived mesenchymal stem cells promote regeneration of crush-injured rat sciatic nerves. Neural Regen. Res. 2012, 7, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Dasari, V.R.; Spomar, D.G.; Gondi, C.S.; Sloffer, C.A.; Saving, K.L.; Gujrati, M.; Rao, J.S.; Dinh, D.H. Axonal remyelination by cord blood stem cells after spinal cord injury. J. Neurotrauma 2007, 24, 391–410. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Li, E.; Yang, B.; Wang, B. Human umbilical cord blood-derived mesenchymal stem cell transplantation for the treatment of spinal cord injury. Exp. Ther. Med. 2014, 7, 1233–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berebichez-Fridman, R.; Montero-Olvera, P.R. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ. Med. J. 2018, 18, e264–e277. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Wang, Y.; Zhang, L.; Zhao, B.; Zhao, Z.; Chen, J.F.; Guo, Q.Y.; Liu, S.Y.; Sui, X.; Xu, W.J.; et al. Human umbilical cord Wharton’s jelly-derived mesenchymal stem cells differentiate into a Schwann-cell phenotype and promote neurite outgrowth in vitro. Brain Res. Bull. 2011. [Google Scholar] [CrossRef]
- Matsuse, D.; Kitada, M.; Kohama, M.; Nishikawa, K.; Makinoshima, H.; Wakao, S.; Fujiyoshi, Y.; Heike, T.; Nakahata, T.; Akutsu, H.; et al. Human umbilical cord-derived mesenchymal stromal cells differentiate into functional Schwann cells that sustain peripheral nerve regeneration. J. Neuropathol. Exp. Neurol. 2010, 69, 973–985. [Google Scholar] [CrossRef] [Green Version]
- Krupa, P.; Vackova, I.; Ruzicka, J.; Zaviskova, K.; Dubisova, J.; Koci, Z.; Turnovcova, K.; Urdzikova, L.M.; Kubinova, S.; Rehak, S.; et al. The Effect of Human Mesenchymal Stem Cells Derived from Wharton’s Jelly in Spinal Cord Injury Treatment Is Dose-Dependent and Can Be Facilitated by Repeated Application. Int. J. Mol. Sci. 2018, 19, 1503. [Google Scholar] [CrossRef] [Green Version]
- Chudickova, M.; Vackova, I.; Machova Urdzikova, L.; Jancova, P.; Kekulova, K.; Rehorova, M.; Turnovcova, K.; Jendelova, P.; Kubinova, S. The Effect of Wharton Jelly-Derived Mesenchymal Stromal Cells and Their Conditioned Media in the Treatment of a Rat Spinal Cord Injury. Int. J. Mol. Sci. 2019, 20, 4516. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Liu, X.; Hua, R.; Dai, G.; Wang, X.; Gao, J.; An, Y. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J. Transl. Med. 2014, 12, 253. [Google Scholar] [CrossRef] [Green Version]
- Qu, J.; Zhang, H. Roles of Mesenchymal Stem Cells in Spinal Cord Injury. Stem Cells Int. 2017, 2017, 5251313. [Google Scholar] [CrossRef]
- Kalaszczynska, I.; Ferdyn, K. Wharton’s jelly derived mesenchymal stem cells: Future of regenerative medicine? Recent findings and clinical significance. Biomed. Res. Int. 2015, 2015, 430847. [Google Scholar] [CrossRef] [PubMed]
- Blanpain, C.; Fuchs, E. Epidermal homeostasis: A balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 2009, 10, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Xu, X. Isolation and Culture of Neural Crest Stem Cells from Human Hair Follicles. J. Vis. Exp. 2013. [Google Scholar] [CrossRef] [PubMed]
- Sieber-Blum, M.; Grim, M.; Hu, Y.F.; Szeder, V. Pluripotent neural crest stem cells in the adult hair follicle. Dev. Dyn. 2004, 231, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Biernaskie, J. Human hair follicles: “bulging” with neural crest-like stem cells. J. Investig. Dermatol. 2010, 130, 1202–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Fang, D.; Kumar, S.M.; Li, L.; Nguyen, T.K.; Acs, G.; Herlyn, M.; Xu, X. Isolation of a novel population of multipotent adult stem cells from human hair follicles. Am. J. Pathol. 2006, 168, 1879–1888. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, K.J.L.; McKenzie, I.A.; Mill, P.; Smith, K.M.; Akhavan, M.; Barnab-Heider, F.; Biernaskie, J.; Junek, A.; Kobayashi, N.R.; Toma, J.G.; et al. A dermal niche for multipotent adult skin-derived precursor cells. Nat. Cell Biol. 2004, 6, 1082–1093. [Google Scholar] [CrossRef]
- Ni, Y.; Zhang, K.; Liu, X.; Yang, T.; Wang, B.; Fu, L.; A, L.; Zhou, Y. miR-21 promotes the differentiation of hair follicle-derived neural crest stem cells into Schwann cells. Neural. Regen. Res. 2014, 9, 828–836. [Google Scholar] [CrossRef]
- Sakaue, M.; Sieber-Blum, M. Human epidermal neural crest stem cells as a source of schwann cells. Development (Cambridge) 2015, 142, 3188–3197. [Google Scholar] [CrossRef] [Green Version]
- Clewes, O.; Narytnyk, A.; Gillinder, K.R.; Loughney, A.D.; Murdoch, A.P.; Sieber-Blum, M. Human epidermal neural crest stem cells (hEPI-NCSC)--characterization and directed differentiation into osteocytes and melanocytes. Stem Cell Rev. Rep. 2011, 7, 799–814. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Chen, H.; Zhou, K.; Jia, X. Quantitative Multimodal Evaluation of Passaging Human Neural Crest Stem Cells for Peripheral Nerve Regeneration. Stem Cell Rev. Rep. 2018, 14, 92–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, I.; Novikov, L.N.; Renardy, M.; Kingham, P.J. Regenerative effects of human embryonic stem cell-derived neural crest cells for treatment of peripheral nerve injury. J. Tissue Eng. Regen. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Ouchi, T.; Shibata, S.; Amemiya, T.; Nagoshi, N.; Nakagawa, T.; Matsumoto, M.; Okano, H.; Nakamura, M.; Sato, K. Stem cells purified from human induced pluripotent stem cell-derived neural crest-like cells promote peripheral nerve regeneration. Sci. Rep. 2018, 8, 10071. [Google Scholar] [CrossRef] [Green Version]
- Amoh, Y.; Kanoh, M.; Niiyama, S.; Hamada, Y.; Kawahara, K.; Sato, Y.; Hoffman, R.M.; Katsuoka, K. Human hair follicle pluripotent stem (hfPS) cells promote regeneration of peripheral-nerve injury: An advantageous alternative to ES and iPS cells. J. Cell. Biochem. 2009, 107, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Amoh, Y.; Li, L.; Katsuoka, K.; Hoffman, R.M. Multipotent nestin-expressing hair follicle stem cells. J. Dermatol. 2009, 36, 1–9. [Google Scholar] [CrossRef]
- Amoh, Y.; Li, L.; Katsuoka, K.; Hoffman, R.M. Multipotent hair follicle stem cells promote repair of spinal cord injury and recovery of walking function. Cell Cycle 2008, 7, 1865–1869. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.F.; Gourab, K.; Wells, C.; Clewes, O.; Schmit, B.D.; Sieber-Blum, M. Epidermal neural crest stem cell (EPI-NCSC)--mediated recovery of sensory function in a mouse model of spinal cord injury. Stem Cell Rev. Rep. 2010, 6, 186–198. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, I.A.; Biernaskie, J.; Toma, J.G.; Midha, R.; Miller, F.D. Skin-derived precursors generate myelinating Schwann cells for the injured and dysmyelinated nervous system. J. Neurosci. 2006, 26, 6651–6660. [Google Scholar] [CrossRef]
- Toma, J.G.; Akhavan, M.; Fernandes, K.J.; Barnabé-Heider, F.; Sadikot, A.; Kaplan, D.R.; Miller, F.D. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat. Cell Biol. 2001, 3, 778–784. [Google Scholar] [CrossRef]
- Toma, J.G.; McKenzie, I.A.; Bagli, D.; Miller, F.D. Isolation and Characterization of Multipotent Skin-Derived Precursors from Human Skin. Stem Cells 2005, 23, 727–737. [Google Scholar] [CrossRef]
- Biernaskie, J.; Paris, M.; Morozova, O.; Fagan, B.M.; Marra, M.; Pevny, L.; Miller, F.D. SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell 2009, 5, 610–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krause, M.P.; Dworski, S.; Feinberg, K.; Jones, K.; Johnston, A.P.W.; Paul, S.; Paris, M.; Peles, E.; Bagli, D.; Forrest, C.R.; et al. Direct genesis of functional rodent and human schwann cells from skin mesenchymal precursors. Stem Cell Rep. 2014, 3, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Khuong, H.T.; Kumar, R.; Senjaya, F.; Grochmal, J.; Ivanovic, A.; Shakhbazau, A.; Forden, J.; Webb, A.; Biernaskie, J.; Midha, R. Skin derived precursor Schwann cells improve behavioral recovery for acute and delayed nerve repair. Exp. Neurol. 2014, 254, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Yandava, B.D.; Billinghurst, L.L.; Snyder, E.Y. "Global" cell replacement is feasible via neural stem cell transplantation: Evidence from the dysmyelinated shiverer mouse brain. Proc. Natl. Acad. Sci. USA 1999, 96, 7029–7034. [Google Scholar] [CrossRef] [Green Version]
- Sparling, J.S.; Bretzner, F.; Biernaskie, J.; Assinck, P.; Jiang, Y.; Arisato, H.; Plunet, W.T.; Borisoff, J.; Liu, J.; Miller, F.D.; et al. Schwann cells generated from neonatal skin-derived precursors or neonatal peripheral nerve improve functional recovery after acute transplantation into the partially injured cervical spinal cord of the rat. J. Neurosci. 2015, 35, 6714–6730. [Google Scholar] [CrossRef] [Green Version]
- Assinck, P.; Sparling, J.S.; Dworski, S.; Duncan, G.J.; Wu, D.L.; Liu, J.; Kwon, B.K.; Biernaskie, J.; Miller, F.D.; Tetzlaff, W. Transplantation of Skin Precursor-Derived Schwann Cells Yields Better Locomotor Outcomes and Reduces Bladder Pathology in Rats with Chronic Spinal Cord Injury. Stem Cell Rep. 2020, 15, 140–155. [Google Scholar] [CrossRef]
- Ruetze, M.; Knauer, T.; Gallinat, S.; Wenck, H.; Achterberg, V.; Maerz, A.; Deppert, W.; Knott, A. A novel niche for skin derived precursors in non-follicular skin. J. Dermatol. Sci. 2013, 69, 132–139. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Naitoh, M.; Kubota, H.; Ishiko, T.; Aya, R.; Yamawaki, S.; Suzuki, S. Multipotent stem cells are effectively collected from adult human cheek skin. Biochem. Biophys. Res. Commun. 2013, 431, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Dai, R.; Hua, W.; Xie, H.; Chen, W.; Xiong, L.; Li, L. The Human Skin-Derived Precursors for Regenerative Medicine: Current State, Challenges, and Perspectives. Stem Cells Int. 2018, 2018, 8637812. [Google Scholar] [CrossRef]
- Okita, K.; Ichisaka, T.; Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 2007, 448, 313–317. [Google Scholar] [CrossRef]
- Maherali, N.; Sridharan, R.; Xie, W.; Utikal, J.; Eminli, S.; Arnold, K.; Stadtfeld, M.; Yachechko, R.; Tchieu, J.; Jaenisch, R.; et al. Directly Reprogrammed Fibroblasts Show Global Epigenetic Remodeling and Widespread Tissue Contribution. Cell Stem Cell 2007, 1, 55–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrier, A.L.; Tabar, V.; Barberi, T.; Rubio, M.E.; Bruses, J.; Topf, N.; Harrison, N.L.; Studer, L. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2004, 101, 12543–12548. [Google Scholar] [CrossRef] [Green Version]
- Pomp, O.; Brokhman, I.; Ziegler, L.; Almog, M.; Korngreen, A.; Tavian, M.; Goldstein, R.S. PA6-induced human embryonic stem cell-derived neurospheres: A new source of human peripheral sensory neurons and neural crest cells. Brain Res. 2008, 1230, 50–60. [Google Scholar] [CrossRef]
- Rathjen, J.; Haines, B.P.; Hudson, K.M.; Nesci, A.; Dunn, S.; Rathjen, P.D. Directed differentiation of pluripotent cells to neural lineages: Homogeneous formation and differentiation of a neurectoderm population. Development 2002, 129, 2649–2661. [Google Scholar]
- Pomp, O.; Brokhman, I.; Ben-Dor, I.; Reubinoff, B.; Goldstein, R.S. Generation of Peripheral Sensory and Sympathetic Neurons and Neural Crest Cells from Human Embryonic Stem Cells. Stem Cells 2005, 23, 923–930. [Google Scholar] [CrossRef]
- Eldridge, C.F.; Bunge, M.B.; Bunge, R.P.; Wood, P.M. Differentiation of axon-related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. J. Cell Biol. 1987, 105, 1023–1034. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Spusta, S.C.; Mi, R.; Lassiter, R.N.T.; Stark, M.R.; Hke, A.; Rao, M.S.; Zeng, X. Human Neural Crest Stem Cells Derived from Human ESCs and Induced Pluripotent Stem Cells: Induction, Maintenance, and Differentiation into Functional Schwann Cells. STEM CELLS Transl. Med. 2012, 1, 266–278. [Google Scholar] [CrossRef]
- Ziegler, L.; Grigoryan, S.; Yang, I.H.; Thakor, N.V.; Goldstein, R.S. Efficient Generation of Schwann Cells from Human Embryonic Stem Cell-Derived Neurospheres. Stem Cell Reviews and Reports 2011, 7, 394–403. [Google Scholar] [CrossRef]
- Lee, G.; Kim, H.; Elkabetz, Y.A. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat. Biotechnol. 2007, 25, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, W.; Zhang, Y.; Wei, W.; Ambasudhan, R.; Xia, P.; Talantova, M.; Lin, T.; Kim, J.; Wang, X.; et al. Rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proc. Natl. Acad. Sci. USA 2011, 108, 8299–8304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.R.; Vallier, L.; Lupo, G.; Alexander, M.; Harris, W.A.; Pedersen, R.A. Inhibition of Activin/Nodal signaling promotes specification of human embryonic stem cells into neuroectoderm. Dev. Biol. 2008, 313, 107–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.S.; Lee, J.; Lee, D.Y.; Kim, Y.D.; Kim, J.Y.; Lim, H.J.; Lim, S.; Cho, Y.S. Schwann Cell Precursors from Human Pluripotent Stem Cells as a Potential Therapeutic Target for Myelin Repair. Stem Cell Reports 2017, 8, 1714–1726. [Google Scholar] [CrossRef] [Green Version]
- Melino, G. P63 is a suppressor of tumorigenesis and metastasis interacting with mutant p53. Cell Death Differ. 2011, 18, 1487–1499. [Google Scholar] [CrossRef] [Green Version]
- Li, J.Y.; Christophersen, N.S.; Hall, V.; Soulet, D.; Brundin, P. Critical issues of clinical human embryonic stem cell therapy for brain repair. Trends Neurosci. 2008, 31, 146–153. [Google Scholar] [CrossRef]
- Sowa, Y.; Kishida, T.; Tomita, K.; Yamamoto, K.; Numajiri, T.; Mazda, O. Direct conversion of human fibroblasts into schwann cells that facilitate regeneration of injured peripheral nerve in vivo. Stem Cells Transl. Med. 2017, 6, 1207–1216. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kishida, T.; Sato, Y.; Nishioka, K.; Ejima, A.; Fujiwara, H.; Kubo, T.; Yamamoto, T.; Kanamura, N.; Mazda, O. Direct conversion of human fibroblasts into functional osteoblasts by defined factors. Proc. Natl. Acad. Sci. USA 2015, 112, 6152–6157. [Google Scholar] [CrossRef] [Green Version]
- Pang, Z.P.; Yang, N.; Vierbuchen, T.; Ostermeier, A.; Fuentes, D.R.; Yang, T.Q.; Citri, A.; Sebastiano, V.; Marro, S.; Sdhof, T.C.; et al. Induction of human neuronal cells by defined transcription factors. Nature 2011, 476, 220-–223. [Google Scholar] [CrossRef]
- Mazzara, P.G.; Massimino, L.; Pellegatta, M.; Ronchi, G.; Ricca, A.; Iannielli, A.; Giannelli, S.G.; Cursi, M.; Cancellieri, C.; Sessa, A.a. Two factor-based reprogramming of rodent and human fibroblasts into Schwann cells. Nature Commun. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Smyth Templeton, N.; Zwaka, T. Use of Genetically Modified Stem Cells in Experimental Gene Therapies. Gene Cell Ther. 2008. [Google Scholar] [CrossRef]
- Sulaiman, O.A.; Gordon, T. Effects of short- and long-term Schwann cell denervation on peripheral nerve regeneration, myelination, and size. Glia 2000, 32, 234–246. [Google Scholar] [CrossRef]
- Mendonça, M.V.; Larocca, T.F.; de Freitas Souza, B.S.; Villarreal, C.F.; Silva, L.F.; Matos, A.C.; Novaes, M.A.; Bahia, C.M.; de Oliveira Melo Martinez, A.C.; Kaneto, C.M.; et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell. Res. Ther. 2014, 5, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.K.; Choi, K.H.; Yoo, J.Y.; Kim, D.Y.; Kim, S.J.; Jeon, S.R. A Phase III Clinical Trial Showing Limited Efficacy of Autologous Mesenchymal Stem Cell Therapy for Spinal Cord Injury. Neurosurgery 2016, 78, 436–447; discussion 447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hopf, A.; Schaefer, D.J.; Kalbermatten, D.F.; Guzman, R.; Madduri, S. Schwann Cell-Like Cells: Origin and Usability for Repair and Regeneration of the Peripheral and Central Nervous System. Cells 2020, 9, 1990. https://doi.org/10.3390/cells9091990
Hopf A, Schaefer DJ, Kalbermatten DF, Guzman R, Madduri S. Schwann Cell-Like Cells: Origin and Usability for Repair and Regeneration of the Peripheral and Central Nervous System. Cells. 2020; 9(9):1990. https://doi.org/10.3390/cells9091990
Chicago/Turabian StyleHopf, Alois, Dirk J. Schaefer, Daniel F. Kalbermatten, Raphael Guzman, and Srinivas Madduri. 2020. "Schwann Cell-Like Cells: Origin and Usability for Repair and Regeneration of the Peripheral and Central Nervous System" Cells 9, no. 9: 1990. https://doi.org/10.3390/cells9091990