Tristetraprolin Posttranscriptionally Downregulates TRAIL Death Receptors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Viability Assay
2.3. Semi-Quantitative RT-PCR and Quantitative Real-Time PCR (qRT-PCR) Analysis
2.4. Quantitative Real-Time PCR (qRT-PCR) Analysis for RNA Kinetics
2.5. Plasmid, siRNAs, Transfection, and Dual-Luciferase Assay
2.6. RNA EMSA
2.7. Western Blot Analysis
2.8. Apoptosis by Annexin V/PI Analysis
2.9. In Vivo Antitumor Activity
2.10. Statistical Analysis
3. Results
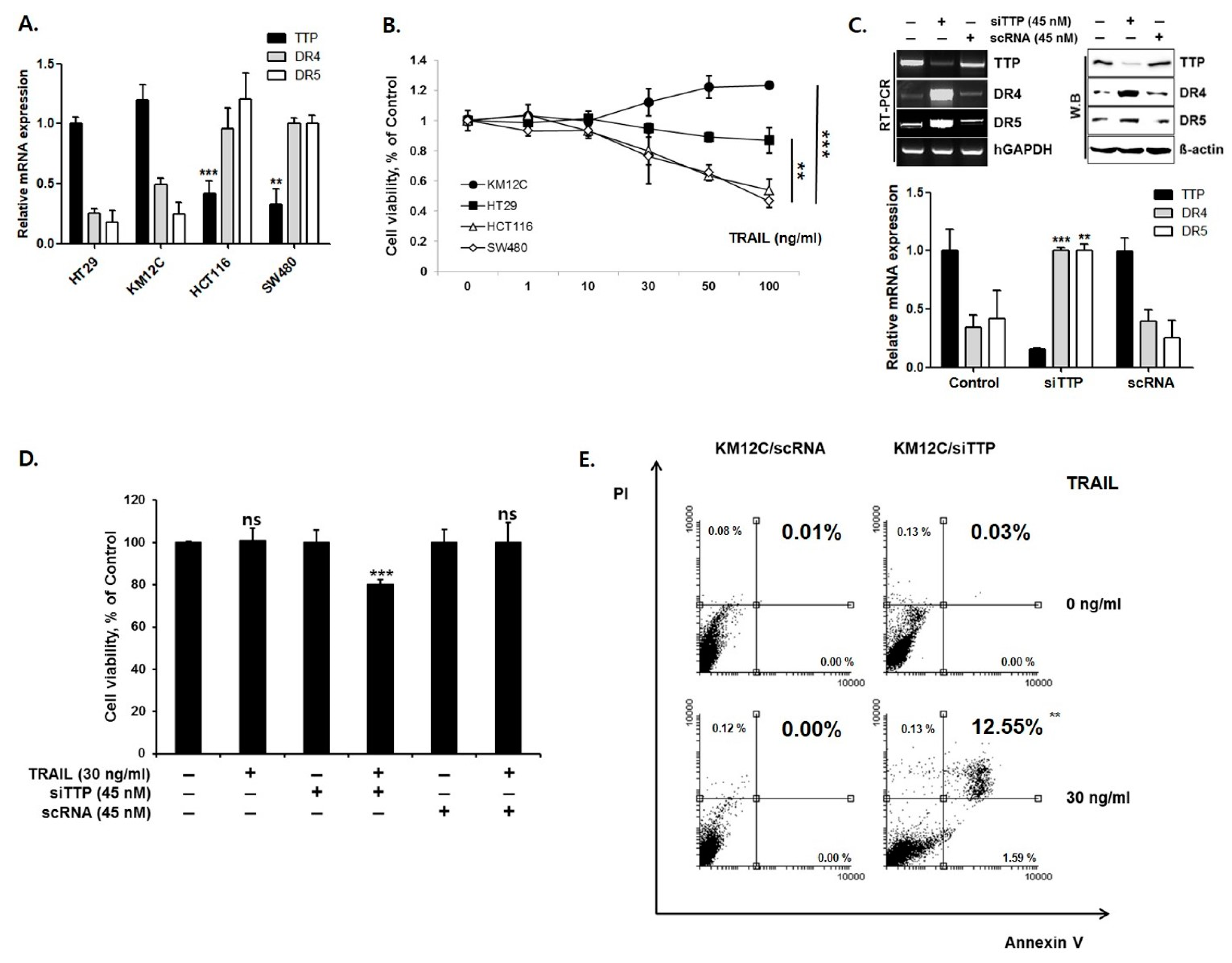
3.1. DR4/5 Expression is Inversely Correlated with TTP Expression in Human Colon Cancer Cell Lines
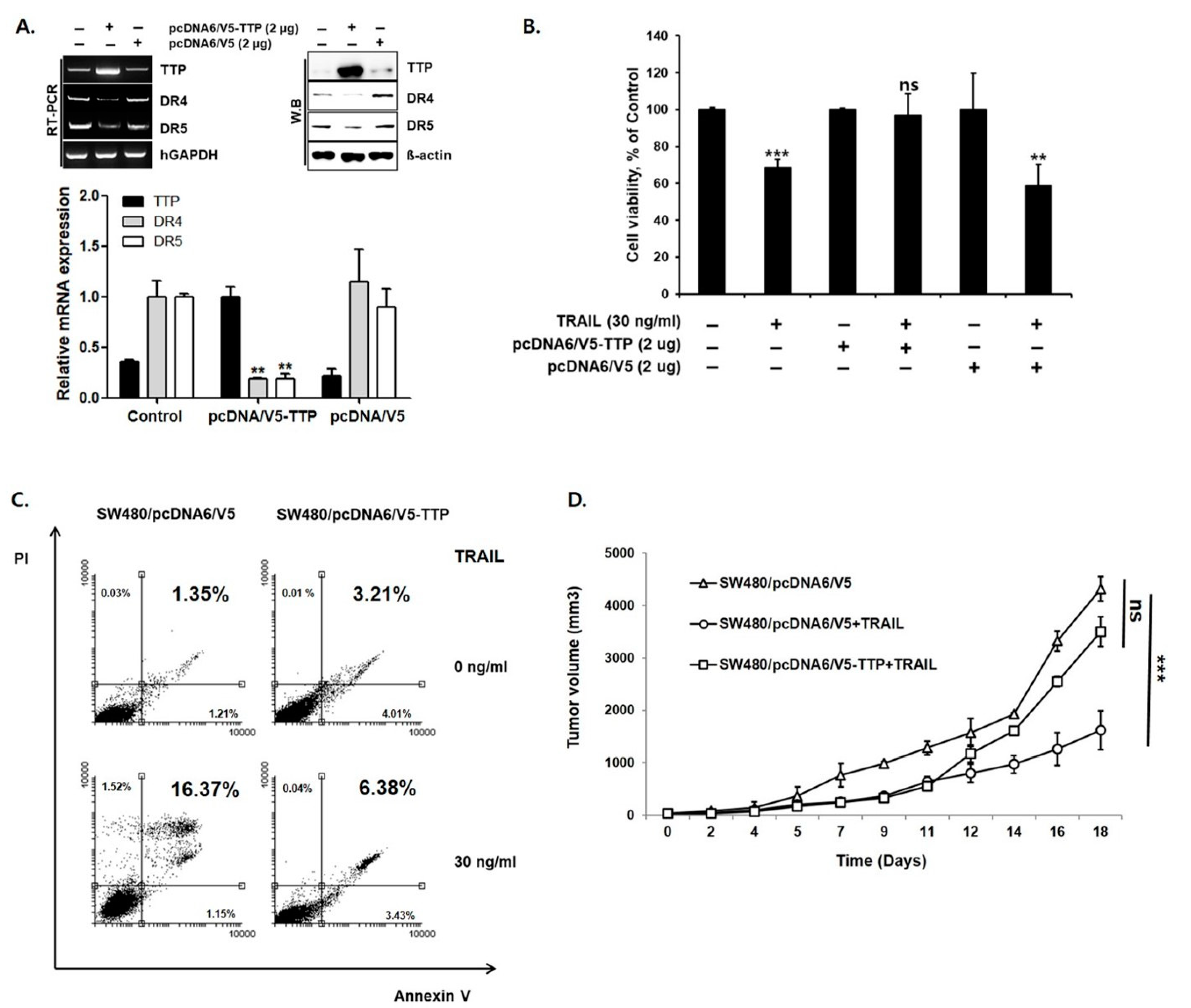
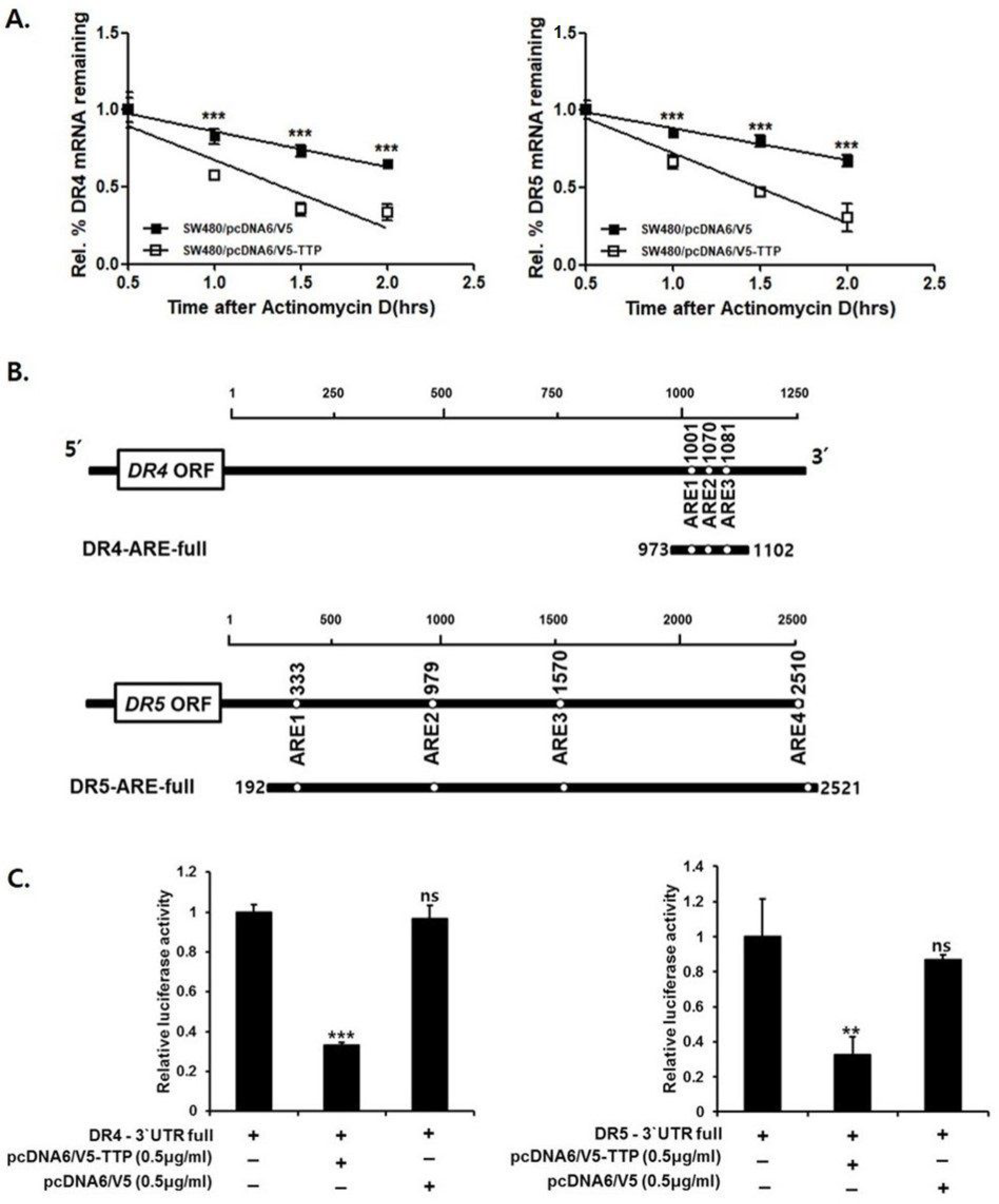
3.2. TTP Destabilized DR4/5 mRNA
3.3. TTP Binds to All Three ARE in DR4 and Only the 3rd ARE in DR5 mRNA 3′-UTR
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Wiley, S.R.; Schooley, K.; Smolak, P.J.; Din, W.S.; Huang, C.-P.; Nicholl, J.K.; Sutherland, G.R.; Smith, T.D.; Rauch, C.; Smith, C.A.; et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 1995, 3, 673–682. [Google Scholar] [CrossRef] [Green Version]
- Pitti, R.M.; Marsters, S.A.; Ruppert, S.; Donahue, C.J.; Moore, A.; Ashkenazi, A. Induction of Apoptosis by Apo-2 Ligand, a New Member of the Tumor Necrosis Factor Cytokine Family. J. Biol. Chem. 1996, 271, 12687–12690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Miguel, D.; Lemke, J.; Anel, A.; Walczak, H.; Martinez-Lostao, L. Onto better TRAILs for cancer treatment. Cell Death Differ. 2016, 23, 733–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Karstedt, S.; Montinaro, A.; Walczak, H. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat. Rev. Cancer 2017, 17, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Mellier, G.; Huang, S.; Shenoy, K.; Pervaiz, S. TRAILing death in cancer. Mol. Asp. Med. 2010, 31, 93–112. [Google Scholar] [CrossRef]
- Horak, P.; Pils, D.; Haller, G.; Pribill, I.; Roessler, M.; Tomek, S.; Horvat, R.; Zeillinger, R.; Zielinski, C.; Krainer, M. Contribution of epigenetic silencing of tumor necrosis factor-related apoptosis inducing ligand receptor 1 (DR4) to TRAIL resistance and ovarian cancer. Mol. Cancer Res. 2005, 3, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.C.; Lee, W.H.; Min, Y.J.; Cha, H.J.; Han, M.W.; Chang, H.W.; Kim, S.A.; Choi, S.H.; Kim, S.W.; Kim, S.Y. Development of TRAIL resistance by radiation-induced hypermethylation of DR4 CpG island in recurrent laryngeal squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 1203–1211. [Google Scholar] [CrossRef]
- Hopkins-Donaldson, S.; Ziegler, A.; Kurtz, S.; Bigosch, C.; Kandioler, D.; Ludwig, C.; Zangemeister-Wittke, U.; Stahel, R. Silencing of death receptor and caspase-8 expression in small cell lung carcinoma cell lines and tumors by DNA methylation. Cell Death Differ. 2003, 10, 356–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Z.; McDonald, E.R., III; Dicker, D.T.; El-Deiry, W.S. Deficient tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor transport to the cell surface in human colon cancer cells selected for resistance to TRAIL-induced apoptosis. J. Biol. Chem. 2004, 279, 35829–35839. [Google Scholar] [CrossRef] [Green Version]
- Pai, S.I.; Wu, G.S.; Ozoren, N.; Wu, L.; Jen, J.; Sidransky, D.; El-Deiry, W.S. Rare loss-of-function mutation of a death receptor gene in head and neck cancer. Cancer Res. 1998, 58, 3513–3518. [Google Scholar]
- Fisher, M.J.; Virmani, A.K.; Wu, L.; Aplenc, R.; Harper, J.C.; Powell, S.M.; Rebbeck, T.R.; Sidransky, D.; Gazdar, A.F.; El-Deiry, W.S. Nucleotide substitution in the ectodomain of trail receptor DR4 is associated with lung cancer and head and neck cancer. Clin. Cancer Res. 2001, 7, 1688–1697. [Google Scholar] [PubMed]
- Lee, S.H.; Shin, M.S.; Kim, H.S.; Lee, H.K.; Park, W.S.; Kim, S.Y.; Lee, J.H.; Han, S.Y.; Park, J.Y.; Oh, R.R.; et al. Somatic mutations of TRAIL-receptor 1 and TRAIL-receptor 2 genes in non-Hodgkin’s lymphoma. Oncogene 2001, 20, 399–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, M.S.; Kim, H.S.; Lee, S.H.; Park, W.S.; Kim, S.Y.; Park, J.Y.; Lee, J.H.; Lee, S.K.; Lee, S.N.; Jung, S.S.; et al. Mutations of tumor necrosis factor-related apoptosis-inducing ligand receptor 1 (TRAIL-R1) and receptor 2 (TRAIL-R2) genes in metastatic breast cancers. Cancer Res. 2001, 61, 4942–4946. [Google Scholar]
- Twomey, J.D.; Kim, S.R.; Zhao, L.; Bozza, W.P.; Zhang, B. Spatial dynamics of TRAIL death receptors in cancer cells. Drug Resist. Updates 2015, 19, 13–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Ortin, J.E.; Alepuz, P.; Chavez, S.; Choder, M. Eukaryotic mRNA decay: Methodologies, pathways, and links to other stages of gene expression. J. Mol. Biol. 2013, 425, 3750–3775. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.; Kamen, R. A conserved AU sequence from the 3 untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell 1986, 46, 659–667. [Google Scholar] [CrossRef]
- Brooks, S.A.; Blackshear, P.J. Tristetraprolin (TTP): Interactions with mRNA and proteins, and current thoughts on mechanisms of action. Biochim. Biophys. Acta 2013, 1829, 666–679. [Google Scholar] [CrossRef] [Green Version]
- Lai, W.S.; Carballo, E.; Thorn, J.M.; Kennington, E.A.; Blackshear, P.J. Interactions of CCCH zinc finger proteins with mRNA. Binding of tristetraprolin-related zinc finger proteins to Au-rich elements and destabilization of mRNA. J. Biol. Chem. 2000, 275, 17827–17837. [Google Scholar] [CrossRef] [Green Version]
- Park, J.M.; Lee, T.H.; Kang, T.H. Roles of Tristetraprolin in Tumorigenesis. Int. J. Mol. Sci. 2018, 19, 3384. [Google Scholar] [CrossRef] [Green Version]
- Daniels, R.A.; Turley, H.; Kimberley, F.C.; Liu, X.S.; Mongkolsapaya, J.; Ch’En, P.; Xu, X.N.; Jin, B.Q.; Pezzella, F.; Screaton, G.R. Expression of TRAIL and TRAIL receptors in normal and malignant tissues. Cell Res. 2005, 15, 430–438. [Google Scholar] [CrossRef]
- Mahalingam, D.; Szegezdi, E.; Keane, M.; Jong, S.d.; Samali, A. TRAIL receptor signalling and modulation: Are we on the right TRAIL? Cancer Treat. Rev. 2009, 35, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Brennan, S.E.; Kuwano, Y.; Alkharouf, N.; Blackshear, P.J.; Gorospe, M.; Wilson, G.M. The mRNA-destabilizing protein tristetraprolin is suppressed in many cancers, altering tumorigenic phenotypes and patient prognosis. Cancer Res. 2009, 69, 5168–5176. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.H.M.; Kong, W.Y.; Fang, C.M.; Loh, H.S.; Chuah, L.H.; Abdullah, S.; Ngai, S.C. The TRAIL to cancer therapy: Hindrances and potential solutions. Crit. Rev. Oncol. Hematol. 2019, 143, 81–94. [Google Scholar] [CrossRef]
- Twomey, J.D.; Zhang, B. Circulating Tumor Cells Develop Resistance to TRAIL-Induced Apoptosis Through Autophagic Removal of Death Receptor 5: Evidence from an In Vitro Model. Cancers 2019, 11, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gundlach, J.P.; Hauser, C.; Schlegel, F.M.; Boger, C.; Roder, C.; Rocken, C.; Becker, T.; Egberts, J.H.; Kalthoff, H.; Trauzold, A. Cytoplasmic TRAIL-R1 is a positive prognostic marker in PDAC. BMC Cancer 2018, 18, 777. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Zhang, Y.; Zhan, Y.; Liu, S.; Lu, J.; Wen, Q.; Fan, S. Expression of DR5 and cFLIP proteins as novel prognostic biomarkers for nonsmall cell lung cancer patients treated with surgical resection and chemotherapy. Oncol. Rep. 2019, 42, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, T.; Vondung, F.; Borzikowsky, C.; Szymczak, S.; Kruger, S.; Alkatout, I.; Wenners, A.; Bauer, M.; Klapper, W.; Rocken, C.; et al. Heterogeneous intracellular TRAIL-receptor distribution predicts poor outcome in breast cancer patients. J. Mol. Med. 2019, 97, 1155–1167. [Google Scholar] [CrossRef] [PubMed]
- Haselmann, V.; Kurz, A.; Bertsch, U.; Hübner, S.; Olempska-Müller, M.; Fritsch, J.; Häsler, R.; Pickl, A.; Fritsche, H.; Annewanter, F.; et al. Nuclear death receptor TRAIL-R2 inhibits maturation of let-7 and promotes proliferation of pancreatic and other tumor cells. Gastroenterology 2014, 146, 278–290. [Google Scholar] [CrossRef]
- Singh, D.; Prasad, C.B.; Biswas, D.; Tewari, M.; Kar, A.G.; Ansari, M.A.; Singh, S.; Narayan, G. TRAIL receptors are differentially regulated and clinically significant in gallbladder cancer. Pathology 2020, 52, 348–358. [Google Scholar] [CrossRef]
- Bertsch, U.; Röder, C.; Kalthoff, H.; Trauzold, A. Compartmentalization of TNF-related apoptosis-inducing ligand (TRAIL) death receptor functions: Emerging role of nuclear TRAIL-R2. Cell Death Dis. 2014, 5, e1390. [Google Scholar] [CrossRef]
- Lu, M.; Lawrence, D.A.; Marsters, S.; Acosta-Alvear, D.; Kimmig, P.; Mendez, A.S.; Paton, A.W.; Paton, J.C.; Walter, P.; Ashkenazi, A. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science 2014, 345, 98–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufour, F.; Rattier, T.; Constantinescu, A.A.; Zischler, L.; Morlé, A.; Ben Mabrouk, H.; Humblin, E.; Jacquemin, G.; Szegezdi, E.; Delacote, F.; et al. TRAIL receptor gene editing unveils TRAIL-R1 as a master player of apoptosis induced by TRAIL and ER stress. Oncotarget 2017, 8, 9974–9985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saini, Y.; Chen, J.; Patial, S. The Tristetraprolin Family of RNA-Binding Proteins in Cancer: Progress and Future Prospects. Cancers 2020, 12, 1539. [Google Scholar] [CrossRef] [PubMed]
- Brewer, B.Y.; Malicka, J.; Blackshear, P.J.; Wilson, G.M. RNA sequence elements required for high affinity binding by the zinc finger domain of tristetraprolin: Conformational changes coupled to the bipartite nature of Au-rich MRNA-destabilizing motifs. J. Biol Chem 2004, 279, 27870–27877. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.H.; Vo, M.T.; Kim, H.J.; Lee, U.H.; Kim, C.W.; Kim, H.K.; Ko, M.S.; Lee, W.H.; Cha, S.J.; Min, Y.J.; et al. Stability of the LATS2 tumor suppressor gene is regulated by tristetraprolin. J. Biol. Chem. 2010, 285, 17329–17337. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Gajan, A.; Chu, Q.; Xiong, H.; Wu, K.; Wu, G.S. Developing TRAIL/TRAIL death receptor-based cancer therapies. Cancer Metastasis Rev. 2018, 37, 733–748. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.H.; Han, M.W.; Kim, S.H.; Seong, D.; An, J.H.; Chang, H.W.; Kim, S.Y.; Kim, S.W.; Lee, J.C. Tristetraprolin Posttranscriptionally Downregulates TRAIL Death Receptors. Cells 2020, 9, 1851. https://doi.org/10.3390/cells9081851
Lee WH, Han MW, Kim SH, Seong D, An JH, Chang HW, Kim SY, Kim SW, Lee JC. Tristetraprolin Posttranscriptionally Downregulates TRAIL Death Receptors. Cells. 2020; 9(8):1851. https://doi.org/10.3390/cells9081851
Chicago/Turabian StyleLee, Won Hyeok, Myung Woul Han, Song Hee Kim, Daseul Seong, Jae Hee An, Hyo Won Chang, Sang Yoon Kim, Seong Who Kim, and Jong Cheol Lee. 2020. "Tristetraprolin Posttranscriptionally Downregulates TRAIL Death Receptors" Cells 9, no. 8: 1851. https://doi.org/10.3390/cells9081851





