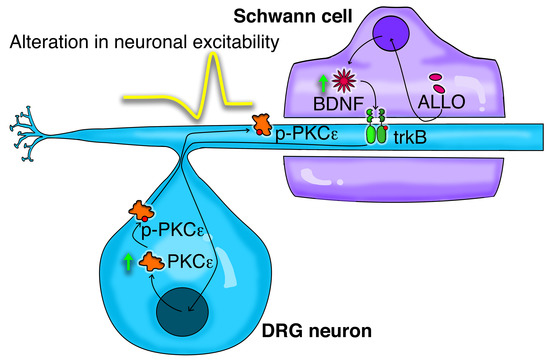
Schwann Cell Autocrine and Paracrine Regulatory Mechanisms, Mediated by Allopregnanolone and BDNF, Modulate PKCε in Peripheral Sensory Neurons
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
2.2. Sensory Neurons and SCs Primary Cultures
2.3. Pharmacological Treatments
2.4. RNA Extraction and qRT-PCR
2.5. Immunofluorescence (IFL)
2.6. Western Blotting
2.7. Quantitative Analysis of ALLO by Using Liquid Chromatography Tandem Mass Spectrometry Analysis (LC-MS/MS)
2.8. ELISA Assay
2.9. Statistic Analysis
3. Results
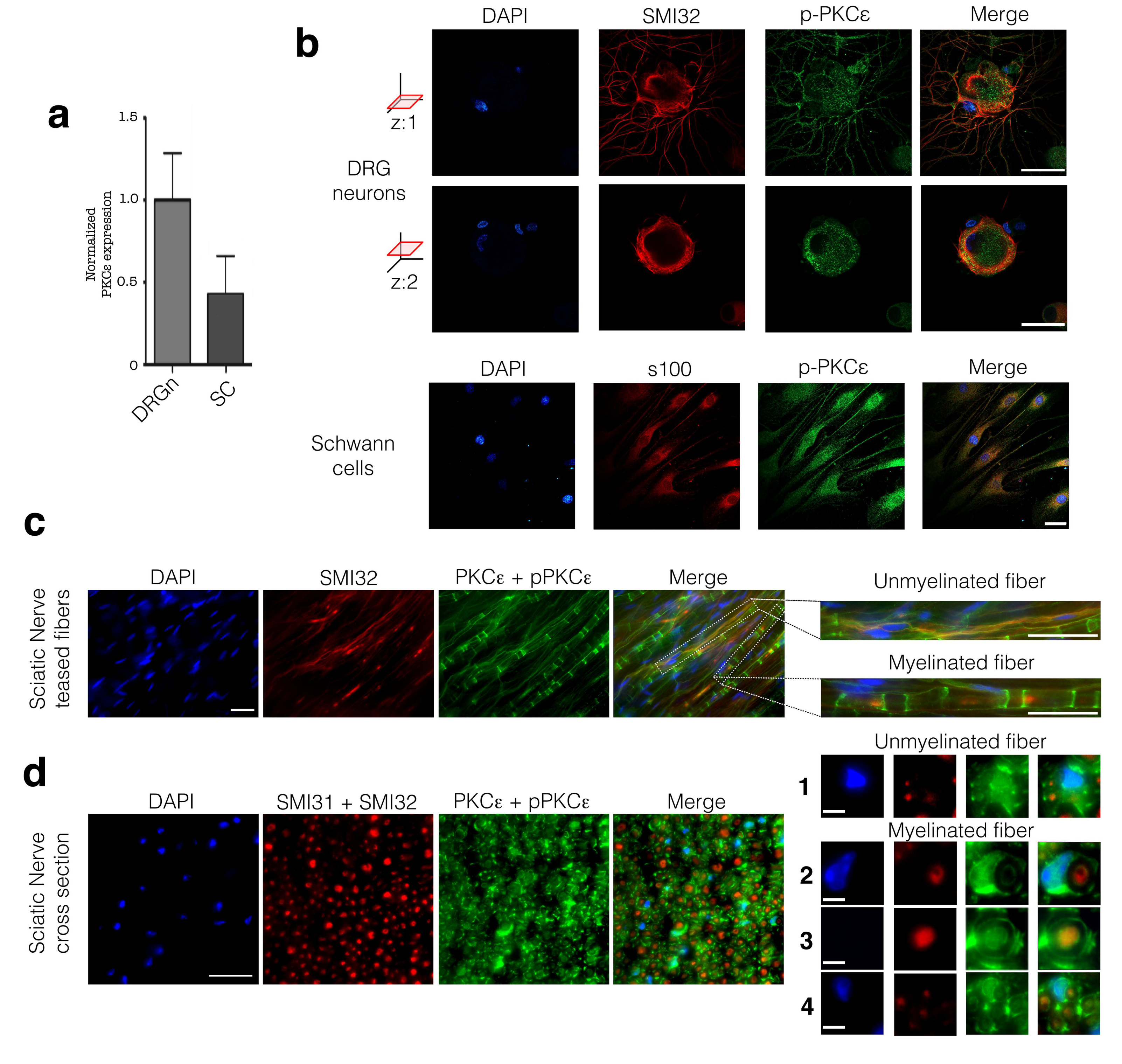
3.1. PKCε is Constitutively Expressed in PNS Cells and Tissue
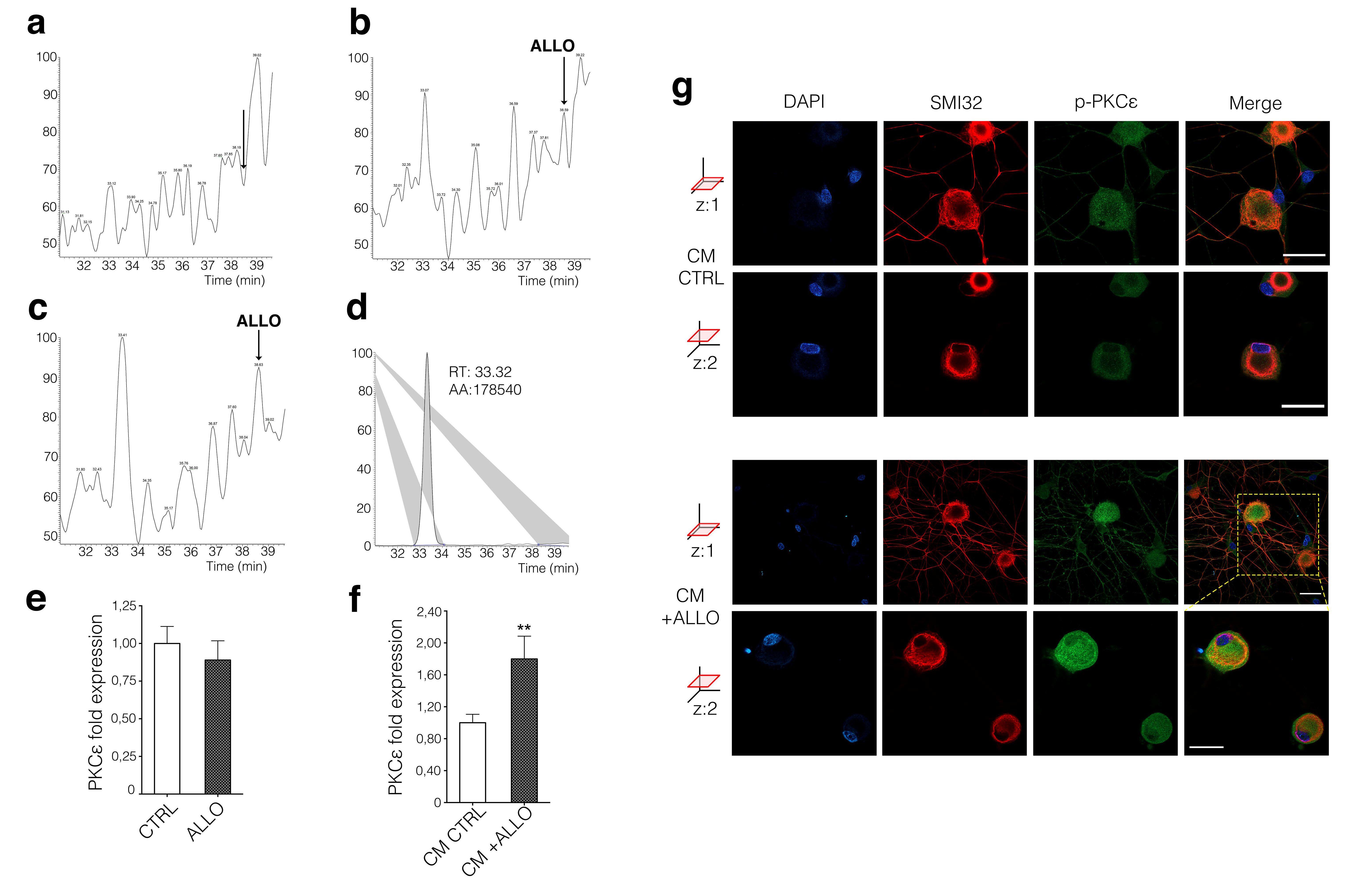
3.2. Neuronal PKCε Is Regulated by an SC’s Humoral Factor
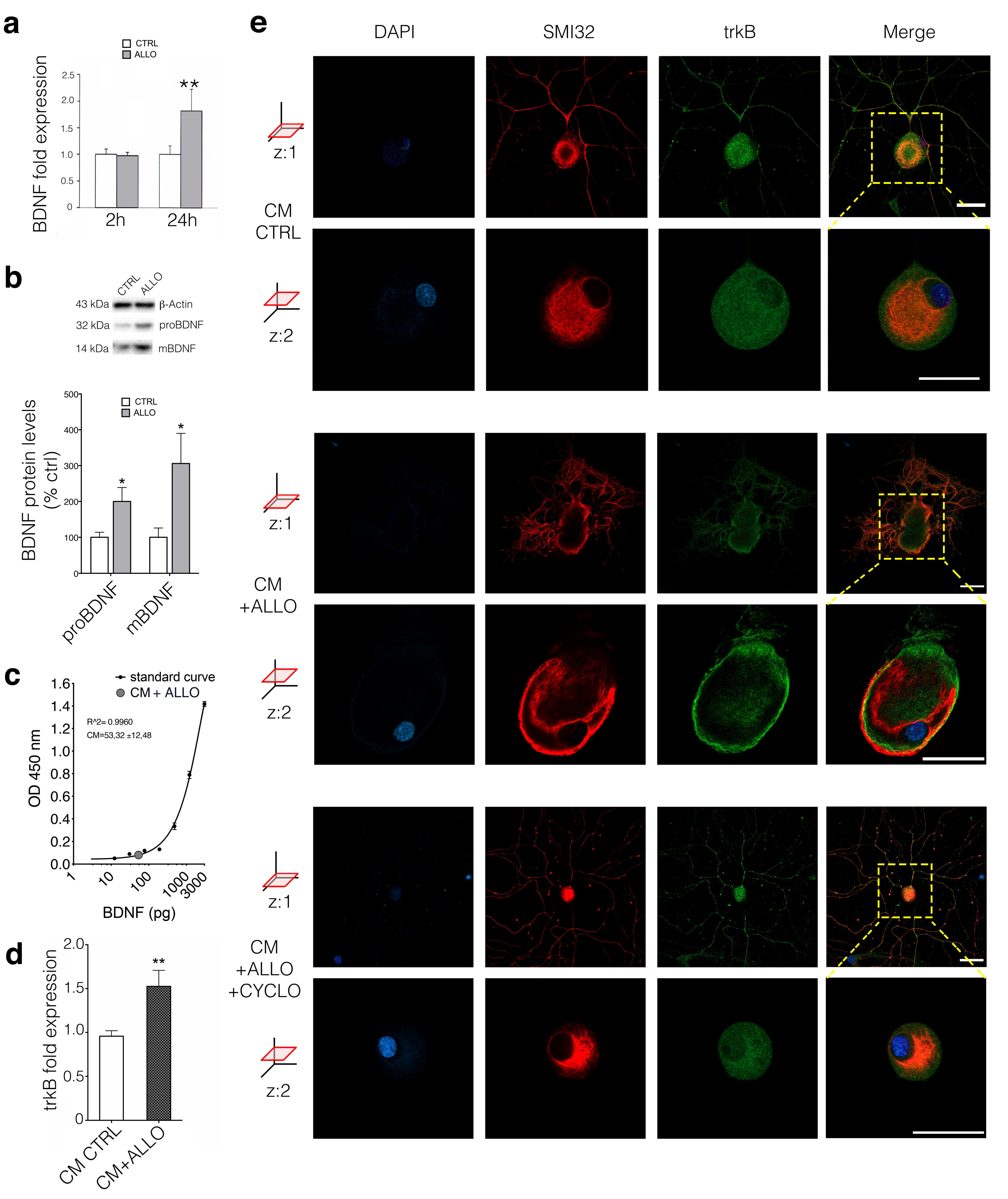
3.3. ALLO Regulates the Production and Release of BDNF by SCs
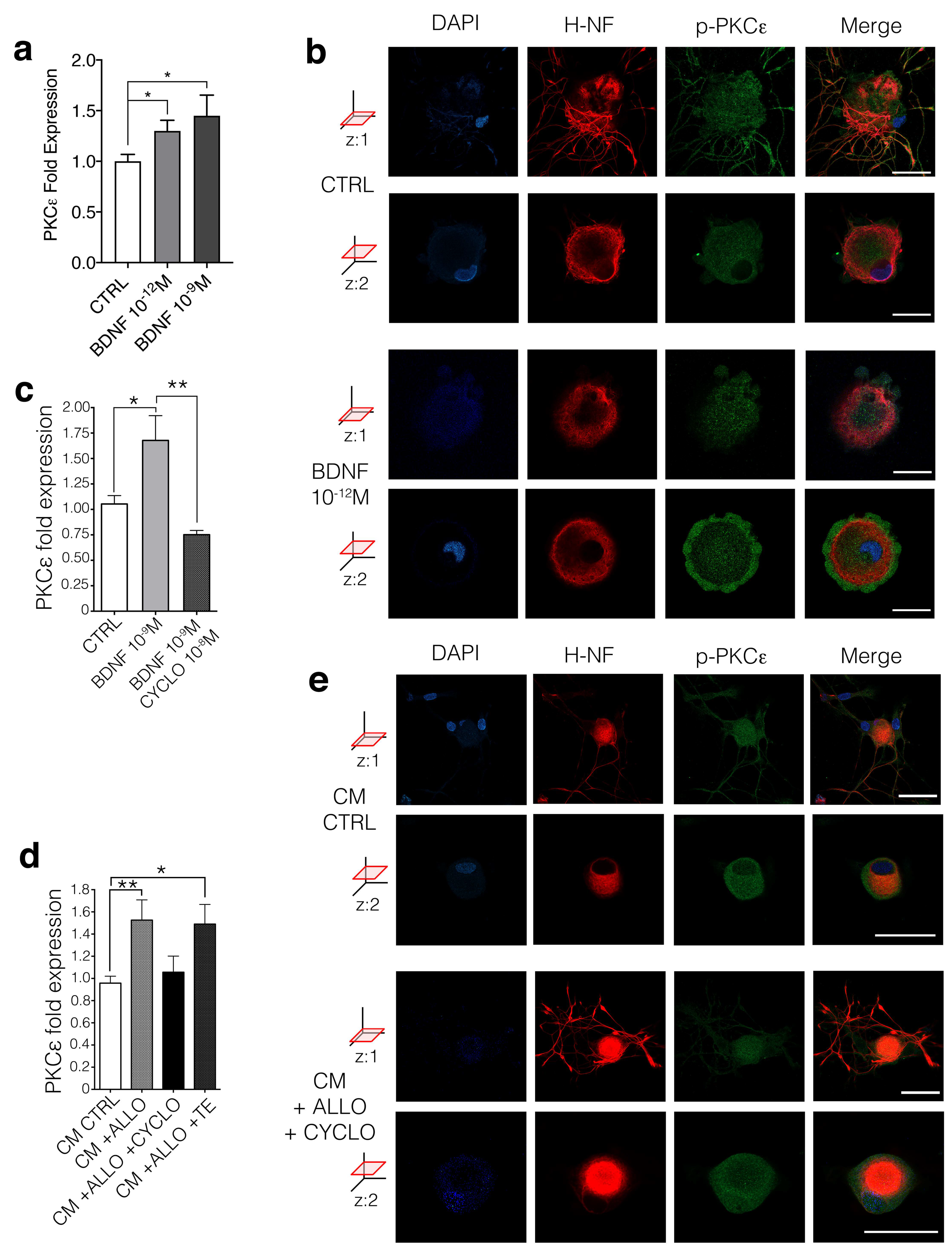
3.4. BDNF Regulates PKCε in DRG Neurons Via trkB Activation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harty, B.L.; Monk, K.R. Unwrapping the unappreciated: Recent progress in Remak Schwann cell biology. Curr. Opin. Neurobiol 2017, 47, 131–137. [Google Scholar] [CrossRef]
- Feltri, M.L.; Poitelon, Y.; Previtali, S.C. How Schwann Cells Sort Axons: New Concepts. Neuroscientist 2016, 22, 252–265. [Google Scholar] [CrossRef]
- Castelnovo, L.F.; Bonalume, V.; Melfi, S.; Ballabio, M.; Colleoni, D.; Magnaghi, V. Schwann cell development, maturation and regeneration: A focus on classic and emerging intracellular signaling pathways. Neural. Regen. Res. 2017, 12, 1013–1023. [Google Scholar] [CrossRef]
- Monk, K.R.; Feltri, M.L.; Taveggia, C. New insights on Schwann cell development. Glia 2015, 63, 1376–1393. [Google Scholar] [CrossRef] [PubMed]
- Yajima, Y.; Narita, M.; Usui, A.; Kaneko, C.; Miyatake, M.; Yamaguchi, T.; Tamaki, H.; Wachi, H.; Seyama, Y.; Suzuki, T. Direct evidence for the involvement of brain-derived neurotrophic factor in the development of a neuropathic pain-like state in mice. J. Neurochem. 2005, 93, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Su, W.F.; Wu, F.; Jin, Z.H.; Gu, Y.; Chen, Y.T.; Fei, Y.; Chen, H.; Wang, Y.X.; Xing, L.Y.; Zhao, Y.Y.; et al. Overexpression of P2X4 receptor in Schwann cells promotes motor and sensory functional recovery and remyelination via BDNF secretion after nerve injury. Glia 2019, 67, 78–90. [Google Scholar] [CrossRef]
- Wei, Z.; Fei, Y.; Su, W.; Chen, G. Emerging Role of Schwann Cells in Neuropathic Pain: Receptors, Glial Mediators and Myelination. Front. Cell Neurosci. 2019, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Baulieu, E.E.; Robel, P. Neurosteroids: A new brain function? J. Steroid Biochem. Mol. Biol. 1990, 37, 395–403. [Google Scholar] [CrossRef]
- Faroni, A.; Magnaghi, V. The neurosteroid allopregnanolone modulates specific functions in central and peripheral glial cells. Front. Endocrinol. 2011, 2, 103. [Google Scholar] [CrossRef]
- Melcangi, R.C.; Magnaghi, V.; Galbiati, M.; Martini, L. Formation and effects of neuroactive steroids in the central and peripheral nervous system. Int. Rev. Neurobiol. 2001, 46, 145–176. [Google Scholar]
- Colciago, A.; Bonalume, V.; Melfi, V.; Magnaghi, V. Genomic and Non-genomic Action of Neurosteroids in the Peripheral Nervous System. Front. Neurosci. 2020, 14. [Google Scholar] [CrossRef]
- Magnaghi, V.; Veiga, S.; Ballabio, M.; Gonzalez, L.C.; Garcia-Segura, L.M.; Melcangi, R.C. Sex-dimorphic effects of progesterone and its reduced metabolites on gene expression of myelin proteins by rat Schwann cells. J. Peripher. Nerv. Syst. 2006, 11, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Magnaghi, V.; Parducz, A.; Frasca, A.; Ballabio, M.; Procacci, P.; Racagni, G.; Bonanno, G.; Fumagalli, F. GABA synthesis in Schwann cells is induced by the neuroactive steroid allopregnanolone. J. Neurochem. 2010, 112, 980–990. [Google Scholar] [CrossRef]
- Melfi, S.; Montt Guevara, M.M.; Bonalume, V.; Ruscica, M.; Colciago, A.; Simoncini, T.; Magnaghi, V. Src and phospho-FAK kinases are activated by allopregnanolone promoting Schwann cell motility, morphology and myelination. J. Neurochem. 2017, 141, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.L.; Meyer, L.; Raggio, M.C.; Taleb, O.; Coronel, M.F.; Patte-Mensah, C.; Mensah-Nyagan, A.G. Allopregnanolone and Progesterone in Experimental Neuropathic Pain: Former and New Insights with a Translational Perspective. Cell Mol. Neurobiol. 2019, 39, 523–537. [Google Scholar] [CrossRef]
- Belelli, D.; Lambert, J.J. Neurosteroids: Endogenous regulators of the GABA(A) receptor. Nat. Rev. Neurosci. 2005, 6, 565–575. [Google Scholar] [CrossRef]
- Shirai, Y.; Adachi, N.; Saito, N. Protein kinase Cepsilon: Function in neurons. Febs. J. 2008, 275, 3988–3994. [Google Scholar] [CrossRef]
- Van Kolen, K.; Pullan, S.; Neefs, J.M.; Dautzenberg, F.M. Nociceptive and behavioural sensitisation by protein kinase Cepsilon signalling in the CNS. J. Neurochem. 2008, 104, 1–13. [Google Scholar] [CrossRef]
- Bogen, O.; Alessandri-Haber, N.; Chu, C.; Gear, R.W.; Levine, J.D. Generation of a pain memory in the primary afferent nociceptor triggered by PKCepsilon activation of CPEB. J. Neurosci. 2012, 32, 2018–2026. [Google Scholar] [CrossRef]
- Ferrari, L.F.; Araldi, D.; Levine, J.D. Distinct terminal and cell body mechanisms in the nociceptor mediate hyperalgesic priming. J. Neurosci. 2015, 35, 6107–6116. [Google Scholar] [CrossRef]
- Sugiura, T.; Tominaga, M.; Katsuya, H.; Mizumura, K. Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1. J. Neurophysiol. 2002, 88, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Amadesi, S.; Cottrell, G.S.; Divino, L.; Chapman, K.; Grady, E.F.; Bautista, F.; Karanjia, R.; Barajas-Lopez, C.; Vanner, S.; Vergnolle, N.; et al. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and A-dependent mechanisms in rats and mice. J. Physiol. 2006, 575, 555–571. [Google Scholar] [CrossRef]
- Qi, Z.H.; Song, M.; Wallace, M.J.; Wang, D.; Newton, P.M.; McMahon, T.; Chou, W.H.; Zhang, C.; Shokat, K.M.; Messing, R.O. Protein kinase C epsilon regulates gamma-aminobutyrate type A receptor sensitivity to ethanol and benzodiazepines through phosphorylation of gamma2 subunits. J. Biol. Chem. 2007, 282, 33052–33063. [Google Scholar] [CrossRef]
- Chou, W.H.; Wang, D.; McMahon, T.; Qi, Z.H.; Song, M.; Zhang, C.; Shokat, K.M.; Messing, R.O. GABAA receptor trafficking is regulated by protein kinase C(epsilon) and the N-ethylmaleimide-sensitive factor. J. Neurosci. 2010, 30, 13955–13965. [Google Scholar] [CrossRef] [PubMed]
- Hodge, C.W.; Mehmert, K.K.; Kelley, S.P.; McMahon, T.; Haywood, A.; Olive, M.F.; Wang, D.; Sanchez-Perez, A.M.; Messing, R.O. Supersensitivity to allosteric GABA(A) receptor modulators and alcohol in mice lacking PKCepsilon. Nat. Neurosci. 1999, 2, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, C.F.; Sachs, D.; Funez, M.I.; Parada, C.A.; de Queiroz Cunha, F.; Ferreira, S.H. The peripheral pro-nociceptive state induced by repetitive inflammatory stimuli involves continuous activation of protein kinase A and protein kinase C epsilon and its Na(V)1.8 sodium channel functional regulation in the primary sensory neuron. Biochem. Pharm. 2009, 77, 867–877. [Google Scholar] [CrossRef]
- Hodge, C.W.; Raber, J.; McMahon, T.; Walter, H.; Sanchez-Perez, A.M.; Olive, M.F.; Mehmert, K.; Morrow, A.L.; Messing, R.O. Decreased anxiety-like behavior, reduced stress hormones, and neurosteroid supersensitivity in mice lacking protein kinase Cepsilon. J. Clin. Investig. 2002, 110, 1003–1010. [Google Scholar] [CrossRef]
- Puia, G.; Ravazzini, F.; Castelnovo, L.F.; Magnaghi, V. PKCepsilon and allopregnanolone: Functional cross-talk at the GABAA receptor level. Front. Cell Neurosci. 2015, 9, 83. [Google Scholar] [CrossRef]
- Mauro, N.; Manfredi, A.; Ranucci, E.; Procacci, P.; Laus, M.; Antonioli, D.; Mantovani, C.; Magnaghi, V.; Ferruti, P. Degradable Poly(amidoamine) Hydrogels as Scaffolds for In Vitro Culturing of Peripheral Nervous System Cells. Macromol. Biosci. 2013, 13, 332–347. [Google Scholar] [CrossRef]
- Colciago, A.; Melfi, S.; Giannotti, G.; Bonalume, V.; Ballabio, M.; Caffino, L.; Fumagalli, F.; Magnaghi, V. Tumor suppressor Nf2/merlin drives Schwann cell changes following electromagnetic field exposure through Hippo-dependent mechanisms. Cell Death Discov. 2015, 1. [Google Scholar] [CrossRef]
- Mottarlini, F.; Racagni, G.; Brambilla, P.; Fumagalli, F.; Caffino, L. Repeated cocaine exposure during adolescence impairs recognition memory in early adulthood: A role for BDNF signaling in the perirhinal cortex. Dev. Cogn. Neurosci. 2020, 43, 100789. [Google Scholar] [CrossRef]
- Caruso, D.; Pesaresi, M.; Abbiati, F.; Calabrese, D.; Giatti, S.; Garcia-Segura, L.M.; Melcangi, R.C. Comparison of plasma and cerebrospinal fluid levels of neuroactive steroids with their brain, spinal cord and peripheral nerve levels in male and female rats. Psychoneuroendocrinology 2013, 38, 2278–2290. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Huang, J.; Lu, L.; Hu, X.; Luo, Z.; Li, M. Electrically induced brain-derived neurotrophic factor release from Schwann cells. J. Neurosci. Res. 2014, 92, 893–903. [Google Scholar] [CrossRef]
- Wilhelm, J.C.; Xu, M.; Cucoranu, D.; Chmielewski, S.; Holmes, T.; Lau, K.S.; Bassell, G.J.; English, A.W. Cooperative roles of BDNF expression in neurons and Schwann cells are modulated by exercise to facilitate nerve regeneration. J. Neurosci. 2012, 32, 5002–5009. [Google Scholar] [CrossRef] [PubMed]
- Verheij, M.M.; Vendruscolo, L.F.; Caffino, L.; Giannotti, G.; Cazorla, M.; Fumagalli, F.; Riva, M.A.; Homberg, J.R.; Koob, G.F.; Contet, C. Systemic Delivery of a Brain-Penetrant TrkB Antagonist Reduces Cocaine Self-Administration and Normalizes TrkB Signaling in the Nucleus Accumbens and Prefrontal Cortex. J. Neurosci. 2016, 36, 8149–8159. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.J.; Hengst, U.; Gurskaya, N.G.; Lukyanov, K.A.; Jaffrey, S.R. Intra-axonal translation and retrograde trafficking of CREB promotes neuronal survival. Nat. Cell Biol. 2008, 10, 149–159. [Google Scholar] [CrossRef]
- Gallant, P.E. Axonal protein synthesis and transport. J. Neurocytol. 2000, 29, 779–782. [Google Scholar] [CrossRef]
- Russell, F.D.; Koishi, K.; Jiang, Y.; McLennan, I.S. Anterograde axonal transport of glial cell line-derived neurotrophic factor and its receptors in rat hypoglossal nerve. Neuroscience 2000, 97, 575–580. [Google Scholar] [CrossRef]
- Patte-Mensah, C.; Meyer, L.; Taleb, O.; Mensah-Nyagan, A.G. Potential role of allopregnanolone for a safe and effective therapy of neuropathic pain. Prog. Neurobiol. 2014, 113, 70–78. [Google Scholar] [CrossRef]
- Xie, W.; Strong, J.A.; Zhang, J.M. Active Nerve Regeneration with Failed Target Reinnervation Drives Persistent Neuropathic Pain. eNeuro 2017, 4. [Google Scholar] [CrossRef]
- Vogelin, E.; Baker, J.M.; Gates, J.; Dixit, V.; Constantinescu, M.A.; Jones, N.F. Effects of local continuous release of brain derived neurotrophic factor (BDNF) on peripheral nerve regeneration in a rat model. Exp. Neurol. 2006, 199, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Thoenen, H. Neurotrophins and neuronal plasticity. Science 1995, 270, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Takei, N.; Sasaoka, K.; Inoue, K.; Takahashi, M.; Endo, Y.; Hatanaka, H. Brain-derived neurotrophic factor increases the stimulation-evoked release of glutamate and the levels of exocytosis-associated proteins in cultured cortical neurons from embryonic rats. J. Neurochem. 1997, 68, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Levine, E.S.; Dreyfus, C.F.; Black, I.B.; Plummer, M.R. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc. Natl. Acad Sci. USA 1995, 92, 8074–8077. [Google Scholar] [CrossRef] [PubMed]
- Suen, P.C.; Wu, K.; Levine, E.S.; Mount, H.T.; Xu, J.L.; Lin, S.Y.; Black, I.B. Brain-derived neurotrophic factor rapidly enhances phosphorylation of the postsynaptic N-methyl-D-aspartate receptor subunit 1. Proc. Natl. Acad Sci. USA 1997, 94, 8191–8195. [Google Scholar] [CrossRef] [PubMed]
- Kafitz, K.W.; Rose, C.R.; Thoenen, H.; Konnerth, A. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature 1999, 401, 918–921. [Google Scholar] [CrossRef]
- Kerr, B.J.; Bradbury, E.J.; Bennett, D.L.; Trivedi, P.M.; Dassan, P.; French, J.; Shelton, D.B.; McMahon, S.B.; Thompson, S.W. Brain-derived neurotrophic factor modulates nociceptive sensory inputs and NMDA-evoked responses in the rat spinal cord. J. Neurosci. 1999, 19, 5138–5148. [Google Scholar] [CrossRef]
- Mannion, R.J.; Costigan, M.; Decosterd, I.; Amaya, F.; Ma, Q.P.; Holstege, J.C.; Ji, R.R.; Acheson, A.; Lindsay, R.M.; Wilkinson, G.A.; et al. Neurotrophins: Peripherally and centrally acting modulators of tactile stimulus-induced inflammatory pain hypersensitivity. Proc. Natl. Acad Sci. USA 1999, 96, 9385–9390. [Google Scholar] [CrossRef]
- Thompson, S.W.; Bennett, D.L.; Kerr, B.J.; Bradbury, E.J.; McMahon, S.B. Brain-derived neurotrophic factor is an endogenous modulator of nociceptive responses in the spinal cord. Proc. Natl. Acad Sci. USA 1999, 96, 7714–7718. [Google Scholar] [CrossRef]
- Naert, G.; Maurice, T.; Tapia-Arancibia, L.; Givalois, L. Neuroactive steroids modulate HPA axis activity and cerebral brain-derived neurotrophic factor (BDNF) protein levels in adult male rats. Psychoneuroendocrinology 2007, 32, 1062–1078. [Google Scholar] [CrossRef]
- Arcourt, A.; Gorham, L.; Dhandapani, R.; Prato, V.; Taberner, F.J.; Wende, H.; Gangadharan, V.; Birchmeier, C.; Heppenstall, P.A.; Lechner, S.G. Touch Receptor-Derived Sensory Information Alleviates Acute Pain Signaling and Fine-Tunes Nociceptive Reflex Coordination. Neuron 2017, 93, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Reynders, A.; Mantilleri, A.; Malapert, P.; Rialle, S.; Nidelet, S.; Laffray, S.; Beurrier, C.; Bourinet, E.; Moqrich, A. Transcriptional Profiling of Cutaneous MRGPRD Free Nerve Endings and C-LTMRs. Cell Rep. 2015, 10, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Usoskin, D.; Furlan, A.; Islam, S.; Abdo, H.; Lonnerberg, P.; Lou, D.; Hjerling-Leffler, J.; Haeggstrom, J.; Kharchenko, O.; Kharchenko, P.V.; et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 2015, 18, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Chiu, I.M.; Barrett, L.B.; Williams, E.K.; Strochlic, D.E.; Lee, S.; Weyer, A.D.; Lou, S.; Bryman, G.S.; Roberson, D.P.; Ghasemlou, N.; et al. Transcriptional profiling at whole population and single cell levels reveals somatosensory neuron molecular diversity. Elife 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.J.; Hovhannisyan, A.H.; Akopian, A.N. Characteristics of sensory neuronal groups in CGRP-cre-ER reporter mice: Comparison to Nav1.8-cre, TRPV1-cre and TRPV1-GFP mouse lines. PLoS ONE 2018, 13, e0198601. [Google Scholar] [CrossRef] [PubMed]
- Salio, C.; Averill, S.; Priestley, J.V.; Merighi, A. Costorage of BDNF and neuropeptides within individual dense-core vesicles in central and peripheral neurons. Dev. Neurobiol. 2007, 67, 326–338. [Google Scholar] [CrossRef]
- Luo, X.G.; Rush, R.A.; Zhou, X.F. Ultrastructural localization of brain-derived neurotrophic factor in rat primary sensory neurons. Neurosci. Res. 2001, 39, 377–384. [Google Scholar] [CrossRef]
- Wetmore, C.; Olson, L. Neuronal and nonneuronal expression of neurotrophins and their receptors in sensory and sympathetic ganglia suggest new intercellular trophic interactions. J. Comp. Neurol. 1995, 353, 143–159. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, G.D.; Zhao, Z.Q. State-dependent phosphorylation of epsilon-isozyme of protein kinase C in adult rat dorsal root ganglia after inflammation and nerve injury. J. Neurochem. 2003, 85, 571–580. [Google Scholar] [CrossRef]

| Primer Name | Forward Primer 5′–3′ | Reverse Primer 5′–3′ | Probe (When Applicable) |
|---|---|---|---|
| PKCε | CCCCTTGTGACCAGGAACTA | AGCTGGCCATCAGTAGACGA | |
| BDNF | AAGTCTGCATTACATTCCTCGA | GTTTTCTGAAAGAGGGACAGTTTAT | GATCAGGTCAGACAAGTCAAGG |
| trkB | GTGGATTCCGGCTTAAAGTTTG | GATCAGGTCAGACAAGTCAAGG | CCTGCGGCACATCAATTTCACTCG |
| α-tubulin | TCGCGCTGTAAGAAGCAACACC | ATGGAGATGCACTCACGATGGT | |
| 18s | CTGCCCTATCAACTTTCGATGGTAG | CCGTTTCTCAGGCTCCCTCTC | |
| β2-microglobulin | ACATACGCCTGCAGAGTTAAGC | TGCTTGATCACATGTCTCGATCCC | |
| β-actin | CACTTTCTACAATGAGCTGCG | CTGGATGGCTACGTACATGG | TCTGGGTCATCTTTTCACGGTTGGC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonalume, V.; Caffino, L.; Castelnovo, L.F.; Faroni, A.; Giavarini, F.; Liu, S.; Caruso, D.; Schmelz, M.; Fumagalli, F.; Carr, R.W.; et al. Schwann Cell Autocrine and Paracrine Regulatory Mechanisms, Mediated by Allopregnanolone and BDNF, Modulate PKCε in Peripheral Sensory Neurons. Cells 2020, 9, 1874. https://doi.org/10.3390/cells9081874
Bonalume V, Caffino L, Castelnovo LF, Faroni A, Giavarini F, Liu S, Caruso D, Schmelz M, Fumagalli F, Carr RW, et al. Schwann Cell Autocrine and Paracrine Regulatory Mechanisms, Mediated by Allopregnanolone and BDNF, Modulate PKCε in Peripheral Sensory Neurons. Cells. 2020; 9(8):1874. https://doi.org/10.3390/cells9081874
Chicago/Turabian StyleBonalume, Veronica, Lucia Caffino, Luca F. Castelnovo, Alessandro Faroni, Flavio Giavarini, Sheng Liu, Donatella Caruso, Martin Schmelz, Fabio Fumagalli, Richard W. Carr, and et al. 2020. "Schwann Cell Autocrine and Paracrine Regulatory Mechanisms, Mediated by Allopregnanolone and BDNF, Modulate PKCε in Peripheral Sensory Neurons" Cells 9, no. 8: 1874. https://doi.org/10.3390/cells9081874
APA StyleBonalume, V., Caffino, L., Castelnovo, L. F., Faroni, A., Giavarini, F., Liu, S., Caruso, D., Schmelz, M., Fumagalli, F., Carr, R. W., & Magnaghi, V. (2020). Schwann Cell Autocrine and Paracrine Regulatory Mechanisms, Mediated by Allopregnanolone and BDNF, Modulate PKCε in Peripheral Sensory Neurons. Cells, 9(8), 1874. https://doi.org/10.3390/cells9081874