Wide Biological Role of Hydroxytyrosol: Possible Therapeutic and Preventive Properties in Cardiovascular Diseases
Abstract
:1. Introduction
2. Methodology
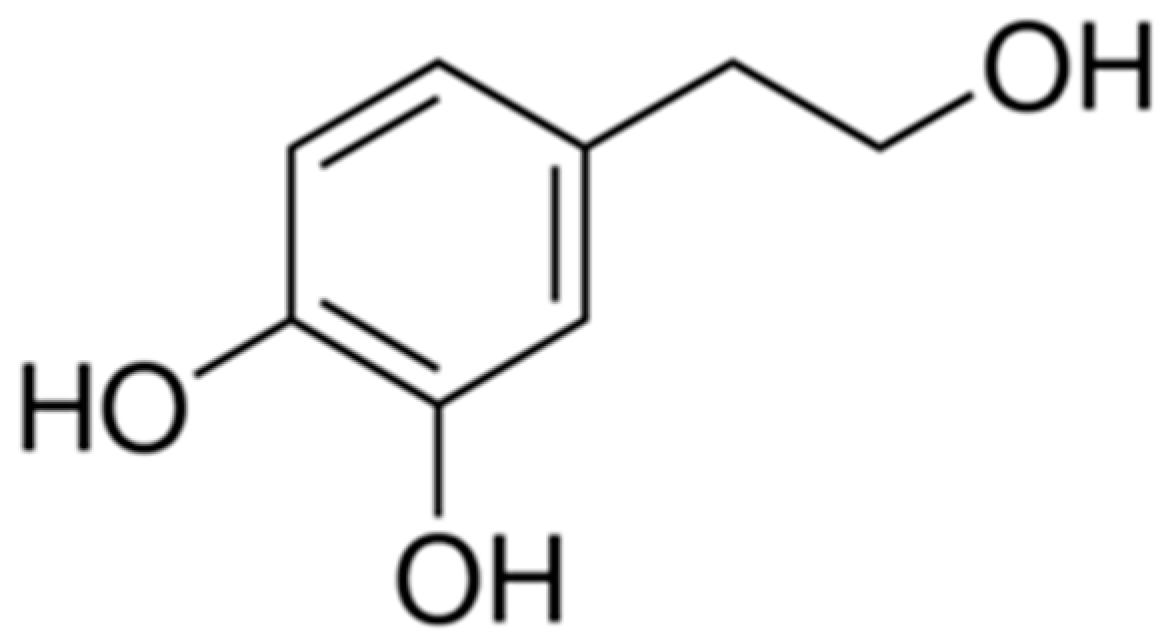
3. Biochemical Properties of Hydroxytyrosol
4. Biological Effects of Hydroxytyrosol in Cellular In Vitro Models
4.1. HT as Radical Species Scavenger: Protective Role against Oxidative Stress Contributing to Atherosclerosis
4.2. Anti-Inflammatory Role of HT
4.3. Modulation of Endothelial and Macrophage Activation in Protection from Atherosclerosis
4.4. Antithrombotic Effect
4.5. Antiadipogenic Role
5. Health Beneficial Effects of Hydroxytyrosol Demonstrated in Animal In Vivo Models
5.1. Protection of Low-Density Lipoprotein (LDL) from Oxidation
5.2. Improvement of Blood Lipid Profile
5.3. Hypoglycemic Ability
5.4. Anti-Inflammatory Activity
5.5. Antithrombotic Effect
6. Health Beneficial Effects Demonstrated Through Clinical Trials in Humans
6.1. Protection of Low-Density Lipoprotein (LDL) from Oxidation
6.2. Modulation of Blood Lipid Profile
6.3. Increased Insulin Sensitivity
6.4. Anti-Inflammatory Activity
6.5. Antithrombotic Effect
7. Conclusions
Funding
Conflicts of Interest
References
- Riccioni, G.; Speranza, L.; Pesce, M.; Cusenza, S.; D’Orazio, N.; Glade, M.J. Novel phytonutrient contributors to antioxidant protection against cardiovascular disease. Nutrition 2012, 28, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Franceschelli, S.; Ferrone, A.; Pesce, M.; Riccioni, G.; Speranza, L. Biological Functional Relevance of Asymmetric Dimethylarginine (ADMA) in Cardiovascular Disease. Int. J. Mol. Sci. 2013, 14, 24412–24421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cervantes Gracia, K.; Llanas-Cornejo, D.; Husi, H. CVD and Oxidative Stress. J. Clin. Med. 2017, 6, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buttar, H.S.; Li, T.; Ravi, N. Prevention of cardiovascular diseases: Role of exercise, dietary interventions, obesity and smoking cessation. Exp. Clin. Cardiol. 2005, 10, 229–249. [Google Scholar]
- Lorber, D. Importance of cardiovascular disease risk management in patients with type 2 diabetes mellitus. Diabetes Metab. Syndr. Obes. 2014, 7, 169–183. [Google Scholar] [CrossRef] [Green Version]
- Riccioni, G.; D’Orazio, N.; Salvatore, C.; Franceschelli, S.; Pesce, M.; Speranza, L. Carotenoids and vitamins C and E in the prevention of cardiovascular disease. Int. J. Vitam. Nutr. Res. 2012, 82, 15–26. [Google Scholar] [CrossRef]
- De Santis, S.; Cariello, M.; Piccinin, E.; Sabbà, C.; Moschetta, A. Extra Virgin Olive Oil: Lesson from Nutrigenomics. Nutrients 2019, 11, 2085. [Google Scholar] [CrossRef] [Green Version]
- Lovren, F.; Teoh, H.; Verma, S. Obesity and atherosclerosis: Mechanistic insights. Can. J. Cardiol. 2015, 31, 177–183. [Google Scholar] [CrossRef]
- Vilaplana-Pérez, C.; Auñón, D.; García-Flores, L.A.; Gil-Izquierdo, A. Hydroxytyrosol and potential uses in cardiovascular diseases, cancer, and AIDS. Front. Nutr. 2014, 1, 18. [Google Scholar] [CrossRef] [Green Version]
- Tejada, S.; Pinya, S.; del Mar Bibiloni, M.; Tur, J.A.; Pons, A.; Sureda, A. Cardioprotective Effects of the Polyphenol Hydroxytyrosol from Olive Oil. Curr. Drug Targets 2017, 18, 1477–1486. [Google Scholar] [CrossRef]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [Green Version]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Pesce, M.; Franceschelli, S.; Ferrone, A.; De Lutiis, M.A.; Patruno, A.; Grilli, A.; Felaco, M.; Speranza, L. Verbascoside down-regulates some pro-inflammatory signal transduction pathways by increasing the activity of tyrosine phosphatase SHP-1 in the U937 cell line. J. Cell. Mol. Med. 2015, 19, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Charoenprasert, S.; Mitchell, A. Factors influencing phenolic compounds in table olives (Olea europaea). J. Agric. Food Chem. 2012, 60, 7081–7095. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C.; Grande, S.; Colonnelli, K.; Patelli, C.; Galli, G.; Caruso, D. Hydroxytyrosol excretion differs between rats and humans and depends on the vehicle of administration. J. Nutr. 2003, 133, 2612–2615. [Google Scholar] [CrossRef]
- Vissers, M.N.; Zock, P.L.; Roodenburg, A.J.C.; Leenen, R.; Katan, M.B. Olive oil phenols are absorbed in humans. J. Nutr. 2002, 132, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Deiana, M. Biological Relevance of Extra Virgin Olive Oil Polyphenols Metabolites. Antioxidants 2018, 7, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suárez, M.; Valls, R.M.; Romero, M.-P.; Macià, A.; Fernández, S.; Giralt, M.; Solà, R.; Motilva, M.-J. Bioavailability of phenols from a phenol-enriched olive oil. Br. J. Nutr. 2011, 106, 1691–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubió, L.; Valls, R.-M.; Macià, A.; Pedret, A.; Giralt, M.; Romero, M.-P.; de la Torre, R.; Covas, M.-I.; Solà, R.; Motilva, M.-J. Impact of olive oil phenolic concentration on human plasmatic phenolic metabolites. Food Chem. 2012, 135, 2922–2929. [Google Scholar] [CrossRef]
- Miro-Casas, E.; Covas, M.-I.; Farre, M.; Fito, M.; Ortuño, J.; Weinbrenner, T.; Roset, P.; de la Torre, R. Hydroxytyrosol disposition in humans. Clin. Chem. 2003, 49, 945–952. [Google Scholar] [CrossRef] [Green Version]
- D’Angelo, S.; Manna, C.; Migliardi, V.; Mazzoni, O.; Morrica, P.; Capasso, G.; Pontoni, G.; Galletti, P.; Zappia, V. Pharmacokinetics and metabolism of hydroxytyrosol, a natural antioxidant from olive oil. Drug Metab. Dispos. 2001, 29, 1492–1498. [Google Scholar] [PubMed]
- Granados-Principal, S.; El-Azem, N.; Pamplona, R.; Ramirez-Tortosa, C.; Pulido-Moran, M.; Vera-Ramirez, L.; Quiles, J.L.; Sanchez-Rovira, P.; Naudí, A.; Portero-Otin, M.; et al. Hydroxytyrosol ameliorates oxidative stress and mitochondrial dysfunction in doxorubicin-induced cardiotoxicity in rats with breast cancer. Biochem. Pharmacol. 2014, 90, 25–33. [Google Scholar] [CrossRef] [PubMed]
- González-Santiago, M.; Fonollá, J.; Lopez-Huertas, E. Human absorption of a supplement containing purified hydroxytyrosol, a natural antioxidant from olive oil, and evidence for its transient association with low-density lipoproteins. Pharmacol. Res. 2010, 61, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Yan, X.; Takenaka, M.; Sekiya, K.; Nagata, T. Determination of Synthetic Hydroxytyrosol in Rat Plasma by GC-MS. J. Agric. Food Chem. 1998, 46, 3998–4001. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Auñón, D.; Ferreres, F.; Gil-Izquierdo, A. Physiological linkage of gender, bioavailable hydroxytyrosol derivatives, and their metabolites with systemic catecholamine metabolism. Food Funct. 2017, 8, 4570–4581. [Google Scholar] [CrossRef]
- Fernández-Ávila, C.; Montes, R.; Castellote, A.I.; Chisaguano, A.M.; Fitó, M.; Covas, M.I.; Muñoz-Aguallo, D.; Nyyssönen, K.; Zunft, H.J.; López-Sabater, M.C. Fast determination of virgin olive oil phenolic metabolites in human high-density lipoproteins. Biomed. Chromatogr. 2015, 29, 1035–1041. [Google Scholar] [CrossRef]
- Serra, A.; Rubió, L.; Borràs, X.; Macià, A.; Romero, M.-P.; Motilva, M.-J. Distribution of olive oil phenolic compounds in rat tissues after administration of a phenolic extract from olive cake. Mol. Nutr. Food Res. 2012, 56, 486–496. [Google Scholar] [CrossRef]
- Chashmi, N.A.; Emadi, S.; Khastar, H. Protective effects of hydroxytyrosol on gentamicin induced nephrotoxicity in mice. Biochem. Biophys. Res. Commun. 2017, 482, 1427–1429. [Google Scholar] [CrossRef]
- Rodríguez-Morató, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Pérez-Mañá, C.; Khymenets, O.; Fitó, M.; Farré, M.; et al. Metabolic disposition and biological significance of simple phenols of dietary origin: Hydroxytyrosol and tyrosol. Drug Metab. Rev. 2016, 48, 218–236. [Google Scholar] [CrossRef] [Green Version]
- Tuck, K.L.; Freeman, M.P.; Hayball, P.J.; Stretch, G.L.; Stupans, I. The in vivo fate of hydroxytyrosol and tyrosol, antioxidant phenolic constituents of olive oil, after intravenous and oral dosing of labeled compounds to rats. J. Nutr. 2001, 131, 1993–1996. [Google Scholar] [CrossRef] [Green Version]
- Speranza, L.; Franceschelli, S.; Pesce, M.; Vinciguerra, I.; De Lutiis, M.A.; Grilli, A.; Felaco, M.; Patruno, A. Phosphodiesterase type-5 inhibitor and oxidative stress. Int. J. Immunopathol. Pharm. 2008, 21, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Li, Y.; Ren, X.; Zhang, X.; Hu, D.; Gao, Y.; Xing, Y.; Shang, H. Oxidative Stress-Mediated Atherosclerosis: Mechanisms and Therapies. Front. Physiol. 2017, 8, 600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschelli, S.; Pesce, M.; Ferrone, A.; Gatta, D.M.P.; Patruno, A.; Lutiis, M.A.D.; Quiles, J.L.; Grilli, A.; Felaco, M.; Speranza, L. Biological Effect of Licochalcone C on the Regulation of PI3K/Akt/eNOS and NF-κB/iNOS/NO Signaling Pathways in H9c2 Cells in Response to LPS Stimulation. Int. J. Mol. Sci. 2017, 18, 690. [Google Scholar] [CrossRef] [PubMed]
- Speranza, L.; Franceschelli, S.; Pesce, M.; Ferrone, A.; Patruno, A.; Riccioni, G.; De Lutiis, M.A.; Felaco, M.; Grilli, A. Negative feedback interaction of HO-1/INOS in PBMC of acute congestive heart failure patients. J. Biol. Regul. Homeost. Agents 2013, 27, 739–748. [Google Scholar] [PubMed]
- Franceschelli, S.; Gatta, D.M.P.; Pesce, M.; Ferrone, A.; Di Martino, G.; Di Nicola, M.; De Lutiis, M.A.; Vitacolonna, E.; Patruno, A.; Grilli, A.; et al. Modulation of the oxidative plasmatic state in gastroesophageal reflux disease with the addition of rich water molecular hydrogen: A new biological vision. J. Cell. Mol. Med. 2018, 22, 2750–2759. [Google Scholar] [CrossRef]
- Napolitano, A.; De Lucia, M.; Panzella, L.; d’Ischia, M. Chapter 134-The Chemistry of Tyrosol and Hydroxytyrosol: Implications for Oxidative Stress. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 1225–1232. ISBN 978-0-12-374420-3. [Google Scholar]
- Fitó, M.; Cladellas, M.; Torre, R.d.l.; Martí, J.; Alcántara, M.; Pujadas-Bastardes, M.; Marrugat, J.; Bruguera, J.; López-Sabater, M.C.; Vila, J.; et al. Antioxidant effect of virgin olive oil in patients with stable coronary heart disease: A randomized, crossover, controlled, clinical trial. Atherosclerosis 2005, 181, 149–158. [Google Scholar] [CrossRef]
- De la Torre-Carbot, K.; Jauregui, O.; Gimeno, E.; Castellote, A.I.; Lamuela-Raventós, R.M.; López-Sabater, M.C. Characterization and quantification of phenolic compounds in olive oils by solid-phase extraction, HPLC-DAD, and HPLC-MS/MS. J. Agric. Food Chem. 2005, 53, 4331–4340. [Google Scholar] [CrossRef]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, J.; Jiang, L.; Zhong, L. Suppressive effects of hydroxytyrosol on oxidative stress and nuclear Factor-kappaB activation in THP-1 cells. Biol. Pharm. Bull. 2009, 32, 578–582. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, C.A.; Handler, N.; Heiss, E.H.; Erker, T.; Dirsch, V.M. No evidence for modulation of endothelial nitric oxide synthase by the olive oil polyphenol hydroxytyrosol in human endothelial cells. Atherosclerosis 2007, 195, e58–e64. [Google Scholar] [CrossRef]
- Wang, W.; Shang, C.; Zhang, W.; Jin, Z.; Yao, F.; He, Y.; Wang, B.; Li, Y.; Zhang, J.; Lin, R. Hydroxytyrosol NO regulates oxidative stress and NO production through SIRT1 in diabetic mice and vascular endothelial cells. Phytomedicine 2019, 52, 206–215. [Google Scholar] [CrossRef]
- Storniolo, C.E.; Roselló-Catafau, J.; Pintó, X.; Mitjavila, M.T.; Moreno, J.J. Polyphenol fraction of extra virgin olive oil protects against endothelial dysfunction induced by high glucose and free fatty acids through modulation of nitric oxide and endothelin-1. Redox Biol. 2014, 2, 971–977. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Khismatullin, D.B. Oxidized low-density lipoprotein contributes to atherogenesis via co-activation of macrophages and mast cells. PLoS ONE 2015, 10, e0123088. [Google Scholar] [CrossRef] [PubMed]
- Salami, M.; Galli, C.; De Angelis, L.; Visioli, F. Formation of F2-isoprostanes in oxidized low density lipoprotein: Inhibitory effect of hydroxytyrosol. Pharmacol. Res. 1995, 31, 275–279. [Google Scholar] [CrossRef]
- Visioli, F.; Bellomo, G.; Montedoro, G.; Galli, C. Low density lipoprotein oxidation is inhibited in vitro by olive oil constituents. Atherosclerosis 1995, 117, 25–32. [Google Scholar] [CrossRef]
- Berrougui, H.; Ikhlef, S.; Khalil, A. Extra Virgin Olive Oil Polyphenols Promote Cholesterol Efflux and Improve HDL Functionality. Evid. Based Complement. Altern. Med. 2015, 2015, 208062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atzeri, A.; Lucas, R.; Incani, A.; Peñalver, P.; Zafra-Gómez, A.; Melis, M.P.; Pizzala, R.; Morales, J.C.; Deiana, M. Hydroxytyrosol and tyrosol sulfate metabolites protect against the oxidized cholesterol pro-oxidant effect in Caco-2 human enterocyte-like cells. Food Funct. 2016, 7, 337–346. [Google Scholar] [CrossRef]
- O’Dowd, Y.; Driss, F.; Dang, P.M.-C.; Elbim, C.; Gougerot-Pocidalo, M.-A.; Pasquier, C.; El-Benna, J. Antioxidant effect of hydroxytyrosol, a polyphenol from olive oil: Scavenging of hydrogen peroxide but not superoxide anion produced by human neutrophils. Biochem. Pharmacol. 2004, 68, 2003–2008. [Google Scholar] [CrossRef]
- Calabriso, N.; Gnoni, A.; Stanca, E.; Cavallo, A.; Damiano, F.; Siculella, L.; Carluccio, M.A. Hydroxytyrosol Ameliorates Endothelial Function under Inflammatory Conditions by Preventing Mitochondrial Dysfunction. Oxid. Med. Cell Longev. 2018, 2018, 9086947. [Google Scholar] [CrossRef]
- Ozbek, N.; Bali, E.B.; Karasu, C. Quercetin and hydroxytyrosol attenuates xanthine/xanthine oxidase-induced toxicity in H9c2 cardiomyocytes by regulation of oxidative stress and stress-sensitive signaling pathways. Gen. Physiol. Biophys. 2015, 34, 407–414. [Google Scholar] [CrossRef]
- Zrelli, H.; Matsuoka, M.; Kitazaki, S.; Zarrouk, M.; Miyazaki, H. Hydroxytyrosol reduces intracellular reactive oxygen species levels in vascular endothelial cells by upregulating catalase expression through the AMPK-FOXO3a pathway. Eur. J. Pharmacol. 2011, 660, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Tagliafierro, L.; Officioso, A.; Sorbo, S.; Basile, A.; Manna, C. The protective role of olive oil hydroxytyrosol against oxidative alterations induced by mercury in human erythrocytes. Food Chem. Toxicol. 2015, 82, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Officioso, A.; Alzoubi, K.; Lang, F.; Manna, C. Hydroxytyrosol inhibits phosphatidylserine exposure and suicidal death induced by mercury in human erythrocytes: Possible involvement of the glutathione pathway. Food Chem. Toxicol. 2016, 89, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Feng, Z.; Li, Y.; Wang, Y.; Wertz, K.; Weber, P.; Fu, Y.; Liu, J. Stimulation of GSH synthesis to prevent oxidative stress-induced apoptosis by hydroxytyrosol in human retinal pigment epithelial cells: Activation of Nrf2 and JNK-p62/SQSTM1 pathways. J. Nutr. Biochem. 2012, 23, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Zrelli, H.; Matsuoka, M.; Kitazaki, S.; Araki, M.; Kusunoki, M.; Zarrouk, M.; Miyazaki, H. Hydroxytyrosol induces proliferation and cytoprotection against oxidative injury in vascular endothelial cells: Role of Nrf2 activation and HO-1 induction. J. Agric. Food Chem. 2011, 59, 4473–4482. [Google Scholar] [CrossRef] [PubMed]
- Zrelli, H.; Kusunoki, M.; Miyazaki, H. Role of Hydroxytyrosol-dependent Regulation of HO-1 Expression in Promoting Wound Healing of Vascular Endothelial Cells via Nrf2 De Novo Synthesis and Stabilization. Phytother. Res. 2015, 29, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, C.; Goldeck, D.; Pawelec, G.; Gaspari, L.; Di Iorio, A.; Paganelli, R. Exploratory study on immune phenotypes in Alzheimer’s Disease and vascular dementia. Eur. J. Neurol. 2020. [Google Scholar] [CrossRef]
- Felaco, P.; Toniato, E.; Castellani, M.L.; Ciampoli, C.; De Amicis, D.; Orso, C.; Cuccurullo, C.; De Lutiis, M.A.; Patruno, A.; Speranza, L.; et al. Infections and mast cells. J. Biol. Regul. Homeost. Agents 2009, 23, 231–238. [Google Scholar]
- D’Angelo, C.; Franch, O.; Fernández-Paredes, L.; Oreja-Guevara, C.; Núñez-Beltrán, M.; Comins-Boo, A.; Reale, M.; Sánchez-Ramón, S. Antiphospholipid Antibodies Overlapping in Isolated Neurological Syndrome and Multiple Sclerosis: Neurobiological Insights and Diagnostic Challenges. Front. Cell Neurosci. 2019, 13, 107. [Google Scholar] [CrossRef]
- Kofler, S.; Nickel, T.; Weis, M. Role of cytokines in cardiovascular diseases: A focus on endothelial responses to inflammation. Clin. Sci. 2005, 108, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Richard, N.; Arnold, S.; Hoeller, U.; Kilpert, C.; Wertz, K.; Schwager, J. Hydroxytyrosol is the major anti-inflammatory compound in aqueous olive extracts and impairs cytokine and chemokine production in macrophages. Planta Med. 2011, 77, 1890–1897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scoditti, E.; Nestola, A.; Massaro, M.; Calabriso, N.; Storelli, C.; De Caterina, R.; Carluccio, M.A. Hydroxytyrosol suppresses MMP-9 and COX-2 activity and expression in activated human monocytes via PKCα and PKCβ1 inhibition. Atherosclerosis 2014, 232, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Bui, V.N.; Iwasaki, K.; Kobayashi, T.; Ogawa, H.; Imai, K. Influence of olive-derived hydroxytyrosol on the toll-like receptor 4-dependent inflammatory response of mouse peritoneal macrophages. Biochem. Biophys. Res. Commun. 2014, 446, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Rosignoli, P.; Fuccelli, R.; Fabiani, R.; Servili, M.; Morozzi, G. Effect of olive oil phenols on the production of inflammatory mediators in freshly isolated human monocytes. J. Nutr. Biochem. 2013, 24, 1513–1519. [Google Scholar] [CrossRef]
- Carluccio, M.A.; Siculella, L.; Ancora, M.A.; Massaro, M.; Scoditti, E.; Storelli, C.; Visioli, F.; Distante, A.; De Caterina, R. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: Antiatherogenic properties of Mediterranean diet phytochemicals. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 622–629. [Google Scholar] [CrossRef] [Green Version]
- Manna, C.; Napoli, D.; Cacciapuoti, G.; Porcelli, M.; Zappia, V. Olive Oil Phenolic Compounds Inhibit Homocysteine-Induced Endothelial Cell Adhesion Regardless of Their Different Antioxidant Activity. J. Agric. Food Chem. 2009, 57, 3478–3482. [Google Scholar] [CrossRef] [PubMed]
- Dell’Agli, M.; Fagnani, R.; Mitro, N.; Scurati, S.; Masciadri, M.; Mussoni, L.; Galli, G.V.; Bosisio, E.; Crestani, M.; De Fabiani, E.; et al. Minor Components of Olive Oil Modulate Proatherogenic Adhesion Molecules Involved in Endothelial Activation. J. Agric. Food Chem. 2006, 54, 3259–3264. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, M.A.; Ancora, M.A.; Massaro, M.; Carluccio, M.; Scoditti, E.; Distante, A.; Storelli, C.; De Caterina, R. Homocysteine induces VCAM-1 gene expression through NF-kappaB and NAD(P)H oxidase activation: Protective role of Mediterranean diet polyphenolic antioxidants. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H2344–H2354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meza-Miranda, E.R.; Rangel-Zúñiga, O.A.; Marín, C.; Pérez-Martínez, P.; Delgado-Lista, J.; Haro, C.; Peña-Orihuela, P.; Jiménez-Morales, A.I.; Malagón, M.M.; Tinahones, F.J.; et al. Virgin olive oil rich in phenolic compounds modulates the expression of atherosclerosis-related genes in vascular endothelium. Eur. J. Nutr. 2016, 55, 519–527. [Google Scholar] [CrossRef]
- Catalán, Ú.; López de Las Hazas, M.-C.; Rubió, L.; Fernández-Castillejo, S.; Pedret, A.; de la Torre, R.; Motilva, M.-J.; Solà, R. Protective effect of hydroxytyrosol and its predominant plasmatic human metabolites against endothelial dysfunction in human aortic endothelial cells. Mol. Nutr. Food Res. 2015, 59, 2523–2536. [Google Scholar] [CrossRef] [Green Version]
- Dell’Agli, M.; Maschi, O.; Galli, G.V.; Fagnani, R.; Dal Cero, E.; Caruso, D.; Bosisio, E. Inhibition of platelet aggregation by olive oil phenols via cAMP-phosphodiesterase. Br. J. Nutr. 2008, 99, 945–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zbidi, H.; Salido, S.; Altarejos, J.; Perez-Bonilla, M.; Bartegi, A.; Rosado, J.A.; Salido, G.M. Olive tree wood phenolic compounds with human platelet antiaggregant properties. Blood Cells Mol. Dis. 2009, 42, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.J.; De La Cruz, J.P.; Muñoz-Marin, J.; Guerrero, A.; Lopez-Villodres, J.A.; Madrona, A.; Espartero, J.L.; Gonzalez-Correa, J.A. Antiplatelet effect of new lipophilic hydroxytyrosol alkyl ether derivatives in human blood. Eur. J. Nutr. 2013, 52, 591–599. [Google Scholar] [CrossRef]
- Scoditti, E.; Massaro, M.; Carluccio, M.A.; Pellegrino, M.; Wabitsch, M.; Calabriso, N.; Storelli, C.; De Caterina, R. Additive regulation of adiponectin expression by the mediterranean diet olive oil components oleic Acid and hydroxytyrosol in human adipocytes. PLoS ONE 2015, 10, e0128218. [Google Scholar] [CrossRef]
- Scoditti, E.; Calabriso, N.; Massaro, M.; Pellegrino, M.; Storelli, C.; Martines, G.; De Caterina, R.; Carluccio, M.A. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: A potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch. Biochem. Biophys. 2012, 527, 81–89. [Google Scholar] [CrossRef]
- Stefanon, B.; Colitti, M. Original Research: Hydroxytyrosol, an ingredient of olive oil, reduces triglyceride accumulation and promotes lipolysis in human primary visceral adipocytes during differentiation. Exp. Biol. Med. (Maywood) 2016, 241, 1796–1802. [Google Scholar] [CrossRef]
- Ochoa, J.J.; Quiles, J.L.; Ramırez-Tortosa, M.C.; Mataix, J.; Huertas, J.R. Dietary oils high in oleic acid but with different unsaponifiable fraction contents have different effects in fatty acid composition and peroxidation in rabbit LDL. Nutrition 2002, 18, 60–65. [Google Scholar] [CrossRef]
- Jemai, H.; Fki, I.; Bouaziz, M.; Bouallagui, Z.; El Feki, A.; Isoda, H.; Sayadi, S. Lipid-lowering and antioxidant effects of hydroxytyrosol and its triacetylated derivative recovered from olive tree leaves in cholesterol-fed rats. J. Agric. Food Chem. 2008, 56, 2630–2636. [Google Scholar] [CrossRef]
- Fki, I.; Sahnoun, Z.; Sayadi, S. Hypocholesterolemic effects of phenolic extracts and purified hydroxytyrosol recovered from olive mill wastewater in rats fed a cholesterol-rich diet. J. Agric. Food Chem. 2007, 55, 624–631. [Google Scholar] [CrossRef]
- Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Zheng, A.; Li, H.; Li, H.; Szeto, I.M.-Y.; Shi, Y.; et al. Hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free Radic. Biol. Med. 2014, 67, 396–407. [Google Scholar] [CrossRef]
- Rietjens, S.J.; Bast, A.; de Vente, J.; Haenen, G.R.M.M. The olive oil antioxidant hydroxytyrosol efficiently protects against the oxidative stress-induced impairment of the NObullet response of isolated rat aorta. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1931–H1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabernero, M.; Sarriá, B.; Largo, C.; Martínez-López, S.; Madrona, A.; Espartero, J.L.; Bravo, L.; Mateos, R. Comparative evaluation of the metabolic effects of hydroxytyrosol and its lipophilic derivatives (hydroxytyrosyl acetate and ethyl hydroxytyrosyl ether) in hypercholesterolemic rats. Food Funct. 2014, 5, 1556–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Santiago, M.; Martín-Bautista, E.; Carrero, J.J.; Fonollá, J.; Baró, L.; Bartolomé, M.V.; Gil-Loyzaga, P.; López-Huertas, E. One-month administration of hydroxytyrosol, a phenolic antioxidant present in olive oil, to hyperlipemic rabbits improves blood lipid profile, antioxidant status and reduces atherosclerosis development. Atherosclerosis 2006, 188, 35–42. [Google Scholar] [CrossRef] [PubMed]
- González-Correa, J.A.; Navas, M.D.; Muñoz-Marín, J.; Trujillo, M.; Fernández-Bolaños, J.; de la Cruz, J.P. Effects of hydroxytyrosol and hydroxytyrosol acetate administration to rats on platelet function compared to acetylsalicylic acid. J. Agric. Food Chem. 2008, 56, 7872–7876. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Casas, R.; Sacanella, E.; Urpí-Sardà, M.; Chiva-Blanch, G.; Ros, E.; Martínez-González, M.-A.; Covas, M.-I.; Lamuela-Raventos, R.M.; Salas-Salvadó, J.; Fiol, M.; et al. The effects of the mediterranean diet on biomarkers of vascular wall inflammation and plaque vulnerability in subjects with high risk for cardiovascular disease. A randomized trial. PLoS ONE 2014, 9, e100084. [Google Scholar] [CrossRef] [Green Version]
- Medina-Remón, A.; Casas, R.; Tressserra-Rimbau, A.; Ros, E.; Martínez-González, M.A.; Fitó, M.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventos, R.M.; Estruch, R.; et al. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: A substudy of the PREDIMED trial. Br. J. Clin. Pharm. 2017, 83, 114–128. [Google Scholar] [CrossRef] [Green Version]
- Castro-Barquero, S.; Tresserra-Rimbau, A.; Vitelli-Storelli, F.; Doménech, M.; Salas-Salvadó, J.; Martín-Sánchez, V.; Rubín-García, M.; Buil-Cosiales, P.; Corella, D.; Fitó, M.; et al. Dietary Polyphenol Intake is Associated with HDL-Cholesterol and A Better Profile of other Components of the Metabolic Syndrome: A PREDIMED-Plus Sub-Study. Nutrients 2020, 12, 689. [Google Scholar] [CrossRef] [Green Version]
- Tresserra-Rimbau, A.; Castro-Barquero, S.; Vitelli-Storelli, F.; Becerra-Tomas, N.; Vázquez-Ruiz, Z.; Díaz-López, A.; Corella, D.; Castañer, O.; Romaguera, D.; Vioque, J.; et al. Associations between Dietary Polyphenols and Type 2 Diabetes in a Cross-Sectional Analysis of the PREDIMED-Plus Trial: Role of Body Mass Index and Sex. Antioxidants 2019, 8, 537. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Rodriguez, E.; Biel-Glesson, S.; Fernandez-Navarro, J.R.; Calleja, M.A.; Espejo-Calvo, J.A.; Gil-Extremera, B.; de la Torre, R.; Fito, M.; Covas, M.-I.; Vilchez, P.; et al. Effects of Virgin Olive Oils Differing in Their Bioactive Compound Contents on Biomarkers of Oxidative Stress and Inflammation in Healthy Adults: A Randomized Double-Blind Controlled Trial. Nutrients 2019, 11, 561. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Rodriguez, E.; Lima-Cabello, E.; Biel-Glesson, S.; Fernandez-Navarro, J.R.; Calleja, M.A.; Roca, M.; Espejo-Calvo, J.A.; Gil-Extremera, B.; Soria-Florido, M.; de la Torre, R.; et al. Effects of Virgin Olive Oils Differing in Their Bioactive Compound Contents on Metabolic Syndrome and Endothelial Functional Risk Biomarkers in Healthy Adults: A Randomized Double-Blind Controlled Trial. Nutrients 2018, 10, 626. [Google Scholar] [CrossRef] [Green Version]
- Biel, S.; Mesa, M.-D.; de la Torre, R.; Espejo, J.-A.; Fernández-Navarro, J.-R.; Fitó, M.; Sánchez-Rodriguez, E.; Rosa, C.; Marchal, R.; Alche, J.d.D.; et al. The NUTRAOLEOUM Study, a randomized controlled trial, for achieving nutritional added value for olive oils. BMC Complement. Altern Med. 2016, 16, 404. [Google Scholar] [CrossRef] [Green Version]
- Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract” (3779), and “contributes to body defences against external agents” (ID 3467) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2033. [CrossRef]
- Covas, M.-I.; Nyyssönen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.-J.F.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Bäumler, H.; et al. The effect of polyphenols in olive oil on heart disease risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 333–341. [Google Scholar] [CrossRef]
- Farré-Albaladejo, M.; Kaikkonen, J.; Fitó, M.; López-Sabater, C.; Pujadas-Bastardes, M.A.; Joglar, J.; Weinbrenner, T.; Lamuela-Raventós, R.M.; de la Torre, R. Postprandial LDL phenolic content and LDL oxidation are modulated by olive oil phenolic compounds in humans. Free Radic. Biol. Med. 2006, 40, 608–616. [Google Scholar] [CrossRef]
- Quirós-Fernández, R.; López-Plaza, B.; Bermejo, L.M.; Palma-Milla, S.; Gómez-Candela, C. Supplementation with Hydroxytyrosol and Punicalagin Improves Early Atherosclerosis Markers Involved in the Asymptomatic Phase of Atherosclerosis in the Adult Population: A Randomized, Placebo-Controlled, Crossover Trial. Nutrients 2019, 11, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colica, C.; Di Renzo, L.; Trombetta, D.; Smeriglio, A.; Bernardini, S.; Cioccoloni, G.; Costa de Miranda, R.; Gualtieri, P.; Sinibaldi Salimei, P.; De Lorenzo, A. Antioxidant Effects of a Hydroxytyrosol-Based Pharmaceutical Formulation on Body Composition, Metabolic State, and Gene Expression: A Randomized Double-Blinded, Placebo-Controlled Crossover Trial. Oxid. Med. Cell Longev. 2017, 2017, 2473495. [Google Scholar] [CrossRef] [PubMed]
- de Bock, M.; Derraik, J.G.B.; Brennan, C.M.; Biggs, J.B.; Morgan, P.E.; Hodgkinson, S.C.; Hofman, P.L.; Cutfield, W.S. Olive (Olea europaea L.) leaf polyphenols improve insulin sensitivity in middle-aged overweight men: A randomized, placebo-controlled, crossover trial. PLoS ONE 2013, 8, e57622. [Google Scholar] [CrossRef]
- Camargo, A.; Ruano, J.; Fernandez, J.M.; Parnell, L.D.; Jimenez, A.; Santos-Gonzalez, M.; Marin, C.; Perez-Martinez, P.; Uceda, M.; Lopez-Miranda, J.; et al. Gene expression changes in mononuclear cells in patients with metabolic syndrome after acute intake of phenol-rich virgin olive oil. BMC Genom. 2010, 11, 253. [Google Scholar] [CrossRef] [Green Version]
- Martínez, N.; Herrera, M.; Frías, L.; Provencio, M.; Pérez-Carrión, R.; Díaz, V.; Morse, M.; Crespo, M.C. A combination of hydroxytyrosol, omega-3 fatty acids and curcumin improves pain and inflammation among early stage breast cancer patients receiving adjuvant hormonal therapy: Results of a pilot study. Clin. Transl. Oncol. 2019, 21, 489–498. [Google Scholar] [CrossRef]
- Ruano, J.; Lopez-Miranda, J.; Fuentes, F.; Moreno, J.A.; Bellido, C.; Perez-Martinez, P.; Lozano, A.; Gómez, P.; Jiménez, Y.; Pérez Jiménez, F. Phenolic Content of Virgin Olive Oil Improves Ischemic Reactive Hyperemia in Hypercholesterolemic Patients. J. Am. Coll. Cardiol. 2005, 46, 1864–1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruano, J.; López-Miranda, J.; de la Torre, R.; Delgado-Lista, J.; Fernández, J.; Caballero, J.; Covas, M.I.; Jiménez, Y.; Pérez-Martínez, P.; Marín, C.; et al. Intake of phenol-rich virgin olive oil improves the postprandial prothrombotic profile in hypercholesterolemic patients. Am. J. Clin. Nutr. 2007, 86, 341–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Study | In Vitro Model | Conditions | HT Health Benefits |
|---|---|---|---|
| Salami et al., 1995 [45] | CuS04-treated LDL samples | 10−5 M HT | ↓ F2-isoprostanes, ↑ Vit E |
| Visioli et al., 1995 [46] | CuS04-treated LDL samples | HT range 10−6–10−4 M | ↑ Vit E, ↓ lipid peroxidation |
| Carluccio et al., 2003 [66] | HUVECs and BAECs + LPS, IL-1β, TNFα, or PMA | 30 μM HT | ↓ VCAM-1, ↓ ICAM-1, ↓ NF-kB, ↓ AP-1, ↓ E-selectin |
| O’Dowd et al., 2004 [49] | TNF and fMLP-stimulated human neutrophils | 10 μM HT | ↓ H2O2 |
| Berrougui et al., 2005 [47] | [3H]-Cholesterol-loaded J774 macrophages | HT range 0–25 μM | ↑ ABCA1, ↑ apoA-I-mediated cholesterol efflux |
| Schmitt et al., 2007 [41] | EA.hy926 | 0.1–100 µM HT for 24 h | No effect on endothelial NO bioavailability and eNOS activity |
| Carluccio et al., 2007 [69] | Hcy-stimulated HUVECs | HT range 0.1–1 μM | ↓ VCAM-1, ↓ ROS, ↓ NF-κB |
| Dell’Agli et al., 2008 [72] | Human platelets | 10 μM HT | ↓ cAMP-PDE, ↓ platelet aggregation |
| Zrelli et al., 2011 [52] | VECs + H2O2 | 10, 30, 50 μM HT for 24 h | ↓ ROS, ↑ CAT, ↑ FOXO3a, ↑ pAMPK |
| Richard et al., 2011 [62] | LPS-stimulated RAW264.7 | 25 μM HT | ↓ NO, ↓ PGE₂, ↓ IL-1α, ↓ IL-1β, ↓ IL-6, ↓ IL-12, ↓ TNFα, ↓ CXCL10/IP-10, ↓ CCL2/MCP-1, ↓ iNOS, ↓ MMP-9 |
| Zou et al., 2012 [55] | VECs + H2O2 | 50 μM HT | ↑ pAkt, ↑ p-p38, ↑ pErK, ↑ Nrf2, ↑ HO-1, ↓ ROS |
| Scoditti et al., 2012 [76] | HUVEC + PMA | 10 μM HT | ↓ MMP-9, ↓ COX-2, ↓ PGE2, ↓ NF-kB |
| Rosignoli et al., 2013 [65] | PMA-activated PBMC | 100 μM HT | ↓ O2−, ↓ COX-2, ↓ PGE2 |
| Scoditti et al., 2014 [63] | PMA-activated PBMC and U937 | HT range 1–10 μM | ↓ MMP-9, ↓ COX-2, ↓ PGE₂, ↓ NF-kB, ↓ PKCβ1, ↓ PKC-α |
| Takeda et al., 2014 [64] | LPS-stimulated mouse peritoneal macrophages | 12.5 μg/mL HT | ↓ iNOS, ↓ NO |
| Storniolo et al., 2014 [43] | HG-stimulated ECV304 | 10 µM HT for 48 h | ↓ ROS, ↑ ET-1, ↑ p-eNOS, ↑ NO |
| Scoditti et al., 2015 [75] | SGBS cells + TNFα | HT range 0.1–20 μM | ↓ pJNK, ↑ adiponectin, ↑ PPARγ |
| Catalán, et al., 2015 [71] | TNF-α-stimulated HAEC | 1, 2, 5, 10 μM HT for 24 h | ↓ E-selectin, ↓ P-selectin, ↓ VCAM-1, ↓ ICAM-1, ↓ MCP-1 |
| Ozbek et al., 2015 [51] | H9c2 + O2− | HT range 0.1-10 µg/mL for 24 h | ↓ ROS, ↓ pMAPKAPK-2, ↓ pErk1/2, ↑ Hsp27, ↓ c-CASP3 |
| Zrelli et al., 2015 [57] | VECs | HT range 10-100 μM | ↑ PI3K/Akt, ↑ pErK, ↑ Nrf2, ↑ HO-1 |
| Tagliaferro et al., 2015 [53] | Hg induced hemolysis of human RBC | HT range 10-80 μM | ↓ ROS, ↑ GSH |
| Officioso et al., 2016 [54] | Hg induced hemolysis of human RBC | HT range 0.1–5 μM | ↓ Eryptosis |
| Atzeri et al., 2016 [48] | Caco-2 + oxidized cholesterol | HT range 2.5–10 μM | ↓ ROS, ↑ GSH, ↓ GPx activity |
| Calabriso et al., 2018 [50] | PMA-stimulated HUVEC and HMEC-1 | HT range 1–30 μM | ↓TNFα, ↓IL-1β, ↓VCAM-1, ↓ ICAM-1, ↓ mtROS, ↓ ROS, ↓ MDA, ↓ MnSOD |
| Manna et al., 2019 [67] | Hcy/TNFα-stimulated EA.hy 926 | HT range 0.5–2.5 μM | ↓ ICAM-1 |
| Wang et al., 2019 [42] | HG-stimulated HUVECs | 25, 50, 100 µM HT-NO for 48 h | ↑ p-eNOS, ↑ NO, ↓ ROS, ↑ SIRT-1 |
| Study | In Vivo Model | Conditions | HT Health Benefits |
|---|---|---|---|
| González-Santiago et al., 2006 [84] | Hyperlipemic rabbits, diet induced for 1 month | 4 mg/kg HT for 1 month | ↓ TC, ↓ TG, ↑ HDL, ↓ atherosclerotic lesion |
| Fki et al., 2007 [80] | Wistar rats fed cholesterol-rich diet for 16 w | 2.5 mg/kg HT + 10 mg/kg OMW | ↓ TC, ↓ LDL, ↑ HDL, ↓ TBARS in liver, heart, kidney, and aorta, ↑ CAT, ↑ SOD1 |
| Rietjens et al., 2007 [82] | Lewis rats, excised aorta | HT range 20–100 μM | Protection against induced impairment of NO-mediated aorta relaxation |
| Jemai et al., 2008 [79] | Wistar rats fed cholesterol-rich diet for 16 w | 3 mg/kg/day HT and triacetylated HT | ↓ TC, ↓ TG, ↓ LDL, ↓ TBARS in liver, heart, kidney, and aorta, ↑ HDL, ↑ CAT, ↑ SOD1 |
| González-Correa et al., 2008 [85] | Wistar rats | 1, 5, 10, 20, 50, 100 mg/kg/day HT, HT-ac or acetylsalicylic acid for 1 w | ↓ platelet aggregation, ↓ thromboxane B2, ↓ prostacyclin, ↑ NO |
| Cao et al., 2014 [81] | C57BL/6J mice high fat diet fed for 17 w | 10 and 50 mg/kg/day HT for 17 w | ↓ body and organs weight, ↓ HOMA-IR index, ↓ leptin, ↓ IL-6, ↓ CRP, ↓ TG, ↓ HDL, ↓ LDL, ↓ SREBP-1c, ↓ FAS, ↑ SOD1 |
| db/db metabolic syndrome mice | 10 mg/kg/day HT or 225mg/kg/day metformin for 8 w | ↓ TG, ↓ TC, ↓ HDL, ↓ LDL, ↓ MDA, ↑ glucose tolerance | |
| Tabernero et al., 2014 [83] | Wistar rats high cholesterol-fed | 25 mg/Kg/day HT, HT-ac, HT-et for 8 w | ↓ TC, ↓ LDL, ↓ glucose, ↓ insulin, ↓ leptin, ↓ MDA, ↓ TNFα, ↓IL-1β, ↓ MCP-1, ↑ ORAC |
| Wang et al., 2019 [42] | KM diabetic mice, streptozotocin-induced | 77 mg/kg/day HT for 4 w | ↓ blood glucose, ↓ MDA, ↑ NO, ↑ SOD1 |
| Study | Population | Conditions | HT Health Benefits |
|---|---|---|---|
| Camargo et al., 2010 [100] | No. 20 metabolic syndrome patients, age range 40–70 | 0.2 μmol g−1 and 45.4 μmol g−1 HT, single dose | ↓ IL-1β, ↓ IL-6, ↓ PTGS2, ↓NF-κB/MAPK/AP-1 pathway, ↓ chemokines |
| de Bock et al., 2013 [99] | No. 46 overweight men, age range 35–55 | 9.7 mg/day HT and 51.1 mg/day oleuropein, for 12 w | ↑ insulin sensitivity, ↑ β-cell responsiveness |
| Colica et al., 2017 [98] | No. 28 healthy subjects, age range 18–65 | 15 mg/day HT for 3 w | ↑ SOD1, ↑ thiol group, ↑ TSA, ↓ MDA, ↓ nitrite, ↓ nitrate, ↓ body fat mass, ↓ suprailiac skinfold, ↓ body weight |
| Quirós-Fernández et al., 2019 [97] | No. 84 healthy subjects, age range 45–65 | 9.9 mg/day HT and 195 mg/day punicalagin, for 20 w | ↓ ox-LDL, ↓ SYS and DIA blood pressure |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Angelo, C.; Franceschelli, S.; Quiles, J.L.; Speranza, L. Wide Biological Role of Hydroxytyrosol: Possible Therapeutic and Preventive Properties in Cardiovascular Diseases. Cells 2020, 9, 1932. https://doi.org/10.3390/cells9091932
D’Angelo C, Franceschelli S, Quiles JL, Speranza L. Wide Biological Role of Hydroxytyrosol: Possible Therapeutic and Preventive Properties in Cardiovascular Diseases. Cells. 2020; 9(9):1932. https://doi.org/10.3390/cells9091932
Chicago/Turabian StyleD’Angelo, Chiara, Sara Franceschelli, José Luis Quiles, and Lorenza Speranza. 2020. "Wide Biological Role of Hydroxytyrosol: Possible Therapeutic and Preventive Properties in Cardiovascular Diseases" Cells 9, no. 9: 1932. https://doi.org/10.3390/cells9091932





