Phosphorus Feast and Famine in Cyanobacteria: Is Luxury Uptake of the Nutrient Just a Consequence of Acclimation to Its Shortage?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgal Strains and Cultivation Conditions
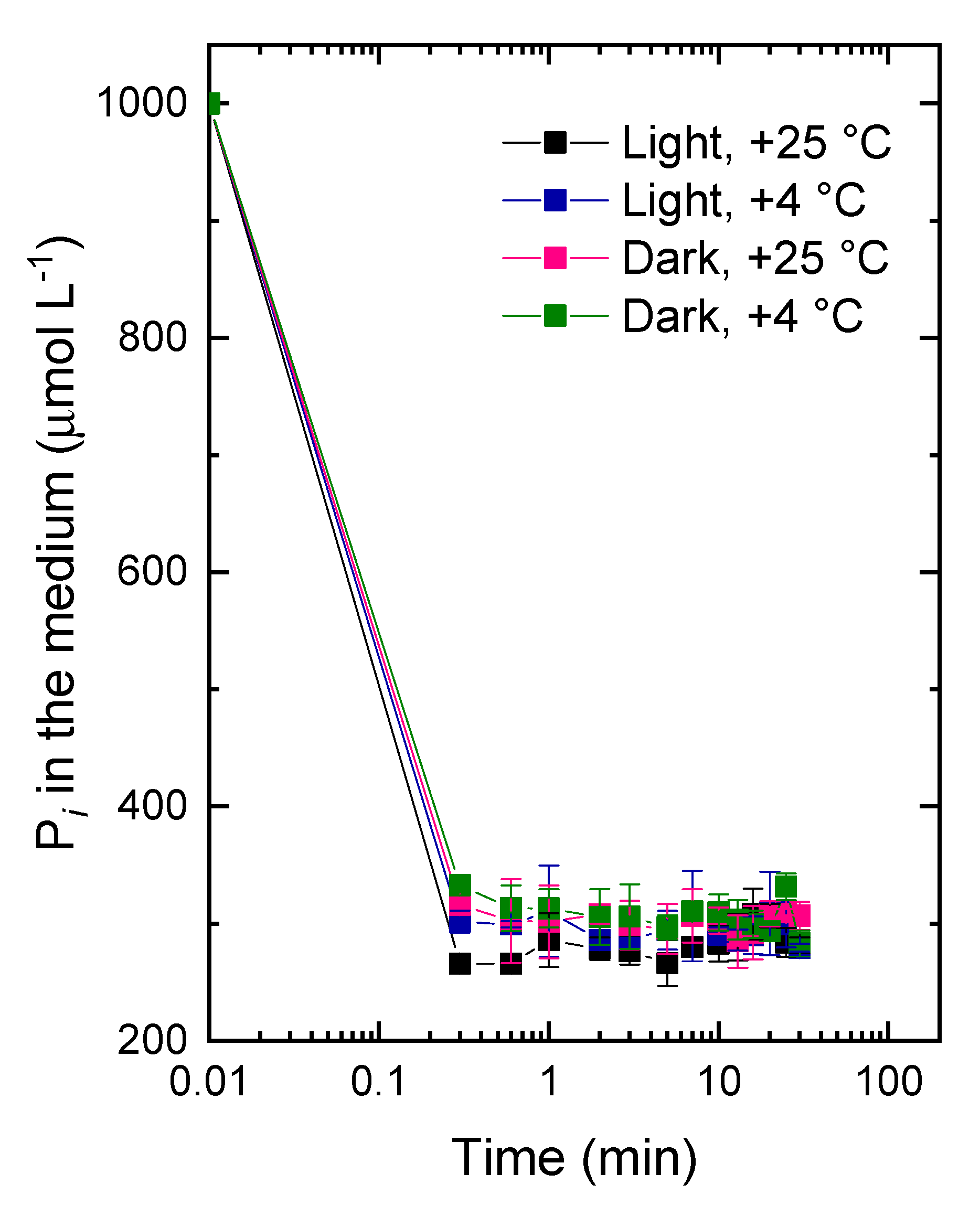
2.2. The Studies of the Fast Pi Uptake Phase
2.3. Spectral Measurements
2.4. Electron Microscopy and Cell Morphometry
2.5. Assay of Phosphate in the Medium: Total Phosphorus and Polyphosphate in the Cells
2.6. Sequencing of Nostoc sp. PCC 7118 Genome
2.7. Studies of Gene Expression
2.8. Statistical Treatment
3. Results
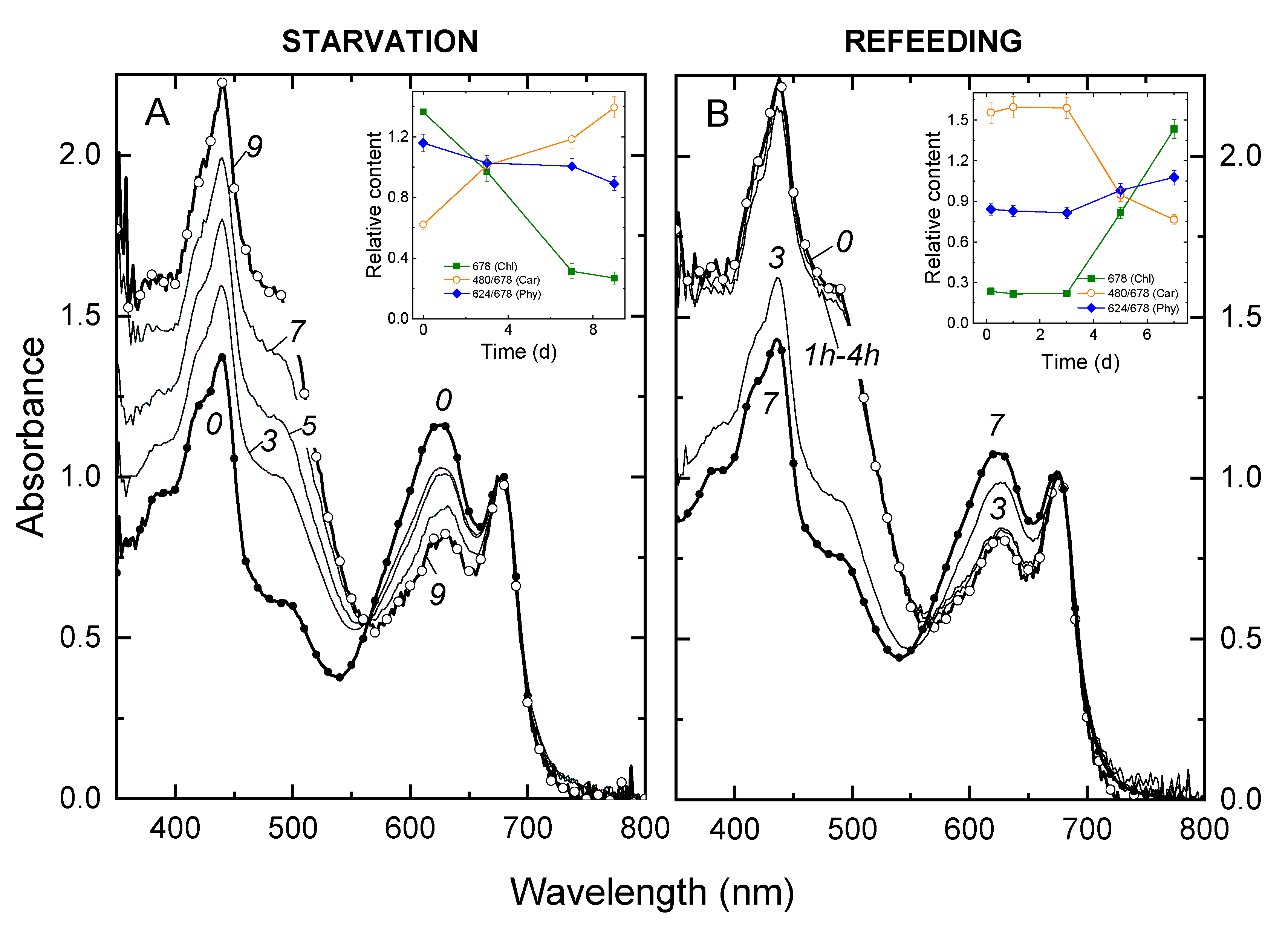
3.1. Changes in the Growth Rate of and Light Absorption by the Culture during P Starvation and Recovery from It
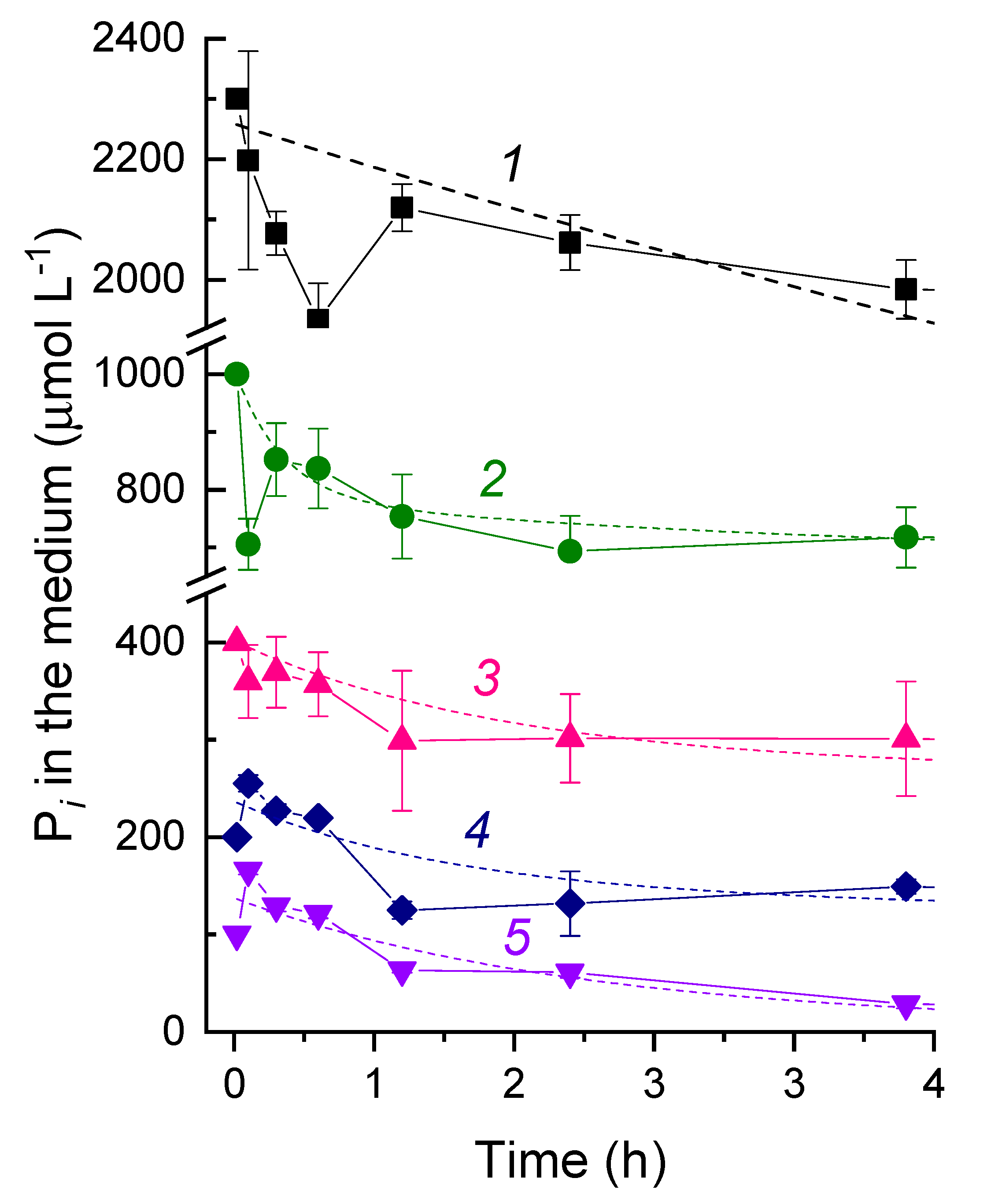
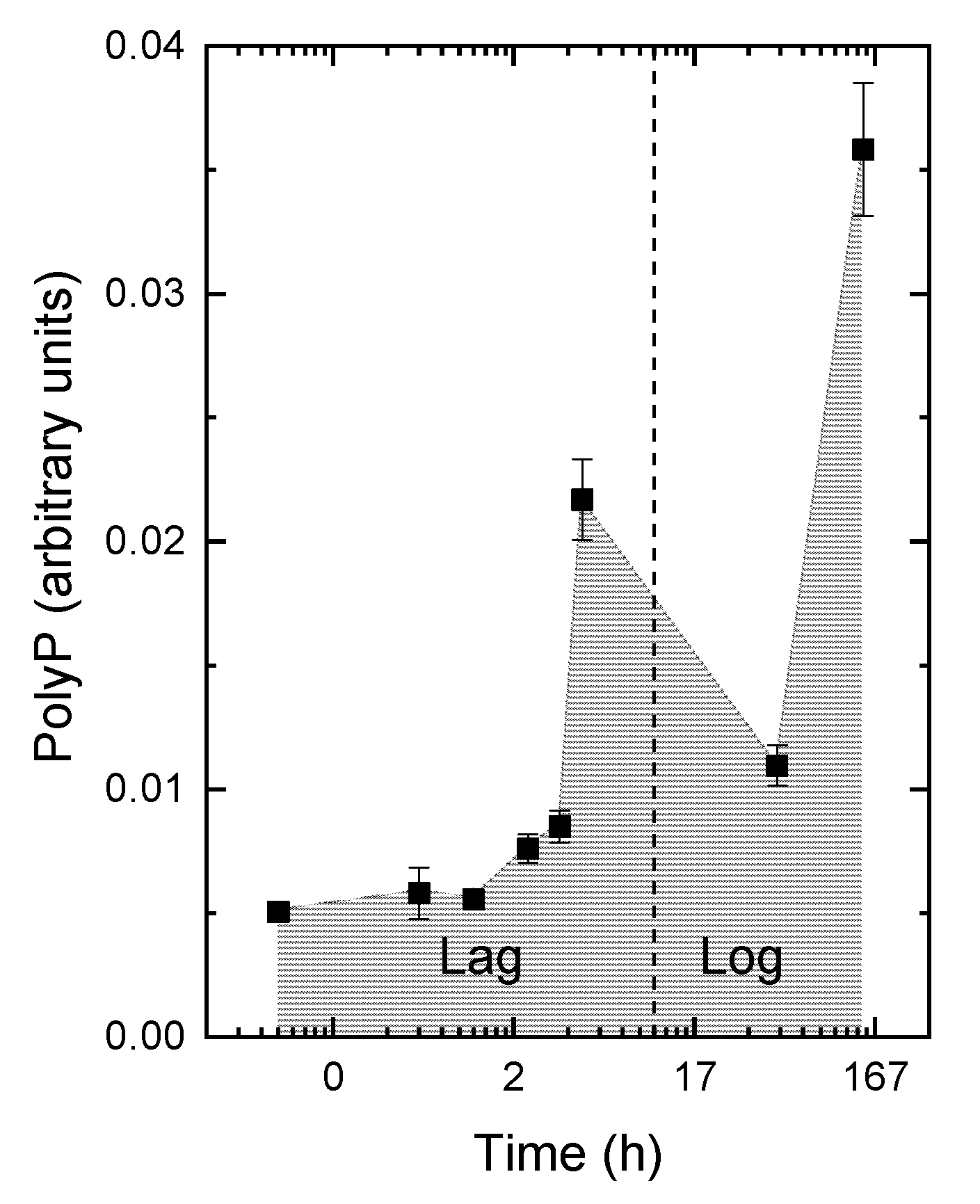
3.2. The Kinetics of Pi Uptake and PolyP Formation during after Refeeding of the P-Starved Cultures
3.3. Ultrastructural Hallmarks of P Starvation, Recovery, and Luxury Uptake of This Nutrient
3.4. Dynamics of Gene Expression during P starvation and Recovery
3.4.1. Overview of the Transcriptome Analysis
3.4.2. Phosphorus Uptake and Intracellular Storage
3.4.3. Photosynthetic Apparatus and Central Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rao, N.N.; Gómez-García, M.R.; Kornberg, A. Inorganic polyphosphate: Essential for growth and survival. Annu. Rev. Biochem. 2009, 78, 605–647. [Google Scholar] [CrossRef] [PubMed]
- Dyhrman, S.T. Nutrients and their acquisition: Phosphorus physiology in microalgae. In The Physiology of Microalgae; Springer: Berlin/Heidelberg, Germany, 2016; pp. 155–183. [Google Scholar]
- Ketchum, B.H. The absorption of phosphate and nitrate by illuminated cultures of Nitzschia closterium. Am. J. Bot. 1939, 26, 399–407. [Google Scholar] [CrossRef]
- Kulaev, I.; Vagabov, I.; Kulakovskaya, T. The Biochemistry of Inorganic Polyphosphates, 2nd ed.; John Wiley & Sons, Ltd: Chichester, UK, 2004. [Google Scholar]
- Droop, M. Some thoughts on nutrient limitation in algae 1. J. Phycol. 1973, 9, 264–272. [Google Scholar] [CrossRef]
- Solovchenko, A.E.; Ismagulova, T.T.; Lukyanov, A.A.; Vasilieva, S.G.; Konyukhov, I.V.; Pogosyan, S.I.; Lobakova, E.S.; Gorelova, O.A. Luxury phosphorus uptake in microalgae. J. Appl. Phycol. 2019, 31, 2755–2770. [Google Scholar] [CrossRef]
- Aitchison, P.; Butt, V. The relation between the synthesis of inorganic polyphosphate and phosphate uptake by Chlorella vulgaris. J. Exp. Bot. 1973, 24, 497–510. [Google Scholar] [CrossRef]
- Miyachi, S.; Kanai, R.; Mihara, S.; Miyachi, S.; Aoki, S. Metabolic roles of inorganic polyphosphates in Chlorella cells. Biochim. Biophys. Acta 1964, 93, 625–634. [Google Scholar] [CrossRef]
- Grillo, J.F.; Gibson, J. Regulation of phosphate accumulation in the unicellular cyanobacterium Synechococcus. J. Bacteriol. 1979, 140, 508–517. [Google Scholar] [CrossRef] [Green Version]
- Jensen, T.E.; Sicko, L.M. Phosphate metabolism in blue-green algae. I. Fine structure of the “polyphosphate overplus” phenomenon in Plectonema boryanum. Can. J. Microbiol. 1974, 20, 1235–1239. [Google Scholar] [CrossRef]
- Allen, M.M. Cyanobacterial cell inclusions. Annu. Rev. Microbiol. 1984, 38, 1–25. [Google Scholar] [CrossRef]
- Rhee, G.-Y. A continuous culture study of phosphate uptake, growth rate and polyphosphate in Scenedesmus sp. J. Phycol. 1973, 9, 495–506. [Google Scholar]
- Falkner, G.; Falkner, R. The complex regulation of the phosphate uptake system of cyanobacteria. In Bioenergetic Processes of Cyanobacteria; Springer: Berlin/Heidelberg, Germany, 2011; pp. 109–130. [Google Scholar]
- Falkner, R.; Falkner, G. Distinct adaptivity during phosphate uptake by the cyanobacterium Anabaena variabilis reflects information processing about preceding phosphate supply. J. Trace Microprobe Tech. 2003, 21, 363–375. [Google Scholar] [CrossRef]
- Sanz-Luque, E.; Bhaya, D.; Grossman, A.R. Polyphosphate: A Multifunctional Metabolite in Cyanobacteria and Algae. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Achbergerová, L.; Nahálka, J. Polyphosphate-an ancient energy source and active metabolic regulator. Microb. Cell Fact. 2011, 10, 14170–14175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baxter, M.; Jensen, T. Uptake of magnesium, strontium, barium, and manganese byPlectonema boryanum (Cyanophyceae) with special reference to polyphosphate bodies. Protoplasma 1980, 104, 81–89. [Google Scholar] [CrossRef]
- Baxter, M.; Jensen, T. A study of methods for in situ X-ray energy dispersive analysis of polyphosphate bodies in Plectonema boryanum. Arch. Microbiol. 1980, 126, 213–215. [Google Scholar] [CrossRef]
- Jacobson, L.; Halmann, M. Polyphosphate metabolism in the blue-green alga Microcystis aeru-ginosa. J. Plankton Res. 1982, 4, 481–488. [Google Scholar] [CrossRef]
- Livingstone, D.; Whitton, B. Influence of phosphorus on morphology of Calothrix parietina (Cyanophyta) in culture. Br. Phycol. J. 1983, 18, 29–38. [Google Scholar] [CrossRef]
- Ritchie, R.J.; Trautman, D.A.; Larkum, A. Phosphate uptake in the cyanobacterium Synechococcus R-2 PCC 7942. Plant Cell Physiol. 1997, 38, 1232–1241. [Google Scholar] [CrossRef]
- Ritchie, R.J.; Trautman, D.A.; Larkum, A.W. Phosphate limited cultures of the cyanobacterium Synechococcus are capable of very rapid, opportunistic uptake of phosphate. New Phytol. 2001, 152, 189–201. [Google Scholar] [CrossRef]
- Su, Z.; Olman, V.; Xu, Y. Computational prediction of Pho regulons in cyanobacteria. BMC Genom. 2007, 8, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, M.M.; Gómez-García, M.R.; Grossman, A.R.; Bhaya, D. Phosphorus deprivation responses and phosphonate utilization in a thermophilic Synechococcus sp. from microbial mats. J. Bacteriol. 2008, 190, 8171–8184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, L.; Chen, X.; Deng, Q.; Yang, L.; Li, X.; Zhang, J.; Song, C.; Zhou, Y.; Cao, X. Phosphorus strategy in bloom-forming cyanobacteria (Dolichospermum and Microcystis) and its role in their succession. Harmful Algae 2019, 84, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Nausch, M.; Nausch, G.; Lass, H.U.; Mohrholz, V.; Nagel, K.; Siegel, H.; Wasmund, N. Phosphorus input by upwelling in the eastern Gotland Basin (Baltic Sea) in summer and its effects on filamentous cyanobacteria. Estuar. Coast. Shelf Sci. 2009, 83, 434–442. [Google Scholar] [CrossRef]
- Solovchenko, A.; Verschoor, A.M.; Jablonowski, N.D.; Nedbal, L. Phosphorus from wastewater to crops: An alternative path involving microalgae. Biotechnol. Adv. 2016, 34, 550–564. [Google Scholar] [CrossRef] [PubMed]
- de Siqueira Castro, J.; Calijuri, M.L.; Ferreira, J.; Assemany, P.P.; Ribeiro, V.J. Microalgae based biofertilizer: A life cycle approach. Sci. Total Environ. 2020. [Google Scholar] [CrossRef]
- Elhai, J.; Wolk, C.P. Developmental regulation and spatial pattern of expression of the structural genes for nitrogenase in the cyanobacterium Anabaena. EMBO J. 1990, 9, 3379–3388. [Google Scholar] [CrossRef]
- Sato, N.; Wada, A. Disruption analysis of the gene for a cold-regulated RNA-Binding protein, rbpA1, in Anabaena: Cold-induced initiation of the heterocyst differentiation pathway. Plant Cell Physiol. 1996, 37, 1150–1160. [Google Scholar] [CrossRef] [Green Version]
- Rippka, R.; Stanier, R. The effects of anaerobiosis on nitrogenase synthesis and heterocyst development by nostocacean cyanobacteria. Microbiology 1978, 105, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Rippka, R. Recognition and identification of cyanobacteria. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1988; Volume 167, pp. 28–67. [Google Scholar]
- Kaneko, T. Complete Genomic Sequence of the Filamentous Nitrogen-fixing Cyanobacterium Anabaena sp. Strain PCC 7120. DNA Res. 2001, 8, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Stanier, R.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Microbiol. Mol. Biol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef] [Green Version]
- Pal, D.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl. Microbiol. Biotechnol. 2011, 90, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Solovchenko, A.; Merzlyak, M.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. Coordinated carotenoid and lipid syntheses induced in Parietochloris incisa (Chlorophyta, Trebouxiophyceae) mutant deficient in Δ5 desaturase by nitrogen starvation and high light. J. Phycol. 2010, 46, 763–772. [Google Scholar] [CrossRef]
- Simis, G.; Peers, S.; Gons, H. Remote sensing of the cyanobacterial pigment phycocyanin in turbid inland water. Limnol. Oceanogr. 2005, 50, 237–245. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Naqvi, K.R. On recording the true absorption spectrum and the scattering spectrum of a turbid sample: Application to cell suspensions of the cyanobacterium Anabaena variabilis. J. Photochem. Photobiol. B 2000, 58, 123–129. [Google Scholar] [CrossRef]
- Shebanova, A.; Ismagulova, T.; Solovchenko, A.; Baulina, O.; Lobakova, E.; Ivanova, A.; Moiseenko, A.; Shaitan, K.; Polshakov, V.; Nedbal, L.; et al. Versatility of the green microalga cell vacuole function as revealed by analytical transmission electron microscopy. Protoplasma 2017, 254, 1323–1340. [Google Scholar] [CrossRef]
- Gorelova, O.; Baulina, O.; Solovchenko, A.; Selyakh, I.; Chivkunova, O.; Semenova, L.; Scherbakov, P.; Burakova, O.; Lobakova, E. Coordinated rearrangements of assimilatory and storage cell compartments in a nitrogen-starving symbiotic chlorophyte cultivated under high light. Arch. Microbiol. 2015, 197, 181–195. [Google Scholar] [CrossRef]
- Solovchenko, A.; Pogosyan, S.; Chivkunova, O.; Selyakh, I.; Semenova, L.; Voronova, E.; Scherbakov, P.; Konyukhov, I.; Chekanov, K.; Kirpichnikov, M.; et al. Phycoremediation of alcohol distillery wastewater with a novel Chlorella sorokiniana strain cultivated in a photobioreactor monitored on-line via chlorophyll fluorescence. Algal Res.-Biomass Biofuels Bioprod. 2014, 6, 234–241. [Google Scholar] [CrossRef]
- Ota, S.; Kawano, S. Extraction and Molybdenum Blue-based Quantification of Total Phosphate and Polyphosphate in Parachlorella. Bio-Protocol 2017, 7, e2539. [Google Scholar] [CrossRef]
- Ota, S.; Yoshihara, M.; Yamazaki, T.; Takeshita, T.; Hirata, A.; Konomi, M.; Oshima, K.; Hattori, M.; Bisova, K.; Zachleder, V.; et al. Deciphering the relationship among phosphate dynamics, electron-dense body and lipid accumulation in the green alga Parachlorella kessleri. Sci. Rep. 2016, 6, 25731. [Google Scholar] [CrossRef]
- Kopf, M.; Klähn, S.; Scholz, I.; Matthiessen, J.K.; Hess, W.R.; Voß, B. Comparative analysis of the primary transcriptome of Synechocystis sp. PCC 6803. DNA Res. 2014, 21, 527–539. [Google Scholar] [CrossRef]
- Hein, S.; Scholz, I.; Voß, B.; Hess, W.R. Adaptation and modification of three CRISPR loci in two closely related cyanobacteria. RNA Biol. 2013, 10, 852–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baulina, O.I. Ultrastructural Plasticity of Cyanobacteria; Springer: Berlin/Heidelberg, Germany, 2012; p. 203. [Google Scholar]
- Ismagulova, T.; Shebanova, A.; Gorelova, O.; Baulina, O.; Solovchenko, A. A new simple method for quantification and locating P and N reserves in microalgal cells based on energy-filtered transmission electron microscopy (EFTEM) elemental maps. PLoS ONE 2018, 13, e0208830. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Ferjani, A.; Suzuki, I.; Murata, N. The SphS-SphR Two Component System Is the Exclusive Sensor for the Induction of Gene Expression in Response to Phosphate Limitation in Synechocystis. J. Biol. Chem. 2004, 279, 13234–13240. [Google Scholar] [CrossRef] [Green Version]
- Peterson, C.N.; Mandel, M.J.; Silhavy, T.J. Escherichia coli starvation diets: Essential nutrients weigh in distinctly. J. Bacteriol. 2005, 187, 7549–7553. [Google Scholar] [CrossRef] [Green Version]
- Scanlan, D.J.; Ostrowski, M.; Mazard, S.; Dufresne, A.; Garczarek, L.; Hess, W.R.; Post, A.F.; Hagemann, M.; Paulsen, I.; Partensky, F. Ecological genomics of marine picocyanobacteria. Microbiol. Mol. Biol. Rev. 2009, 73, 249–299. [Google Scholar] [CrossRef] [Green Version]
- Ault-Riché, D.; Fraley, C.D.; Tzeng, C.-M.; Kornberg, A. Novel assay reveals multiple pathways regulating stress-induced accumulations of inorganic polyphosphate in Escherichia coli. J. Bacteriol. 1998, 180, 1841–1847. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Sivonen, K.; Rouhiainen, L.; Fewer, D.P.; Lyra, C.; Rantala-Ylinen, A.; Vestola, J.; Jokela, J.; Rantasärkkä, K.; Li, Z. Genome-derived insights into the biology of the hepatotoxic bloom-forming cyanobacterium Anabaena sp. strain 90. BMC Genom. 2012, 13, 613. [Google Scholar] [CrossRef] [Green Version]
- Harke, M.J.; Gobler, C.J. Global transcriptional responses of the toxic cyanobacterium, Microcystis aeruginosa, to nitrogen stress, phosphorus stress, and growth on organic matter. PLoS ONE 2013, 8, 69834. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, M.; Bryant, D.A. Acclimation of the global transcriptome of the cyanobacterium Synechococcus sp. strain PCC 7002 to nutrient limitations and different nitrogen sources. Front. Microbiol. 2012, 3, 145. [Google Scholar] [CrossRef] [Green Version]
- Longhurst, A. A major seasonal phytoplankton bloom in the Madagascar Basin. Deep-Sea Res. Part I 2001, 48, 2413–2422. [Google Scholar] [CrossRef]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Barlow, D.J.; van Rensburl, W.; Pieterse, A.; Eloff, J. Effect of phosphate concentration on the fine structure of the cyanobacterium, Microcystis aeruginosa Kütz. emend. Elenkin. J. Limnol. Soc. South. Africa 1979, 5, 79–83. [Google Scholar] [CrossRef]
- Solovchenko, A.; Khozin-Goldberg, I.; Selyakh, I.; Semenova, L.; Ismagulova, T.; Lukyanov, A.; Mamedov, I.; Vinogradova, E.; Karpova, O.; Konyukhov, I.; et al. Phosphorus starvation and luxury uptake in green microalgae revisited. Algal Res. 2019, 43, 101651. [Google Scholar] [CrossRef]
- Bougaran, G.; Bernard, O.; Sciandra, A. Modeling continuous cultures of microalgae colimited by nitrogen and phosphorus. J. Theor. Biol. 2010, 265, 443–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tetu, S.G.; Brahamsha, B.; Johnson, D.A.; Tai, V.; Phillippy, K.; Palenik, B.; Paulsen, I.T. Microarray analysis of phosphate regulation in the marine cyanobacterium Synechococcus sp. WH8102. ISME J. 2009, 3, 835–849. [Google Scholar] [CrossRef] [Green Version]
- Teikari, J.; Österholm, J.; Kopf, M.; Battchikova, N.; Wahlsten, M.; Aro, E.-M.; Hess, W.R.; Sivonen, K. Transcriptomic and proteomic profiling of Anabaena sp. strain 90 under inorganic phosphorus stress. Appl. Environ. Microbiol. 2015, 81, 5212–5222. [Google Scholar] [CrossRef] [Green Version]
- Hudek, L.; Premachandra, D.; Webster, W.; Bräu, L. Role of phosphate transport system component PstB1 in phosphate internalization by Nostoc punctiforme. Appl. Environ. Microbiol. 2016, 82, 6344–6356. [Google Scholar] [CrossRef] [Green Version]
- Martiny, A.C.; Coleman, M.L.; Chisholm, S.W. Phosphate acquisition genes in Prochlorococcus ecotypes: Evidence for genome-wide adaptation. Proc. Natl. Acad. Sci. USA 2006, 103, 12552–12557. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Martín, M.Á.; Mateo, P.; Leganés, F.; Fernández-Piñas, F. Novel cyanobacterial bioreporters of phosphorus bioavailability based on alkaline phosphatase and phosphate transporter genes of Anabaena sp. PCC 7120. Anal. Bioanal. Chem. 2011, 400, 3573–3584. [Google Scholar] [CrossRef]
- Dyhrman, S.; Chappell, P.; Haley, S.; Moffett, J.; Orchard, E.; Waterbury, J.; Webb, E. Phosphonate utilization by the globally important marine diazotroph Trichodesmium. Nature 2006, 439, 68. [Google Scholar] [CrossRef] [PubMed]
- Fuszard, M.A.; Ow, S.Y.; Gan, C.S.; Noirel, J.; Ternan, N.G.; McMullan, G.; Biggs, C.A.; Reardon, K.F.; Wright, P.C. The quantitative proteomic response of Synechocystis sp. PCC6803 to phosphate acclimation. Aquat. Biosyst. 2013, 9, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collier, J.L.; Grossman, A. Chlorosis induced by nutrient deprivation in Synechococcus sp. strain PCC 7942: Not all bleaching is the same. J. Bacteriol. 1992, 174, 4718–4726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collier, J.L.; Herbert, S.K.; Fork, D.C.; Grossman, A.R. Changes in the cyanobacterial photosynthetic apparatus during acclimation to macronutrient deprivation. Photosynth. Res. 1994, 42, 173–183. [Google Scholar] [CrossRef]
- Voronkov, A.; Sinetova, M. Polyphosphate accumulation dynamics in a population of Synechocystis sp. PCC 6803 cells under phosphate overplus. Protoplasma 2019. [Google Scholar] [CrossRef]
- Li, Q.; Fu, L.; Wang, Y.; Zhou, D.; Rittmann, B.E. Excessive phosphorus caused inhibition and cell damage during heterotrophic growth of Chlorella regularis. Bioresour. Technol. 2018, 268, 266–270. [Google Scholar] [CrossRef]
- Gerasimaite, R.; Sharma, S.; Desfougeres, Y.; Schmidt, A.; Mayer, A. Coupled synthesis and translocation restrains polyphosphate to acidocalcisome-like vacuoles and prevents its toxicity. J. Cell Sci. 2014, 127, 5093–5104. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Yang, L.; Wu, J.; Xiao, L.; Wang, X.; Jiang, L. Simultaneous removal of phosphorus and nitrogen in a sequencing batch biofilm reactor with transgenic bacteria expressing polyphosphate kinase. Appl. Microbiol. Biotechnol. 2012, 96, 265–272. [Google Scholar] [CrossRef]
- Ishige, K.; Zhang, H.; Kornberg, A. Polyphosphate kinase (PPK2), a potent, polyphosphate-driven generator of GTP. Proc. Natl. Acad. Sci. USA 2002, 99, 16684–16688. [Google Scholar] [CrossRef] [Green Version]
- Gómez-García, M.a.R.; Losada, M.; Serrano, A. Concurrent transcriptional activation of ppa and ppx genes by phosphate deprivation in the cyanobacterium Synechocystis sp. strain PCC 6803. Biochem. Biophys. Res. Commun. 2003, 302, 601–609. [Google Scholar] [CrossRef]
- Albi, T.; Serrano, A. Two strictly polyphosphate-dependent gluco (manno) kinases from diazotrophic Cyanobacteria with potential to phosphorylate hexoses from polyphosphates. Appl. Microbiol. Biotechnol. 2015, 99, 3887–3900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klemke, F.; Beyer, G.; Sawade, L.; Saitov, A.; Korte, T.; Maldener, I.; Lockau, W.; Nürnberg, D.J.; Volkmer, T. All1371 is a polyphosphate-dependent glucokinase in Anabaena sp. PCC 7120. Microbiology 2014, 160, 2807. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.; Shilton, A. Luxury uptake of phosphorus by microalgae in waste stabilisation ponds: Current understanding and future direction. Rev. Environ. Sci. Bio/Technol. 2014, 13, 321–328. [Google Scholar] [CrossRef]
- Ray, K.; Mukherjee, C.; Ghosh, A.N. A way to curb phosphorus toxicity in the environment: Use of polyphosphate reservoir of cyanobacteria and microalga as a safe alternative phosphorus biofertilizer for Indian agriculture. Environ. Sci. Technol. 2013, 47, 11378–11379. [Google Scholar] [CrossRef] [PubMed]
- Powell, N.; Shilton, A.; Pratt, S.; Chisti, Y. Phosphate release from waste stabilisation pond sludge: Significance and fate of polyphosphate. Water Sci. Technol. 2011, 63, 1689. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, U.; Heiss, A.A.; Roth, R.; Rusch, J.; Lee, J.-H. Acidocalcisomes: Ultrastructure, Biogenesis, and Distribution in Microbial Eukaryotes. Protist 2019, 170, 287–313. [Google Scholar] [CrossRef]
- Wykoff, D.D.; Grossman, A.R.; Weeks, D.P.; Usuda, H.; Shimogawara, K. Psr1, a nuclear localized protein that regulates phosphorus metabolism in Chlamydomonas. Proc. Natl. Acad. Sci. USA 1999, 96, 15336–15341. [Google Scholar] [CrossRef] [Green Version]




| ORF | Name (Description) | Conditions | |||
|---|---|---|---|---|---|
| Preculture (+P) | P-Starved (−P) | Recovery (+P) | |||
| 1 d | 7 d | ||||
| all4573 | pstA1 | −1.92 | −0.43 | −0.47 | −0.1 |
| all4572 | pstB1 | −1.56 | −0.28 | −0.1 | −0.58 |
| all4574 | pstC1 | −2.76 | −0.13 | −0.29 | −0.09 |
| all4575 | pstS1 | −3.74 | −0.18 | −0.37 | −0.69 |
| all0909 | pstA2 | −2.76 | 0.88 | −0.16 | −1.92 |
| all0908 | pstB2 | −0.94 | −0.4 | −0.9 | −0.45 |
| all0910 | pstC2 | −3.62 | −0.37 | −0.58 | −0.79 |
| all0911 | pstS2 | −6.09 | 0.95 | −0.03 | −0.78 |
| ORF | Name (Description) | Conditions | |||
|---|---|---|---|---|---|
| Preculture (+P) | P-Starved (–P) | Recovery (+P) | |||
| 1 d | 7 d | ||||
| alr2234 | phoD (extracellular phosphatase) | −5.04 | −0.4 | −0.89 | −2.55 |
| all1686 | phoA (alkaline phosphatase) | −0.16 | 0.91 | 0.36 | 0.01 |
| all2843 | alkaline phosphatase | −4.23 | 1.86 | 0.92 | 0.74 |
| alr0276 | nucH (extracellular nuclease) | −2.18 | 0.08 | −0.17 | 0.31 |
| all1371 | ppgK (PolyP-dependent glucokinase) | 0.38 | −0.24 | 0.61 | 0.17 |
| alr3593 | ppk1 (ATP-dependent PolyP kinase) | 0.71 | 2.00 | 0.63 | 0.45 |
| all2191 | ppk2 (GTP-dependent PolyP kinase) | −1.29 | 0.90 | 1.23 | 1.28 |
| – | ppa (pyrophosphatase) | 0.67 | −0.99 | 0.83 | −0.20 |
| all3552 | ppx (exopolyphosphatase) | 0.06 | −0.93 | 0.44 | 0.08 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solovchenko, A.; Gorelova, O.; Karpova, O.; Selyakh, I.; Semenova, L.; Chivkunova, O.; Baulina, O.; Vinogradova, E.; Pugacheva, T.; Scherbakov, P.; et al. Phosphorus Feast and Famine in Cyanobacteria: Is Luxury Uptake of the Nutrient Just a Consequence of Acclimation to Its Shortage? Cells 2020, 9, 1933. https://doi.org/10.3390/cells9091933
Solovchenko A, Gorelova O, Karpova O, Selyakh I, Semenova L, Chivkunova O, Baulina O, Vinogradova E, Pugacheva T, Scherbakov P, et al. Phosphorus Feast and Famine in Cyanobacteria: Is Luxury Uptake of the Nutrient Just a Consequence of Acclimation to Its Shortage? Cells. 2020; 9(9):1933. https://doi.org/10.3390/cells9091933
Chicago/Turabian StyleSolovchenko, Alexei, Olga Gorelova, Olga Karpova, Irina Selyakh, Larisa Semenova, Olga Chivkunova, Olga Baulina, Elizaveta Vinogradova, Tatiana Pugacheva, Pavel Scherbakov, and et al. 2020. "Phosphorus Feast and Famine in Cyanobacteria: Is Luxury Uptake of the Nutrient Just a Consequence of Acclimation to Its Shortage?" Cells 9, no. 9: 1933. https://doi.org/10.3390/cells9091933





