Abstract
Recent studies conducted over the past 10 years evidence the intriguing role of the tumor suppressor gene Phosphatase and Tensin Homolog deleted on Chromosome 10 PTEN in the regulation of cellular energy expenditure, together with its capability to modulate proliferation and survival, thus expanding our knowledge of its physiological functions. Transgenic PTEN mice models are resistant to oncogenic transformation, present decreased adiposity and reduced cellular glucose and glutamine uptake, together with increased mitochondrial oxidative phosphorylation. These acquisitions led to a novel understanding regarding the role of PTEN to counteract cancer cell metabolic reprogramming. Particularly, PTEN drives an “anti-Warburg state” in which less glucose is taken up, but it is more efficiently directed to the mitochondrial Krebs cycle. The maintenance of cellular homeostasis together with reduction of metabolic stress are controlled by specific pathways among which autophagy, a catabolic process strictly governed by mTOR and PTEN. Besides, a role of PTEN in metabolic reprogramming and tumor/stroma interactions in cancer models, has recently been established. The genetic inactivation of PTEN in stromal fibroblasts of mouse mammary glands, accelerates breast cancer initiation and progression. This review will discuss our novel understanding in the molecular connection between cell metabolism and autophagy by PTEN, highlighting novel implications regarding tumor/stroma/immune system interplay. The newly discovered action of PTEN opens innovative avenues for investigations relevant to counteract cancer development and progression.
1. Introduction
The tumor suppressor gene Phosphatase and Tensin Homolog deleted on Chromosome 10 (PTEN) is often mutated in human tumors with germline mutations causing cancer-predisposition syndromes [1]. Genetic inactivation of PTEN is frequently found in glioblastomas, melanomas, endometrial, prostate, colon, and bladder cancers, and reduced PTEN expression has also been observed in lung and breast cancer [2]. Loss of PTEN function can occur by mutations or deletions, epigenetic silencing, transcriptional repression or by micro RNA (miRNA) regulation [3].
PTEN is a protein–phosphatase and a lipid–phosphatase. As a lipid–phosphatase, PTEN decreases the cellular amount of phosphatidylinositol-3,4,5-phosphate (PIP3) which is an important second messenger mediating different signaling pathways [4]. Inactivation of PTEN enzymatic activity leads to induction of cell proliferation and inhibition of cell death, causing cancer development and progression [5]. Several studies carried out in both human samples and hypomorphic PTEN mice indicate a continuum model of PTEN tumor suppression, rather than a stepwise alteration of PTEN levels [6]. Indeed, even partial loss of PTEN function is sufficient to promote some cancer types and a reduction in PTEN levels below 50% further accelerates cancer progression [7]. Notably, studies carried out in vitro and in PTEN mouse models (see Table 1) show that even subtle reductions in PTEN enzymatic activity influence cancer susceptibility, demonstrating the existence of PTEN tumor suppressor pathways [5]. Nevertheless, it is reported that complete loss of PTEN, resulting in a tumor suppressor p53 increase can counteract tumor growth by activating an effective fail-safe mechanism—cellular senescence—in the prostate cells [8]. The above described effect has important relevance, since it indicates that both genes need to be ablated for prostate cancer progression.

Table 1.
List of PTEN alteration, observable effects, and experimental models.
New insights into molecular mechanisms of cancer demonstrate dysregulation of cellular metabolism, increase of glucose uptake and fermentation of glucose to lactate. In this concern, a metabolic point of view of PTEN function emerges: PTEN inactivation produces fatty acid accumulation which leads to non-alcoholic fatty liver disease and long-latent liver tumorigenesis [9].
Moreover, it is accepted that mechanisms for PTEN dimerization and inactivation could be deregulated in cancer [10]. PTEN is secreted into the extracellular environment for uptake by recipient cells, thus also working as a tumor suppressor in a cell non-autonomous manner [11].
Interestingly, a role of PTEN in tumor/stroma interactions in cancer models is increasingly supported [12]. Genetic inactivation of PTEN in stromal fibroblasts of mouse mammary glands accelerates breast cancer initiation and progression. Specifically, the tumor suppressor activity of PTEN in the stroma is mediated by the regulation of multiple signaling pathways, such as Ras proto-oncogenes, Protein kinase B (PKB), also known as AKT and c-Jun N-terminal kinase (Jnk) networks, which modulate the transcription factor Ets2 and was able to reduce tumor growth and progression [13].
The above reported findings delineate a complex scenario of tumor suppressor function of PTEN. The classical role of PTEN in human disease is played through the modulation of the phosphoinositide 3-kinase (PI3K) activity. Indeed PTEN, dimethylated at arginine 159 (R159) is mutated at R159 in cancers, losing the capability to inhibit the PI3K–AKT pathway targeting the mammalian target of rapamycin (mTOR) [14,15,16]. The role of mTOR is critical for sensing the nutritional status of the cell, regulating initiation of autophagy and the energy expenditure [17]. Cells reprogram their metabolism very early during carcinogenesis, and this event is critical for the establishment of other cancer hallmarks. Thus, PTEN is emerging as a crucial sorter of a metabolic network, controlling specific gene expression and pathways. These new findings suggest an intriguing point of view of PTEN biology and function.
Here, we will outline PTEN as an essential determinant of a tumor suppressor metabolic state influencing the complex interplay between the tumor and immune system. First, the biochemical functions of PTEN on cell metabolism and autophagy will be discussed. Then, the role of PTEN in tumor microenvironment remodeling will be underlined. The recent advances in our understanding of PTEN biological roles may help to identify new opportunities to improve PTEN function for cancer therapy.
2. Biochemical Functions of PTEN and Cancer Metabolism
PTEN as lipid–phosphatase [27,28] acts as negative regulator of the class I phosphatidylinositol 3-kinases (PI3Ks) which phosphorylates phosphatidylinositol-4,5-bisphosphate (PIP2) to generate the second messenger phosphatidylinositol-3,4,5-trisphosphate (PIP3). The PIP3 induces molecular signaling, such as the activation of AKT kinases, which act on molecular targets relevant for different biological roles, like regulation of cell growth, cell proliferation, vesicle trafficking, angiogenesis, anabolic metabolism and cancer [29]. Thus, PTEN is relevant for the control of the nutrient-responsive signaling involved in protein synthesis and transcription [30].
2.1. PTEN Intercepts AKT-Dependent Metabolic Pathways
Activated AKT is a central regulator of oncogenic metabolism. It is accepted that AKT stimulation pushes the glycolytic metabolism of tumor cells [31,32]. The activation of AKT, resulting from PTEN loss, stabilizes the enzyme phosphofructokinase-1 (PFK1) [33] (see Figure 1, point 4) thus promoting glycolysis, cellular proliferation, and brain tumor growth. Specific phosphorylation of AKT rises cellular glucose uptake and is crucial to induce glucose transporter 1 (Glut1) or Glut4 translocation to the plasma membrane of adipocytes (see Figure 1, point 3) [34]. In addition, AKT stimulates the mTOR, a serine/threonine kinase that is part of two distinct complexes, mTOR Complex (TORC) 1, and TORC2 which directly control cell metabolism and growth in response to environmental signals [35].

Figure 1.
A schematic summary of metabolic pathways (upper panel) determining the tumor suppressive activities (lower panel) for PTEN. See the text for details. (1) PTEN through AMPK induces Krebs cycle and OXFOS. (2) PTEN suppresses glycolysis through PGK1 inhibition. (3) PTEN blocks AKT induced Glut1 translocation and glucose uptake. (4) PTEN blocks mTOR-induced HIF1, PFK1, and glycolysis. (5) PTEN blocks mTOR-induced PKM2 transcription. (6) PTEN decreases PPP flux. (7) PTEN blocks mTOR-induced SREBP transcription factor and lipid synthesis. (8) PTEN reduces pyrimidine synthesis.
2.2. PTEN Intercepts mTOR Dependent Metabolic Pathways
The mTOR pathway is one of the most deregulated signaling pathways in human cancer, and constitutive activation of mTORC1 is very frequently observed in different tumors [36]. Indeed, mTOR acts on many anabolic pathways sustaining cell proliferation, such as glycolysis and the pentose phosphate pathway (PPP), through regulation of hypoxia-inducible factor (HIF)1 (see Figure 1, points 5, 6) [37]. It also stimulates lipid synthesis by activating the transcription factor sterol regulatory element-binding protein (SREBP)1 [38] (see Figure 1, point 7) and nucleotide through regulation of the PPP and by activation of an enzyme of pyrimidine synthesis (see Figure 1 point 8) [39].
The PI3K–AKT–mTOR pathways play a crucial role in cancer development through an elevated number of components within the cascade, whose level or activity is found altered, and recent studies suggest that tumor suppressive activities for PTEN are exerted at biochemical metabolic pathways. The phosphatase action of PTEN regulates phosphoglycerate kinase 1 (PGK1) glycolytic enzyme (see Figure 1, point 2) capable of autophosphorylation. Loss of PTEN function in cancer cells causes augmented PGK1 autophosphorylation, glycolysis, and ATP production, induction of cancer cell proliferation, and tumorigenesis. [40,41]. Thus, PTEN exerts multiple functions that occur in different cellular compartments for which the phosphatase domain is required to inhibit cancer development [18,42]. Consequently, PTEN may represent one of the main targets for cancer therapy.
3. The Multifaceted Action of PTEN on Cell Metabolism
Cancer cells reprogram their metabolism to sustain abnormal cell proliferation, survival, and long-term maintenance [43]. The common feature of this altered metabolism is increased glucose uptake and fermentation of glucose to lactate. This phenomenon is observed even in the presence of completely functioning mitochondria and is known as the Warburg effect, the most important metabolic hallmark of cancer cells [44] which prefer the glycolysis pathway even in the presence of normal or high oxygen tension. Although aerobic glycolysis is an inefficient mean of generating ATP compared to the amount obtained by mitochondrial respiration, cancer cells adopt the metabolic reprogramming as approach for energy compensation [45]. Indeed, the rate of glucose metabolism through aerobic glycolysis is higher such that the production of lactate from glucose occurs 10–100 times faster, compared with the complete oxidation of glucose in the mitochondria. In cancer cells, the amount of ATP synthesized over any given period of time is comparable when either form of glucose metabolism is utilized [46]. In this concern, microarray analysis shows that genes of the glycolysis pathway are overexpressed in the majority of clinically relevant cancers. Besides, the upregulation of plasma membrane glucose transporters and changes in key enzymes involved in glucose utilization have been observed in many tumor types [46] likely contributes to the avid uptake of glucose, even when its availability is becoming insufficient, because of the continuous growth of the tumor. Some tumors show increased expression and activity levels of hexokinase (HK) isoforms, PFK1, PFK2, aldolase (ADO), phosphoglycerate kinase (PGK), enolase (ENO), and pyruvate kinase (PK) [47] which increase pyruvate production from glucose breakdown. Inhibition of glycolysis in cancer cells is considered an alternative therapeutic strategy for cancer patients; thus, drugs targeting the abovementioned controlling enzymes in tumor glycolysis could have new promising applications.
3.1. Regulation of Glucose Metabolism
Multiple oncogenic pathways, such as Ras-dependent, Myc or PI3K, favor glycolysis over oxidative phosphorylation, while many tumor suppressors such as p53, Von Hippel–Lindau (VHL), or liver kinase B1 (LKB1) negate the “Warburg effect” [48,49]. Accordingly, in vivo models with tumor suppressor PTEN expression elevated to varying levels (PTEN tg mice) have revealed mouse embryonic fibroblast (MEF) metabolic changes in which less glucose is taken up, but it is more efficiently directed to the mitochondrial Krebs cycle thus consistent with an “anti-Warburg effect” (see Figure 1, point 1). Specifically, PTEN tg MEFs present higher levels of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α), a transcriptional coactivator which regulates mitochondrial biogenesis and energy metabolism [42,50]. These mice exhibit increased oxygen consumption and energy expenditure [51]. Moreover, MEFs show an augmented number of mitochondria, together with ATP production and oxygen consumption and lower levels of lactate secretion. All these features indicate that PTEN decreases the glycolytic rate and favors oxidative phosphorylation. Thus, in summary, PTEN tg mice exhibit an unexpected cancer-resistant and very unique metabolic state which is the outcome of the ability of PTEN to regulate metabolism at multiple levels both from the cytosol and from the nucleus.
Glucose consumption is limted by PTEN in cancer cells, by preventing the expression of Glut1 on plasma membrane. At a molecular level, PTEN blocks AKT activation which controls the localization of Glut1 in the plasma membrane [46]. The regulator of glycolysis, HK2, is increased by the combined loss of PTEN and p53. The mechanism of PTEN deletion is dependent on activation of the AKT–mTORC1 and HK2 protein synthesis. In prostate cancer models with PTEN/p53-deficiency, aerobic glycolysis dependent on HK2 drives tumor growth [52]. Very recently, it has been reported that the regulation of PTEN/AKT/HK2 could be targeted to overcome cancer resistance to cisplatin treatment [53]. Moreover, PTEN reduces the levels of pyruvate kinase muscle isozyme (PKM) 2 which catalyzes the last step of glycolysis (see Figure 1, point 5) and its expression is associated to the “Warburg effect” of cancer cells [49]. The transcription of PKM2 is induced by mTOR and lower levels of PKM2 are found in PTEN transgenic cells [42]. Remarkably, PTEN counteracts the glyoxalase dependent PI3K/AKT/mTOR/p-PKM2(Y105)-axis inducing an elevated glycolytic rate and cell proliferation in prostate cancer.
Loss of PTEN, via suppressive effects on anaphase-promoting complex (APC) and its coactivator Cdh1-mediated ubiquitination, could stabilize 6-phosphofructo-1-kinase/fructose-2,6-biphosphatase isoform 3 (PFKFB3) family member [54] which is critical for the first commitment step of glycolysis and whose activity has been implicated in cancer [44].
3.2. Regulation of Glutamine Metabolism
Glutaminolys, as well as glycolysis, is another mainstay for energy production and anabolism in cancer cells. A recent elegant study demonstrates that PTEN loss brings a hyperglycolytic phenotype which would render T-cell acute lymphoblastic leukemia (T-ALL) cells resistant to Notch signaling pathway inhibition [55], given the aberrant activation of Notch in over 60% of T-ALL cases. Besides, the same T-cells are less sensitive to inhibition of glutaminolysis as result of increased glucose-derived carbon input to the Krebs cycle. Notably, PTEN can affect glutaminolysis as a crucial point in metabolic reprogramming, influenced by Notch. Glutaminases (GLS1 and GLS2) produce glutamate from glutamine and activate step one in the glutaminolytic pathway. Glutamine consumption is reduced by PTEN s due to the concomitant degradation of GLS1 [50] which is pro-oncogenic [56]; however, GLS2 is anti-oncogenic [57]. The oncogene Myc upregulates only GLS1 while the onco-suppressor p53 stimulates GLS2 [58]. In agreement with these data, PTEN inhibits the glutaminase GLS1, further supporting the tumor-suppressive activity of PTEN in cancer metabolism.
Notably, studies on the effects of suppression of PTEN expression by a specific miRNA, such as miR-181a, evidence increased AKT phosphorylation and lactate production, causing cell proliferation [59]. Specifically, miR-181a via PTEN is a crucial determinant of metabolic reprogramming in colon cancer, while no significant changes in the critical components of mTORC2 are observed.
3.3. Regulation of Krebs Cycle and Oxidative Phosphorylation
The Krebs cycle occupies a central position in metabolism and meets most of cell energy requirement by the complete oxidation of acetyl-CoA, a key product in the catabolism of carbohydrates, fatty acids and amino acids, to CO2. Recently in non-transformed thyrocytes of a PTEN-deficient mouse model, the constitutive PTEN loss affects Krebs cycle and oxidative phosphorylation, with defective mitochondria and compensatory metabolic switch to glycolysis [18]. Furthermore, impairment of the Krebs cycle is associated to pathological conditions including cancer, whereas genetic and epigenetic alterations of Krebs cycle enzymes favor the shift of cancer cells from oxidative phosphorylation to anaerobic glycolysis. Conversely, recent data from transgenic mice models carrying additional copies of PTEN (PTEN tg) indicate that elevation of this gene induces a tumor suppressive metabolic state [42]. The PTEN tg mice resulted in homogeneous and systemic PTEN overexpression (2–3 fold higher than normal mice) and shared remarkably overlapping phenotypes. The elevation of PTEN results in healthy metabolism characterized by increased energy expenditure and reduced body fat accumulation. Cells derived from these mice are resistant to oncogenic transformation and show reduced glucose and glutamine uptake and increased mitochondrial oxidative phosphorylation. These results demonstrate that PTEN is a crucial node for the control of tumorigenesis related to dysregulated cell metabolism (see Figure 1, point 1).
Notably, PTEN plays a key role in insulin-mediated oxidative stress and genomic damage in a human hepatocyte cell line and in vivo models. Increased reactive oxygen species (ROS), stress-proteins, and genomic damage in the liver of PTEN haplo-deficient mice maintained with a high fat diet, is reported [19] further supporting a causative role of PTEN in hepatic and extrahepatic carcinogenesis observed in obese subjects. Surprisingly, in PTEN tg mice, oxidative phosphorylation is augmented together with the ROS amount [42]. Since PTEN overexpression is associated with cancer protection, this increase in ROS levels is not sufficient to exert relevant effects on DNA. Besides, it should be evidenced that PTEN induces the transcription of genes mediating antioxidant activity through the Forkhead box O (FOXO)3 transcription factors [60].
4. PTEN, Autophagy, and Cancer
Recent acquisitions demonstrate that cell metabolism is tightly connected with autophagic pathways [61,62]. Specific enzymes such as AMP-activated protein kinase (AMPK), protein kinase A (PK) A, and mTOR play a role in cellular energy homeostasis and control autophagy process together with respiration amending energy requests for cellular behavior. Cell proliferation is possible in particular energy conditions influenced by cellular ATP demands. Autophagy, which is a catabolic process, produces glucogenic and ketogenic amino acids and is enabled to fuel the Krebs cycle at multiple entry points thus contributing to the ATP supply. During autophagy, lysosomes degrade damaged cell components thus precursor molecules, energy for neo-synthesis, and metabolic requests are generated. Consequently, autophagy retains an adaptive response through which cells tolerate unfavorable conditions. Moreover, autophagy is a protective mechanism able to avoid hazardous situations in the cell (e.g., increasing ROS or DNA damage), preventing cancer initiation and progression.
Interestingly, genetic manipulation causing impaired autophagy in mice demonstrates that tumor formation is prevented by autophagy process. For instance, mice with allelic loss of BECLIN 1, the master autophagic gene, show augmented susceptibility to tumor development compared to wild-type mice [63]. Although autophagy sustains tumor metabolism and growth during Ras-induced transformation and tumorigenesis [64], compelling evidence suggests that onco-suppressors mediate autophagy process [63] particularly targeting mTOR. Specifically, AMPK, LKB1, ttuberous sclerosis proteins (TSC) ½, and PTEN induce autophagy, equally, oncogenes that activate mTOR block autophagy.
4.1. Interaction between PTEN Signaling and Autophagic Alterations
In glioblastomas PTEN is frequently mutated s and ectopic expression of functional PTEN in U87MG glioma cells, induces the autophagic flux and the lysosomal mass. However, proteasome activity and protein ubiquitination are inhibited. Interestingly, the effects are independent of PTEN lipid phosphatase activity on the PI3K/AKT/mTOR signaling pathway [20]. These results propose a novel mTOR-independent signaling pathway by which PTEN can act on intracellular protein degradation influencing autophagy. The molecular components by which the tumor suppressor PTEN regulates proteolytic systems related to cancer development could represent innovative therapeutic targets for patient therapy.
The oncogene RAS and p53 loss are crucial determinants of pancreatic cancer, and loss of autophagy contributes to the progression of the disease [65]. Besides, animals with deletion of the key modulator Autophagy related (Atg) 7 and hemizygous for PTEN, develop pancreatic ductal adenocarcinoma [66]. Indeed, blocking of autophagy together with PTEN hemizygosity permit tumor development and an early death related to pancreatic cancer matched to autophagy-competent mice. Specifically, autophagy-deficient tumors are also PTEN-deficient but notably wild-type for p53, further strengthening the crucial protective role of PTEN.
Published findings show that PTEN can determine autophagy’s contribution to tumor development by DNA damage, increased inflammation, metabolic reprogramming, and oxidative stress increase. For instance, molecular mechanisms controlling autophagy are influenced by the cellular messenger nitric oxide (NO) formed by distinct isoforms of nitric oxide synthase (NOS). Specifically, NOS1 stimulates the survival of nasopharyngeal carcinoma cells through S-nitrosylation of PTEN proteins, induction of AKT/mTOR, and block of the autophagic flux [67].
Generally, autophagy effects are context dependent to exert a tumor suppressive or oncogenic action; however, a very elegant study showed an association of a casein kinase 1 alpha 1 (CK1α)-dependent autophagic mechanism and the tumor-suppressor role exerted by PTEN/Atg7 signal in xenograft models as well as in lung cancers. [68]. Specifically, CK1α increased PTEN stability and activity counteracting PTEN polyubiquitination and abrogating PTEN phosphorylation. These events account for AKT inhibition and FOXO3a-induced transcription of Atg7. The effects of PTEN deficiency are investigated also in hepatocyte of PTEN-deficient mice. The authors describe a reduction of autophagosomes formation and maturation, inhibition of Atg conjugation reactions, and induction of insulin pathway, suggesting that hepatic PTEN loss causes relevant action on the whole mice metabolism [21]. However further characterization of Atg proteins is necessary to improve the comprehension of autophagy defects in the PTEN loss and cancer.
4.2. Regulation of Autophagy and Cell Growth by PTEN in Endocrine Related Cancer
Given the abovementioned phenotypes associated with increased PTEN dosage, some studies investigate the role of PTEN through steroid receptors in endocrine-related cancer [15,69]. In estrogen receptor (ER) +/progesterone receptor (PR) + breast cancer cells, a novel functional connection between PTEN and autophagy is described as a tumor suppressor pathway (see Figure 2, point 1). We show that progesterone (OHPg)/PR-B induce a genomic mechanism involving PTEN gene transcription. The PTEN gene is located on the human chromosome 10 q23.3, consisting of nine exons. The 5′-UTR sequence contains a strong promoter mapped to the region between −551 and −220 bases upstream of the translation start codon [70] PR-B recruits at an Sp1-rich region within the PTEN gene promoter. Increase of PTEN levels reduces PI3K/AKT signals, switching on the autophagic flux by enhancing the expression of the regulator of membrane trafficking in autophagy (UVRAG) driving a reduction of breast cancer cell proliferation [15].
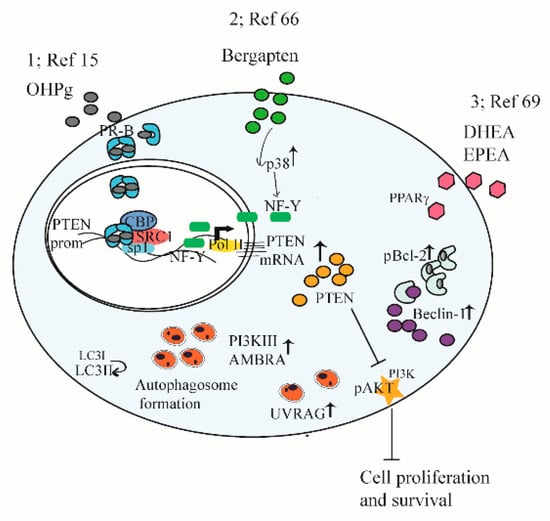
Figure 2.
A schematic summary illustrating mechanisms targeting PTEN and autophagy in cancer cells. See the text for details. (1) The functional interplay between progesterone receptor-B and PTEN, via AKT, modulates autophagy in breast cancer cells. (2) PTEN as a key target of BergaPTEN action in breast cancer cells for the induction of autophagy. (3) DHEA and EPEA through PPARγ-increased expression PTEN, resulting in the inhibition of AKT–mTOR pathways, induction of Bcl-2 phosphorylation, its dissociation from Beclin-1, and autophagy induction.
Additional studies address how induction of PTEN expression causes autophagy in hormone-dependent breast cancer cells. For instance, the psoralen bergapten reveals anti-survival effects by inducing PTEN through p38 mitogen-activated protein kinase (p38MAPK), nuclear transcription factor Y (NF-Y) axis, and autophagy [71]. Indeed, autophagy regulators, such as BECLIN 1, Class III PI 3-kinase, UVRAG, and aativating molecule in Beclin 1-regulated autophagy (AMBRA) 1 protein expression increase, together with conversion of the microtubule-associated protein 1A/1B-light chain 3 (LC3) I to LC3-II. Moreover, autophagic vesicles are evidenced in treated cells. The autophagy process is crucially related to the increase of PTEN as demonstrated by specific siRNA studies (see Figure 2, point 2).
In agreement with these results, a functional molecular crosstalk between steroid receptors, PTEN and autophagy is demonstrated in testicular germ cells. In TCAM2 cell line, estradiol through ERβ increases PTEN gene expression and promoter activity. Both ERβ and PTEN are responsible for the reduction of cell survival, suggesting a molecular liaison, pAKT is inhibited, and autophagy-related markers increase, indicating the autophagy induction by estradiol [72]. Through DNA fragmentation, cleavages of caspase 9 and Poly (ADP-ribose) polymerase (PARP1), involved in DNA repair processes, are not evidenced; thus, authors [72] propose that necroptosis and/or parthanatos occur, instead of apoptosis. These data demonstrate that ERβ/PTEN play a protective action in testicular germ cells.
In prostate cancer, PTEN deletions are associated with abnormal induction of AKT–mTOR and androgen receptor (AR) signaling pathways. A recent study evidenced that conditional homozygous deletion of histone deacetylase (HDAC) 3, AKT inhibition, and AR reduction in PTEN-deficient mouse models suppresses prostate tumorigenesis and progression [73], suggesting in the HDAC 3 inhibition together with PTEN inducers new possible therapeutic approaches for prostate cancer patients.
Protein levels of PTEN are induced by activation of peroxisome proliferator-activated receptor gamma (PPARγ) which mediates the interplay between cancer and metabolic syndromes, diabetes, and obesity. The studies signify the importance of PPARγ and PTEN’s interaction in cancer prevention [74]. In ER+ PR+ breast cancer cells, dietary omega-3 long-chain polyunsaturated fatty acids, docosahexaenoic acid (DHEA), and eicosapentaenoic acid (EPEA) enhance PTEN via PPARγ, blocking AKT–mTOR pathways. Moreover, DHEA and EPEA stimuli increase B-cell lymphoma 2 (Bcl-2)-phosphorylation causing its dissociation from BECLIN 1 which results in autophagy induction [75]. In summary (see Figure 2, point 3), data show the anti-proliferative action of two omega-3 ethanolamides involving PTEN-mediated induction of autophagy, suggesting their potential use as breast cancer preventive and/or therapeutic agents.
4.3. Regulation of EMT and Invasion through Autophagy by PTEN
Recent findings in cancer progression investigate new possible molecular mechanisms regulating invasion, epithelial mesenchymal transition (EMT) [76,77], and the possible link with autophagy. It is reported that (synaptojanin 2 binding protein) SYNJ2BP, crucially involved in the pathogenesis of metastatic breast tumors, is responsible of the degradation of PTEN through the lysosomes; particularly, SYNJ2BP stimulates the recruitment of PTEN at autophagy-lysosomes, the autophagosome cargo (p62) expression, and LC3-I to LC3-II conversion. Collectively, these data evidence that SYNJ2BP causes the autophagic degradation of PTEN which is related to the activation of Snail, a zinc-finger transcriptional repressor controlling EMT thus promoting both EMT and invasion in breast cancer models [78]. Further study investigates the effects of PTEN, lncRNA growth arrest-specific 5 (GAS5), its target genes, and microRNA-222-3p (miR-222-3p) on motility, invasive capacity, and autophagic flux in colorectal cancer cells (CRCs). The results indicate, with elegant experimental design, that lncRNA GAS5 exerts an anti-oncogenic action in CRCs counteracting the inhibitory effects of miR-222-3p on PTEN [79] which acquires novel relevance for patient’s treatment.
5. PTEN, Immune System, and Cancer
Through autophagy PTEN determines the fate of the tumor [61]. In addition, the biology of the immune system dictates tumor initiation and progression [80] through the balance between effector and tolerogenic response modulated by autophagy [81]. Autophagy influences different biological functions of different cell types of the immune system such as natural killer cells, dendritic cells, macrophages, and T and B lymphocytes. It could modulate the secretion of cytokines and antibodies which also have effects on the autophagic process itself. Transforming growth factor -β, interferon-γ, and several interleukins (IL) are stimulators, while IL-4, IL-10, and IL-13 counteract autophagy [82]. Autophagy can be stimulated by the activity of innate immune receptors, such as Toll-like receptors [83], and in adaptive immunity, it is determinant for antigen presentation, lymphocyte differentiation, and cytokines secretion with onco-suppressor activity [84]. Therefore, an ideal therapeutic portfolio could be integrated by autophagy-based inducers, namely, PTEN inducers together with existing therapeutic strategies to elicit cancer cell death and patients’ responses.
Unfortunately, malignant tumors can avoid immune surveillance through the expression of components sustaining immune tolerance. One example is the programmed cell death-ligand-1 (PD-L1) secreted by cancer cells, which interacts with specific receptor, such as programmed cell death-1 (PD-1), expressed by lymphoid and non-lymphoid immune cells. The interaction between PD-L1 and PD-1 causes reduction of lymphocyte response [80]. Indeed, interference of PD-L1/PD-1 axis by blocking molecules, produces durable responses in numerous cancer types, such as advanced melanoma, bladder cancer, kidney cancer, and glioblastoma [85]. However, often the therapy fails and patients relapse. In this concern autophagy inducers could improve the action of immunotherapy by warranting an ideal secretion of immune-stimulatory factors, providing antigens to the immune cells and eventually modulating immune responses for tumor recognition and rejection [86]. Conversely, an autophagic process, can hinder the immune responses thus decreasing immunotherapy effects. Although the molecular basis of immune resistance is still to be defined, a number of studies demonstrate the role played by PTEN.
5.1. Role of PTEN in T Cells Function
Loss of PTEN crucially contributes to immune resistance in cancer disease. For instance, in cancer cells and in mouse models of melanoma, PTEN knockout counteracts T cells’ action on tumor cells and reduces T cell trafficking into cancer tissue [22]. Loss of PTEN induces a mechanism regulated by immunosuppressive cytokines, determining a reduction of T cells’ infiltration into tumor tissue and inhibition of autophagy and consequently T cell–mediated cell death. In patients, PTEN loss correlates with inferior outcomes with PD-1 inhibitor therapy. The use of selective PI3Kβ inhibitor ameliorates the efficacy of immunotherapy in murine models [22].
In humans, cancer development is frequently associated with chronic immunosuppression and inflammatory diseases. For instance, head and neck squamous cancers are characterized by neutrophils in inflammatory infiltrates. Neutrophils found in mouse tumors antagonize effector T cell function, support the generation of immunosuppressive T cell populations, and inhibit the lysis of tumor cells by cytotoxic T cells or natural killer (NK) cells [87]. In mouse, biallelic inactivation of LKB1, which is involved in starvation-induced autophagy, and PTEN (so called LP mice), causes lung squamous cell carcinoma, exhibiting histologic pattern and gene expression showed in human disease. These tumors are characterized by tumor-associated neutrophils and by epithelial cell populations expressing high levels of the CXC chemokines which regulate the motility and adhesion of neutrophils. Moreover, cancer stem cells population show enhanced tumor-propagating capability as well as elevated expression of the immune evasion marker (PD-L1) indicating that these cells display immune evasion capacity [88]. The results also indicate the inefficacy of checkpoint inhibitors in these models, suggesting that PTEN deletion could be retained, a strong indicator of non-responder tumors. Notably, a meta-analysis of the data of non-small-cell lung cancer (NSCLC) patients. treated by immunotherapy showed that while p53, epidermal growth factor receptor (EGFR), and LKB mutations are not correlated with the immune response, PTEN was associated with resistance to anti-PD-1 therapy [89].
Interestingly, further studies established that PD-1 may be retained an haploinsufficient tumor suppressor in T cell lymphoma. The molecular mechanism appears to be related to PD-1 activity which increases PTEN levels and reduces AKT and protein kinase C signals in pre-malignant cells. Therefore, reinforcing PTEN activity could be a potential strategy for treatment of these types of cancers; conversely, checkpoint inhibitors could reactivate T cell in limphoma patients [90].
Systemic immunosuppression was evidenced in patients with glioblastoma and in glioma animal models [91]. Specifically, glioblastomas had a reduced amount of infiltrating T cells and harbored a quite small number of somatic mutations in respect to other tumors. However, a significant augmentation of PTEN mutations, correlated with immunosuppressive expression profile in glioblastomas patients resistant to anti-PD-1 immunotherapy [91]. Meanwhile, a further study in glioblastoma shows that expression of the PD-L1 increases in human glioma in the case of PTEN deletion as well as dysregulated PI3K signal [92]. In summary, the effects of specific drugs need further investigations, although, in most cases, T cells’ functions might be improved by employing specific agents, such as PTEN inducers or PI3K inhibitors, in different cancer patients.
5.2. Role of PTEN in Macrophages Function
Similarly, immune response involves macrophages. These cell types after pathogens and other noxious stimuli, become activated and initiate immune responses. The different phases of macrophage activation are defined as M1 and M2 polarization, characterized by specific phenotypes induced by inflammatory stimuli and influenced by the cellular context. Macrophages isolated from metastatic human cancers usually present an M2-like phenotype, consistent with the cancer-related inflammation. The PI3K/AKT pathway and its downstream targets play crucial role in the activated macrophages consequently PTEN, acting on various converging pathways, controls macrophage biology. It is reported that PTEN regulates macrophages activation by increase of Arginase I release [93] leading to a hypoinflammatory environment. Further evidences demonstrate that a potent inhibitor of PTEN, VO-OHpic, inhibits adverse cardiac remodeling due to the macrophages polarization. Pro-inflammatory M1 macrophages are reduced while anti-inflammatory M2 macrophages are increased in animal models treated with doxorubicin which unfortunately can stimulate cardiomyopathy [94].
6. PTEN and Tumor Microenvironment
Tumor growth and metastatic process rely on intrinsic characteristics, the response of host tissue and signals coming from tumor microenvironment (TME). The TME consists of blood vessels close to cancer cells, the extracellular matrix (ECM), and other non-cancer cells [95]. These stromal cells comprise fibroblasts (CAFs), immune cells such as T and B lymphocytes, natural killer cells, tumor-associated macrophages (TAM) but rarely adipocytes. Tumor cells stimulate the infiltration of immune cells inside the TME [96]. Tumor-infiltrating immune cells and tumor cells interact with each other, then immune responses act to inhibit tumor growth. To this aim different stromal cells, immune cells, and CAFs closely interacting with cancer cells produce an inflammatory response, secrete growth factors and chemokines which unfortunately can promote tumor development, progression, and metastasis [97].
Over the last years, it has been proposed that the changes regarding tumor stroma metabolism are potent inducers of tumor growth [98]. In the phases of cancer induction and progression, normal fibroblasts, intimately connected to tumor cells undergo metabolic reprogramming altering phenotype. Cancer associated fibroblasts sustain the growth of neighboring epithelial cancer cells, causing in turn oxidative stress and senescence in adjacent CAFs [98]. In senescent CAFs, induction of autophagy and mitophagy potentiate a change on aerobic glycolysis producing biochemical molecules that drive oxidative phosphorylation anabolic growth in the tumor cells.
Recent studies outline the onco-suppressor role of PTEN in the tumor microenvironment regulation, affecting metabolic reprogramming and autophagy. Indeed, specific genetic alterations including those of PTEN in cancer cells may affect the immune composition of the TME (see Figure 3 for a schematic representation), and such infiltrating immune cells may in turn act to inhibit or sustain cancer cells proliferation [99].

Figure 3.
A schematic summary illustrating PTEN role in tumor/stroma interplay. See the text for details. (1) PTEN blocks oncogenic drivers of tumors including PI3K/AKT/mTOR and JAK2/STAT3. (2) PTEN reduces immunosuppressive cytokines, determining induction of T cells’ infiltration into tumor tissue. (3) PTEN reduces tumor-associated neutrophils (4) PTEN in macrophages reduces cytokines and M2 macrophage polarization in TME. (5) In tumor cells PTEN decreases PD-L1 expression which is responsible for T cell inactivation in the tumor microenvironment. (6) PTEN induces a condensed layer of SMA-positive stroma. (7) PTEN blocks PI3K-dependent activation of SHP 2 which acts as a negative regulator to inhibit IFN-γ.
6.1. Regulation of TME Immune Response by PTEN
Mouse melanoma model carrying the frequent mutation of the BRAF oncogene (V600E) and PTEN deletion shows the repression of a protective immune response in the TME which was counteracted by an autophagy inducer, metformin, which determines tumor growth inhibition. Metformin causes the increase in the number of lung CD8-effector-memory T and CD4+ IL-10+ T cells and reduces metastasis in B16F10 melanoma cells transplanted mice [23]. These findings indicate that autophagy and PTEN inducers may contribute to treating melanoma carrying BRAF mutations, improving TME response. In drug-resistant ovarian cancer PTEN may cooperate with BECLIN 1, to block signaling inducing macrophage activity modification. Particularly, reduced expression of PTEN and BECLIN 1 was revealed in ovarian cancer tissues [100].
Furthermore, it is reported that PTEN deletion induces TME remodeling and is associated with immunosuppressive infrastructure in the TME. In the prostate of PTEN-knockout mice, much relaxed layers of smooth muscle actin (SMA)-positive malignant epithelial cells surrounding stroma are demonstrated, while a highly condensed layer of SMA-positive stroma, useful against the invasion of tumor cells into the adjacent stromal tissue, was detected in the prostate of wild-type mice (see Figure 3, point 6) [24].
Cross-talk between different signals during TME reprogramming is greatly influenced by genetic alterations of the PTEN/PI3K pathway. For instance, the stimulation of the Janus kinase (JAK) 2/Signal transducer and activator of transcription (STAT) 3 pathway as well as the secretion of such chemokines activating the infiltration of myeloid-derived suppressor cells contribute to PTEN-null prostate tumor growth and chemo-resistance (see Figure 3, points 1, 2) [101].
In glioblastoma (GBM) datasets, Chen and co-workers [25] evidence that poor outcomes and high stromal and immune signatures are related to PTEN loss and PI3K dysregulation but not with other pathway modifications. Deletion or mutation of PTEN are associated with higher infiltration of macrophage, a common cell type in GBM TME. The mechanism is dependent by PTEN deficiency promoting lysyl oxidase (LOX) expression, a potent macrophage chemoattractant in glioma cells. Lysyl oxidase, in turn, activates the β1 integrin dependent pathway in macrophages to promote their infiltration into GBM TME. This very elegant study evidences that these infiltrated macrophages secrete osteopontin (SPP1) promoting glioma cell survival and angiogenesis (see Figure 3, point 4).
In hepatocytes PTEN inactivation causes chronic damage that could remodel the hepatic microenvironment, recruiting inflammatory cells secreting cytokines and chemokines, driving hepatic cancer initiation and progression [9].
In vitro models simulating tumor under a nutrient depletion stress in the microenvironment show that miR-224 levels are inversely related to the expression of PTEN. Elevated miR-224 in tumor tissues compared with the adjacent normal tissues is correlated with tumor growth, decreased apoptosis, and autophagy [102].
All together these studies indicate that PTEN crucially contributes to immune-surveillance action in the complex interplay between tumor and stroma. This is confirmed in different cell contexts as the fibroblast-like synoviocytes increased concentrations of pro-inflammatory cytokines, chemokines, and VEGF expression are observed with PTEN inhibitor or PTEN-RNAi [103].
Thus, PTEN plays a critical role in TME, blocking a vicious cycle of positive feedback, responsible of aggressive local cell proliferation and metastatic spread to distant organs.
6.2. Cross-Talk Tumor–Stroma, PTEN Expression, and Function
In addition, PTEN loss-driven tumorigenesis is influenced by stromal cells inhibiting PTEN expression itself, in the tumor. [12]. In metastatic tumors exosomes containing anti-PTEN miRNAs secreted by stromal cells suppress PTEN expression in the tumor cells. PTEN reduction causes CCL2 chemokine secretion further increasing the outgrowth of metastatic tumor cells. Notably, stroma cells are effectors of PTEN loss-driven tumorigenesis through the secretion of factors potentiating the tumorigenic potential of cells. Loss of PTEN in macrophages, causes over-expression of cytokines that stimulate M2 macrophage to polarize. This is related to the increased infiltration of M2 macrophage, increased angiogenesis due to augmented vascular endothelial growth factor (VEGF) A and immune suppression in TME [104]. In high-grade serous ovarian carcinoma study, the number and/or localization of CD8+, CD45RO+, and CD68+ leucocytes, frequently associated with a patient’s overall survival [105], was not related with PTEN expression in the TME. This discrepancy could be related to a persistent low expression level of PTEN in these cases. Additional evidence supports the idea that the link between PTEN and the anti-tumor reprogramming of the stroma is mediated by the interferon signaling pathway. It was published that lung adenocarcinoma cells express low levels of PTEN and are interferon (IFN) γ insensitive and restoring PTEN expression reverses those effects [26]. The signal transduction of IFN γ is related with glycogen synthase kinase (GSK)-3β upregulation and autophagic induction. Conversely, abnormal PI3K stimulation and reduction of PTEN is accompanied by GSK-3β inactivation and the activation of Src homology 2-containing phosphatase (SHP) 2 which act as negative regulator to inhibit IFN-γ (see Figure 3, point 7).
7. Conclusions
In conclusion PTEN is a powerful tumor suppressor and its loss of function is often detected in heritable and sporadic cancers. Subtle changes in PTEN levels drive cancer predisposition as well as tumor progression, emphasizing the essential role of PTEN regulated mechanisms in cellular homeostasis and tumorigenesis. In recent years in vitro and in vivo studies have delineated a new position of PTEN function within the cell, such as key controller of metabolic states, also through the activation of autophagic phenotype and anti-Warburg effect, for a tumor suppressor action. Given the fact that energy request tightly determines cell proliferation and survival of cancer cells, the elucidation of the mechanisms governing PTEN expression and function acquires important therapeutic implication for an extensive selection of human cancers and inheritable syndromes associated with abnormal signals regulated by PTEN. Notably, PTEN contributes to the regulation of reciprocal interplay between cancer cells and the TME, exerting in such a way a “tumor-suppressive/immune-protective effect”. For instance, PTEN genetic alterations in several type of cancers influences the immune composition of the TME and infiltrating immune cells function to modulate the growth of tumor cells. These evidence strongly highlights the potential clinical benefit of a therapeutic action targeting PTEN which functions inside the interaction between cancer cells and immune cells. Several other questions persist to be elucidated and additional investigations could identify the best strategies for the restoration of PTEN function in cancer prevention and treatment.
Author Contributions
Conceptualization, S.A. and F.D.A.; Resources, S.A., M.S., A.C.; Writing and Figures preparation, F.D.A., M.S., A.C.; Supervision, M.L.P., V.P., F.D.A.; Funding acquisition, M.L.P., F.D.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a special award (Department of Excellence, Italian Law 232/2016) from the Italian Ministry of Research and University (MIUR) to the Department of Pharmacy, Health and Nutritional Sciences of University of Calabria (Italy), and by MIUR ex 60% (FDA, MLP).
Acknowledgments
To Domenico Sturino for English Language editing and review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, J.; Yen, C.; Liaw, D.; Podsypanina, K.; Bose, S.; Wang, S.I.; Puc, J.; Miliaresis, C.; Rodgers, L.; McCombie, R.; et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997, 275, 1943–1947. [Google Scholar] [CrossRef] [PubMed]
- Pulido, R. PTEN Inhibition in Human Disease Therapy. Molecules 2018, 23, 285. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.-H.; Zhou, F.; Shi, C.; Xiang, T.; Zhou, C.-K.; Wang, Q.-Q.; Jiang, Y.-S.; Gao, S.-F. miRNA-221 promotes cutaneous squamous cell carcinoma progression by targeting PTEN. Cell. Mol. Biol. Lett. 2019, 24, 9. [Google Scholar] [CrossRef] [PubMed]
- Mighell, T.L.; Evans-Dutson, S.; O’Roak, B.J. A Saturation Mutagenesis Approach to Understanding PTEN Lipid Phosphatase Activity and Genotype-Phenotype Relationships. Am. J. Hum. Genet. 2018, 102, 943–955. [Google Scholar] [CrossRef]
- Milella, M.; Falcone, I.; Conciatori, F.; Incani, U.C.; Del Curatolo, A.; Inzerilli, N.; Nuzzo, C.M.; Vaccaro, V.; Vari, S.; Cognetti, F.; et al. PTEN: Multiple Functions in Human Malignant Tumors. Front. Oncol. 2015, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Alimonti, A.; Carracedo, A.; Clohessy, J.G.; Trotman, L.C.; Nardella, C.; Egia, A.; Salmena, L.; Sampieri, K.; Haveman, W.J.; Brogi, E.; et al. Subtle variations in PTEN dose determine cancer susceptibility. Nat. Genet. 2010, 42, 454–458. [Google Scholar] [CrossRef]
- Berger, A.H.; Knudson, A.G.; Pandolfi, P.P. A continuum model for tumour suppression. Nature 2011, 476, 163–169. [Google Scholar] [CrossRef]
- Chen, Z.; Trotman, L.C.; Shaffer, D.; Lin, H.K.; Dotan, Z.A.; Niki, M.; Koutcher, J.A.; Scher, H.I.; Ludwig, T.; Gerald, W.; et al. Crucial role of p53-dependent cellular senescence in suppression of PTEN-deficient tumorigenesis. Nature 2005, 436, 725–730. [Google Scholar] [CrossRef]
- Galicia, V.A.; He, L.; Dang, H.; Kanel, G.; Vendryes, C.; French, B.A.; Zeng, N.; Bayan, J.A.; Ding, W.; Wang, K.S.; et al. Expansion of hepatic tumor progenitor cells in PTEN-null mice requires liver injury and is reversed by loss of AKT2. Gastroenterology 2010, 139, 2170–2182. [Google Scholar] [CrossRef]
- Yu-Ru, L.; Ming, C.; Jonathan, D.L.; Jinfang, Z.; Shu-Yu, L.; Tian-Min, F.; Hao, C.; Tomoki, I.; Shang-Yin, C.; Jesse, K.; et al. Reactivation of PTEN tumor suppressor for cancer treatment through inhibition of a MYC-WWP1 inhibitory pathway. Science 2019, 364, eaau0159. [Google Scholar]
- Hopkins, B.D.; Fine, B.; Steinbach, N.; Dendy, M.; Rapp, Z.; Shaw, J.; Pappas, K.; Yu, J.S.; Hodakoski, C.; Mense, S.; et al. A secreted PTEN phosphatase that enters cells to alter signaling and survival. Science 2013, 341, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, S.; Yao, J.; Lowery, F.J.; Zhang, Q.; Huang, W.C.; Li, P.; Li, M.; Wang, X.; Zhang, C.; et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 2015, 527, 100–104. [Google Scholar] [CrossRef]
- Trimboli, A.J.; Cantemir-Stone, C.Z.; Li, F.; Wallace, J.A.; Merchant, A.; Creasap, N.; Thompson, J.C.; Caserta, E.; Wang, H.; Chong, J.L.; et al. PTEN in stromal fibroblasts suppresses mammary epithelial tumours. Nature 2009, 461, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Dang, Y.; Zhang, W.; Zhao, X.; Zhang, C.; Hou, Z.; Jin, Y.; McNutt, M.A.; Marks, A.R.; Yin, Y. PTEN arginine methylation by PRMT6 suppresses PI3K-AKT signaling and modulates pre-mRNA splicing. Proc. Natl. Acad. Sci. USA 2019, 116, 6868–6877. [Google Scholar] [CrossRef] [PubMed]
- De Amicis, F.; Guido, C.; Santoro, M.; Lanzino, M.; Panza, S.; Avena, P.; Panno, M.L.; Perrotta, I.; Aquila, S.; Andò, S. A novel functional interplay between Progesterone Receptor-B and PTEN, via AKT, modulates autophagy in breast cancer cells. J. Cell. Mol. Med. 2014, 18, 2252–2265. [Google Scholar] [CrossRef]
- Lynch, J.T.; Polanska, U.M.; Hancox, U.; Delpuech, O.; Maynard, J.; Trigwell, C.; Eberlein, C.; Lenaghan, C.; Polanski, R.; Avivar-Valderas, A.; et al. Combined Inhibition of PI3Kβ and mTOR Inhibits Growth of PTEN-null Tumors. Mol. Cancer Ther. 2018, 17, 2309–2319. [Google Scholar] [CrossRef]
- Kim, J.; Guan, K.L. mTOR as a central hub of nutrient signalling and cell growth. Nat. Cell Biol. 2019, 21, 63–71. [Google Scholar] [CrossRef]
- Antico Arciuch, V.G.; Russo, M.A.; Kang, K.S.; Di Cristofano, A. Inhibition of AMPK and Krebs cycle gene expression drives metabolic remodeling of PTEN-deficient preneoplastic thyroid cells. Cancer Res. 2013, 73, 5459–5472. [Google Scholar] [CrossRef]
- Bankoglu, E.E.; Tschopp, O.; Schmitt, J.; Burkard, P.; Jahn, D.; Geier, A.; Stopper, H. Role of PTEN in Oxidative Stress and DNA Damage in the Liver of Whole-Body PTEN Haplodeficient Mice. PLoS ONE 2016, 11, e0166956. [Google Scholar] [CrossRef]
- Errafiy, R.; Aguado, C.; Ghislat, G.; Esteve, J.M.; Gil, A.; Loutfi, M.; Knecht, E. PTEN increases autophagy and inhibits the ubiquitin-proteasome pathway in glioma cells independently of its lipid phosphatase activity. PLoS ONE 2013, 8, e83318. [Google Scholar] [CrossRef]
- Ueno, T.; Sato, W.; Horie, Y.; Komatsu, M.; Tanida, I.; Yoshida, M.; Ohshima, S.; Wah Mak, T.; Watanabe, S.; Kominami, E. Loss of PTEN, a tumor suppressor, causes the strong inhibition of autophagy without affecting LC3 lipidation. Autophagy 2008, 4, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Chen, J.Q.; Liu, C.; Malu, S.; Creasy, C.; Tetzlaff, M.T.; Xu, C.; McKenzie, J.A.; Zhang, C.; Liang, X.; et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov. 2016, 6, 202–216. [Google Scholar] [CrossRef]
- Pereira, F.V.; Melo, A.C.L.; Low, J.S.; de Castro, Í.A.; Braga, T.T.; Almeida, D.C.; Batista de Lima, A.G.U.; Hiyane, M.I.; Correa-Costa, M.; Andrade-Oliveira, V.; et al. Metformin exerts antitumor activity via induction of multiple death pathways in tumor cells and activation of a protective immune response. Oncotarget 2018, 9, 25808–25825. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Bai, Y.; He, Y.; Zhao, Y.; Chen, J.; Ma, L.; Pan, Y.; Hinten, M.; Zhang, J.; Jeffrey Karnes, R.; et al. Loss Promotes Intratumoral Androgen Synthesis and Tumor Microenvironment Remodeling via Aberrant Activation of RUNX2 in Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2018, 24, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhao, D.; Li, J.; Liang, X.; Li, J.; Chang, A.; Henry, V.K.; Lan, Z.; Spring, D.J.; Rao, Y.G.; et al. Symbiotic Macrophage-Glioma Cell Interactions Reveal Synthetic Lethality in PTEN-Null Glioma. Cancer Cell 2019, 35, 868–884. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Chiang, T.H.; Tseng, P.C.; Wang, Y.C.; Lin, C.F. Loss of PTEN causes SHP2 activation, making lung cancer cells unresponsive to IFN-gamma. Biochem. Biophys. Res. Commun. 2015, 466, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, L.; Leslie, N.R. Assays to Measure PTEN Lipid Phosphatase Activity In Vitro from Purified Enzyme or Immunoprecipitates. Methods Mol. Biol. 2016, 1447, 95–105. [Google Scholar]
- Yao, L.; Shi, W.; Gu, J. Micro-RNA 205-5p is Involved in the Progression of Gastric Cancer and Targets Phosphatase and Tensin Homolog (PTEN) in SGC-7901 Human Gastric Cancer Cells. Med. Sci. Monit. 2019, 25, 6367–6377. [Google Scholar] [CrossRef]
- Revathidevi, S.; Munirajan, A.K. Akt in cancer: Mediator and more. Semin. Cancer Biol. 2019, 59, 80–91. [Google Scholar] [CrossRef]
- Song, M.S.; Salmena, L.; Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor. Nat. Rev. Mol. Cell Biol. 2012, 13, 283–296. [Google Scholar] [CrossRef]
- Robey, R.B.; Hay, N. Is Akt the “Warburg kinase”?-Akt-energy metabolism interactions and oncogenesis. Semin. Cancer Biol. 2009, 19, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Fu, X.L.; Wang, J.J.; Guan, R.; Sun, Y.; To Tony, S.S. Inhibition of glycolytic metabolism in glioblastoma cells by Pt3glc combinated with PI3K inhibitor via SIRT3-mediated mitochondrial and PI3K/Akt-MAPK pathway. J. Cell. Physiol. 2019, 234, 5888–5903. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Liu, R.; Li, J.; Zhang, C.; Wang, Y.; Cai, Q.; Qian, X.; Xia, Y.; Zheng, Y.; Piao, Y.; et al. Stabilization of phosphofructokinase 1 platelet isoform by AKT promotes tumorigenesis. Nat. Commun. 2017, 8, 949. [Google Scholar] [CrossRef] [PubMed]
- Beg, M.; Abdullah, N.; Thowfeik, F.S.; Altorki, N.K.; McGraw, T.E. Distinct Akt phosphorylation states are required for insulin regulated Glut4 and Glut1-mediated glucose uptake. eLife 2017, 6, e26896. [Google Scholar] [CrossRef]
- Mossmann, D.; Park, S.; Hall, M.N. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat. Rev. Cancer 2018, 18, 744–757. [Google Scholar] [CrossRef]
- Kelsey, I.; Manning, B.D. mTORC1 status dictates tumor response to targeted therapeutics. Sci. Signal. 2013, 6, pe31. [Google Scholar] [CrossRef]
- Gharibi, B.; Ghuman, M.; Hughes, F.J. DDIT4 regulates mesenchymal stem cell fate by mediating between HIF1α and mTOR signalling. Sci. Rep. 2016, 6, 36889. [Google Scholar] [CrossRef]
- Düvel, K.; Yecies, J.L.; Menon, S.; Raman, P.; Lipovsky, A.I.; Souza, A.L.; Triantafellow, E.; Ma, Q.; Gorski, R.; Cleaver, S.; et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 2010, 39, 171–183. [Google Scholar] [CrossRef]
- Ben-Sahra, I.; Howell, J.J.; Asara, J.M.; Manning, B.D. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science 2013, 339, 1323–1328. [Google Scholar] [CrossRef]
- Qian, X.; Li, X.; Shi, Z.; Xia, Y.; Cai, Q.; Xu, D.; Tan, L.; Du, L.; Zheng, Y.; Zhao, D.; et al. PTEN Suppresses Glycolysis by Dephosphorylating and Inhibiting Autophosphorylated PGK1. Mol. Cell. 2019, 76, 516–527.e7. [Google Scholar] [CrossRef]
- Chalhoub, N.; Baker, S.J. PTEN and the PI3-Kinase Pathway in Cancer. Annu. Rev. Pathol. 2009, 4, 127–150. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cao, I.; Song, M.S.; Hobbs, R.M.; Laurent, G.; Giorgi, C.; de Boer, V.C.; Anastasiou, D.; Ito, K.; Sasaki, A.T.; Rameh, L.; et al. Systemic elevation of PTEN induces a tumor-suppressive metabolic state. Cell 2012, 149, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Guido, C.; De Amicis, F.; Sisci, D.; Cione, E.; Vincenza, D.; Donà, A.; Panno, M.L.; Aquila, S. BergaPTEN induces metabolic reprogramming in breast cancer cells. Oncol. Rep. 2016, 35, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Unterlass, J.E.; Curtin, N.J. Warburg and Krebs and related effects in cancer. Expert Rev. Mol. Med. 2019, 21, e4. [Google Scholar] [CrossRef] [PubMed]
- Lyssiotis, C.A.; Nagrath, D. Metabolic Reprogramming and Vulnerabilities in Cancer. Cancers 2019, 12, 90. [Google Scholar] [CrossRef]
- Phadngam, S.; Castiglioni, A.; Ferraresi, A.; Morani, F.; Follo, C.; Isidoro, C. PTEN dephosphorylates AKT to prevent the expression of GLUT1 on plasmamembrane and to limit glucose consumption in cancer cells. Oncotarget 2016, 7, 84999–85020. [Google Scholar] [CrossRef]
- Marin-Hernández, A.; Gallardo-Pérez, J.C.; Rodríguez-Enríquez, S.; Encalada, R.; Moreno-Sánchez, R.; Soovedra, E. Modeling cancer glycolysis. Biochim. Biophys. Acta 2011, 1807, 755–767. [Google Scholar] [CrossRef]
- Tramontano, D.; De Amicis, F. Is the secret for a successful aging to keep track of cancer pathways? J. Cell. Physiol. 2018, 233, 8467–8476. [Google Scholar] [CrossRef]
- Talesa, V.N.; Ferri, I.; Bellezza, G.; Love, H.D.; Sidoni, A.; Antognelli, C. Glyoxalase 2 Is Involved in Human Prostate Cancer Progression as Part of a Mechanism Driven by PTEN/PI3K/AKT/mTOR Signaling with Involvement of PKM2 and ERα. Prostate 2017, 77, 196–210. [Google Scholar] [CrossRef]
- Ortega-Molina, A.; Serrano, M. PTEN in cancer, metabolism, and aging. TEM 2012, 24, 184–189. [Google Scholar] [CrossRef]
- Qiao, X.; Kim, D.I.; Jun, H.; Ma, Y.; Knights, A.J.; Park, M.J.; Zhu, K.; Lipinski, J.H.; Liao, J.; Li, Y.; et al. Protein Arginine Methyltransferase 1 Interacts with PGC1α and Modulates Thermogenic Fat Activation. Endocrinology 2019, 160, 2773–2786. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiong, H.; Wu, F.; Zhang, Y.; Wang, J.; Zhao, L.; Guo, X.; Chang, L.J.; Zhang, Y.; You, M.J.; et al. Hexokinase 2-mediated Warburg effect is required for PTEN- and p53-deficiency-driven prostate cancer growth. Cell Rep. 2014, 8, 1461–1474. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Liu, H. TRIM59 knockdown blocks cisplatin resistance in A549/DDP cells through regulating PTEN/AKT/HK2. Gene 2020, 747, 144553. [Google Scholar] [CrossRef] [PubMed]
- Klarer, A.C.; O’Neal, J.; Imbert-Fernandez, Y.; Clem, A.; Ellis, S.R.; Clark, J.; Clem, B.; Chesney, J.; Telang, S. Inhibition of 6-phosphofructo-2-kinase (PFKFB3) induces autophagy as a survival mechanism. Cancer Metab. 2014, 2, 2. [Google Scholar] [CrossRef]
- Herranz, D.; Ambesi-Impiombato, A.; Sudderth, J.; Sánchez-Martín, M.; Belver, L.; Tosello, V.; Xu, L.; Wendorff, A.A.; Castillo, M.; Haydu, J.E.; et al. Metabolic reprogramming induces resistance to anti-NOTCH1 therapies in T cell acute lymphoblastic leukemia. Nat. Med. 2015, 21, 1182–1189. [Google Scholar] [CrossRef]
- Qu, X.; Sun, J.; Zhang, Y.; Li, J.; Hu, J.; Li, K.; Gao, L.; Shen, L. c-Myc-driven glycolysis via TXNIP suppression is dependent on glutaminase-MondoA axis in prostate cancer. Biochem. Biophys. Res. Commun. 2018, 504, 415–421. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, C.; Wu, R.; Sun, Y.; Levine, A.; Feng, Z. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc. Natl. Acad. Sci. USA 2010, 107, 7455–7460. [Google Scholar] [CrossRef]
- Suzuki, S.; Tanaka, T.; Poyurovsky, M.V.; Nagano, H.; Mayama, T.; Ohkubo, S.; Lokshin, M.; Hosokawa, H.; Nakayama, T.; Suzuki, Y.; et al. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proc. Natl. Acad. Sci. USA 2010, 107, 7461–7466. [Google Scholar] [CrossRef]
- Wei, Z.; Cui, L.; Mei, Z.; Liu, M.; Zhang, D. miR-181a mediates metabolic shift in colon cancer cells via the PTEN/AKT pathway. FEBS Lett. 2014, 588, 1773–1779. [Google Scholar] [CrossRef]
- Wang, K.C.; Liu, Y.C.; El-Shazly, M.; Shih, S.P.; Du, Y.C.; Hsu, Y.; Lin, H.Y.; Chen, Y.C.; Wu, Y.C.; Yang, S.C.; et al. The Antioxidant from Ethanolic Extract of Rosa cymosa Fruits Activates Phosphatase and Tensin Homolog in Vitro and in Vivo: A New Insight on Its Antileukemic Effect. Int. J. Mol. Sci. 2019, 20, 1935. [Google Scholar] [CrossRef]
- Wen, X.; Klionsky, D.J. At a glance: A history of autophagy and cancer. Semin. Cancer Biol. 2019, S1044-579X(19)30167-1. [Google Scholar] [CrossRef] [PubMed]
- Conte, A.; Kisslinger, A.; Procaccini, C.; Paladino, S.; Oliviero, O.; de Amicis, F.; Faicchia, D.; Fasano, D.; Caputo, M.; Matarese, G.; et al. Convergent Effects of Resveratrol and PYK2 on Prostate Cells. Int. J. Mol. Sci. 2016, 17, 1542. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Yu, J.; Bhagat, G.; Furuya, N.; Hibshoosh, H.; Troxel, A.; Rosen, J.; Eskelinen, E.L.; Mizushima, N.; Ohsumi, Y.; et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J. Clin. Investig. 2003, 112, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Lock, R.; Roy, S.; Kenific, C.M.; Su, J.S.; Salas, E.; Ronen, S.M.; Debnath, J. Autophagy facilitates glycolysis during Ras-mediated oncogenic transformation. Mol. Biol. Cell 2011, 22, 165–178. [Google Scholar] [CrossRef]
- Rosenfeldt, M.T.; O’Prey, J.; Morton, J.P.; Nixon, C.; MacKay, G.; Mrowinska, A.; Au, A.; Rai, T.S.; Zheng, L.; Ridgway, R.; et al. p53 status determines the role of autophagy in pancreatic tumour development. Nature 2013, 504, 296–300. [Google Scholar] [CrossRef]
- Yang, A.; Rajeshkumar, N.V.; Wang, X.; Yabuuchi, S.; Alexander, B.M.; Chu, G.C.; Von Hoff, D.D.; Maitra, A.; Kimmelman, A.C. Autophagy is critical for pancreatic tumor growth and progression in tumors with p53 alterations. Cancer Discov. 2014, 4, 905–913. [Google Scholar] [CrossRef]
- Zhu, L.; Li, L.; Zhang, Q.; Yang, X.; Zou, Z.; Hao, B.; Marincola, F.M.; Liu, Z.; Zhong, Z.; Wang, M.; et al. NOS1 S-nitrosylates PTEN and inhibits autophagy in nasopharyngeal carcinoma cells. Cell Death Discov. 2017, 3, 17011. [Google Scholar] [CrossRef]
- Cai, J.; Li, R.; Xu, X.; Zhang, L.; Lian, R.; Fang, L.; Huang, Y.; Feng, X.; Liu, X.; Li, X.; et al. CK1α suppresses lung tumour growth by stabilizing PTEN and inducing autophagy. Nat. Cell Biol. 2018, 20, 465–478. [Google Scholar] [CrossRef]
- Choi, J.P.; Zheng, Y.; Handelsman, D.J.; Simanainen, U. Glandular epithelial AR inactivation enhances PTEN deletion-induced uterine pathology. Endocr. Relat. Cancer 2016, 23, 377–390. [Google Scholar] [CrossRef][Green Version]
- Han, B.; Dong, Z.; Liu, Y.; Chen, Q.; Hashimoto, K.; Zhang, J.-T. Regulation of constitutive expression of mouse PTEN by the 5′-untranslated region. Oncogene 2003, 22, 5325–5337. [Google Scholar] [CrossRef][Green Version]
- De Amicis, F.; Aquila, S.; Morelli, C.; Guido, C.; Santoro, M.; Perrotta, I.; Mauro, L.; Giordano, F.; Nigro, A.; Andò, S.; et al. Bergaptendrives autophagy through the up-regulation of PTEN expression in breast cancer cells. Mol. Cancer 2015, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Guido, C.; Panza, S.; Santoro, M.; Avena, P.; Panno, M.L.; Perrotta, I.; Giordano, F.; Casaburi, I.; Catalano, S.; De Amicis, F.; et al. Estrogen receptor beta (ERβ) produces autophagy and necroptosis in human seminoma cell line through the binding of the Sp1 on the phosphatase and tensin homolog deleted from chromosome 10 (PTEN) promoter gene. Cell Cycle 2012, 11, 2911–2921. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; An, J.; Yang, Y.; Wu, D.; Bai, Y.; Cao, W.; Ma, L.; Chen, J.; Yu, Z.; He, Y.; et al. Dual inhibition of AKT-mTOR and AR signaling by targeting HDAC3 in PTEN- or SPOP-mutated prostate cancer. EMBO Mol. Med. 2018, 10, e8478. [Google Scholar] [CrossRef] [PubMed]
- Teresi, R.E.; Waite, K.A. PPARγ, PTEN, and the Fight against Cancer. PPAR Res. 2008, 2008, 932632. [Google Scholar] [CrossRef]
- Rovito, D.; Giordano, C.; Vizza, D.; Plastina, P.; Barone, I.; Casaburi, I.; Lanzino, M.; De Amicis, F.; Sisci, D.; Mauro, L.; et al. Omega-3 PUFA ethanolamides DHEA and EPEA induce autophagy through PPARγ activation in MCF-7 breast cancer cells. J. Cell. Physiol. 2013, 228, 1314–1322. [Google Scholar] [CrossRef]
- Montalto, F.I.; Giordano, F.; Chiodo, C.; Marsico, S.; Mauro, L.; Sisci, D.; Aquila, S.; Lanzino, M.; Panno, M.L.; Andò, S.; et al. Progesterone Receptor B signaling Reduces Breast Cancer Cell Aggressiveness: Role of Cyclin-D1/Cdk4 Mediating Paxillin Phosphorylation. Cancers 2019, 11, 1201. [Google Scholar] [CrossRef]
- De Amicis, F.; Lanzino, M.; Kisslinger, A.; Calì, G.; Chieffi, P.; Andò, S.; Mancini, F.P.; Tramontano, D. Loss of proline-rich tyrosine kinase 2 function induces spreading and motility of epithelial prostate cells. J. Cell. Physiol. 2006, 209, 74–80. [Google Scholar] [CrossRef]
- Wang, M.; Wu, H.; Li, S.; Xu, Z.; Li, X.; Yang, Y.; Li, B.; Li, Y.; Guo, J.; Chen, H. SYNJ2BP promotes the degradation of PTEN through the lysosome-pathway and enhances breast tumor metastasis via PI3K/AKT/SNAI1 signaling. Oncotarget 2017, 8, 89692–89706. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.J.; Meng, T.; Lei, C.; Yang, X.; Wang, Q.; Jin, B.; Zhu, J.-F. lncRNA GAS5 Inhibits Cell Migration and Invasion and Promotes Autophagy by Targeting miR-222-3p via the GAS5/PTEN-Signaling Pathway in CRC. Mol. Ther Nucleic Acids 2019, 17, 644–656. [Google Scholar] [CrossRef]
- Jang, Y.J.; Kim, J.H.; Byun, S. Modulation of Autophagy for Controlling Immunity. Cells 2019, 8, 138. [Google Scholar] [CrossRef]
- Hagerling, C.; Casbon, A.J.; Werb, Z. Balancing the innate immune system in tumor development. Trends Cell Biol. 2015, 25, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Monkkonen, T.; Debnath, J. Inflammatory signaling cascades and autophagy in cancer. Autophagy 2018, 14, 190–198. [Google Scholar] [CrossRef]
- Pan, H.; Chen, L.; Xu, Y.; Han, W.; Lou, F.; Fei, W.; Liu, S.; Jing, Z.; Sui, X. Autophagy-associated immune responses and cancer immunotherapy. Oncotarget 2016, 7, 21235–21246. [Google Scholar] [CrossRef] [PubMed]
- Shibutani, S.T.; Saitoh, T.; Nowag, H.; Münz, C.; Yoshimori, T. Autophagy and autophagy-related proteins in the immune system. Nat. Immunol. 2015, 16, 1014–1024. [Google Scholar] [CrossRef]
- Wurz, G.T.; Kao, C.J.; DeGregorio, M.W. Novel cancer antigens for personalized immunotherapies: Latest evidence and clinical potential. Ther. Adv. Med. Oncol. 2016, 8, 4–31. [Google Scholar] [CrossRef]
- Jiang, G.M.; Tan, Y.; Wang, H.; Peng, L.; Chen, H.T.; Meng, X.J.; Li, L.L.; Liu, Y.; Li, W.F.; Shan, H. The relationship between autophagy and the immune system and its applications for tumor immunotherapy. Mol. Cancer 2019, 18, 17. [Google Scholar] [CrossRef]
- Dumitru, C.A.; Moses, K.; Trellakis, S.; Lang, S.; Brandau, S. Neutrophils and granulocytic myeloid-derived suppressor cells: Immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol. Immunother. 2012, 61, 1155–1167. [Google Scholar] [CrossRef]
- Xu, C.; Fillmore, C.M.; Koyama, S.; Wu, H.; Zhao, Y.; Chen, Z.; Herter-Sprie, G.S.; Akbay, E.A.; Tchaicha, J.H.; Altabef, A.; et al. Loss of Lkb1 and PTEN leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer Cell 2014, 25, 590–604. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chong, W.; Teng, C.; Yao, Y.; Wang, X.; Li, X. The immune response-related mutational signatures and driver genes in non-small-cell lung cancer. Cancer Sci. 2019, 110, 2348–2356. [Google Scholar] [CrossRef]
- Wartewig, T.; Kurgyis, Z.; Keppler, S.; Pechloff, K.; Hameister, E.; Ollinger, R.; Maresch, R.; Buch, T.; Steiger, K.; Winter, C.; et al. PD-1 is a haploinsufficient suppressor of T cell lymphomagenesis. Nature 2017, 552, 121–125. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, A.X.; Gartrell, R.D.; Silverman, A.M.; Aparicio, L.; Chu, T.; Bordbar, D.; Shan, D.; Samanamud, J.; Mahajan, A.; et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat. Med. 2019, 25, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Cretella, D.; Digiacomo, G.; Giovannetti, E.; Cavazzoni, A. PTEN Alterations as a Potential Mechanism for Tumor Cell Escape from PD-1/PD-L1 Inhibition. Cancers 2019, 11, 1318. [Google Scholar] [CrossRef] [PubMed]
- Sahin, E.; Haubenwallner, S.; Kuttke, M.; Kollmann, I.; Halfmann, A.; Dohnal, A.M.; Chen, L.; Cheng, P.; Hoesel, B.; Einwallner, E.; et al. Macrophage PTEN regulates expression and secretion of arginase I modulating innate and adaptive immune responses. J. Immunol. 2014, 193, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.A.; Singla, D.K. PTEN Inhibitor VO-OHpic Attenuates Inflammatory M1 Macrophages and Cardiac Remodeling in Doxorubicin-Induced Cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2018, 15, H1236–H1249. [Google Scholar] [CrossRef]
- Junttila, M.R.; de Sauvage, F.J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 2013, 501, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Zamarron, B.F.; Chen, W. Dual roles of immune cells and their factors in cancer development and progression. Int. J. Biol. Sci. 2011, 7, 651–658. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef]
- Martinez-Outschoorn, U.E.; Lisanti, M.P.; Sotgia, F. Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Semin. Cancer Biol. 2014, 25, 47–60. [Google Scholar] [CrossRef]
- Thies, K.A.; Lefler, J.E.; Leone, G.; Ostrowski, M.C. PTEN in the Stroma. Cold Spring Harb. Perspect. Med. 2019, 9, a036111. [Google Scholar] [CrossRef]
- Ying, H.; Qu, D.; Liu, C.; Ying, T.; Lv, J.; Jin, S.; Xu, H. Chemoresistance is associated with Beclin-1 and PTEN expression in epithelial ovarian cancers. Oncol. Lett. 2015, 9, 1759–1763. [Google Scholar] [CrossRef]
- Toso, A.; Revandkar, A.; Di Mitri, D.; Guccini, I.; Proietti, M.; Sarti, M.; Pinton, S.; Zhang, J.; Kalathur, M.; Civenni, G.; et al. Enhancing chemotherapy efficacy in PTEN-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Rep. 2014, 9, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Han, J.; Zhuang, L.; Li, S.; Gong, Q.; Chen, Y. Serum starvation induces cell death in NSCLC via miR-224. Onco Targets Ther. 2019, 2019, 3955–3964. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Chen, X.; Bao, J.; Xu, L.; Zhang, L.; Huang, C.; Meng, X.M.; Li, J. PTEN negatively regulates the expression of pro-inflammatory cytokines and chemokines of fibroblast-like synoviocytes in adjuvant-induced arthritis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3687–3696. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Qin, J.; Lan, L.; Zhang, H.; Liu, F.; Wu, Z.; Ni, H.; Wang, Y. PTEN inhibits macrophage polarization from M1 to M2 through CCL2 and VEGF-A reduction and NHERF-1 synergism. Cancer Biol. Ther. 2015, 16, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Montfort, A.; Owen, S.; Piskorz, A.M.; Supernat, A.; Moore, L.; Al-Khalidi, S.; Böhm, S.; Pharoah, P.; McDermott, J.; Balkwill, F.R.; et al. Combining measures of immune infiltration shows additive effect on survival prediction in high-grade serous ovarian carcinoma. Br. J. Cancer 2020, 122, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).