Non-Muscle Myosin 2A (NM2A): Structure, Regulation and Function
Abstract
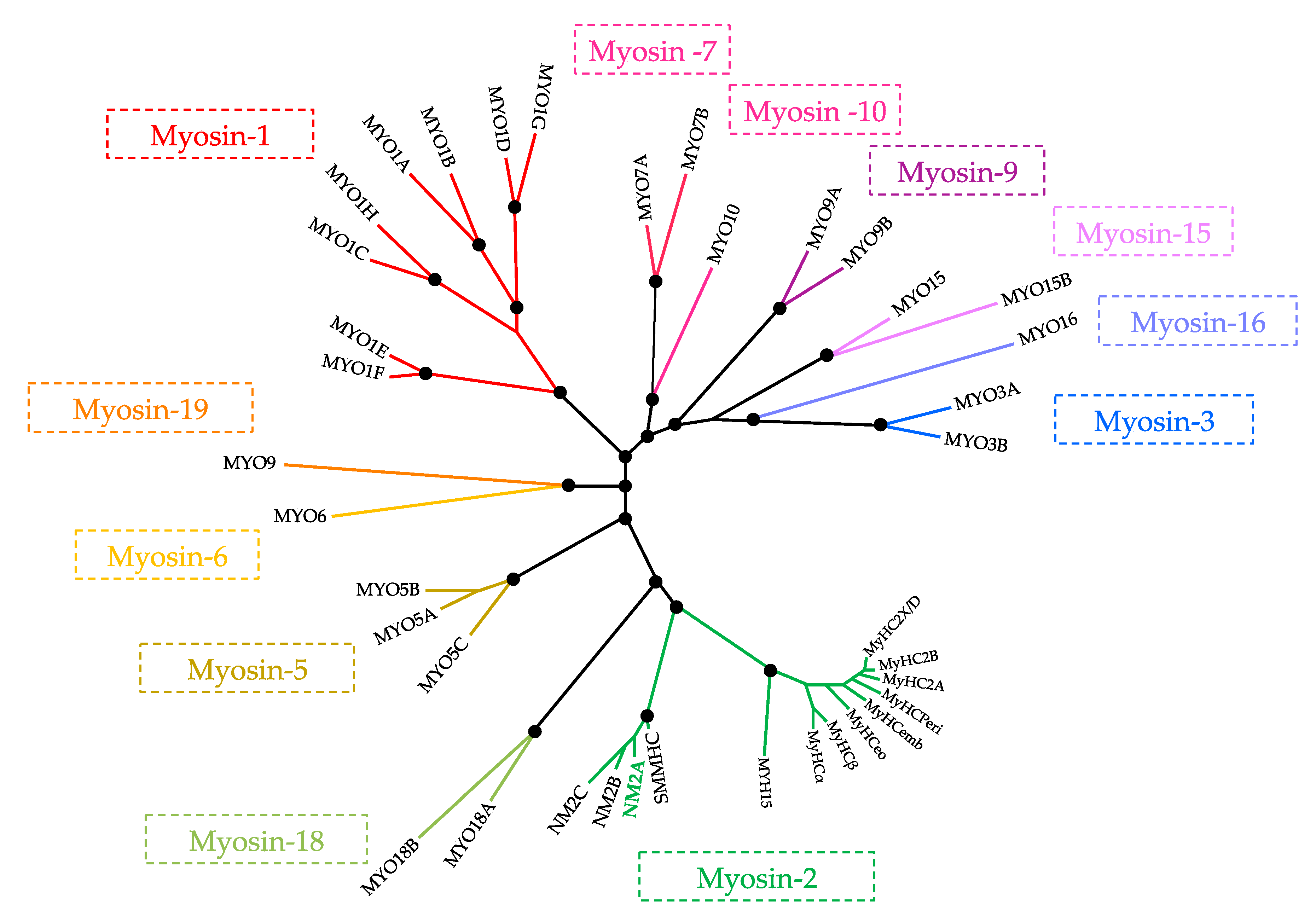
1. Superfamily of Myosins
2. Myosin-2 Subfamily
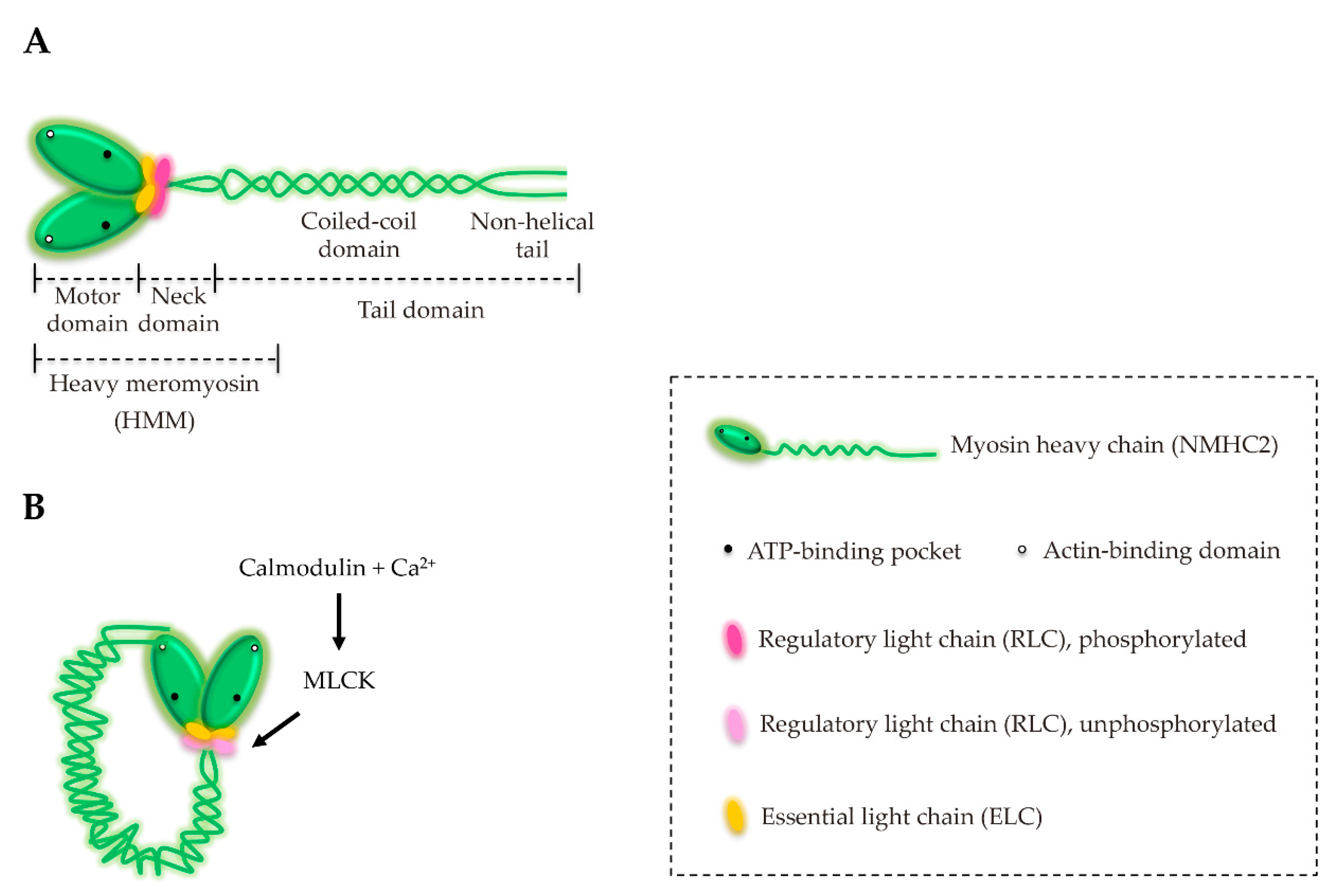
3. Structure and Characterization of NM2
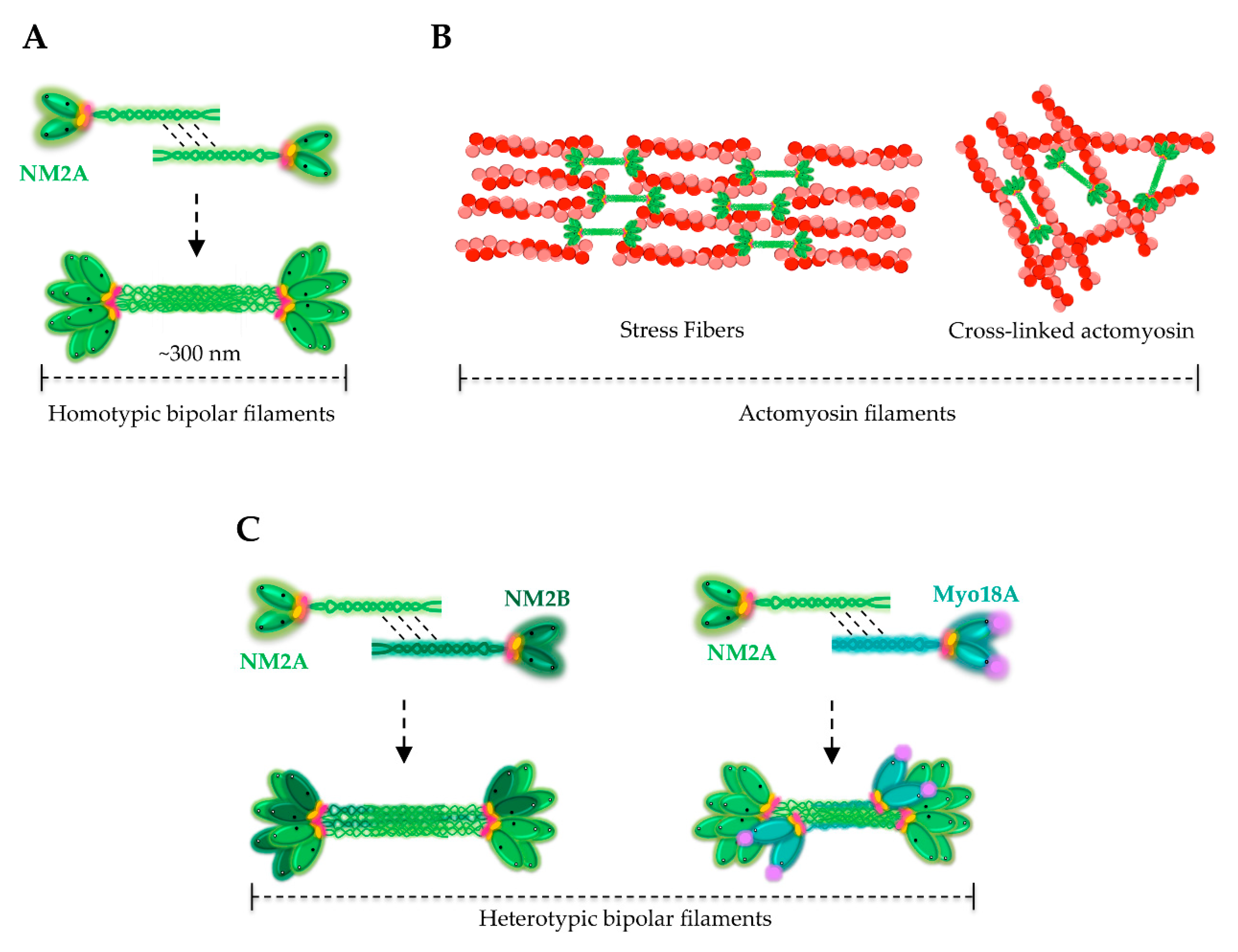
4. Assembly of NM2A Filaments
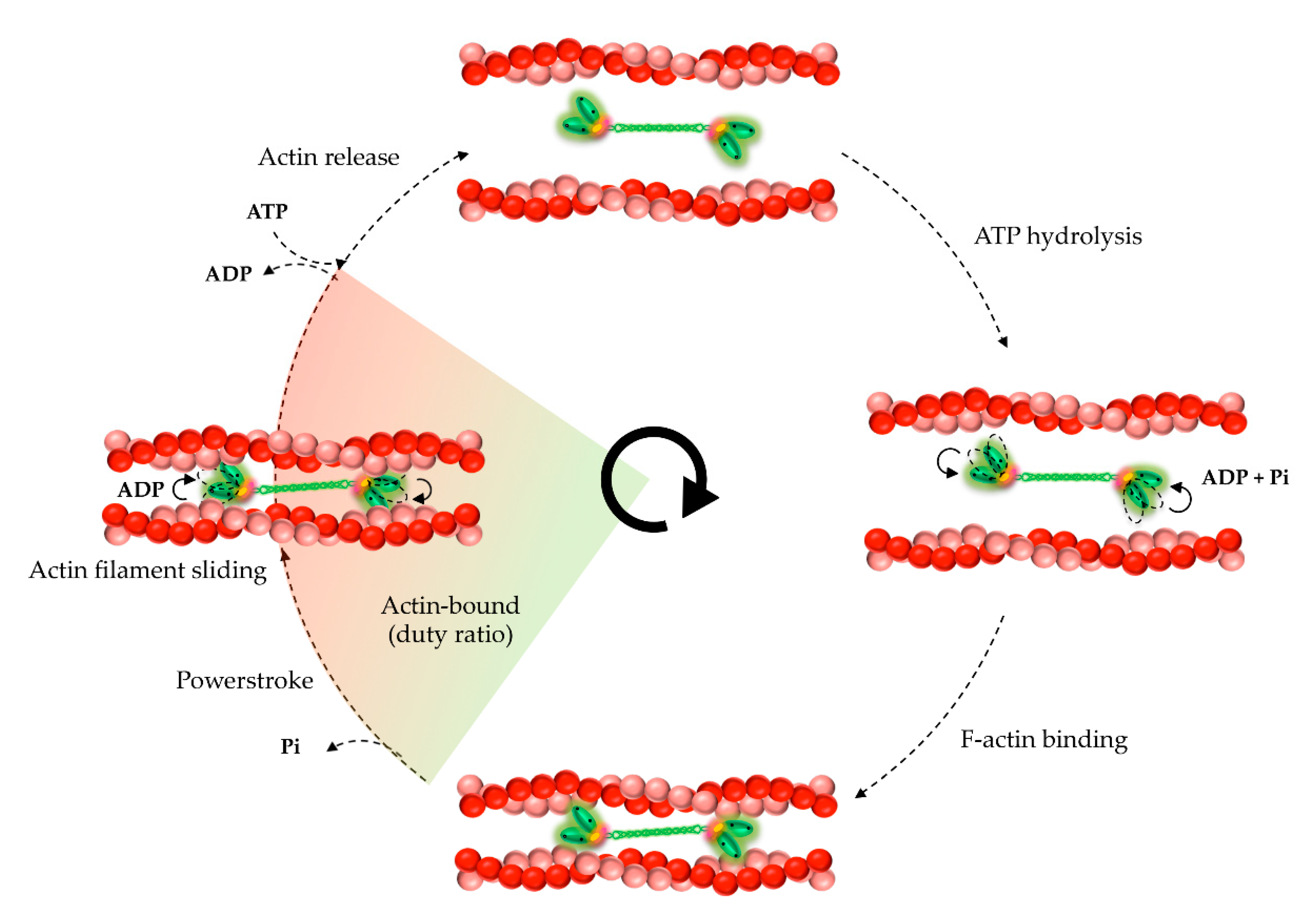
5. Kinetic and Mechanical Properties of NM2A
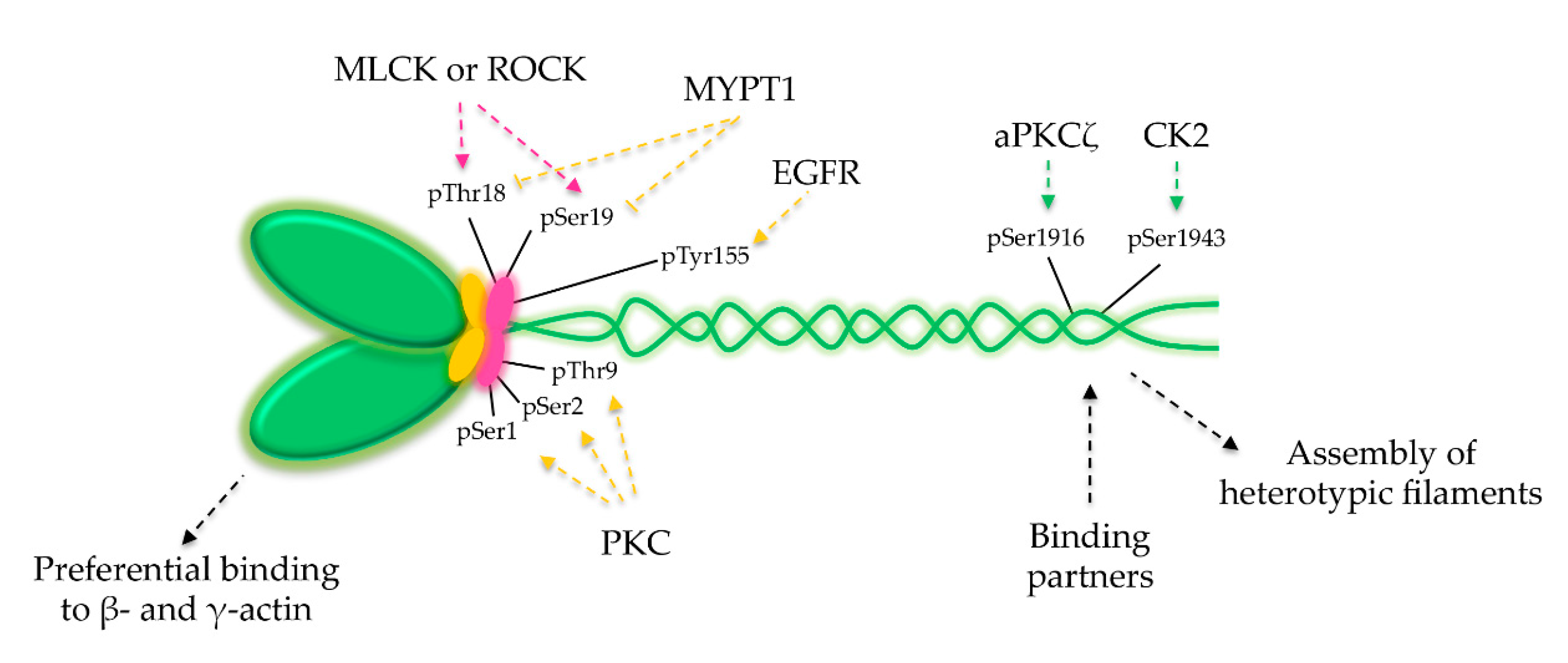
6. NM2A Regulation
7. NM2A in Development
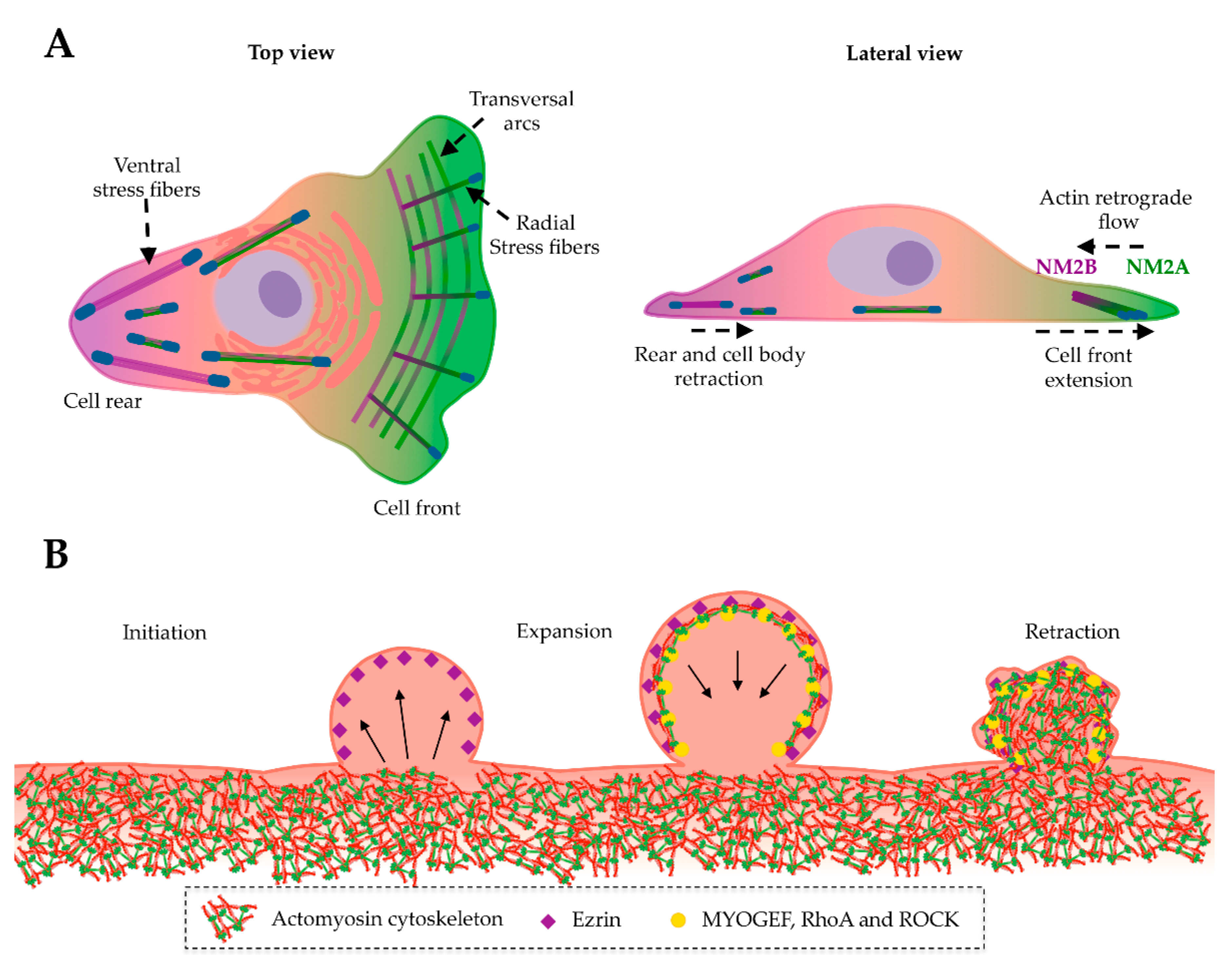
8. NM2A in Cell Division, Adhesion and Migration
9. NM2A in Membrane Blebbing
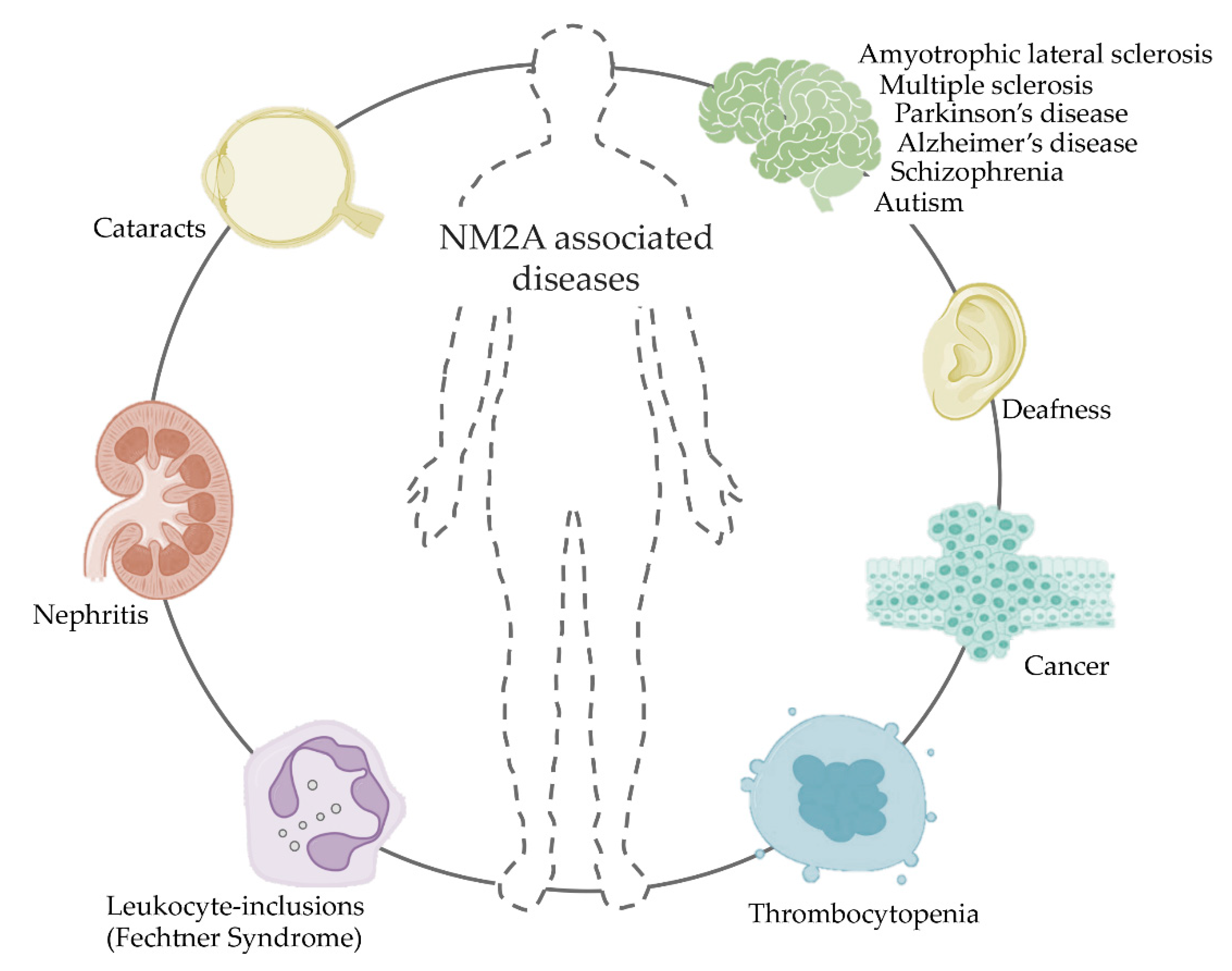
10. NM2A in Disease
11. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef]
- Ray, P.D.; Fry, R.C. Chapter 2-The Cell: The Fundamental Unit in Systems Biology. In Systems Biology in Toxicology and Environmental Health; Fry, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 11–42. [Google Scholar] [CrossRef]
- Ross, J.L.; Ali, M.Y.; Warshaw, D.M. Cargo transport: Molecular motors navigate a complex cytoskeleton. Curr. Opin. Cell Biol. 2008, 20, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, M.A.; Hyman, A.A.; Bahler, M. Motor proteins of the eukaryotic cytoskeleton. Proc. Natl. Acad. Sci. USA 1997, 94, 12747–12748. [Google Scholar] [CrossRef] [PubMed]
- Sellers, J.R. Myosins: A diverse superfamily. Biochim. Biophys. Acta 2000, 1496, 3–22. [Google Scholar] [CrossRef]
- Richards, T.A.; Cavalier-Smith, T. Myosin domain evolution and the primary divergence of eukaryotes. Nature 2005, 436, 1113–1118. [Google Scholar] [CrossRef]
- Odronitz, F.; Kollmar, M. Drawing the tree of eukaryotic life based on the analysis of 2269 manually annotated myosins from 328 species. Genome Biol. 2007, 8, R196. [Google Scholar] [CrossRef]
- Zhang, N.; Li, X.-D. Directional Transportation of Assembled Molecular Linear Motors. In Supramolecular Chemistry of Biomimetic Systems; Li, J., Ed.; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Hartman, M.A.; Spudich, J.A. The myosin superfamily at a glance. J. Cell Sci. 2012, 125, 1627–1632. [Google Scholar] [CrossRef]
- Heissler, S.M.; Sellers, J.R. Kinetic Adaptations of Myosins for Their Diverse Cellular Functions. Traffic 2016, 17, 839–859. [Google Scholar] [CrossRef]
- Masters, T.A.; Kendrick-Jones, J.; Buss, F. Myosins: Domain Organisation, Motor Properties, Physiological Roles and Cellular Functions. Handb. Exp. Pharmacol. 2017, 235, 77–122. [Google Scholar] [CrossRef]
- Vicente-Manzanares, M.; Ma, X.; Adelstein, R.S.; Horwitz, A.R. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 2009, 10, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Krendel, M.; Mooseker, M.S. Myosins: Tails (and heads) of functional diversity. Physiology (Bethesda) 2005, 20, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Loubery, S.; Coudrier, E. Myosins in the secretory pathway: Tethers or transporters? Cell Mol. Life Sci. 2008, 65, 2790–2800. [Google Scholar] [CrossRef] [PubMed]
- Breshears, L.M.; Wessels, D.; Soll, D.R.; Titus, M.A. An unconventional myosin required for cell polarization and chemotaxis. Proc. Natl. Acad. Sci. USA 2010, 107, 6918–6923. [Google Scholar] [CrossRef] [PubMed]
- Woolner, S.; Bement, W.M. Unconventional myosins acting unconventionally. Trends Cell Biol. 2009, 19, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Heissler, S.M.; Sellers, J.R. Various Themes of Myosin Regulation. J. Mol. Biol. 2016, 428, 1927–1946. [Google Scholar] [CrossRef]
- Guzik-Lendrum, S.; Heissler, S.M.; Billington, N.; Takagi, Y.; Yang, Y.; Knight, P.J.; Homsher, E.; Sellers, J.R. Mammalian myosin-18A, a highly divergent myosin. J. Biol. Chem. 2013, 288, 9532–9548. [Google Scholar] [CrossRef]
- Cao, Y.; White, H.D.; Li, X.D. Drosophila myosin-XX functions as an actin-binding protein to facilitate the interaction between Zyx102 and actin. Biochemistry 2014, 53, 350–360. [Google Scholar] [CrossRef]
- Bloemink, M.J.; Geeves, M.A. Shaking the myosin family tree: Biochemical kinetics defines four types of myosin motor. Semin. Cell Dev. Biol. 2011, 22, 961–967. [Google Scholar] [CrossRef]
- Billington, N.; Wang, A.; Mao, J.; Adelstein, R.S.; Sellers, J.R. Characterization of three full-length human nonmuscle myosin II paralogs. J. Biol. Chem. 2013, 288, 33398–33410. [Google Scholar] [CrossRef]
- Sanger, J.W.; Wang, J.; Fan, Y.; White, J.; Sanger, J.M. Assembly and dynamics of myofibrils. J. Biomed. Biotechnol. 2010, 2010, 858606. [Google Scholar] [CrossRef]
- Yuen, S.L.; Ogut, O.; Brozovich, F.V. Nonmuscle myosin is regulated during smooth muscle contraction. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H191–H199. [Google Scholar] [CrossRef] [PubMed]
- Dasbiswas, K.; Hu, S.; Schnorrer, F.; Safran, S.A.; Bershadsky, A.D. Ordering of myosin II filaments driven by mechanical forces: Experiments and theory. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.S. Myosins in yeast. Curr. Opin. Cell Biol. 1997, 9, 44–48. [Google Scholar] [CrossRef]
- Mansfield, S.G.; al-Shirawi, D.Y.; Ketchum, A.S.; Newbern, E.C.; Kiehart, D.P. Molecular organization and alternative splicing in zipper, the gene that encodes the Drosophila non-muscle myosin II heavy chain. J. Mol. Biol. 1996, 255, 98–109. [Google Scholar] [CrossRef]
- Soldati, T.; Geissler, H.; Schwarz, E.C. How many is enough? Exploring the myosin repertoire in the model eukaryote Dictyostelium discoideum. Cell Biochem. Biophys. 1999, 30, 389–411. [Google Scholar] [CrossRef]
- Golomb, E.; Ma, X.; Jana, S.S.; Preston, Y.A.; Kawamoto, S.; Shoham, N.G.; Goldin, E.; Conti, M.A.; Sellers, J.R.; Adelstein, R.S. Identification and characterization of nonmuscle myosin II-C, a new member of the myosin II family. J. Biol. Chem. 2004, 279, 2800–2808. [Google Scholar] [CrossRef]
- Simons, M.; Wang, M.; McBride, O.W.; Kawamoto, S.; Yamakawa, K.; Gdula, D.; Adelstein, R.S.; Weir, L. Human nonmuscle myosin heavy chains are encoded by two genes located on different chromosomes. Circ. Res. 1991, 69, 530–539. [Google Scholar] [CrossRef]
- Shohet, R.V.; Conti, M.A.; Kawamoto, S.; Preston, Y.A.; Brill, D.A.; Adelstein, R.S. Cloning of the cDNA encoding the myosin heavy chain of a vertebrate cellular myosin. Proc. Natl. Acad. Sci. USA 1989, 86, 7726–7730. [Google Scholar] [CrossRef]
- Katsuragawa, Y.; Yanagisawa, M.; Inoue, A.; Masaki, T. Two distinct nonmuscle myosin-heavy-chain mRNAs are differentially expressed in various chicken tissues. Identification of a novel gene family of vertebrate non-sarcomeric myosin heavy chains. Eur. J. Biochem. 1989, 184, 611–616. [Google Scholar] [CrossRef]
- Leal, A.; Endele, S.; Stengel, C.; Huehne, K.; Loetterle, J.; Barrantes, R.; Winterpacht, A.; Rautenstrauss, B. A novel myosin heavy chain gene in human chromosome 19q13.3. Gene 2003, 312, 165–171. [Google Scholar] [CrossRef]
- Toothaker, L.E.; Gonzalez, D.A.; Tung, N.; Lemons, R.S.; Le Beau, M.M.; Arnaout, M.A.; Clayton, L.K.; Tenen, D.G. Cellular myosin heavy chain in human leukocytes: Isolation of 5’ cDNA clones, characterization of the protein, chromosomal localization, and upregulation during myeloid differentiation. Blood 1991, 78, 1826–1833. [Google Scholar] [CrossRef]
- Wang, A.; Ma, X.; Conti, M.A.; Adelstein, R.S. Distinct and redundant roles of the non-muscle myosin II isoforms and functional domains. Biochem. Soc. Trans. 2011, 39, 1131–1135. [Google Scholar] [CrossRef]
- Burridge, K.; Bray, D. Purification and structural analysis of myosins from brain and other non-muscle tissues. J. Mol. Biol. 1975, 99, 1–14. [Google Scholar] [CrossRef]
- Conti, M.A.; Adelstein, R.S. Nonmuscle myosin II moves in new directions. J. Cell Sci. 2008, 121, 11–18. [Google Scholar] [CrossRef]
- Li, Y.; Lalwani, A.K.; Mhatre, A.N. Alternative splice variants of MYH9. DNA Cell Biol. 2008, 27, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Kawamoto, S.; Adelstein, R.S. Evidence for inserted sequences in the head region of nonmuscle myosin specific to the nervous system. Cloning of the cDNA encoding the myosin heavy chain-B isoform of vertebrate nonmuscle myosin. J. Biol. Chem. 1992, 267, 17864–17871. [Google Scholar] [PubMed]
- Nagy, A.; Takagi, Y.; Billington, N.; Sun, S.A.; Hong, D.K.; Homsher, E.; Wang, A.; Sellers, J.R. Kinetic characterization of nonmuscle myosin IIb at the single molecule level. J. Biol. Chem. 2013, 288, 709–722. [Google Scholar] [CrossRef]
- Wang, A.; Ma, X.; Conti, M.A.; Liu, C.; Kawamoto, S.; Adelstein, R.S. Nonmuscle myosin II isoform and domain specificity during early mouse development. Proc. Natl. Acad. Sci. USA 2010, 107, 14645–14650. [Google Scholar] [CrossRef]
- Kovacs, M.; Wang, F.; Hu, A.; Zhang, Y.; Sellers, J.R. Functional divergence of human cytoplasmic myosin II: Kinetic characterization of the non-muscle IIA isoform. J. Biol. Chem. 2003, 278, 38132–38140. [Google Scholar] [CrossRef]
- Kolega, J. Cytoplasmic dynamics of myosin IIA and IIB: Spatial ‘sorting’ of isoforms in locomoting cells. J. Cell Sci. 1998, 111, 2085–2095. [Google Scholar]
- Maupin, P.; Phillips, C.L.; Adelstein, R.S.; Pollard, T.D. Differential localization of myosin-II isozymes in human cultured cells and blood cells. J. Cell Sci. 1994, 107, 3077–3090. [Google Scholar] [PubMed]
- Bao, J.; Jana, S.S.; Adelstein, R.S. Vertebrate nonmuscle myosin II isoforms rescue small interfering RNA-induced defects in COS-7 cell cytokinesis. J. Biol. Chem. 2005, 280, 19594–19599. [Google Scholar] [CrossRef]
- Heissler, S.M.; Manstein, D.J. Nonmuscle myosin-2: Mix and match. Cell Mol. Life Sci. 2013, 70, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Heissler, S.M.; Sellers, J.R. Myosin light chains: Teaching old dogs new tricks. Bioarchitecture 2014, 4, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Han, C.; Jin, S.; Lee, B.; Choi, H.; Kwon, J.T.; Kim, D.; Kim, J.; Lifirsu, E.; Park, W.J.; et al. Myosin regulatory light chains are required to maintain the stability of myosin II and cellular integrity. Biochem. J. 2011, 434, 171–180. [Google Scholar] [CrossRef]
- Maliga, Z.; Junqueira, M.; Toyoda, Y.; Ettinger, A.; Mora-Bermudez, F.; Klemm, R.W.; Vasilj, A.; Guhr, E.; Ibarlucea-Benitez, I.; Poser, I.; et al. A genomic toolkit to investigate kinesin and myosin motor function in cells. Nat. Cell Biol. 2013, 15, 325–334. [Google Scholar] [CrossRef]
- Berg, J.S.; Powell, B.C.; Cheney, R.E. A millennial myosin census. Mol. Biol. Cell 2001, 12, 780–794. [Google Scholar] [CrossRef]
- Kim, K.Y.; Kovacs, M.; Kawamoto, S.; Sellers, J.R.; Adelstein, R.S. Disease-associated mutations and alternative splicing alter the enzymatic and motile activity of nonmuscle myosins II-B and II-C. J. Biol. Chem. 2005, 280, 22769–22775. [Google Scholar] [CrossRef]
- Scholey, J.M.; Taylor, K.A.; Kendrick-Jones, J. Regulation of non-muscle myosin assembly by calmodulin-dependent light chain kinase. Nature 1980, 287, 233–235. [Google Scholar] [CrossRef]
- Beach, J.R.; Shao, L.; Remmert, K.; Li, D.; Betzig, E.; Hammer, J.A., 3rd. Nonmuscle Myosin II Isoforms Coassemble in Living Cells. Curr. Biol. 2015, 25, 402. [Google Scholar] [CrossRef]
- Shutova, M.S.; Spessott, W.A.; Giraudo, C.G.; Svitkina, T. Endogenous species of mammalian nonmuscle myosin IIA and IIB include activated monomers and heteropolymers. Curr. Biol. 2014, 24, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Shutova, M.S.; Asokan, S.B.; Talwar, S.; Assoian, R.K.; Bear, J.E.; Svitkina, T.M. Self-sorting of nonmuscle myosins IIA and IIB polarizes the cytoskeleton and modulates cell motility. J. Cell Biol. 2017, 216, 2877–2889. [Google Scholar] [CrossRef]
- Billington, N.; Beach, J.R.; Heissler, S.M.; Remmert, K.; Guzik-Lendrum, S.; Nagy, A.; Takagi, Y.; Shao, L.; Li, D.; Yang, Y.; et al. Myosin 18A coassembles with nonmuscle myosin 2 to form mixed bipolar filaments. Curr. Biol. 2015, 25, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Dasbiswas, K.; Guo, Z.; Tee, Y.H.; Thiagarajan, V.; Hersen, P.; Chew, T.L.; Safran, S.A.; Zaidel-Bar, R.; Bershadsky, A.D. Long-range self-organization of cytoskeletal myosin II filament stacks. Nat. Cell Biol. 2017, 19, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Fenix, A.M.; Taneja, N.; Buttler, C.A.; Lewis, J.; Van Engelenburg, S.B.; Ohi, R.; Burnette, D.T. Expansion and concatenation of non-muscle myosin IIA filaments drive cellular contractile system formation during interphase and mitosis. Mol. Biol. Cell 2016. [Google Scholar] [CrossRef]
- Beach, J.R.; Bruun, K.S.; Shao, L.; Li, D.; Swider, Z.; Remmert, K.; Zhang, Y.; Conti, M.A.; Adelstein, R.S.; Rusan, N.M.; et al. Actin dynamics and competition for myosin monomer govern the sequential amplification of myosin filaments. Nat. Cell Biol. 2017, 19, 85–93. [Google Scholar] [CrossRef]
- Melli, L.; Billington, N.; Sun, S.A.; Bird, J.E.; Nagy, A.; Friedman, T.B.; Takagi, Y.; Sellers, J.R. Bipolar filaments of human nonmuscle myosin 2-A and 2-B have distinct motile and mechanical properties. Elife 2018, 7. [Google Scholar] [CrossRef]
- Mori, K.; Furusawa, T.; Okubo, T.; Inoue, T.; Ikawa, S.; Yanai, N.; Mori, K.J.; Obinata, M. Genome structure and differential expression of two isoforms of a novel PDZ-containing myosin (MysPDZ) (Myo18A). J. Biochem. 2003, 133, 405–413. [Google Scholar] [CrossRef]
- Jiu, Y.; Kumari, R.; Fenix, A.M.; Schaible, N.; Liu, X.; Varjosalo, M.; Krishnan, R.; Burnette, D.T.; Lappalainen, P. Myosin-18B Promotes the Assembly of Myosin II Stacks for Maturation of Contractile Actomyosin Bundles. Curr. Biol. 2019, 29, 81–92.e85. [Google Scholar] [CrossRef]
- Pato, M.D.; Sellers, J.R.; Preston, Y.A.; Harvey, E.V.; Adelstein, R.S. Baculovirus expression of chicken nonmuscle heavy meromyosin II-B. Characterization of alternatively spliced isoforms. J. Biol. Chem. 1996, 271, 2689–2695. [Google Scholar] [CrossRef]
- Heissler, S.M.; Manstein, D.J. Comparative kinetic and functional characterization of the motor domains of human nonmuscle myosin-2C isoforms. J. Biol. Chem. 2011, 286, 21191–21202. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Kovacs, M.; Hu, A.; Limouze, J.; Harvey, E.V.; Sellers, J.R. Kinetic mechanism of non-muscle myosin IIB: Functional adaptations for tension generation and maintenance. J. Biol. Chem. 2003, 278, 27439–27448. [Google Scholar] [CrossRef] [PubMed]
- Juanes-García, A.; Llorente-González, C.; Vicente-Manzanares, M. Nonmuscle Myosin II. In Encyclopedia of Signaling Molecules; Choi, S., Ed.; Springer: Cham, Switzerland, 2018; pp. 3541–3553. [Google Scholar] [CrossRef]
- Sellers, J.R.; Pato, M.D.; Adelstein, R.S. Reversible phosphorylation of smooth muscle myosin, heavy meromyosin, and platelet myosin. J. Biol. Chem. 1981, 256, 13137–13142. [Google Scholar] [PubMed]
- Umemoto, S.; Bengur, A.R.; Sellers, J.R. Effect of multiple phosphorylations of smooth muscle and cytoplasmic myosins on movement in an in vitro motility assay. J. Biol. Chem. 1989, 264, 1431–1436. [Google Scholar]
- Ikebe, M.; Hartshorne, D.J.; Elzinga, M. Identification, phosphorylation, and dephosphorylation of a second site for myosin light chain kinase on the 20,000-dalton light chain of smooth muscle myosin. J. Biol. Chem. 1986, 261, 36–39. [Google Scholar]
- Beach, J.R.; Licate, L.S.; Crish, J.F.; Egelhoff, T.T. Analysis of the role of Ser1/Ser2/Thr9 phosphorylation on myosin II assembly and function in live cells. BMC Cell Biol. 2011, 12, 52. [Google Scholar] [CrossRef]
- Komatsu, S.; Ikebe, M. The phosphorylation of myosin II at the Ser1 and Ser2 is critical for normal platelet-derived growth factor induced reorganization of myosin filaments. Mol. Biol. Cell 2007, 18, 5081–5090. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Sellers, J.R.; Adelstein, R.S.; Hidaka, H. Protein kinase C modulates in vitro phosphorylation of the smooth muscle heavy meromyosin by myosin light chain kinase. J. Biol. Chem. 1984, 259, 8808–8814. [Google Scholar]
- Griffith, L.M.; Downs, S.M.; Spudich, J.A. Myosin light chain kinase and myosin light chain phosphatase from Dictyostelium: Effects of reversible phosphorylation on myosin structure and function. J. Cell Biol. 1987, 104, 1309–1323. [Google Scholar] [CrossRef]
- Aguilar-Cuenca, R.; Llorente-Gonzalez, C.; Chapman, J.R.; Talayero, V.C.; Garrido-Casado, M.; Delgado-Arevalo, C.; Millan-Salanova, M.; Shabanowitz, J.; Hunt, D.F.; Sellers, J.R.; et al. Tyrosine Phosphorylation of the Myosin Regulatory Light Chain Controls Non-muscle Myosin II Assembly and Function in Migrating Cells. Curr. Biol. 2020. [Google Scholar] [CrossRef]
- Hunter, T. Tyrosine phosphorylation: Thirty years and counting. Curr. Opin. Cell Biol. 2009, 21, 140–146. [Google Scholar] [CrossRef]
- Almeida, M.T.; Mesquita, F.S.; Cruz, R.; Osorio, H.; Custodio, R.; Brito, C.; Vingadassalom, D.; Martins, M.; Leong, J.M.; Holden, D.W.; et al. Src-dependent tyrosine phosphorylation of non-muscle myosin heavy chain-IIA restricts Listeria monocytogenes cellular infection. J. Biol. Chem. 2015, 290, 8383–8395. [Google Scholar] [CrossRef] [PubMed]
- Breckenridge, M.T.; Dulyaninova, N.G.; Egelhoff, T.T. Multiple regulatory steps control mammalian nonmuscle myosin II assembly in live cells. Mol. Biol. Cell 2009, 20, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Dulyaninova, N.G.; Malashkevich, V.N.; Almo, S.C.; Bresnick, A.R. Regulation of myosin-IIA assembly and Mts1 binding by heavy chain phosphorylation. Biochemistry 2005, 44, 6867–6876. [Google Scholar] [CrossRef]
- Elliott, P.R.; Irvine, A.F.; Jung, H.S.; Tozawa, K.; Pastok, M.W.; Picone, R.; Badyal, S.K.; Basran, J.; Rudland, P.S.; Barraclough, R.; et al. Asymmetric mode of Ca(2)(+)-S100A4 interaction with nonmuscle myosin IIA generates nanomolar affinity required for filament remodeling. Structure 2012, 20, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Dahan, I.; Yearim, A.; Touboul, Y.; Ravid, S. The tumor suppressor Lgl1 regulates NMII-A cellular distribution and focal adhesion morphology to optimize cell migration. Mol. Biol. Cell 2012, 23, 591–601. [Google Scholar] [CrossRef]
- Even-Faitelson, L.; Ravid, S. PAK1 and aPKCzeta regulate myosin II-B phosphorylation: A novel signaling pathway regulating filament assembly. Mol. Biol. Cell 2006, 17, 2869–2881. [Google Scholar] [CrossRef]
- Conti, M.A.; Sellers, J.R.; Adelstein, R.S.; Elzinga, M. Identification of the serine residue phosphorylated by protein kinase C in vertebrate nonmuscle myosin heavy chains. Biochemistry 1991, 30, 966–970. [Google Scholar] [CrossRef]
- Murakami, N.; Chauhan, V.P.; Elzinga, M. Two nonmuscle myosin II heavy chain isoforms expressed in rabbit brains: Filament forming properties, the effects of phosphorylation by protein kinase C and casein kinase II, and location of the phosphorylation sites. Biochemistry 1998, 37, 1989–2003. [Google Scholar] [CrossRef]
- Dulyaninova, N.G.; House, R.P.; Betapudi, V.; Bresnick, A.R. Myosin-IIA heavy-chain phosphorylation regulates the motility of MDA-MB-231 carcinoma cells. Mol. Biol. Cell 2007, 18, 3144–3155. [Google Scholar] [CrossRef]
- Ford, H.L.; Silver, D.L.; Kachar, B.; Sellers, J.R.; Zain, S.B. Effect of Mts1 on the structure and activity of nonmuscle myosin II. Biochemistry 1997, 36, 16321–16327. [Google Scholar] [CrossRef]
- Li, Z.H.; Bresnick, A.R. The S100A4 metastasis factor regulates cellular motility via a direct interaction with myosin-IIA. Cancer Res. 2006, 66, 5173–5180. [Google Scholar] [CrossRef][Green Version]
- Du, M.; Wang, G.; Ismail, T.M.; Gross, S.; Fernig, D.G.; Barraclough, R.; Rudland, P.S. S100P dissociates myosin IIA filaments and focal adhesion sites to reduce cell adhesion and enhance cell migration. J. Biol. Chem. 2012, 287, 15330–15344. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Diensthuber, R.P.; Chizhov, I.; Claus, P.; Heissler, S.M.; Preller, M.; Taft, M.H.; Manstein, D.J. Distinct functional interactions between actin isoforms and nonsarcomeric myosins. PLoS ONE 2013, 8, e70636. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Bao, J.; Adelstein, R.S. Loss of cell adhesion causes hydrocephalus in nonmuscle myosin II-B-ablated and mutated mice. Mol. Biol. Cell 2007, 18, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.A.; Even-Ram, S.; Liu, C.; Yamada, K.M.; Adelstein, R.S. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J. Biol. Chem. 2004, 279, 41263–41266. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Kemphues, K.J. A non-muscle myosin required for embryonic polarity in Caenorhabditis elegans. Nature 1996, 382, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Osorio, D.S.; Chan, F.Y.; Saramago, J.; Leite, J.; Silva, A.M.; Sobral, A.F.; Gassmann, R.; Carvalho, A.X. Crosslinking activity of non-muscle myosin II is not sufficient for embryonic cytokinesis in C. elegans. Development 2019, 146. [Google Scholar] [CrossRef]
- Zhang, Y.; Conti, M.A.; Malide, D.; Dong, F.; Wang, A.; Shmist, Y.A.; Liu, C.; Zerfas, P.; Daniels, M.P.; Chan, C.C.; et al. Mouse models of MYH9-related disease: Mutations in nonmuscle myosin II-A. Blood 2012, 119, 238–250. [Google Scholar] [CrossRef]
- Marigo, V.; Nigro, A.; Pecci, A.; Montanaro, D.; Di Stazio, M.; Balduini, C.L.; Savoia, A. Correlation between the clinical phenotype of MYH9-related disease and tissue distribution of class II nonmuscle myosin heavy chains. Genomics 2004, 83, 1125–1133. [Google Scholar] [CrossRef]
- Lienkamp, S.S.; Liu, K.; Karner, C.M.; Carroll, T.J.; Ronneberger, O.; Wallingford, J.B.; Walz, G. Vertebrate kidney tubules elongate using a planar cell polarity-dependent, rosette-based mechanism of convergent extension. Nat. Genet. 2012, 44, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Kurihara, H.; Horita, S.; Chikamoto, H.; Hattori, M.; Harita, Y.; Tsurumi, H.; Kajiho, Y.; Sawada, Y.; Sasaki, S.; et al. Podocyte expression of nonmuscle myosin heavy chain-IIA decreases in idiopathic nephrotic syndrome, especially in focal segmental glomerulosclerosis. Nephrol. Dial. Transplant. 2013, 28, 2993–3003. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Andreeva, A.; Sipe, C.W.; Liu, L.; Cheng, A.; Lu, X. PTK7 regulates myosin II activity to orient planar polarity in the mammalian auditory epithelium. Curr. Biol. 2012, 22, 956–966. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamamoto, N.; Okano, T.; Ma, X.; Adelstein, R.S.; Kelley, M.W. Myosin II regulates extension, growth and patterning in the mammalian cochlear duct. Development 2009, 136, 1977–1986. [Google Scholar] [CrossRef]
- Straight, A.F.; Cheung, A.; Limouze, J.; Chen, I.; Westwood, N.J.; Sellers, J.R.; Mitchison, T.J. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science 2003, 299, 1743–1747. [Google Scholar] [CrossRef]
- De Lozanne, A.; Spudich, J.A. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science 1987, 236, 1086–1091. [Google Scholar] [CrossRef]
- Mabuchi, I.; Okuno, M. The effect of myosin antibody on the division of starfish blastomeres. J. Cell Biol. 1977, 74, 251–263. [Google Scholar] [CrossRef]
- Fujiwara, K.; Pollard, T.D. Fluorescent antibody localization of myosin in the cytoplasm, cleavage furrow, and mitotic spindle of human cells. J. Cell Biol. 1976, 71, 848–875. [Google Scholar] [CrossRef]
- Green, R.A.; Paluch, E.; Oegema, K. Cytokinesis in animal cells. Annu. Rev. Cell Dev. Biol. 2012, 28, 29–58. [Google Scholar] [CrossRef]
- Tsankova, A.; Pham, T.T.; Garcia, D.S.; Otte, F.; Cabernard, C. Cell Polarity Regulates Biased Myosin Activity and Dynamics during Asymmetric Cell Division via Drosophila Rho Kinase and Protein Kinase N. Dev. Cell 2017, 42, 143–155.e145. [Google Scholar] [CrossRef]
- Sugioka, K.; Bowerman, B. Combinatorial Contact Cues Specify Cell Division Orientation by Directing Cortical Myosin Flows. Dev. Cell 2018, 46, 257–270. [Google Scholar] [CrossRef]
- Descovich, C.P.; Cortes, D.B.; Ryan, S.; Nash, J.; Zhang, L.; Maddox, P.S.; Nedelec, F.; Maddox, A.S. Cross-linkers both drive and brake cytoskeletal remodeling and furrowing in cytokinesis. Mol. Biol. Cell 2018, 29, 622–631. [Google Scholar] [CrossRef]
- Reymann, A.C.; Staniscia, F.; Erzberger, A.; Salbreux, G.; Grill, S.W. Cortical flow aligns actin filaments to form a furrow. Elife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.; Jordan, S.N.; Chand, V.; Sees, J.A.; Laband, K.; Carvalho, A.X.; Shirasu-Hiza, M.; Kovar, D.R.; Dumont, J.; Canman, J.C. High-resolution temporal analysis reveals a functional timeline for the molecular regulation of cytokinesis. Dev. Cell 2014, 30, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Adelstein, R.S. The role of vertebrate nonmuscle Myosin II in development and human disease. Bioarchitecture 2014, 4, 88–102. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, S.S.; Fassler, R. Integrin-mediated mechanotransduction. J. Cell Biol. 2016, 215, 445–456. [Google Scholar] [CrossRef]
- Thomas, D.G.; Yenepalli, A.; Denais, C.M.; Rape, A.; Beach, J.R.; Wang, Y.L.; Schiemann, W.P.; Baskaran, H.; Lammerding, J.; Egelhoff, T.T. Non-muscle myosin IIB is critical for nuclear translocation during 3D invasion. J. Cell Biol. 2015, 210, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Biais, N.; Giannone, G.; Tanase, M.; Jiang, G.; Hofman, J.M.; Wiggins, C.H.; Silberzan, P.; Buguin, A.; Ladoux, B.; et al. Nonmuscle myosin IIA-dependent force inhibits cell spreading and drives F-actin flow. Biophys. J. 2006, 91, 3907–3920. [Google Scholar] [CrossRef]
- Hartsock, A.; Nelson, W.J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 2008, 1778, 660–669. [Google Scholar] [CrossRef]
- Mui, K.L.; Chen, C.S.; Assoian, R.K. The mechanical regulation of integrin-cadherin crosstalk organizes cells, signaling and forces. J. Cell Sci. 2016, 129, 1093–1100. [Google Scholar] [CrossRef]
- Pasapera, A.M.; Schneider, I.C.; Rericha, E.; Schlaepfer, D.D.; Waterman, C.M. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J. Cell Biol. 2010, 188, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Curran, S.; Strandkvist, C.; Bathmann, J.; de Gennes, M.; Kabla, A.; Salbreux, G.; Baum, B. Myosin II Controls Junction Fluctuations to Guide Epithelial Tissue Ordering. Dev. Cell 2017, 43, 480–492.e486. [Google Scholar] [CrossRef] [PubMed]
- Ladoux, B.; Mege, R.M. Mechanobiology of collective cell behaviours. Nat. Rev. Mol. Cell Biol. 2017, 18, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Smutny, M.; Cox, H.L.; Leerberg, J.M.; Kovacs, E.M.; Conti, M.A.; Ferguson, C.; Hamilton, N.A.; Parton, R.G.; Adelstein, R.S.; Yap, A.S. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat. Cell Biol. 2010, 12, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Manzanares, M.; Zareno, J.; Whitmore, L.; Choi, C.K.; Horwitz, A.F. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J. Cell Biol. 2007, 176, 573–580. [Google Scholar] [CrossRef]
- Heuze, M.L.; Sankara Narayana, G.H.N.; D’Alessandro, J.; Cellerin, V.; Dang, T.; Williams, D.S.; Van Hest, J.C.; Marcq, P.; Mege, R.M.; Ladoux, B. Myosin II isoforms play distinct roles in adherens junction biogenesis. Elife 2019, 8. [Google Scholar] [CrossRef]
- Ivanov, A.I.; Bachar, M.; Babbin, B.A.; Adelstein, R.S.; Nusrat, A.; Parkos, C.A. A unique role for nonmuscle myosin heavy chain IIA in regulation of epithelial apical junctions. PLoS ONE 2007, 2, e658. [Google Scholar] [CrossRef]
- Ruprecht, V.; Wieser, S.; Callan-Jones, A.; Smutny, M.; Morita, H.; Sako, K.; Barone, V.; Ritsch-Marte, M.; Sixt, M.; Voituriez, R.; et al. Cortical contractility triggers a stochastic switch to fast amoeboid cell motility. Cell 2015, 160, 673–685. [Google Scholar] [CrossRef]
- Shutova, M.S.; Svitkina, T.M. Common and Specific Functions of Nonmuscle Myosin II Paralogs in Cells. Biochemistry (Mosc) 2018, 83, 1459–1468. [Google Scholar] [CrossRef]
- Barbier, L.; Saez, P.J.; Attia, R.; Lennon-Dumenil, A.M.; Lavi, I.; Piel, M.; Vargas, P. Myosin II Activity Is Selectively Needed for Migration in Highly Confined Microenvironments in Mature Dendritic Cells. Front. Immunol. 2019, 10, 747. [Google Scholar] [CrossRef]
- Fournier, M.F.; Sauser, R.; Ambrosi, D.; Meister, J.J.; Verkhovsky, A.B. Force transmission in migrating cells. J. Cell Biol. 2010, 188, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Schaub, S.; Bohnet, S.; Laurent, V.M.; Meister, J.J.; Verkhovsky, A.B. Comparative maps of motion and assembly of filamentous actin and myosin II in migrating cells. Mol. Biol. Cell 2007, 18, 3723–3732. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.; Te Lindert, M.; Krause, M.; Alexander, S.; Te Riet, J.; Willis, A.L.; Hoffman, R.M.; Figdor, C.G.; Weiss, S.J.; Friedl, P. Physical limits of cell migration: Control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J. Cell Biol. 2013, 201, 1069–1084. [Google Scholar] [CrossRef]
- Gardel, M.L.; Sabass, B.; Ji, L.; Danuser, G.; Schwarz, U.S.; Waterman, C.M. Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. J. Cell Biol. 2008, 183, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Alexandrova, A.Y.; Arnold, K.; Schaub, S.; Vasiliev, J.M.; Meister, J.J.; Bershadsky, A.D.; Verkhovsky, A.B. Comparative dynamics of retrograde actin flow and focal adhesions: Formation of nascent adhesions triggers transition from fast to slow flow. PLoS ONE 2008, 3, e3234. [Google Scholar] [CrossRef]
- Lo, C.M.; Buxton, D.B.; Chua, G.C.; Dembo, M.; Adelstein, R.S.; Wang, Y.L. Nonmuscle myosin IIb is involved in the guidance of fibroblast migration. Mol. Biol. Cell 2004, 15, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Betapudi, V.; Licate, L.S.; Egelhoff, T.T. Distinct roles of nonmuscle myosin II isoforms in the regulation of MDA-MB-231 breast cancer cell spreading and migration. Cancer Res. 2006, 66, 4725–4733. [Google Scholar] [CrossRef]
- Liu, Z.; Ho, C.H.; Grinnell, F. The different roles of myosin IIA and myosin IIB in contraction of 3D collagen matrices by human fibroblasts. Exp. Cell Res. 2014, 326, 295–306. [Google Scholar] [CrossRef][Green Version]
- Even-Ram, S.; Doyle, A.D.; Conti, M.A.; Matsumoto, K.; Adelstein, R.S.; Yamada, K.M. Myosin IIA regulates cell motility and actomyosin-microtubule crosstalk. Nat. Cell Biol. 2007, 9, 299–309. [Google Scholar] [CrossRef]
- Liu, Y.J.; Le Berre, M.; Lautenschlaeger, F.; Maiuri, P.; Callan-Jones, A.; Heuze, M.; Takaki, T.; Voituriez, R.; Piel, M. Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell 2015, 160, 659–672. [Google Scholar] [CrossRef]
- Svitkina, T.M.; Verkhovsky, A.B.; McQuade, K.M.; Borisy, G.G. Analysis of the actin-myosin II system in fish epidermal keratocytes: Mechanism of cell body translocation. J. Cell Biol. 1997, 139, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Zaidel-Bar, R. Diverse roles of non-muscle myosin II contractility in 3D cell migration. Essays Biochem. 2019, 63, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Nagy, S.; Ricca, B.L.; Norstrom, M.F.; Courson, D.S.; Brawley, C.M.; Smithback, P.A.; Rock, R.S. A myosin motor that selects bundled actin for motility. Proc. Natl. Acad. Sci. USA 2008, 105, 9616–9620. [Google Scholar] [CrossRef] [PubMed]
- Hind, L.E.; Vincent, W.J.; Huttenlocher, A. Leading from the Back: The Role of the Uropod in Neutrophil Polarization and Migration. Dev. Cell 2016, 38, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Soldati, T. Dissection of amoeboid movement into two mechanically distinct modes. J. Cell Sci. 2006, 119, 3833–3844. [Google Scholar] [CrossRef]
- Charras, G.; Paluch, E. Blebs lead the way: How to migrate without lamellipodia. Nat. Rev. Mol. Cell Biol. 2008, 9, 730–736. [Google Scholar] [CrossRef]
- Sanchez-Madrid, F.; Serrador, J.M. Bringing up the rear: Defining the roles of the uropod. Nat. Rev. Mol. Cell Biol. 2009, 10, 353–359. [Google Scholar] [CrossRef]
- Eddy, R.J.; Pierini, L.M.; Matsumura, F.; Maxfield, F.R. Ca2+-dependent myosin II activation is required for uropod retraction during neutrophil migration. J. Cell Sci. 2000, 113 Pt 7, 1287–1298. [Google Scholar]
- Wong, K.; Van Keymeulen, A.; Bourne, H.R. PDZRhoGEF and myosin II localize RhoA activity to the back of polarizing neutrophil-like cells. J. Cell Biol. 2007, 179, 1141–1148. [Google Scholar] [CrossRef]
- Conrad, G.W.; Davis, S.E. Polar lobe formation and cytokinesis in fertilized eggs of Ilyanassa obsoleta. III. Large bleb formation caused by Sr2+, ionophores X537A and A23187, and compound 48/80. Dev. Biol. 1980, 74, 152–172. [Google Scholar] [CrossRef]
- Charras, G.T. A short history of blebbing. J. Microsc. 2008, 231, 466–478. [Google Scholar] [CrossRef]
- Fackler, O.T.; Grosse, R. Cell motility through plasma membrane blebbing. J. Cell Biol. 2008, 181, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.; Cabanes, D.; Sarmento Mesquita, F.; Sousa, S. Mechanisms protecting host cells against bacterial pore-forming toxins. Cell Mol. Life Sci. 2019, 76, 1319–1339. [Google Scholar] [CrossRef] [PubMed]
- Rottner, K.; Schaks, M. Assembling actin filaments for protrusion. Curr. Opin. Cell Biol. 2019, 56, 53–63. [Google Scholar] [CrossRef] [PubMed]
- de Lucas, B.; Bernal, A.; Perez, L.M.; San Martin, N.; Galvez, B.G. Membrane Blebbing Is Required for Mesenchymal Precursor Migration. PLoS ONE 2016, 11, e0150004. [Google Scholar] [CrossRef][Green Version]
- Charras, G.T.; Yarrow, J.C.; Horton, M.A.; Mahadevan, L.; Mitchison, T.J. Non-equilibration of hydrostatic pressure in blebbing cells. Nature 2005, 435, 365–369. [Google Scholar] [CrossRef]
- Trinkaus, J.P. Modes of cell locomotion in vivo. Ciba Found. Symp 1973, 14, 233–249. [Google Scholar] [CrossRef]
- Ikenouchi, J.; Aoki, K. Membrane bleb: A seesaw game of two small GTPases. Small GTPases 2017, 8, 85–89. [Google Scholar] [CrossRef]
- Tinevez, J.Y.; Schulze, U.; Salbreux, G.; Roensch, J.; Joanny, J.F.; Paluch, E. Role of cortical tension in bleb growth. Proc. Natl. Acad. Sci. USA 2009, 106, 18581–18586. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A. Blebs on the move. Dev. Cell 2011, 20. [Google Scholar] [CrossRef]
- Gong, X.; Didan, Y.; Lock, J.G.; Stromblad, S. KIF13A-regulated RhoB plasma membrane localization governs membrane blebbing and blebby amoeboid cell migration. EMBO J. 2018, 37. [Google Scholar] [CrossRef] [PubMed]
- Norman, L.; Sengupta, K.; Aranda-Espinoza, H. Blebbing dynamics during endothelial cell spreading. Eur. J. Cell Biol. 2011, 90, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Charras, G.T.; Coughlin, M.; Mitchison, T.J.; Mahadevan, L. Life and times of a cellular bleb. Biophys. J. 2008, 94, 1836–1853. [Google Scholar] [CrossRef]
- Charras, G.T.; Hu, C.K.; Coughlin, M.; Mitchison, T.J. Reassembly of contractile actin cortex in cell blebs. J. Cell Biol. 2006, 175, 477–490. [Google Scholar] [CrossRef]
- Sheetz, M.P.; Sable, J.E.; Dobereiner, H.G. Continuous membrane-cytoskeleton adhesion requires continuous accommodation to lipid and cytoskeleton dynamics. Annu. Rev. Biophys. Biomol. Struct. 2006, 35, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.; Dantzig, J.A.; Hollingworth, S.; Baylor, S.M.; Goldman, Y.E.; Mitchison, T.J.; Straight, A.F. A small-molecule inhibitor of skeletal muscle myosin II. Nat. Cell Biol. 2002, 4, 83–88. [Google Scholar] [CrossRef]
- Taneja, N.; Burnette, D.T. Myosin IIA drives membrane bleb retraction. Mol. Biol. Cell 2019, 30, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Fritzsche, M.; Lewalle, A.; Duke, T.; Kruse, K.; Charras, G. Analysis of turnover dynamics of the submembranous actin cortex. Mol. Biol. Cell 2013, 24, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, F.S.; Brito, C.; Cabanes, D.; Sousa, S. Control of cytoskeletal dynamics during cellular responses to pore forming toxins. Commun. Integr. Biol. 2017, 10, e1349582. [Google Scholar] [CrossRef]
- Mesquita, F.S.; Brito, C.; Mazon Moya, M.J.; Pinheiro, J.C.; Mostowy, S.; Cabanes, D.; Sousa, S. Endoplasmic reticulum chaperone Gp96 controls actomyosin dynamics and protects against pore-forming toxins. EMBO Rep. 2017, 18, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, A.J.; Perez, F. Plasma membrane repair: The adaptable cell life-insurance. Curr. Opin. Cell Biol. 2017, 47, 99–107. [Google Scholar] [CrossRef]
- Andrews, N.W.; Corrotte, M. Plasma membrane repair. Curr. Biol. 2018, 28, R392–R397. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.; Mesquita, F.S.; Bleck, C.K.E.; Sellers, J.R.; Cabanes, D.; Sousa, S. Perfringolysin O-Induced Plasma Membrane Pores Trigger Actomyosin Remodeling and Endoplasmic Reticulum Redistribution. Toxins (Basel) 2019, 11, 419. [Google Scholar] [CrossRef] [PubMed]
- Pecci, A.; Ma, X.; Savoia, A.; Adelstein, R.S. MYH9: Structure, functions and role of non-muscle myosin IIA in human disease. Gene 2018, 664, 152–167. [Google Scholar] [CrossRef]
- Newell-Litwa, K.A.; Horwitz, R.; Lamers, M.L. Non-muscle myosin II in disease: Mechanisms and therapeutic opportunities. Dis. Model. Mech. 2015, 8, 1495–1515. [Google Scholar] [CrossRef] [PubMed]
- Kunishima, S.; Matsushita, T.; Kojima, T.; Amemiya, N.; Choi, Y.M.; Hosaka, N.; Inoue, M.; Jung, Y.; Mamiya, S.; Matsumoto, K.; et al. Identification of six novel MYH9 mutations and genotype-phenotype relationships in autosomal dominant macrothrombocytopenia with leukocyte inclusions. J. Hum. Genet. 2001, 46, 722–729. [Google Scholar] [CrossRef]
- Seri, M.; Cusano, R.; Gangarossa, S.; Caridi, G.; Bordo, D.; Lo Nigro, C.; Ghiggeri, G.M.; Ravazzolo, R.; Savino, M.; Del Vecchio, M.; et al. Mutations in MYH9 result in the May-Hegglin anomaly, and Fechtner and Sebastian syndromes. The May-Heggllin/Fechtner Syndrome Consortium. Nat. Genet. 2000, 26, 103–105. [Google Scholar] [CrossRef]
- Lalwani, A.K.; Goldstein, J.A.; Kelley, M.J.; Luxford, W.; Castelein, C.M.; Mhatre, A.N. Human nonsyndromic hereditary deafness DFNA17 is due to a mutation in nonmuscle myosin MYH9. Am. J. Hum. Genet. 2000, 67, 1121–1128. [Google Scholar] [CrossRef]
- Kelley, M.J.; Jawien, W.; Ortel, T.L.; Korczak, J.F. Mutation of MYH9, encoding non-muscle myosin heavy chain A, in May-Hegglin anomaly. Nat. Genet. 2000, 26, 106–108. [Google Scholar] [CrossRef]
- Asensio-Juarez, G.; Llorente-Gonzalez, C.; Vicente-Manzanares, M. Linking the Landscape of MYH9-Related Diseases to the Molecular Mechanisms that Control Non-Muscle Myosin II-A Function in Cells. Cells 2020, 9, 1458. [Google Scholar] [CrossRef]
- Pecci, A.; Panza, E.; Pujol-Moix, N.; Klersy, C.; Di Bari, F.; Bozzi, V.; Gresele, P.; Lethagen, S.; Fabris, F.; Dufour, C.; et al. Position of nonmuscle myosin heavy chain IIA (NMMHC-IIA) mutations predicts the natural history of MYH9-related disease. Hum. Mutat. 2008, 29, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Kunishima, S. [May-Hegglin anomaly--from genome research to clinical laboratory]. Rinsho Byori. 2003, 51, 898–904. [Google Scholar] [PubMed]
- Kunishima, S.; Matsushita, T.; Kojima, T.; Sako, M.; Kimura, F.; Jo, E.K.; Inoue, C.; Kamiya, T.; Saito, H. Immunofluorescence analysis of neutrophil nonmuscle myosin heavy chain-A in MYH9 disorders: Association of subcellular localization with MYH9 mutations. Lab. Invest. 2003, 83, 115–122. [Google Scholar] [CrossRef]
- Saposnik, B.; Binard, S.; Fenneteau, O.; Nurden, A.; Nurden, P.; Hurtaud-Roux, M.F.; Schlegel, N.; French, M.Y.H.n. Mutation spectrum and genotype-phenotype correlations in a large French cohort of MYH9-Related Disorders. Mol. Genet. Genom. Med. 2014, 2, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Pecci, A.; Verver, E.J.; Schlegel, N.; Canzi, P.; Boccio, C.M.; Platokouki, H.; Krause, E.; Benazzo, M.; Topsakal, V.; Greinacher, A. Cochlear implantation is safe and effective in patients with MYH9-related disease. Orphanet J. Rare Dis. 2014, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Heath, K.E.; Campos-Barros, A.; Toren, A.; Rozenfeld-Granot, G.; Carlsson, L.E.; Savige, J.; Denison, J.C.; Gregory, M.C.; White, J.G.; Barker, D.F.; et al. Nonmuscle myosin heavy chain IIA mutations define a spectrum of autosomal dominant macrothrombocytopenias: May-Hegglin anomaly and Fechtner, Sebastian, Epstein, and Alport-like syndromes. Am. J. Hum. Genet. 2001, 69, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Seri, M.; Pecci, A.; Di Bari, F.; Cusano, R.; Savino, M.; Panza, E.; Nigro, A.; Noris, P.; Gangarossa, S.; Rocca, B.; et al. MYH9-related disease: May-Hegglin anomaly, Sebastian syndrome, Fechtner syndrome, and Epstein syndrome are not distinct entities but represent a variable expression of a single illness. Medicine (Baltimore) 2003, 82, 203–215. [Google Scholar] [CrossRef]
- Pecci, A.; Panza, E.; De Rocco, D.; Pujol-Moix, N.; Girotto, G.; Podda, L.; Paparo, C.; Bozzi, V.; Pastore, A.; Balduini, C.L.; et al. MYH9 related disease: Four novel mutations of the tail domain of myosin-9 correlating with a mild clinical phenotype. Eur. J. Haematol. 2010, 84, 291–297. [Google Scholar] [CrossRef]
- Pecci, A.; Malara, A.; Badalucco, S.; Bozzi, V.; Torti, M.; Balduini, C.L.; Balduini, A. Megakaryocytes of patients with MYH9-related thrombocytopenia present an altered proplatelet formation. Thromb. Haemost 2009, 102, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Heynen, M.J.; Blockmans, D.; Verwilghen, R.L.; Vermylen, J. Congenital macrothrombocytopenia, leucocyte inclusions, deafness and proteinuria: Functional and electron microscopic observations on platelets and megakaryocytes. Br. J. Haematol. 1988, 70, 441–448. [Google Scholar] [CrossRef]
- Chen, Z.; Naveiras, O.; Balduini, A.; Mammoto, A.; Conti, M.A.; Adelstein, R.S.; Ingber, D.; Daley, G.Q.; Shivdasani, R.A. The May-Hegglin anomaly gene MYH9 is a negative regulator of platelet biogenesis modulated by the Rho-ROCK pathway. Blood 2007, 110, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Pecci, A.; Biino, G.; Fierro, T.; Bozzi, V.; Mezzasoma, A.; Noris, P.; Ramenghi, U.; Loffredo, G.; Fabris, F.; Momi, S.; et al. Alteration of liver enzymes is a feature of the MYH9-related disease syndrome. PLoS ONE 2012, 7, e35986. [Google Scholar] [CrossRef] [PubMed]
- Pecci, A.; Klersy, C.; Gresele, P.; Lee, K.J.; De Rocco, D.; Bozzi, V.; Russo, G.; Heller, P.G.; Loffredo, G.; Ballmaier, M.; et al. MYH9-related disease: A novel prognostic model to predict the clinical evolution of the disease based on genotype-phenotype correlations. Hum. Mutat. 2014, 35, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, T.; Jiang, Y.; Chen, H.; He, X.; Chen, C.; Li, X.; Shao, Q.; Ran, X.; Li, Z.; et al. Genes with de novo mutations are shared by four neuropsychiatric disorders discovered from NPdenovo database. Mol. Psychiatry 2016, 21, 298. [Google Scholar] [CrossRef]
- Weber, M.; Kim, S.; Patterson, N.; Rooney, K.; Searles, C.D. MiRNA-155 targets myosin light chain kinase and modulates actin cytoskeleton organization in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1192–H1203. [Google Scholar] [CrossRef]
- Moore, C.S.; Rao, V.T.; Durafourt, B.A.; Bedell, B.J.; Ludwin, S.K.; Bar-Or, A.; Antel, J.P. miR-155 as a multiple sclerosis-relevant regulator of myeloid cell polarization. Ann. Neurol. 2013, 74, 709–720. [Google Scholar] [CrossRef]
- Parisi, C.; Arisi, I.; D’Ambrosi, N.; Storti, A.E.; Brandi, R.; D’Onofrio, M.; Volonte, C. Dysregulated microRNAs in amyotrophic lateral sclerosis microglia modulate genes linked to neuroinflammation. Cell Death Dis. 2013, 4, e959. [Google Scholar] [CrossRef]
- DePoy, L.M.; Noble, B.; Allen, A.G.; Gourley, S.L. Developmentally divergent effects of Rho-kinase inhibition on cocaine- and BDNF-induced behavioral plasticity. Behav. Brain Res. 2013, 243, 171–175. [Google Scholar] [CrossRef]
- Roland, A.B.; Ricobaraza, A.; Carrel, D.; Jordan, B.M.; Rico, F.; Simon, A.; Humbert-Claude, M.; Ferrier, J.; McFadden, M.H.; Scheuring, S.; et al. Cannabinoid-induced actomyosin contractility shapes neuronal morphology and growth. Elife 2014, 3, e03159. [Google Scholar] [CrossRef] [PubMed]
- Tonges, L.; Frank, T.; Tatenhorst, L.; Saal, K.A.; Koch, J.C.; Szego, E.M.; Bahr, M.; Weishaupt, J.H.; Lingor, P. Inhibition of rho kinase enhances survival of dopaminergic neurons and attenuates axonal loss in a mouse model of Parkinson’s disease. Brain 2012, 135, 3355–3370. [Google Scholar] [CrossRef] [PubMed]
- Couch, B.A.; DeMarco, G.J.; Gourley, S.L.; Koleske, A.J. Increased dendrite branching in AbetaPP/PS1 mice and elongation of dendrite arbors by fasudil administration. J. Alzheimers Dis. 2010, 20, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.F.; Zhang, Q.; Xi, J.Y.; Li, Y.H.; Ma, C.G.; Xiao, B.G. Multitarget intervention of Fasudil in the neuroprotection of dopaminergic neurons in MPTP-mouse model of Parkinson’s disease. J. Neurol. Sci. 2015, 353, 28–37. [Google Scholar] [CrossRef]
- Tonges, L.; Gunther, R.; Suhr, M.; Jansen, J.; Balck, A.; Saal, K.A.; Barski, E.; Nientied, T.; Gotz, A.A.; Koch, J.C.; et al. Rho kinase inhibition modulates microglia activation and improves survival in a model of amyotrophic lateral sclerosis. Glia 2014, 62, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Chen, X.; Wang, L.Y.; Gao, W.; Zhu, M.J. Rho kinase inhibitor fasudil protects against beta-amyloid-induced hippocampal neurodegeneration in rats. CNS Neurosci. Ther. 2013, 19, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Huentelman, M.J.; Stephan, D.A.; Talboom, J.; Corneveaux, J.J.; Reiman, D.M.; Gerber, J.D.; Barnes, C.A.; Alexander, G.E.; Reiman, E.M.; Bimonte-Nelson, H.A. Peripheral delivery of a ROCK inhibitor improves learning and working memory. Behav. Neurosci. 2009, 123, 218–223. [Google Scholar] [CrossRef]
- Si, J.; Ge, Y.; Zhuang, S.; Gong, R. Inhibiting nonmuscle myosin II impedes inflammatory infiltration and ameliorates progressive renal disease. Lab. Invest. 2010, 90, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.J.; Xu, J.Z.; Wu, Y.J.; Jean-Charles, L.; Xiao, B.; Gao, P.J.; Zhu, D.L. Effects of fasudil on early atherosclerotic plaque formation and established lesion progression in apolipoprotein E-knockout mice. Atherosclerosis 2009, 207, 68–73. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zheng, H.M.; Zhan, Y.T.; Fan, S.Q. An emerging tumor invasion mechanism about the collective cell migration. Am. J. Transl. Res. 2019, 11, 5301–5312. [Google Scholar]
- Salhia, B.; Hwang, J.H.; Smith, C.A.; Nakada, M.; Rutka, F.; Symons, M.; Rutka, J.T. Role of myosin II activity and the regulation of myosin light chain phosphorylation in astrocytomas. Cell Motil. Cytoskelet. 2008, 65, 12–24. [Google Scholar] [CrossRef]
- Beadle, C.; Assanah, M.C.; Monzo, P.; Vallee, R.; Rosenfeld, S.S.; Canoll, P. The role of myosin II in glioma invasion of the brain. Mol. Biol. Cell 2008, 19, 3357–3368. [Google Scholar] [CrossRef]
- Ye, G.; Huang, K.; Yu, J.; Zhao, L.; Zhu, X.; Yang, Q.; Li, W.; Jiang, Y.; Zhuang, B.; Liu, H.; et al. MicroRNA-647 Targets SRF-MYH9 Axis to Suppress Invasion and Metastasis of Gastric Cancer. Theranostics 2017, 7, 3338–3353. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Li, R.; Zhou, R.; Pan, Z.; Xu, L.; Ding, Y.; Zhao, L. LIM kinase 1 interacts with myosin-9 and alpha-actinin-4 and promotes colorectal cancer progression. Br. J. Cancer 2017, 117, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Katono, K.; Sato, Y.; Jiang, S.X.; Kobayashi, M.; Nagashio, R.; Ryuge, S.; Fukuda, E.; Goshima, N.; Satoh, Y.; Saegusa, M.; et al. Prognostic significance of MYH9 expression in resected non-small cell lung cancer. PLoS ONE 2015, 10, e0121460. [Google Scholar] [CrossRef] [PubMed]
- Derycke, L.; Stove, C.; Vercoutter-Edouart, A.S.; De Wever, O.; Dolle, L.; Colpaert, N.; Depypere, H.; Michalski, J.C.; Bracke, M. The role of non-muscle myosin IIA in aggregation and invasion of human MCF-7 breast cancer cells. Int. J. Dev. Biol. 2011, 55, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Schramek, D.; Sendoel, A.; Segal, J.P.; Beronja, S.; Heller, E.; Oristian, D.; Reva, B.; Fuchs, E. Direct in Vivo RNAi Screen Unveils Myosin IIa as a Tumor Suppressor of Squamous Cell Carcinomas. Science 2014, 343, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yuan, X.; Liu, Y.; Cai, X.; Guo, S.; Wang, A. Non-muscle Myosin II: Role in Microbial Infection and Its Potential as a Therapeutic Target. Front. Microbiol. 2019, 10, 401. [Google Scholar] [CrossRef]
- Valiya Veettil, M.; Sadagopan, S.; Kerur, N.; Chakraborty, S.; Chandran, B. Interaction of c-Cbl with myosin IIA regulates Bleb associated macropinocytosis of Kaposi’s sarcoma-associated herpesvirus. PLoS Pathog. 2010, 6, e1001238. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brito, C.; Sousa, S. Non-Muscle Myosin 2A (NM2A): Structure, Regulation and Function. Cells 2020, 9, 1590. https://doi.org/10.3390/cells9071590
Brito C, Sousa S. Non-Muscle Myosin 2A (NM2A): Structure, Regulation and Function. Cells. 2020; 9(7):1590. https://doi.org/10.3390/cells9071590
Chicago/Turabian StyleBrito, Cláudia, and Sandra Sousa. 2020. "Non-Muscle Myosin 2A (NM2A): Structure, Regulation and Function" Cells 9, no. 7: 1590. https://doi.org/10.3390/cells9071590
APA StyleBrito, C., & Sousa, S. (2020). Non-Muscle Myosin 2A (NM2A): Structure, Regulation and Function. Cells, 9(7), 1590. https://doi.org/10.3390/cells9071590





