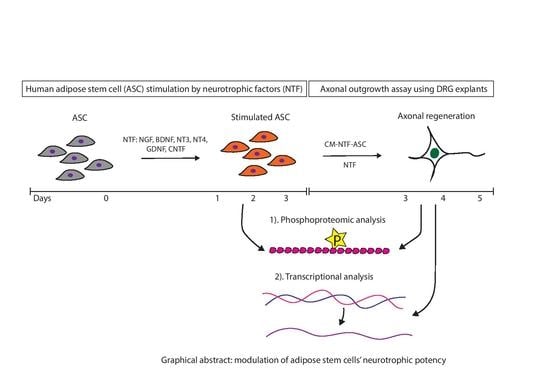
Modulation of Human Adipose Stem Cells’ Neurotrophic Capacity Using a Variety of Growth Factors for Neural Tissue Engineering Applications: Axonal Growth, Transcriptional, and Phosphoproteomic Analyses In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of Human Adipose Stem Cells (ASC)
2.2. Human ASC Characterization
2.3. Isolation of Chicken Embryonic Dorsal Root Ganglia (DRG)
2.4. Quantitative Measurement of NT3 by ELISA
2.5. Experimental Design for the Axonal Outgrowth Assay
2.6. Immunocytochemistry of DRG Cultures
2.7. Quantitative Analysis of Axonal Outgrowth
2.8. Quantitative RT-PCR for Regeneration-Associated Genes (RAG) in DRG
2.9. Phosphoproteomic Analysis
2.10. Data Availability
2.11. Statistical Analysis
3. Results
3.1. Human ASC Characterization
3.2. Distinct Effects of NTF on Axonal Outgrowth
3.3. Conditioned Medium Derived from Stimulated Stem Cells
3.4. NT3 Content
3.5. Transcriptional Analysis of RAG in Treated DRG Explants
3.6. Quantitative Phosphoproteomic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosberg, H.E.; Carlsson, K.S.; Hjgard, S.; Lindgren, B.; Lundborg, G.; Dahlin, L.B. Injury to the human median and ulnar nerves in the forearm–analysis of costs for treatment and rehabilitation of 69 patients in southern Sweden. J. Hand Surg. 2005, 30B, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Christie, K.J.; Zochodne, D. Peripheral axon regrowth: New molecular approaches. Neuroscience 2013, 240, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Grinsell, D.; Keating, C.P. Peripheral nerve reconstruction after injury: A review of clinical and experimental therapies. BioMed Res. Int. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muheremu, A.; Ao, Q. Past, Present, and Future of Nerve Conduits in the Treatment of Peripheral Nerve Injury. BioMed Res. Int. 2015. [Google Scholar] [CrossRef] [Green Version]
- Raza, C.; Riaz, H.A.; Anjum, R.; Shakeel, A. Repair strategies for injured peripheral nerve: Review. Life Sci. 2020, 243, 117308. [Google Scholar] [CrossRef]
- Lundborg, G. Alternatives to Autologous Nerve Grafts. Handchir. Mikrochir. Plast. Chir. 2004, 10. [Google Scholar] [CrossRef]
- Allodi, I.; Udina, E.; Navarro, X. Specificity of peripheral nerve regeneration: Interactions at the axon level. Prog. Neurobiol. 2012, 98, 16–37. [Google Scholar] [CrossRef]
- Hall, S. The response to injury in the peripheral nervous system. J. Bone Jt. Surg. 2005, 87, 1309–1319. [Google Scholar] [CrossRef]
- Carvalho, C.R.; Oliveira, J.M.; Reis, R.L. Modern Trends for Peripheral Nerve Repair and Regeneration: Beyond the Hollow Nerve Guidance Conduit. Front. Bioeng. Biotechnol. 2019, 7, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Webber, C.; Zochodne, D. The nerve regenerative microenvironment: Early behavior and partnership of axons and Schwann cells. Exp. Neurol. 2010, 223, 51–59. [Google Scholar] [CrossRef]
- Bennett, D.L.H.; Boucher, T.J.; Armanini, M.P.; Poulsen, K.T.; Michael, G.J.; Priestley, J.V.; Phillips, H.S.; McMahon, S.B.; Shelton, D.L. The Glial Cell Line-Derived Neurotrophic Factor Family Receptor Components Are Differentially Regulated within Sensory Neurons after Nerve Injury. J. Neurosci. 2000, 20, 427–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessen, K.R.; Mirsky, R. The Success and Failure of the Schwann Cell Response to Nerve Injury. Front. Cell. Neurosci. 2019, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Benito, C.; Davis, C.M.; Gomez-sanchez, J.A.; Turmaine, M.; Meijer, D.; Poli, V. STAT3 controls the long-term survival and phenotype of repair Schwann cells during nerve regeneration. J. Neurosci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Frisen, J.; Verge, V.M.K.; Friedt, K.A.J.; Rislingt, M.; Perssonii, H.; Trotter, J.; Hokfelt, T.; Lindholm, D.A.N. Characterization of glial trkB receptors: Differential response to injury in the central and peripheral nervous systems. Proc. Natl. Acad. Sci. USA 1993, 90, 4971–4975. [Google Scholar] [CrossRef] [Green Version]
- Cosgaya, M.; Chan, J.R.; Shooter, E.M. The Neurotrophin Receptor p75 NTR as a Positive Modulator of Myelination. Science 2002, 298, 1245–1248. [Google Scholar] [CrossRef]
- Funakoshi, H.; Frisen, J.; Barbany, G.; Timmusk, T.; Zachrisson, O.; Verge, V.M.K.; Persson, H. Differential Expression of mRNAs for Neurotrophins and Their Receptors after Axotomy of the Sciatic Nerve. J. Cell Biol. 1993, 123, 455–465. [Google Scholar] [CrossRef]
- Hase, A.; Saito, F.; Yamada, H.; Arai, K.; Shimizu, T.; Matsumura, K. Characterization of glial cell line-derived neurotrophic factor family receptor a -1 in peripheral nerve Schwann cells. J. Neurochem. 2005, 95, 537–543. [Google Scholar] [CrossRef]
- Soilu-Hnninen, M.; Ekert, P.; Bucci, T.; Syroid, D.; Bartlett, P.F.; Kilpatrick, T.J. Nerve Growth Factor Signaling through p75 Induces Apoptosis in Schwann Cells via a Bcl-2-Independent Pathway. J. Neurosci. 1999, 19, 4828–4838. [Google Scholar] [CrossRef] [Green Version]
- Trupp, M.; Belluardo, N.; Funakoshi, H.; Iba, C.F. Complementary and Overlapping Expression of Glial Cell Line- Derived Neurotrophic Factor (GDNF), c-ret Proto-Oncogene, and GDNF Receptor-α Indicates Multiple Mechanisms of Trophic Actions in the Adult Rat CNS. J. Neurosci. 1997, 17, 3554–3567. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Kilpatrick, J.; Murray, S. The Role of Neurotrophins in the Regulation of Myelin Development. Neurosignals 2009, 3010, 265–276. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Luo, X.-G.; Xian, C.J.; Liu, Z.-H.; Zhou, X.-F. Endogenous BDNF is required for myelination and regeneration of injured sciatic nerve in rodents. Eur. J. Neurosci. 2000, 12, 4171–4180. [Google Scholar] [PubMed]
- Ching, R.C.; Wiberg, M.; Kingham, P.J. Schwann cell-like differentiated adipose stem cells promote neurite outgrowth via secreted exosomes and RNA transfer. Stem Cell Res. Ther. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Godinho, M.J.; Teh, L.; Pollett, M.A.; Goodman, D.; Hodgetts, S.I.; Sweetman, I.; Walters, M.; Verhaagen, J.; Plant, G.W.; Harvey, A.R. Immunohistochemical, Ultrastructural and Functional Analysis of Axonal Regeneration through Peripheral Nerve Grafts Containing Schwann Cells Expressing BDNF, CNTF or NT3. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Han, G.-H.; Peng, J.; Liu, P.; Ding, X.; Wei, S.; Lu, S.; Wang, Y. Therapeutic strategies for peripheral nerve injury: Decellularized nerve conduits and Schwann cell transplantation. Neural Regen. Res. 2019, 14, 1343. [Google Scholar] [CrossRef]
- Mantovani, C.; Mahay, D.; Kingham, M.; Terenghi, G.; Shawcross, S.G.; Wiberg, M. Bone marrow- and adipose-derived stem cells show expression of myelin mRNAs and proteins. Regen. Med. 2010, 5, 403–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tohill, M.; Terenghi, G. Stem-cell plasticity and therapy for injuries of the peripheral nervous system Bioengineered nerve grafts. Biotechnol. Appl. Biochem. 2004, 24, 17–24. [Google Scholar]
- Di Summa, P.G.; Kalbermatten, D.F.; Pralong, E.; Raffoul, W.; Kingham, P.J.; Terenghi, G. Long-term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience 2011, 181, 278–291. [Google Scholar] [CrossRef]
- Teng, Y.D. Functional multipotency of stem cells: Biological traits gleaned from neural progeny studies. Semin. Cell Dev. Biol. 2019, 95, 74–83. [Google Scholar] [CrossRef]
- Thakor, D.K.; Wang, L.; Benedict, D.; Kabatas, S.; Zafonte, R.D.; Teng, Y.D. Establishing an Organotypic System for Investigating Multimodal Neural Repair Effects of Human Mesenchymal Stromal Stem Cells. Curr. Protoc. Stem Cell Biol. 2018, 47, e58. [Google Scholar] [CrossRef]
- Nakagami, H.; Maeda, K.; Morishita, R.; Iguchi, S.; Nishikawa, T.; Takami, Y.; Kikuchi, Y.; Saito, Y.; Tamai, K.; Ogihara, T.; et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2542–2547. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Kluter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells (Dayt. Ohio) 2006, 24, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Strem, B.M.; Hicok, K.C.; Zhu, M.; Wulur, I.; Alfonso, Z.; Schreiber, R.E.; Fraser, J.K.; Hedrick, M.H. Multipotential differentiation of adipose tissue-derived stem cells. Keio J. Med. 2005, 54, 132–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aust, L.; Devlin, B.; Foster, S.J.; Halvorsen, Y.D.; Hicok, K.; du Laney, T.; Sen, A.; Willingmyre, G.D.; Gimble, J.M. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy 2004, 6, 7–14. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, K.R.; Lopez, M.J.; Borneman, J.N.; Spencer, N.D.; Anderson, P.A.; Gimble, J.M. Immunogenicity of allogeneic adipose-derived stem cells in a rat spinal fusion model. Tissue Eng. Part A 2009, 15, 2677–2686. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.J.; Reis, R.L.; Sousa, N.J.; Gimble, J.M. Adipose tissue derived stem cells secretome: Soluble factors and their roles in regenerative medicine. Curr. Stem Cell Res. Ther. 2010, 5, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Kaewkhaw, R.; Scutt, A.M.; Haycock, J.W. Anatomical Site Influences the Differentiation of Adipose-Derived Stem Cells for Schwann-Cell Phenotype and Function. Glia 2011, 749, 734–749. [Google Scholar] [CrossRef]
- Kalbermatten, D.F.; Schaakxs, D.; Kingham, P.J.; Wiberg, M. Neurotrophic activity of human adipose stem cells isolated from deep and superficial layers of abdominal fat. Cell Tissue Res. 2011, 344. [Google Scholar] [CrossRef]
- Marconi, S.; Castiglione, G.; Turano, E.; Bissolotti, G.; Angiari, S.; Farinazzo, A.; Constantin, G.; Bedogni, G.; Bedogni, A.; Bonetti, B. Human adipose-derived mesenchymal stem cells systemically injected promote peripheral nerve regeneration in the mouse model of sciatic crush. Tissue Eng. Part A 2012, 18, 1264–1272. [Google Scholar] [CrossRef]
- Kingham, P.J.; Kalbermatten, D.F.; Mahay, D.; Armstrong, S.J.; Wiberg, M.; Terenghi, G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp. Neurol. 2007, 207. [Google Scholar] [CrossRef]
- Faroni, A.; Mobasseri, S.A.; Kingham, P.J.; Reid, A.J. Peripheral nerve regeneration: Experimental strategies and future perspectives. Adv. Drug Deliv. Rev. 2015, 82, 160–167. [Google Scholar] [CrossRef]
- Orbay, H.; Uysal, A.C.; Hyakusoku, H.; Mizuno, H. Differentiated and undifferentiated adipose-derived stem cells improve function in rats with peripheral nerve gaps. J. Plast. Reconstr. Aesthetic Surg. 2012, 65, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Erba, P.; Mantovani, C.; Kalbermatten, D.F.; Pierer, G.; Terenghi, G.; Kingham, P.J. Regeneration potential and survival of transplanted undifferentiated adipose tissue-derived stem cells in peripheral nerve conduits. J. Plast. Reconstr. Aesthetic Surg. Jpras 2010, 63, e811–e817. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Sasaki, R.; Matsumine, H.; Yamato, M.; Okano, T. Undifferentiated and differentiated adipose-derived stem cells improve nerve regeneration in a rat model of facial nerve defect. J. Tissue Eng. Regen. Med. 2017, 11, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Rosen, J.M. The role of undifferentiated adipose-derived stem cells in peripheral nerve repair. Neural Regen. Res. 2018, 13, 757–763. [Google Scholar] [CrossRef]
- McGregor, C.E.; English, A.W. The Role of BDNF in Peripheral Nerve Regeneration: Activity-Dependent Treatments and Val66Met. Front. Cell. Neurosci. 2019, 12. [Google Scholar] [CrossRef] [Green Version]
- Belanger, K.; Dinis, T.M.; Taourirt, S.; Vidal, G.; Kaplan, D.L.; Egles, C. Recent Strategies in Tissue Engineering for Guided Peripheral Nerve Regeneration. Macromol. Biosci. 2016, 16, 472–481. [Google Scholar] [CrossRef]
- Duraikannu, A.; Krishnan, A.; Chandrasekhar, A.; Zochodne, D.W. Beyond Trophic Factors: Exploiting the Intrinsic Regenerative Properties of Adult Neurons. Front. Cell. Neurosci. 2019, 13. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Chang, W.; Chen, Y.; Dai, F.; Anwar, A. Biological and Medical Applications of Materials and Interfaces Nanofibrous Nerve Conduits with Nerve Growth Factors and Bone Marrow Stromal Cells Pre-Cultured in Bioreactors for Peripheral Nerve Regeneration Nanofibrous Nerve Conduits with Nerve Growth, F. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef]
- Madduri, S.; Gander, B. Growth factor delivery systems and repair strategies for damaged peripheral nerves. J. Control. Release 2012, 161, 274–282. [Google Scholar] [CrossRef]
- Sharp, K.G.; Yee, K.M.; Steward, O. A re-assessment of long distance growth and connectivity of neural stem cells after severe spinal cord injury. Exp. Neurol. 2014, 257, 186–204. [Google Scholar] [CrossRef] [Green Version]
- Prautsch, K.M.; Degrugillier, L.; Schaefer, D.J.; Guzman, R.; Kalbermatten, D.F.; Madduri, S. Ex-Vivo Stimulation of Adipose Stem Cells by Growth Factors and Fibrin-Hydrogel Assisted Delivery Strategies for Treating Nerve Gap-Injuries. Bioengineering 2020, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Meier, C.; Parmantier, E.; Brennan, A.; Mirsky, R.; Jessen, K.R. Developing Schwann cells acquire the ability to survive without axons by establishing an autocrine circuit involving insulin-like growth factor, neurotrophin-3, and platelet-derived growth factor-BB. J. Neurosci. Off. J. Soc. Neurosci. 1999, 19, 3847–3859. [Google Scholar] [CrossRef] [Green Version]
- Faroni, A.; Smith, R.J.P.; Lu, L.; Reid, A.J. Human Schwann-like cells derived from adipose-derived mesenchymal stem cells rapidly de-differentiate in the absence of stimulating medium. Eur. J. Neurosci. 2016, 43, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, W.; Geloso, M.C.; Saulnier, N.; Giannetti, S.; Puglisi, M.A.; Corvino, V.; Gasbarrini, A.; Michetti, F. Neurotrophic Features of Human Adipose Tissue-Derived Stromal Cells: In Vitro and In Vivo Studies. J. Biomed. Biotechnol. 2011. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, N.; Akamatsu, H.; Hasegawa, S. Isolation of multipotent stem cells from mouse adipose tissue. J. Dermatol. Sci. 2007. [Google Scholar] [CrossRef]
- Zarrinpour, V.; Hajebrahimi, Z.; Jafarinia, M. Expression pattern of neurotrophins and their receptors during neuronal differentiation of adipose—Derived stem cells in simulated microgravity condition. Iran. J. Basic Med Sci. 2016, 27–29. [Google Scholar]
- Boyd, J.G.; Gordon, T. Neurotrophic Factors and Their Receptors in Axonal Regeneration and Functional Recovery After Peripheral Nerve Injury. Mol. Neurobiol. 2003, 27, 277–323. [Google Scholar] [CrossRef]
- Powell, S.; Vinod, A.; Lemons, M.L. Isolation and Culture of Dissociated Sensory Neurons From Chick Embryos. JoVE (J. Vis. Exp.) 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lelkes, P.I.; Unsworth, B.R.; Saporta, S.; Cameron, D.F.; Gallo, G. Culture of Neuroendocrine and Neuronal Cells for Tissue Engineering. Cult. Cell. Tissue Eng. 2006, 14, 375–415. [Google Scholar] [CrossRef]
- Madduri, S.; Papaloizos, M.; Gander, B. Synergistic effect of GDNF and NGF on axonal branching and elongation in vitro. Neurosci. Res. 2009, 65, 88–97. [Google Scholar] [CrossRef]
- UniProt Database for Protein Sequence and Functional Information. Available online: https://www.uniprot.org (accessed on 7 March 2019).
- UniProt Database for Protein Sequence and Functional Information. Available online: https://www.uniprot.org (accessed on 14 April 2020).
- Hock, C.; Heese, K.; Müller-Spahn, F.; Huber, P.; Riesen, W.; Nitsch, R.M.; Otten, U. Increased cerebrospinal fluid levels of neurotrophin 3 (NT-3) in elderly patients with major depression. Mol. Psychiatry 2000, 5, 510–513. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.; Dong, J.; Lu, T.; Lv, H.; Yang, P.; Cheng, Z.; Li, J.; Liang, B.; Xu, J.; Li, H.; et al. Effects of different sera conditions on olfactory ensheathing cells in vitro. Int. J. Mol. Sci. 2014, 16, 420–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.B.; Chen, Y.; Kang, W.; Guo, J.; Payne, R.; Li, H.; Wei, Q.; Baker, J.; Dong, C.; Zhang, S.; et al. The critical chemical and mechanical regulation of folic acid on neural engineering. Biomaterials 2018, 178, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.Y.; Gordon, T. The cellular and molecular basis of peripheral nerve regeneration. Mol. Neurobiol. 1997, 14, 67–116. [Google Scholar] [CrossRef] [PubMed]
- Alvites, R.; Caseiro, A.R.; Pedrosa, S.S.; Branquinho, M.V.; Ronchi, G.; Geuna, S.; Varejo, A.S.P.; Maurcio, A.C. Peripheral nerve injury and axonotmesis: State of the art and recent advances. Cogent Med. 2018, 20, 1–45. [Google Scholar] [CrossRef]
- Lischer, M.; di Summa, P.G.; Oranges, C.M.; Schaefer, D.J.; Kalbermatten, D.F.; Guzman, R.; Madduri, S. Human platelet lysate stimulated adipose stem cells exhibit strong neurotrophic potency for nerve tissue engineering applications. Regen. Med. 2020, 15, 1399–1408. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, Y.; Zhou, J.; Cao, K. Adipose-derived mesenchymal stem cell exosomes: A novel pathway for tissues repair. Cell Tissue Bank. 2019, 20, 153–161. [Google Scholar] [CrossRef]
- Bellei, B.; Migliano, E.; Tedesco, M.; Caputo, S.; Papaccio, F.; Lopez, G.; Picardo, M. Adipose tissue-derived extracellular fraction characterization: Biological and clinical considerations in regenerative medicine. Stem Cell Res. Ther. 2018, 9. [Google Scholar] [CrossRef]
- Santiago, L.Y.; Clavijo-Alvarez, J.; Brayfield, C.; Rubin, J.P.; Marra, K.G. Delivery of adipose-derived precursor cells for peripheral nerve repair. Cell Transplant. 2009, 18, 145–158. [Google Scholar] [CrossRef]
- Vasilev, G.; Ivanova, M.; Ivanova-Todorova, E.; Tumangelova-Yuzeir, K.; Krasimirova, E.; Stoilov, R.; Kyurkchiev, D. Secretory factors produced by adipose mesenchymal stem cells downregulate Th17 and increase Treg cells in peripheral blood mononuclear cells from rheumatoid arthritis patients. Rheumatol. Int. 2019, 39, 819–826. [Google Scholar] [CrossRef]
- Newman, S.A. Inherency of Form and Function in Animal Development and Evolution. Front. Physiol. 2019, 10, 702. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.M.; Han, J.Y. The early development of germ cells in chicken. Int. J. Dev. Biol. 2018, 62, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Homma, S.; Oppenheim, R.W.; Yaginuma, H.; Kimura, S. Expression pattern of GDNF, c-ret, and GFRalphas suggests novel roles for GDNF ligands during early organogenesis in the chick embryo. Dev. Biol. 2000, 217, 121–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebendal, T.; Larhammar, D.; Persson, H. Structure and expression of the chicken beta nerve growth factor gene. EMBO J. 1986, 5, 1483–1487. [Google Scholar] [CrossRef] [PubMed]
- Madduri, S.; Papaloizos, M.; Gander, B. Trophically and topographically functionalized silk fibroin nerve conduits for guided peripheral nerve regeneration. Biomaterials 2010, 31, 2323–2334. [Google Scholar] [CrossRef] [PubMed]
- Heslop, J.A.; Hammond, T.G.; Santeramo, I.; Tort Piella, A.; Hopp, I.; Zhou, J.; Baty, R.; Graziano, E.I.; Proto Marco, B.; Caron, A.; et al. Concise review: Workshop review: Understanding and assessing the risks of stem cell-based therapies. Stem Cells Transl. Med. 2015, 4, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yee, W.-C.; Hwang, P.Y.K.; Yu, H.; Wan, A.C.A.; Gao, S.; Boon, K.-L.; Mao, H.-Q.; Leong, K.W.; Wang, S. Peripheral nerve regeneration with sustained release of poly (phosphoester) microencapsulated nerve growth factor within nerve guide conduits. Biomaterials 2003, 24, 2405–2412. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, C.; Yu, Z.; Li, W.; Huang, Z.; Zhang, W. GDNF pretreatment overcomes Schwann cell phenotype mismatch to promote motor axon regeneration via sensory graft. Exp. Neurol. 2019, 318, 258–266. [Google Scholar] [CrossRef]
- Hke, A.; Ho, T.; Crawford, T.O.; Lebel, C.; Hilt, D.; Griffin, J.W. Glial Cell Line-Derived Neurotrophic Factor Alters Axon Schwann Cell Units and Promotes Myelination in Unmyelinated Nerve Fibers. J. Neurosci. 2003, 23, 561–567. [Google Scholar] [CrossRef]
- Hke, A.; Redett, R.; Hameed, H.; Jari, R.; Zhou, C.; Li, Z.B.; Griffin, J.W.; Brushart, T.M. Schwann Cells Express Motor and Sensory Phenotypes That Regulate Axon Regeneration. J. Neurosci. 2006, 26, 9646–9655. [Google Scholar] [CrossRef] [Green Version]
- Boyd, J.G.; Gordon, T. Differentially Regulate Motor Axonal Regeneration. J. Neurobiol. 2001, 49, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Vgelin, E.; Baker, J.M.; Gates, J.; Dixit, V.; Constantinescu, M.A.; Jones, N.F. Effects of local continuous release of brain derived neurotrophic factor (BDNF) on peripheral nerve regeneration in a rat model. Exp. Neurol. 2006, 199, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Poo, M.-M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013, 14. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Su, C. Review Progesterone, Brain-Derived Neurotrophic Factor and Neuroprotection. Neuroscience 2013, 239, 84–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ernfors, P.; Rosario, C.M.; Grant, G.; Aldskogius, H.; Persson, H. Expression of mRNAs for neurotrophin receptors in the dorsal root ganglion and spinal cord during development and following peripheral or central axotomy. Mol. Brain Res. 1993, 17, 217–226. [Google Scholar] [CrossRef]
- Rabinovsky, E.D.; Smith, G.M.; Browder, D.P.; Shine, H.D.; McManaman, J.L. Peripheral Nerve Injury Down-Regulates CNTF Expression in Adult Rat Sciatic Nerves. J. Neurosci. Res. 1992, 192, 188–192. [Google Scholar] [CrossRef]
- Henderson, C.E.; Camu, W.; Mettling, C.; Gouin, A.; Poulsen, K.; Karihaloo, M.; Rullamas, J.; Evans, T.; McMahon, S.B.; Armanini, M.P.; et al. Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature 1993, 363, 266–270. [Google Scholar] [CrossRef]
- Simon, M.; Porter, R.; Brown, R.; Coulton, G.R.; Terenghi, G. Effect of NT-4 and BDNF delivery to damaged sciatic nerves on phenotypic recovery of fast and slow muscles fibres. Eur. J. Neurosci. 2003, 18, 2460–2466. [Google Scholar] [CrossRef]
- Airaksinen, M.S.; Koltzenburg, M.; Lewin, G.R.; Masu, Y.; Helbig, C.; Wolf, E.; Brem, G.; Toyka, K.V.; Thoenen, H.; Meyer, M. Specific Subtypes of Cutaneous Mechanoreceptors Require Neurotrophin-3 Following Peripheral Target Innervation. Neuron 1996, 16, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Sterne, G.D.; Brown, R.A.; Green, C.J.; Terenghi, G. Neurotrophin-3 Delivered Locally via Fibronectin Mats Enhances Peripheral Nerve Regeneration. Eur. J. Neurosci. 1997, 9, 1388–1396. [Google Scholar] [CrossRef]
- Künzel, U.; Grieve, A.G.; Meng, Y.; Sieber, B.; Cowley, S.A.; Freeman, M. FRMD8 promotes inflammatory and growth factor signalling by stabilising the iRhom/ADAM17 sheddase complex. Elife 2018, 7. [Google Scholar] [CrossRef]
- Grieve, A.G.; Xu, H.; Künzel, U.; Bambrough, P.; Sieber, B.; Freeman, M. Phosphorylation of iRhom2 at the plasma membrane controls mammalian TACE-dependent inflammatory and growth factor signalling. Elife 2017, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahin, U.; Blobel, C.P. Ectodomain shedding of the EGF-receptor ligand epigen is mediated by ADAM17. FEBS Lett. 2007, 581, 41–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falk, R.; Falk, A.; Dyson, M.R.; Melidoni, A.N.; Parthiban, K.; Young, J.L.; Roake, W.; McCafferty, J. Generation of anti-Notch antibodies and their application in blocking Notch signalling in neural stem cells. Methods 2012, 58, 69–78. [Google Scholar] [CrossRef]
- Engler, A.; Rolando, C.; Giachino, C.; Saotome, I.; Erni, A.; Brien, C.; Zhang, R.; Zimber-Strobl, U.; Radtke, F.; Artavanis-Tsakonas, S.; et al. Notch2 Signaling Maintains NSC Quiescence in the Murine Ventricular-Subventricular Zone. Cell Rep. 2018, 22, 992–1002. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Boareto, M.; Engler, A.; Louvi, A.; Giachino, C.; Iber, D.; Taylor, V. Id4 Downstream of Notch2 Maintains Neural Stem Cell Quiescence in the Adult Hippocampus. Cell Rep. 2019, 28, 1485–1498.e1486. [Google Scholar] [CrossRef]
- Drees, F.; Gertler, F.B. Ena/VASP: Proteins at the tip of the nervous system. Curr. Opin. Neurobiol. 2008, 18, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowski, A.V.; Rubinson, D.A.; Dent, E.W.; Edward van Veen, J.; Leslie, J.D.; Zhang, J.; Mebane, L.M.; Philippar, U.; Pinheiro, E.M.; Burds, A.A.; et al. Ena/VASP Is Required for neuritogenesis in the developing cortex. Neuron 2007, 56, 441–455. [Google Scholar] [CrossRef] [Green Version]
- Lebrand, C.; Dent, E.W.; Strasser, G.A.; Lanier, L.M.; Krause, M.; Svitkina, T.M.; Borisy, G.G.; Gertler, F.B. Critical role of Ena/VASP proteins for filopodia formation in neurons and in function downstream of netrin-1. Neuron 2004, 42, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Szebenyi, G.; Bollati, F.; Bisbal, M.; Sheridan, S.; Faas, L.; Wray, R.; Haferkamp, S.; Nguyen, S.; Caceres, A.; Brady, S.T. Activity-driven dendritic remodeling requires microtubule-associated protein 1A. Curr. Biol. 2005, 15, 1820–1826. [Google Scholar] [CrossRef] [Green Version]
- Gu, T.; Zhao, T.; Kohli, U.; Hewes, R.S. The large and small SPEN family proteins stimulate axon outgrowth during neurosecretory cell remodeling in Drosophila. Dev. Biol. 2017, 431, 226–238. [Google Scholar] [CrossRef]
- Curtis, R.; Stewart, H.J.S.; Hall, S.M.; Wilkin, G.P.; Mirsky, R.; Jessent, K.R. GAP43 Is Expressed by Nonmyelin-forming Schwann Cells of the Peripheral Nervous System. J. Cell Biol. 1992, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woolf, C.J.; Chong, M.S.; Emson, P.; Reynolds, M.L. Denervation by Terminal of the Motor Endplate Results in the Rapid Expression Schwann Cells of the Growth-associated Protein GAP-43. J. Neurosci. 1992, 12. [Google Scholar] [CrossRef] [Green Version]
- Tedeschi, A. Tuning the orchestra: Transcriptional pathways controlling axon regeneration. Front. Mol. Neurosci. 2012, 4, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quarta, S.; Baeumer, B.E.; Scherbakov, N.; Andratsch, M.; Rose-John, S.; Dechant, G.; Bandtlow, C.E.; Kress, M. Peripheral Nerve Regeneration and NGF-Dependent Neurite Outgrowth of Adult Sensory Neurons Converge on STAT3 Phosphorylation Downstream of Neuropoietic Cytokine Receptor gp130. J. Neurosci. 2014, 34, 13222–13233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bierlein, M.; Rosa, D.; Kozik, E.M.; Sakaguchi, D.S. Adult Stem Cell-Based Strategies for Peripheral Nerve Regeneration. Cell Biol. Transl. Med. 2018, 4. [Google Scholar]
- Madduri, S.; Gander, B. Schwann cell delivery of neurotrophic factors for peripheral nerve regeneration. J. Peripher. Nerv. Syst. 2010, 15, 93–103. [Google Scholar] [CrossRef]
- Dezawa, M.; Takahashi, I.; Esaki, M.; Takano, M.; Sawada, H. Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. Eur. J. Neurosci. 2001, 14, 1771–1776. [Google Scholar] [CrossRef]
- Hu, Y.; Leaver, S.G.; Plant, G.W.; Hendriks, W.T.J.; Niclou, S.P.; Verhaagen, J.; Harvey, A.R.; Cui, Q. Lentiviral-Mediated Transfer of CNTF to Schwann Cells within Reconstructed Peripheral Nerve Grafts Enhances Adult Retinal Ganglion Cell Survival and Axonal Regeneration. Mol. Ther. 2005, 11. [Google Scholar] [CrossRef]
- Wan, W.; Zhang, L.; Blanchard, S.; Bigou, S.; Bohl, D.; Wang, C.; Liu, S. Combination of hypoglossal-facial nerve surgical reconstruction and neurotrophin-3 gene therapy for facial palsy. J. Neurosurg. 2013, 119, 739–750. [Google Scholar] [CrossRef]
- Li, H.; Terenghi, G.; Hall, S.M. Effects of delayed re-innervation on the expression of c-erbB receptors by chronically denervated rat Schwann cells in vivo. Glia 1997, 20, 333–347. [Google Scholar] [CrossRef]
- Sulaiman, O.A.; Gordon, T. Effects of short- and long-term Schwann cell denervation on peripheral nerve regeneration, myelination, and size. Glia 2000, 32, 234–246. [Google Scholar] [CrossRef]
- Sahenk, Z.; Oblinger, J.; Edwards, C. Neurotrophin-3 deficient Schwann cells impair nerve regeneration. Exp. Neurol. 2008, 212, 552–556. [Google Scholar] [CrossRef] [PubMed]





| Group | Axonal Length (μm) | Axonal Area (mm2) |
|---|---|---|
| NGF | 413 ± 182 | 1.903 ± 1.239 |
| GDNF | 405 ± 116 | 1.252 ± 0.486 |
| BDNF | 419 ± 73 | 1.566 ± 0.479 |
| CNTF | 352 ± 74 | 0.766 ± 0.286 |
| NT3 | 463 ± 121 | 1.819 ± 0.700 |
| NT4 | 291 ± 51 | 0.674 ± 0.123 |
| GM | 282 ± 41 | 0.616 ± 0.125 |
| CM-ASC | 354 ± 31 | 0.999 ± 0.296 |
| CM-NGF-ASC | 526 ± 87 | 2.460 ± 0.586 |
| CM-GDNF-ASC | 505 ± 75 | 1.512 ± 0.334 |
| CM-BDNF-ASC | 598 ± 118 | 1.918 ± 0.547 |
| CM-CNTF-ASC | 599 ± 58 | 2.032 ± 0.643 |
| CM-NT3-ASC | 765 ± 134 | 3.423 ± 0.798 |
| CM-NT4-ASC | 522 ± 80 | 1.617 ± 0.427 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prautsch, K.M.; Schmidt, A.; Paradiso, V.; Schaefer, D.J.; Guzman, R.; Kalbermatten, D.F.; Madduri, S. Modulation of Human Adipose Stem Cells’ Neurotrophic Capacity Using a Variety of Growth Factors for Neural Tissue Engineering Applications: Axonal Growth, Transcriptional, and Phosphoproteomic Analyses In Vitro. Cells 2020, 9, 1939. https://doi.org/10.3390/cells9091939
Prautsch KM, Schmidt A, Paradiso V, Schaefer DJ, Guzman R, Kalbermatten DF, Madduri S. Modulation of Human Adipose Stem Cells’ Neurotrophic Capacity Using a Variety of Growth Factors for Neural Tissue Engineering Applications: Axonal Growth, Transcriptional, and Phosphoproteomic Analyses In Vitro. Cells. 2020; 9(9):1939. https://doi.org/10.3390/cells9091939
Chicago/Turabian StylePrautsch, Katharina M., Alexander Schmidt, Viola Paradiso, Dirk J. Schaefer, Raphael Guzman, Daniel F. Kalbermatten, and Srinivas Madduri. 2020. "Modulation of Human Adipose Stem Cells’ Neurotrophic Capacity Using a Variety of Growth Factors for Neural Tissue Engineering Applications: Axonal Growth, Transcriptional, and Phosphoproteomic Analyses In Vitro" Cells 9, no. 9: 1939. https://doi.org/10.3390/cells9091939
APA StylePrautsch, K. M., Schmidt, A., Paradiso, V., Schaefer, D. J., Guzman, R., Kalbermatten, D. F., & Madduri, S. (2020). Modulation of Human Adipose Stem Cells’ Neurotrophic Capacity Using a Variety of Growth Factors for Neural Tissue Engineering Applications: Axonal Growth, Transcriptional, and Phosphoproteomic Analyses In Vitro. Cells, 9(9), 1939. https://doi.org/10.3390/cells9091939