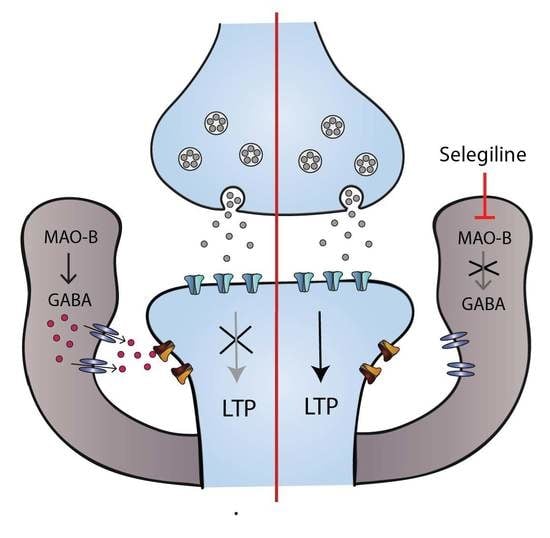
Blocking Astrocytic GABA Restores Synaptic Plasticity in Prefrontal Cortex of Rat Model of Depression
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Recordings
2.2. Patch-Clamp Recordings
2.3. Immunohistochemistry
3. Results
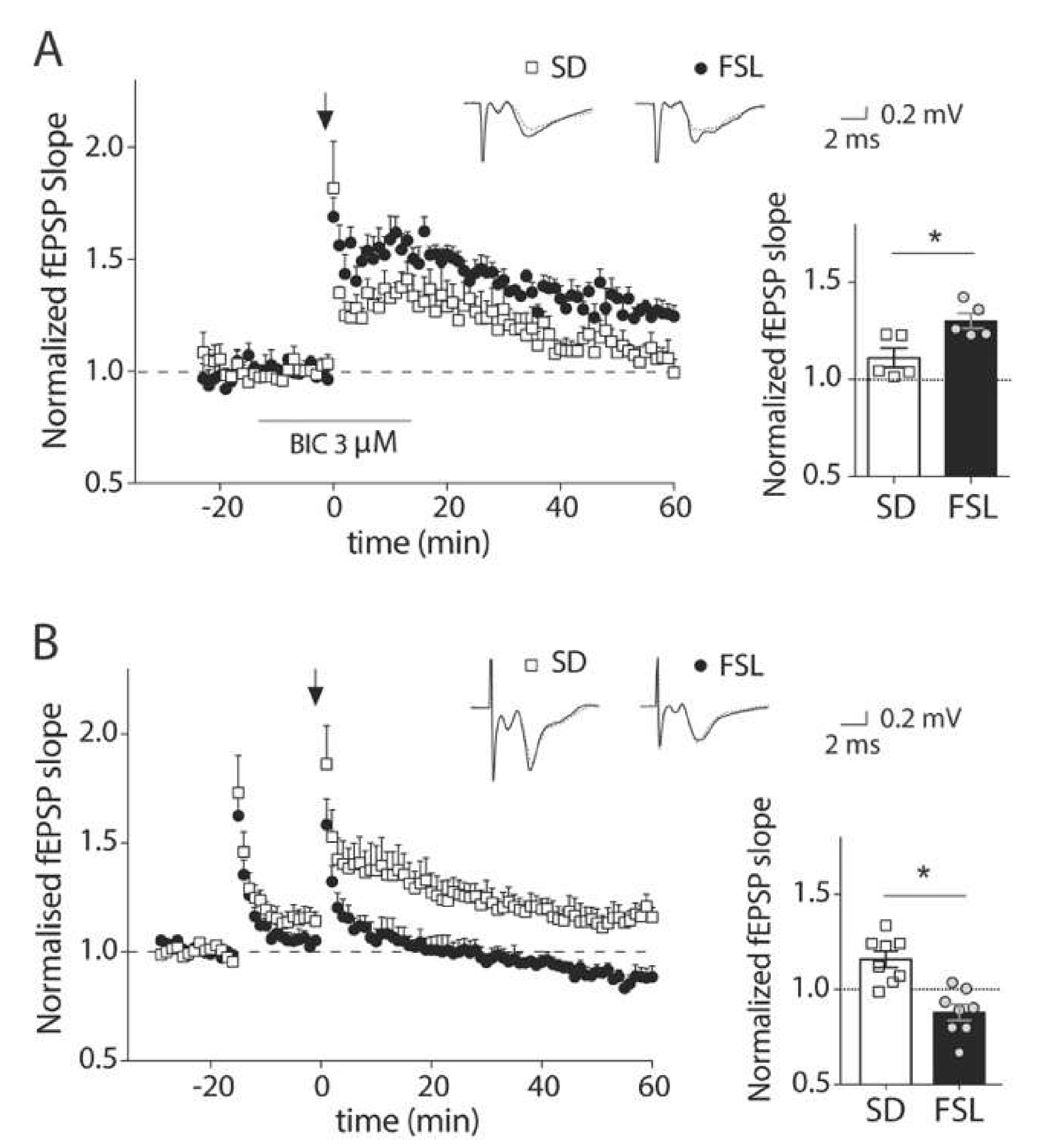
3.1. GABAA Receptor Blockage During LTP Induction Unmasks a Dysfunction of Inhibition in the Prefrontal Cortex of FSL Rats
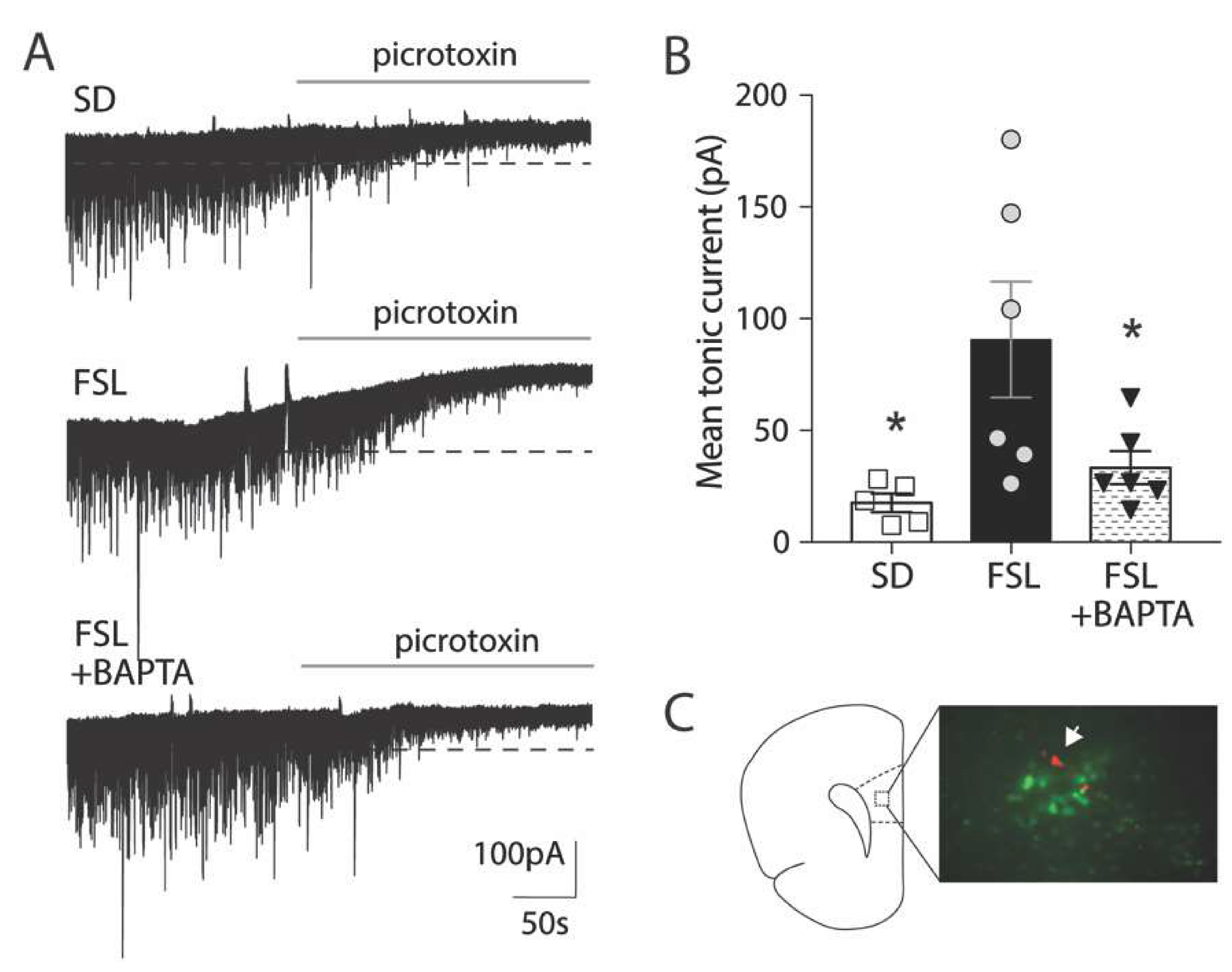
3.2. Astrocytes in the FSL Rat are Atrophic and Contain Higher Levels of GABA
3.3. Astrocytes Mediate Increased Tonic GABA Inhibition in FSL Rats Compared to SD
3.4. Impaired LTP in the FSL Rat is Restored by Pretreatment with Selegiline
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Biselli, T.; Lange, S.S.; Sablottny, L.; Steffen, J.; Walther, A. Optogenetic and chemogenetic insights into the neurocircuitry of depression-like behaviour: A systematic review. Eur. J. Neurosci. 2019. [Google Scholar] [CrossRef]
- Femenia, T.; Gomez-Galan, M.; Lindskog, M.; Magara, S. Dysfunctional hippocampal activity affects emotion and cognition in mood disorders. Brain Res. 2012, 1476, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Belleau, E.L.; Treadway, M.T.; Pizzagalli, D.A. The Impact of Stress and Major Depressive Disorder on Hippocampal and Medial Prefrontal Cortex Morphology. Biol. Psychiatry 2019, 85, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, L.; Silva, C.; Cheniaux, E.; Telles-Correia, D. Neuroimaging Correlates of Depression-Implications to Clinical Practice. Front. Psychiatry 2019, 10, 703. [Google Scholar] [CrossRef]
- Price, J.L.; Drevets, W.C. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn. Sci. 2012, 16, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Brunoni, A.R.; Chaimani, A.; Moffa, A.H.; Razza, L.B.; Gattaz, W.F.; Daskalakis, Z.J.; Carvalho, A.F. Repetitive Transcranial Magnetic Stimulation for the Acute Treatment of Major Depressive Episodes: A Systematic Review With Network Meta-analysis. JAMA Psychiatry 2017, 74, 143–152. [Google Scholar] [CrossRef]
- Covington, H.E., 3rd; Lobo, M.K.; Maze, I.; Vialou, V.; Hyman, J.M.; Zaman, S.; LaPlant, Q.; Mouzon, E.; Ghose, S.; Tamminga, C.A.; et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J. Neurosci. 2010, 30, 16082–16090. [Google Scholar] [CrossRef]
- Page, C.E.; Coutellier, L. Prefrontal excitatory/inhibitory balance in stress and emotional disorders: Evidence for over-inhibition. Neurosci. Biobehav. Rev. 2019, 105, 39–51. [Google Scholar] [CrossRef]
- Voineskos, D.; Blumberger, D.M.; Zomorrodi, R.; Rogasch, N.C.; Farzan, F.; Foussias, G.; Rajji, T.K.; Daskalakis, Z.J. Altered Transcranial Magnetic Stimulation-Electroencephalographic Markers of Inhibition and Excitation in the Dorsolateral Prefrontal Cortex in Major Depressive Disorder. Biol. Psychiatry 2019, 85, 477–486. [Google Scholar] [CrossRef]
- Bai, T.; Wei, Q.; Zu, M.; Xie, W.; Wang, J.; Gong-Jun, J.; Yu, F.; Tian, Y.; Wang, K. Functional plasticity of the dorsomedial prefrontal cortex in depression reorganized by electroconvulsive therapy: Validation in two independent samples. Hum. Brain Mapp. 2019, 40, 465–473. [Google Scholar] [CrossRef]
- Gomez-Galan, M.; De Bundel, D.; Van Eeckhaut, A.; Smolders, I.; Lindskog, M. Dysfunctional astrocytic regulation of glutamate transmission in a rat model of depression. Mol. Psychiatry 2013, 18, 582–594. [Google Scholar] [CrossRef]
- Liu, W.; Ge, T.; Leng, Y.; Pan, Z.; Fan, J.; Yang, W.; Cui, R. The Role of Neural Plasticity in Depression: From Hippocampus to Prefrontal Cortex. Neural Plast. 2017, 2017, 6871089. [Google Scholar] [CrossRef] [PubMed]
- Segev, A.; Rubin, A.S.; Abush, H.; Richter-Levin, G.; Akirav, I. Cannabinoid receptor activation prevents the effects of chronic mild stress on emotional learning and LTP in a rat model of depression. Neuropsychopharmacology 2014, 39, 919–933. [Google Scholar] [CrossRef]
- Sousa, V.C.; Vital, J.; Costenla, A.R.; Batalha, V.L.; Sebastiao, A.M.; Ribeiro, J.A.; Lopes, L.V. Maternal separation impairs long term-potentiation in CA1-CA3 synapses and hippocampal-dependent memory in old rats. Neurobiol. Aging 2014, 35, 1680–1685. [Google Scholar] [CrossRef]
- Bjorkholm, C.; Monteggia, L.M. BDNF - a key transducer of antidepressant effects. Neuropharmacology 2016, 102, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Galan, M.; Femenia, T.; Aberg, E.; Graae, L.; Van Eeckhaut, A.; Smolders, I.; Brene, S.; Lindskog, M. Running Opposes the Effects of Social Isolation on Synaptic Plasticity and Transmission in a Rat Model of Depression. PLoS ONE 2016, 11, e0165071. [Google Scholar] [CrossRef]
- Moda-Sava, R.N.; Murdock, M.H.; Parekh, P.K.; Fetcho, R.N.; Huang, B.S.; Huynh, T.N.; Witztum, J.; Shaver, D.C.; Rosenthal, D.L.; Alway, E.J.; et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science 2019, 364. [Google Scholar] [CrossRef]
- Nosyreva, E.; Autry, A.E.; Kavalali, E.T.; Monteggia, L.M. Age dependence of the rapid antidepressant and synaptic effects of acute NMDA receptor blockade. Front. Mol. Neurosci. 2014, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Umemori, J.; Winkel, F.; Didio, G.; Llach Pou, M.; Castren, E. iPlasticity: Induced juvenile-like plasticity in the adult brain as a mechanism of antidepressants. Psychiatry Clin. Neurosci. 2018, 72, 633–653. [Google Scholar] [CrossRef]
- Prevot, T.; Sibille, E. Altered GABA-mediated information processing and cognitive dysfunctions in depression and other brain disorders. Mol. Psychiatry 2020. [Google Scholar] [CrossRef]
- Farrant, M.; Nusser, Z. Variations on an inhibitory theme: Phasic and tonic activation of GABA(A) receptors. Nat. Rev. Neurosci. 2005, 6, 215–229. [Google Scholar] [CrossRef]
- Jo, S.; Yarishkin, O.; Hwang, Y.J.; Chun, Y.E.; Park, M.; Woo, D.H.; Bae, J.Y.; Kim, T.; Lee, J.; Chun, H.; et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat. Med. 2014, 20, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yoon, B.E.; Berglund, K.; Oh, S.J.; Park, H.; Shin, H.S.; Augustine, G.J.; Lee, C.J. Channel-mediated tonic GABA release from glia. Science 2010, 330, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.E.; Woo, J.; Chun, Y.E.; Chun, H.; Jo, S.; Bae, J.Y.; An, H.; Min, J.O.; Oh, S.J.; Han, K.S.; et al. Glial GABA, synthesized by monoamine oxidase B, mediates tonic inhibition. J. Physiol. 2014, 592, 4951–4968. [Google Scholar] [CrossRef] [PubMed]
- Cobb, J.A.; O’Neill, K.; Milner, J.; Mahajan, G.J.; Lawrence, T.J.; May, W.L.; Miguel-Hidalgo, J.; Rajkowska, G.; Stockmeier, C.A. Density of GFAP-immunoreactive astrocytes is decreased in left hippocampi in major depressive disorder. Neuroscience 2016, 316, 209–220. [Google Scholar] [CrossRef]
- Gittins, R.A.; Harrison, P.J. A morphometric study of glia and neurons in the anterior cingulate cortex in mood disorder. J. Affect. Disord. 2011, 133, 328–332. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Steardo, L.; Peng, L.; Parpura, V. Astroglia, Glutamatergic Transmission and Psychiatric Diseases. Adv. Neurobiol. 2016, 13, 307–326. [Google Scholar] [CrossRef]
- Santello, M.; Toni, N.; Volterra, A. Astrocyte function from information processing to cognition and cognitive impairment. Nat. Neurosci. 2019, 22, 154–166. [Google Scholar] [CrossRef]
- Overstreet, D.H.; Wegener, G. The flinders sensitive line rat model of depression--25 years and still producing. Pharmacol. Rev. 2013, 65, 143–155. [Google Scholar] [CrossRef]
- Soderlund, J.; Lindskog, M. Relevance of Rodent Models of Depression in Clinical Practice: Can We Overcome the Obstacles in Translational Neuropsychiatry? Int. J. Neuropsychopharmacol. 2018, 21, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Gemperle, A.; Olpe, H.R. Effects of subchronic clozapine treatment on long-term potentiation in rat prefrontal cortex. Eur. Neuropsychopharmacol. 2004, 14, 340–346. [Google Scholar] [CrossRef]
- Arima-Yoshida, F.; Watabe, A.M.; Manabe, T. The mechanisms of the strong inhibitory modulation of long-term potentiation in the rat dentate gyrus. Eur. J. Neurosci. 2011, 33, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.; Neupane, C.; Woo, J.; Sharma, R.; Nam, M.H.; Lee, G.S.; Yi, M.H.; Shin, N.; Kim, D.W.; Cho, H.; et al. Bestrophin1-mediated tonic GABA release from reactive astrocytes prevents the development of seizure-prone network in kainate-injected hippocampi. Glia 2020, 68, 1065–1080. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.E.; Jo, S.; Woo, J.; Lee, J.H.; Kim, T.; Kim, D.; Lee, C.J. The amount of astrocytic GABA positively correlates with the degree of tonic inhibition in hippocampal CA1 and cerebellum. Mol. Brain 2011, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Guo, Z.; Gearing, M.; Chen, G. Tonic inhibition in dentate gyrus impairs long-term potentiation and memory in an Alzheimer’s [corrected] disease model. Nat. Commun. 2014, 5, 4159. [Google Scholar] [CrossRef] [PubMed]
- Ekblom, J.; Jossan, S.S.; Bergstrom, M.; Oreland, L.; Walum, E.; Aquilonius, S.M. Monoamine oxidase-B in astrocytes. Glia 1993, 8, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Mallajosyula, J.K.; Kaur, D.; Chinta, S.J.; Rajagopalan, S.; Rane, A.; Nicholls, D.G.; Di Monte, D.A.; Macarthur, H.; Andersen, J.K. MAO-B elevation in mouse brain astrocytes results in Parkinson’s pathology. PLoS ONE 2008, 3, e1616. [Google Scholar] [CrossRef]
- Nakamura, S.; Kawamata, T.; Akiguchi, I.; Kameyama, M.; Nakamura, N.; Kimura, H. Expression of monoamine oxidase B activity in astrocytes of senile plaques. Acta Neuropathol. 1990, 80, 419–425. [Google Scholar] [CrossRef]
- Fowler, C.J.; Wiberg, A.; Oreland, L.; Marcusson, J.; Winblad, B. The effect of age on the activity and molecular properties of human brain monoamine oxidase. J. Neural Transm. 1980, 49, 1–20. [Google Scholar] [CrossRef]
- Kitani, K.; Minami, C.; Isobe, K.; Maehara, K.; Kanai, S.; Ivy, G.O.; Carrillo, M.C. Why (--)deprenyl prolongs survivals of experimental animals: Increase of anti-oxidant enzymes in brain and other body tissues as well as mobilization of various humoral factors may lead to systemic anti-aging effects. Mech. Ageing Dev. 2002, 123, 1087–1100. [Google Scholar] [CrossRef]
- Niittykoski, M.; Haapalinna, A.; Sirvio, J. Selegiline reduces N-methyl-D-aspartic acid induced perturbation of neurotransmission but it leaves NMDA receptor dependent long-term potentiation intact in the hippocampus. J. Neural Transm. (Vienna) 2003, 110, 1225–1240. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Simpson, E.; Kellendonk, C.; Kandel, E.R. Genetic evidence for the bidirectional modulation of synaptic plasticity in the prefrontal cortex by D1 receptors. Proc. Natl. Acad. Sci. USA 2004, 101, 3236–3241. [Google Scholar] [CrossRef] [PubMed]
- Otani, S.; Blond, O.; Desce, J.M.; Crepel, F. Dopamine facilitates long-term depression of glutamatergic transmission in rat prefrontal cortex. Neuroscience 1998, 85, 669–676. [Google Scholar] [CrossRef]
- Amsterdam, J.D.; Bodkin, J.A. Selegiline transdermal system in the prevention of relapse of major depressive disorder: A 52-week, double-blind, placebo-substitution, parallel-group clinical trial. J. Clin. Psychopharmacol. 2006, 26, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Asnis, G.M.; Henderson, M.A. EMSAM (deprenyl patch): How a promising antidepressant was underutilized. Neuropsychiatr. Dis. Treat. 2014, 10, 1911–1923. [Google Scholar] [CrossRef] [PubMed]
- Cristancho, M.A.; Thase, M.E. Critical appraisal of selegiline transdermal system for major depressive disorder. Expert Opin. Drug Deliv. 2016, 13, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Vose, L.R.; Stanton, P.K. Synaptic Plasticity, Metaplasticity and Depression. Curr. Neuropharmacol. 2017, 15, 71–86. [Google Scholar] [CrossRef]
- Simard, S.; Coppola, G.; Rudyk, C.A.; Hayley, S.; McQuaid, R.J.; Salmaso, N. Profiling changes in cortical astroglial cells following chronic stress. Neuropsychopharmacology 2018, 43, 1961–1971. [Google Scholar] [CrossRef]
- Bentea, E.; Demuyser, T.; Van Liefferinge, J.; Albertini, G.; Deneyer, L.; Nys, J.; Merckx, E.; Michotte, Y.; Sato, H.; Arckens, L.; et al. Absence of system xc- in mice decreases anxiety and depressive-like behavior without affecting sensorimotor function or spatial vision. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 59, 49–58. [Google Scholar] [CrossRef]
- Bechtholt-Gompf, A.J.; Walther, H.V.; Adams, M.A.; Carlezon, W.A., Jr.; Ongur, D.; Cohen, B.M. Blockade of astrocytic glutamate uptake in rats induces signs of anhedonia and impaired spatial memory. Neuropsychopharmacology 2010, 35, 2049–2059. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, I.; Vazquez-Juarez, E.; Henning, L.; Gómez-Galán, M.; Lindskog, M. Blocking Astrocytic GABA Restores Synaptic Plasticity in Prefrontal Cortex of Rat Model of Depression. Cells 2020, 9, 1705. https://doi.org/10.3390/cells9071705
Srivastava I, Vazquez-Juarez E, Henning L, Gómez-Galán M, Lindskog M. Blocking Astrocytic GABA Restores Synaptic Plasticity in Prefrontal Cortex of Rat Model of Depression. Cells. 2020; 9(7):1705. https://doi.org/10.3390/cells9071705
Chicago/Turabian StyleSrivastava, Ipsit, Erika Vazquez-Juarez, Lukas Henning, Marta Gómez-Galán, and Maria Lindskog. 2020. "Blocking Astrocytic GABA Restores Synaptic Plasticity in Prefrontal Cortex of Rat Model of Depression" Cells 9, no. 7: 1705. https://doi.org/10.3390/cells9071705
APA StyleSrivastava, I., Vazquez-Juarez, E., Henning, L., Gómez-Galán, M., & Lindskog, M. (2020). Blocking Astrocytic GABA Restores Synaptic Plasticity in Prefrontal Cortex of Rat Model of Depression. Cells, 9(7), 1705. https://doi.org/10.3390/cells9071705