T-Cell Gene Therapy in Cancer Immunotherapy: Why It Is No Longer Just CARs on The Road
Abstract
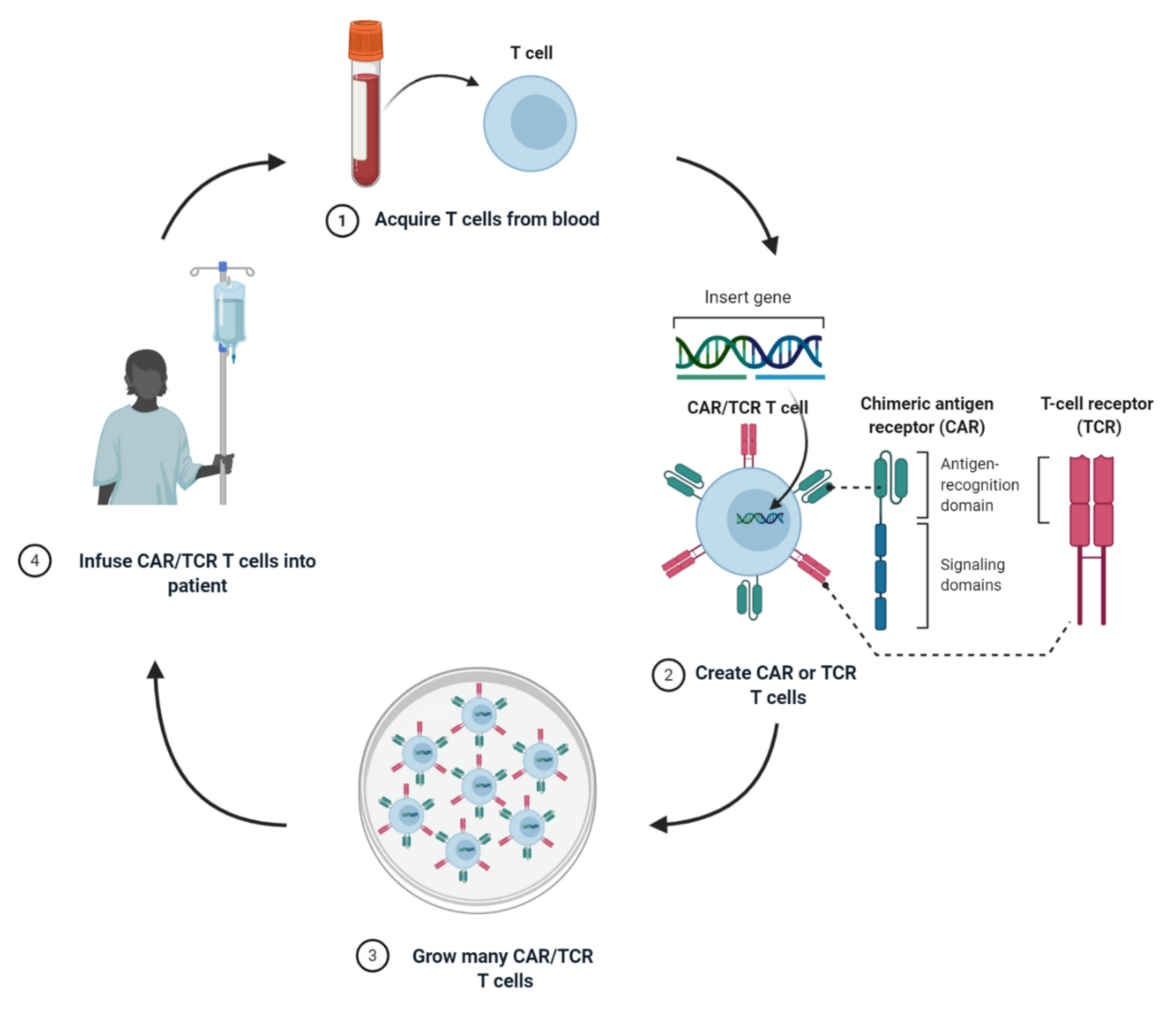
1. Introduction
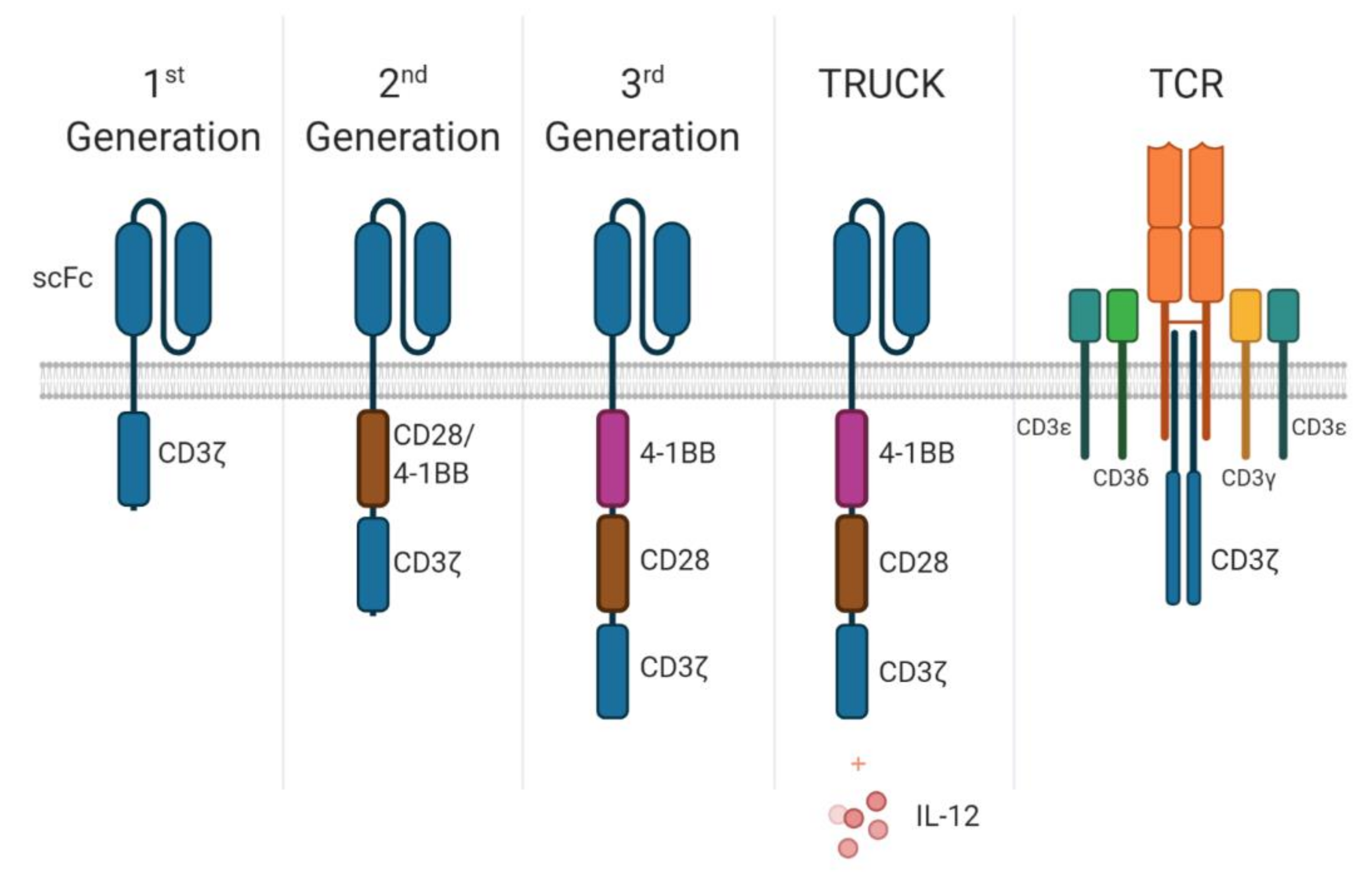
2. When CARs Become TRUCKs
3. Loss of CAR Antigen
4. TCR-T, the Place to Be?
5. Examples of TCR-T
6. TCR-T Limitations
7. Target Cells
8. When to Turn Engineered Cells On and Off
9. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Baitsch, L.; Legat, A.; Barba, L.; Marraco, S.A.F.; Rivals, J.-P.; Baumgaertner, P.; Christiansen-Jucht, C.; Bouzourene, H.; Rimoldi, D.; Pircher, H.; et al. Extended Co-Expression of Inhibitory Receptors by Human CD8 T-Cells Depending on Differentiation, Antigen-Specificity and Anatomical Localization. PLoS ONE 2012, 7, e30852. [Google Scholar] [CrossRef] [PubMed]
- Sawada, M.; Goto, K.; Morimoto-Okazawa, A.; Haruna, M.; Yamamoto, K.; Yamamoto, Y.; Nakagawa, S.; Hiramatsu, K.; Matsuzaki, S.; Kobayashi, E.; et al. PD-1+ Tim3+ Tumor-Infiltrating CD8 T Cells Sustain The Potential for IFN-γ Production, but Lose Cytotoxic Activity in Ovarian Cancer. Int. Immunol. 2020, 32, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, S.D.; Shin, H.; Haining, W.N.; Zou, T.; Workman, C.J.; Polley, A.; Betts, M.R.; Freeman, G.J.; Vignali, D.A.A.; Wherry, E.J. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2008, 10, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Sauce, D.; De Almeida, J.R.; Larsen, M.; Haro, L.; Autran, B.; Freeman, G.J.; Appay, V. PD-1 expression on human CD8 T cells depends on both state of differentiation and activation status. AIDS 2007, 21, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Guedan, S.; Ruella, M.; June, C.H. Emerging Cellular Therapies for Cancer. Annu. Rev. Immunol. 2019, 37, 145–171. [Google Scholar] [CrossRef]
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Yang, J.C.; Sherry, R.M.; Kammula, U.S.; Hughes, M.S.; Phan, G.Q.; Citrin, D.E.; Restifo, N.P.; Robbins, P.F.; Wunderlich, J.R.; et al. Durable Complete Responses in Heavily Pretreated Patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 2011, 17, 4550–4557. [Google Scholar] [CrossRef]
- Özcan, M.; Jensen, K.M.; Chamberlain, C.; Donia, M.; Svane, I.M. Principles of adoptive T cell therapy in cancer. Semin. Immunopathol. 2018, 41, 49–58. [Google Scholar] [CrossRef]
- Kuwana, Y.; Asakura, Y.; Utsunomiya, N.; Nakanishi, M.; Arata, Y.; Itoh, S.; Nagase, F.; Kurosawa, Y. Expression of chimeric receptor composed of immunoglobulin-derived V resions and T-cell receptor-derived C regions. Biochem. Biophys. Res. Commun. 1987, 149, 960–968. [Google Scholar] [CrossRef]
- Kalos, M.; Levine, B.L.; Porter, D.L.; Katz, S.I.; Grupp, S.A.; Bagg, A.; June, C.H. T Cells with Chimeric Antigen Receptors Have Potent Antitumor Effects and Can Establish Memory in Patients with Advanced Leukemia. Sci. Transl. Med. 2011, 3, 95ra73. [Google Scholar] [CrossRef]
- Walseng, E.; Köksal, H.; Sektioglu, I.M.; Fåne, A.; Skorstad, G.; Kvalheim, G.; Gaudernack, G.; Inderberg, E.M.; Wälchli, S. A TCR-based Chimeric Antigen Receptor. Sci. Rep. 2017, 7, 10713. [Google Scholar] [CrossRef]
- Chmielewski, M.; Abken, H. TRUCKs: The fourth generation of CARs. Expert Opin. Boil. Ther. 2015, 15, 1–10. [Google Scholar] [CrossRef]
- Luo, H.; Wu, X.; Sun, R.; Su, J.; Wang, Y.; Dong, Y.; Shi, B.; Sun, Y.; Jiang, H.; Li, Z. Target-Dependent Expression of IL12 by synNotch Receptor-Engineered NK92 Cells Increases the Antitumor Activities of CAR-T Cells. Front. Oncol. 2019, 9, 1448. [Google Scholar] [CrossRef]
- Mancikova, V.; Peschelova, H.; Kozlova, V.; Ledererova, A.; Ladungova, A.; Verner, J.; Loja, T.; Folber, F.; Mayer, J.; Pospisilova, S.; et al. Performance of anti-CD19 chimeric antigen receptor T cells in genetically defined classes of chronic lymphocytic leukemia. J. Immunother. Cancer 2020, 8, e000471. [Google Scholar] [CrossRef] [PubMed]
- Pehlivan, K.C.; Duncan, B.B.; Lee, D.W. CAR-T Cell Therapy for Acute Lymphoblastic Leukemia: Transforming the Treatment of Relapsed and Refractory Disease. Curr. Hematol. Malig. Rep. 2018, 13, 396–406. [Google Scholar] [CrossRef]
- Roberts, Z.J.; Better, M.; Bot, A.; Roberts, M.R.; Ribas, A. Axicabtagene ciloleucel, a first-in-class CAR T cell therapy for aggressive NHL. Leuk. Lymphoma 2017, 59, 1785–1796. [Google Scholar] [CrossRef] [PubMed]
- Ko, R.H.; Ji, L.; Barnette, P.; Bostrom, B.; Hutchinson, R.; Raetz, E.; Seibel, N.L.; Twist, C.J.; Eckroth, E.; Sposto, R.; et al. Outcome of Patients Treated for Relapsed or Refractory Acute Lymphoblastic Leukemia: A Therapeutic Advances in Childhood Leukemia Consortium Study. J. Clin. Oncol. 2010, 28, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.T.; Maude, S.L.; Porter, D.L.; Frey, N.; Wood, P.; Han, X.; Waldron, E.; Chakraborty, A.; Awasthi, R.; Levine, B.L.; et al. Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood 2017, 130, 2317–2325. [Google Scholar] [CrossRef]
- Locke, F.; Ghobadi, A.; A Jacobson, C.; Miklos, D.B.; Lekakis, L.J.; O Oluwole, O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A Single-Arm, Multicentre, Phase 1–2 Trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef]
- U.S National Library of Medicine. ClinicalTrials.gov. Available online: https://clinicaltrials.gov (accessed on 25 March 2020).
- Mackay, M.; Afshinnekoo, E.; Rub, J.; Hassan, C.; Khunte, M.; Baskaran, N.; Owens, B.; Liu, L.; Roboz, G.J.; Guzman, M.L.; et al. The therapeutic landscape for cells engineered with chimeric antigen receptors. Nat. Biotechnol. 2020, 38, 233–244. [Google Scholar] [CrossRef]
- Ahmed, N.; Brawley, V.S.; Hegde, M.; Robertson, C.; Ghazi, A.; Gerken, C.; Liu, E.; Dakhova, O.; Ashoori, A.; Corder, A.; et al. Human Epidermal Growth Factor Receptor 2 (HER2) –Specific Chimeric Antigen Receptor–Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J. Clin. Oncol. 2015, 33, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Morales, A.J.; Cargill, M.J.; Towlerton, A.M.H.; Coffey, D.G.; Warren, E.H.; Tykodi, S.S. Preclinical development of T-cell receptor-engineered T-cell therapy targeting the 5T4 tumor antigen on renal cell carcinoma. Cancer Immunol. Immunother. 2019, 68, 1979–1993. [Google Scholar] [CrossRef] [PubMed]
- Kato, D.; Yaguchi, T.; Iwata, T.; Katoh, Y.; Morii, K.; Tsubota, K.; Takise, Y.; Tamiya, M.; Kamada, H.; Akiba, H.; et al. GPC1 specific CAR-T cells eradicate established solid tumor without adverse effects and synergize with anti-PD-1 Ab. eLife 2020, 9, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Chinnasamy, D.; Yu, Z.; Kerkar, S.P.; Zhang, L.; Morgan, R.A.; Restifo, N.P.; Rosenberg, S.A. Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clin. Cancer Res. 2012, 18, 1672–1683. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; He, T.; Wang, X.; Zheng, W.; Lin, N.; Tu, M.; Xie, Y.; Ping, L.; Zhang, C.; Liu, W.; et al. Parallel Comparison of 4-1BB or CD28 Co-stimulated CD19-Targeted CAR-T Cells for B Cell Non-Hodgkin’s Lymphoma. Mol. Ther. Oncolytics 2019, 15, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Grosser, R.; Cherkassky, L.; Chintala, N.; Adusumilli, P. Combination Immunotherapy with CAR T Cells and Checkpoint Blockade for the Treatment of Solid Tumors. Cancer Cell 2019, 36, 471–482. [Google Scholar] [CrossRef]
- Orlando, E.J.; Han, X.; Tribouley, C.; Wood, P.A.; Leary, R.J.; Riester, M.; Levine, J.E.; Qayed, M.; Grupp, S.A.; Boyer, M.; et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat. Med. 2018, 24, 1504–1506. [Google Scholar] [CrossRef]
- Ruella, M.; Xu, J.; Barrett, D.M.; Fraietta, J.A.; Reich, T.; Ambrose, D.E.; Klichinsky, M.; Shestova, O.; Patel, P.R.; Kulikovskaya, I.; et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat. Med. 2018, 24, 1499–1503. [Google Scholar] [CrossRef]
- Qin, H.; Dong, Z.; Wang, X.; Cheng, W.A.; Wen, F.; Xue, W.; Sun, H.; Walter, M.; Wei, G.; Smith, D.L.; et al. CAR T cells targeting BAFF-R can overcome CD19 antigen loss in B cell malignancies. Sci. Transl. Med. 2019, 11, eaaw9414. [Google Scholar] [CrossRef]
- Cho, J.H.; Collins, J.J.; Wong, W.W. Universal Chimeric Antigen Receptors for Multiplexed and Logical Control of T Cell Responses. Cell 2018, 173, 1426–1438. [Google Scholar] [CrossRef]
- Zugmaier, G.; Gökbuget, N.; Klinger, M.; Viardot, A.; Stelljes, M.; Neumann, S.; Horst, H.-A.; Marks, R.; Faul, C.; Diedrich, H.; et al. Long-term survival and T-cell kinetics in relapsed/refractory ALL patients who achieved MRD response after blinatumomab treatment. Blood 2015, 126, 2578–2584. [Google Scholar] [CrossRef] [PubMed]
- Majzner, R.G.; Mackall, C.L. Tumor Antigen Escape from CAR T-cell Therapy. Cancer Discov. 2018, 8, 1219–1226. [Google Scholar] [CrossRef]
- Du, J.; Zhang, Y. Sequential anti-CD19, 22, and 20 autologous chimeric antigen receptor T-cell (CAR-T) treatments of a child with relapsed refractory Burkitt lymphoma: A case report and literature review. J. Cancer Res. Clin. Oncol. 2020, 146, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Fousek, K.; Watanabe, J.; Joseph, S.K.; George, A.; An, X.; Byrd, T.T.; Morris, J.S.; Luong, A.; Martínez-Paniagua, M.A.; Sanber, K.; et al. CAR T-cells that target acute B-lineage leukemia irrespective of CD19 expression. Leukemia 2020, 1–15. [Google Scholar] [CrossRef]
- Kudo, K.; Imai, C.; Lorenzini, P.; Kamiya, T.; Kono, K.; Davidoff, A.M.; Chng, W.J.; Campana, D. T Lymphocytes Expressing a CD16 Signaling Receptor Exert Antibody-Dependent Cancer Cell Killing. Cancer Res. 2013, 74, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Ochi, F.; Fujiwara, H.; Tanimoto, K.; Asai, H.; Miyazaki, Y.; Okamoto, S.; Mineno, J.; Kuzushima, K.; Shiku, H.; Barrett, J.; et al. Gene-Modified Human α/β-T Cells Expressing a Chimeric CD16-CD3 Receptor as Adoptively Transferable Effector Cells for Anticancer Monoclonal Antibody Therapy. Cancer Immunol. Res. 2014, 2, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Lohmueller, J.J.; Ham, J.D.; Kvorjak, M.; Finn, O.J. mSA2 affinity-enhanced biotin-binding CAR T cells for universal tumor targeting. OncoImmunology 2017, 7, e1368604. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, Y.; Xiang, J.; Long, L.; Green, S.; Yang, Z.; Zimdahl, B.; Lu, J.; Cheng, N.; Horan, L.; et al. Targeting Alpha-Fetoprotein (AFP)–MHC Complex with CAR T-Cell Therapy for Liver Cancer. Clin. Cancer Res. 2016, 23, 478–488. [Google Scholar] [CrossRef]
- Jurtz, V.; Paul, S.; Andreatta, M.; Marcatili, P.; Peters, B.; Nielsen, M. NetMHCpan-4.0: Improved Peptide-MHC Class I Interaction Predictions Integrating Eluted Ligand and Peptide Binding Affinity Data. J. Immunol. 2017, 199, 3360–3368. [Google Scholar] [CrossRef]
- Bentzen, A.K.; Marquard, A.; Lyngaa, R.; Saini, S.K.; Ramskov, S.; Donia, M.; Such, L.; Furness, A.J.S.; McGranahan, N.; Rosenthal, R.; et al. Large-scale detection of antigen-specific T cells using peptide-MHC-I multimers labeled with DNA barcodes. Nat. Biotechnol. 2016, 34, 1037–1045. [Google Scholar] [CrossRef]
- Shitaoka, K.; Hamana, H.; Kishi, H.; Hayakawa, Y.; Kobayashi, E.; Sukegawa, K.; Piao, X.; Lyu, F.; Nagata, T.; Sugiyama, D.; et al. Identification of Tumoricidal TCRs from Tumor-Infiltrating Lymphocytes by Single-Cell Analysis. Cancer Immunol. Res. 2018, 6, 378–388. [Google Scholar] [CrossRef]
- Janssen, A.; Hidalgo, J.V.; Beringer, D.X.; Van Dooremalen, S.; Fernando, F.; Van Diest, E.; Terrizzi, A.R.; Bronsert, P.; Kock, S.; Schmitt-Graeff, A.; et al. γδ T-cell Receptors Derived from Breast Cancer–Infiltrating T Lymphocytes Mediate Antitumor Reactivity. Cancer Immunol. Res. 2020, 8, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Parkhurst, M.R.; Gros, A.; Pasetto, A.; Prickett, T.; Crystal, J.S.; Robbins, P.; Rosenberg, S.A. Isolation of T-Cell Receptors Specifically Reactive with Mutated Tumor-Associated Antigens from Tumor-Infiltrating Lymphocytes Based on CD137 Expression. Clin. Cancer Res. 2016, 23, 2491–2505. [Google Scholar] [CrossRef]
- Rapoport, A.P.; Stadtmauer, E.A.; Binder-Scholl, G.K.; Goloubeva, O.; Vogl, D.T.; Lacey, S.F.; Badros, A.Z.; Garfall, A.; Weiss, B.; Finklestein, J.; et al. NY-ESO-1–specific TCR–engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat. Med. 2015, 21, 914–921. [Google Scholar] [CrossRef]
- Crowther, M.D.; Dolton, G.; Legut, M.; Caillaud, M.E.; Lloyd, A.; Attaf, M.; Galloway, S.A.E.; Rius, C.; Farrell, C.P.; Szomolay, B.; et al. Genome-wide CRISPR–Cas9 screening reveals ubiquitous T cell cancer targeting via the monomorphic MHC class I-related protein MR1. Nat. Immunol. 2020, 21, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Legut, M.; Dolton, G.; Mian, A.A.; Ottmann, O.G.; Sewell, A.K. CRISPR-mediated TCR replacement generates superior anticancer transgenic T cells. Blood 2018, 131, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Lepore, M.; De Lalla, C.; Gundimeda, S.R.; Gsellinger, H.; Consonni, M.; Garavaglia, C.; Sansano, S.; Piccolo, F.; Scelfo, A.; Häussinger, D.; et al. A novel self-lipid antigen targets human T cells against CD1c+ leukemias. J. Exp. Med. 2014, 211, 1363–1377. [Google Scholar] [CrossRef]
- Westergaard, M.; Andersen, R.; Chong, C.; Kjeldsen, J.W.; Pedersen, M.; Friese, C.; Hasselager, T.; Lajer, H.; Coukos, G.; Bassani-Sternberg, M.; et al. Tumour-reactive T cell subsets in the microenvironment of ovarian cancer. Br. J. Cancer 2019, 120, 424–434. [Google Scholar] [CrossRef]
- Lu, Y.; Yao, X.; Crystal, J.S.; Li, Y.F.; El-Gamil, M.; Gross, C.; Davis, L.; Dudley, M.E.; Yang, J.C.; Samuels, Y.; et al. Efficient identification of mutated cancer antigens recognized by T cells associated with durable tumor regressions. Clin. Cancer Res. 2014, 20, 3401–3410. [Google Scholar] [CrossRef]
- Prieto, P.A.; Durflinger, K.H.; Wunderlich, J.R.; Rosenberg, S.A.; Dudley, M.E. Enrichment of CD8+ Cells From Melanoma Tumor-infiltrating Lymphocyte Cultures Reveals Tumor Reactivity for Use in Adoptive Cell Therapy. J. Immunother. 2010, 33, 547–556. [Google Scholar] [CrossRef]
- Giraldo, N.; Becht, E.; Vano, Y.; Petitprez, F.; Lacroix, L.; Validire, P.; Sanchez-Salas, R.; Ingels, A.; Oudard, S.; Moatti, A.; et al. Tumor-Infiltrating and Peripheral Blood T-cell Immunophenotypes Predict Early Relapse in Localized Clear Cell Renal Cell Carcinoma. Clin. Cancer Res. 2017, 23, 4416–4428. [Google Scholar] [CrossRef]
- Kuball, J.; Hauptrock, B.; Malina, V.; Antunes, E.; Voss, R.-H.; Wolfl, M.; Strong, R.; Theobald, M.; Greenberg, P.D. Increasing functional avidity of TCR-redirected T cells by removing defined N-glycosylation sites in the TCR constant domain. J. Exp. Med. 2009, 206, 463–475. [Google Scholar] [CrossRef]
- Hunder, N.N.; Wallen, H.; Cao, J.; Hendricks, D.W.; Reilly, J.Z.; Rodmyre, R.; Jungbluth, A.; Gnjatic, S.; Thompson, J.A.; Yee, C. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N. Engl. J. Med. 2008, 358, 2698–2703. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Melchiori, L.; Merchant, M.S.; Bernstein, D.B.; Glod, J.; Kaplan, R.N.; Grupp, S.A.; Tap, W.D.; Chagin, K.; Binder, G.K.; et al. Antitumor Activity Associated with Prolonged Persistence of Adoptively Transferred NY-ESO-1 c259T Cells in Synovial Sarcoma. Cancer Discov. 2018, 8, 944–957. [Google Scholar] [CrossRef] [PubMed]
- Robbins, P.F.; Kassim, S.H.; Tran, T.L.N.; Crystal, J.S.; Morgan, R.A.; Feldman, S.A.; Yang, J.C.; Dudley, M.E.; Wunderlich, J.R.; Sherry, R.M.; et al. A Pilot Trial Using Lymphocytes Genetically Engineered with an NY-ESO-1-Reactive T-cell Receptor: Long-term Follow-up and Correlates with Response. Clin. Cancer Res. 2014, 21, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Simon, B.; Harrer, D.C.; Schuler-Thurner, B.; Schuler, G.; Uslu, U. Arming T Cells with a gp100-Specific TCR and a CSPG4-Specific CAR Using Combined DNA- and RNA-Based Receptor Transfer. Cancers 2019, 11, 696. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, S.; Watanabe, N.; Bajgain, P.; Raja, K.; Mohammed, S.; Fisher, W.E.; Brenner, M.K.; Leen, A.M.; Vera, J.F. Enhancing the Potency and Specificity of Engineered T Cells for Cancer Treatment. Cancer Discov. 2018, 8, 972–987. [Google Scholar] [CrossRef]
- Van Loenen, M.M.; De Boer, R.; Amir, A.L.; Hagedoorn, R.S.; Volbeda, G.L.; Willemze, R.; Van Rood, J.J.; Falkenburg, J.H.F.; Heemskerk, M.H.M. Mixed T cell receptor dimers harbor potentially harmful neoreactivity. Proc. Natl. Acad. Sci. USA 2010, 107, 10972–10977. [Google Scholar] [CrossRef]
- Harris, D.T.; Hager, M.V.; Smith, S.; Cai, Q.; Stone, J.; Kruger, P.; Lever, M.; Dushek, O.; Schmitt, T.M.; Greenberg, P.D.; et al. Comparison of T Cell Activities Mediated by Human TCRs and CARs That Use the Same Recognition Domains. J. Immunol. 2017, 200, 1088–1100. [Google Scholar] [CrossRef]
- Oh, J.; Warshaviak, D.T.; Mkrtichyan, M.; Munguia, M.L.; Lin, A.; Chai, F.; Pigott, C.; Kang, J.; Gallo, M.; Kamb, A. Single variable domains from the T cell receptor β chain function as mono- and bifunctional CARs and TCRs. Sci. Rep. 2019, 9, 17291. [Google Scholar] [CrossRef]
- Schober, K.; Müller, T.R.; Gökmen, F.; Grassmann, S.; Effenberger, M.; Poltorak, M.; Stemberger, C.; Schumann, K.; Roth, T.L.; Marson, A.; et al. Orthotopic replacement of T-cell receptor α- and β-chains with preservation of near-physiological T-cell function. Nat. Biomed. Eng. 2019, 3, 974–984. [Google Scholar] [CrossRef]
- Ahmadi, M.; King, J.W.; Xue, S.-A.; Voisine, C.; Holler, A.; Wright, G.; Waxman, J.; Morris, E.; Stauss, H.J. CD3 limits the efficacy of TCR gene therapy in vivo. Blood 2011, 118, 3528–3537. [Google Scholar] [CrossRef]
- Stadtmauer, E.A.; Fraietta, J.A.; Davis, M.M.; Cohen, A.D.; Weber, K.L.; Lancaster, E.; Mangan, P.A.; Kulikovskaya, I.; Gupta, M.; Chen, F.; et al. CRISPR-engineered T cells in patients with refractory cancer. Science 2020, 367, eaba7365. [Google Scholar] [CrossRef] [PubMed]
- Garber, K. γδ T cells bring unconventional cancer-targeting to the clinic—Again. Nat. Biotechnol. 2020, 38, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Willcox, C.R.; Pitard, V.; Netzer, S.; Couzi, L.; Salim, M.; Silberzahn, T.; Moreau, J.-F.; Hayday, A.; Willcox, B.E.; Déchanet-Merville, J. Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein C receptor. Nat. Immunol. 2012, 13, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.I.; Le Nours, J.; Andrews, D.; Uldrich, A.P.; Rossjohn, J. Unconventional T Cell Targets for Cancer Immunotherapy. Immunity 2018, 48, 453–473. [Google Scholar] [CrossRef] [PubMed]
- Brandes, M.; Willimann, K.; Bioley, G.; Lévy, N.; Eberl, M.; Luo, M.; Tampé, R.; Lévy, F.; Romero, P.; Moser, B. Cross-presenting human T cells induce robust CD8+ T cell responses. Proc. Natl. Acad. Sci. USA 2009, 106, 2307–2312. [Google Scholar] [CrossRef]
- Liu, E.; Tong, Y.; Dotti, G.; Shaim, H.; Savoldo, B.; Mukherjee, M.; Orange, J.; Wan, X.; Lu, X.; Reynolds, A.; et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 2017, 32, 520–531. [Google Scholar] [CrossRef]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Kerbauy, L.N.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef]
- Bachanova, V.; Cooley, S.; DeFor, T.E.; Verneris, M.R.; Zhang, B.; McKenna, D.H.; Curtsinger, J.; Panoskaltsis-Mortari, A.; Lewis, D.; Hippen, K.; et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood 2014, 123, 3855–3863. [Google Scholar] [CrossRef]
- Romee, R.; Rosario, M.; Berrien-Elliott, M.M.; Wagner, J.A.; Jewell, B.A.; Schappe, T.; Leong, J.W.; Abdel-Latif, S.; Schneider, S.E.; Willey, S.; et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 2016, 8, 357ra123. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; DeFor, T.; Burns, L.J.; et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef] [PubMed]
- Klichinsky, M.; Ruella, M.; Shestova, O.; Lu, X.M.; Best, A.; Zeeman, M.; Schmierer, M.; Gabrusiewicz, K.; Anderson, N.R.; Petty, N.E.; et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 2020, 1–7. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Tummala, S.; Kebriaei, P.; Wierda, W.; Gutierrez, C.; Locke, F.; Komanduri, K.V.; Lin, Y.; Jain, N.; Daver, N.; et al. Chimeric antigen receptor T-cell therapy—Assessment and management of toxicities. Nat. Rev. Clin. Oncol. 2017, 15, 47–62. [Google Scholar] [CrossRef]
- Bueno, C.; Velasco-Hernández, T.; Gutiérrez-Agüera, F.; Zanetti, S.R.; Baroni, M.L.; Sanchez, D.; Molina, O.; Closa, A.; Agraz-Doblás, A.; Marín, P.; et al. CD133-directed CAR T-cells for MLL leukemia: On-target, off-tumor myeloablative toxicity. Leukemia 2019, 33, 2090–2125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L. The Emerging World of TCR-T Cell Trials Against Cancer: A Systematic Review. Technol. Cancer Res. Treat. 2019, 18, 1–13. [Google Scholar] [CrossRef]
- Marotte, L.; Simon, S.; Vignard, V.; Dupre, E.; Gantier, M.; Cruard, J.; Alberge, J.-B.; Hussong, M.; Deleine, C.; Heslan, J.-M.; et al. Increased antitumor efficacy of PD-1-deficient melanoma-specific human lymphocytes. J. Immunother. Cancer 2020, 8, e000311. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, T.; Natsume, A.; Nishimura, F.; Morimoto, T.; Matsuda, R.; Nakamura, M.; Yamada, S.; Nakagawa, I.; Motoyama, Y.; Park, Y.-S.; et al. Effect of CRISPR/Cas9-Mediated PD-1-Disrupted Primary Human Third-Generation CAR-T Cells Targeting EGFRvIII on In Vitro Human Glioblastoma Cell Growth. Cells 2020, 9, 998. [Google Scholar] [CrossRef]
- Jung, I.-Y.; Kim, Y.-Y.; Yu, H.-S.; Lee, M.; Kim, S.; Lee, J. CRISPR/Cas9-Mediated Knockout of DGK Improves Antitumor Activities of Human T Cells. Cancer Res. 2018, 78, 4692–4703. [Google Scholar] [CrossRef]
- Roth, T.L.; Li, P.J.; Blaeschke, F.; Nies, J.F.; Apathy, R.; Mowery, C.; Yu, R.; Nguyen, M.L.; Lee, Y.; Truong, A.; et al. Pooled Knockin Targeting for Genome Engineering of Cellular Immunotherapies. Cell 2020, 181, 728–744. [Google Scholar] [CrossRef]
- Qasim, W.; Zhan, H.; Samarasinghe, S.; Adams, S.; Amrolia, P.; Stafford, S.; Butler, K.; Rivat, C.; Wright, G.; Somana, K.; et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci. Transl. Med. 2017, 9, eaaj2013. [Google Scholar] [CrossRef] [PubMed]
| Cancer Type | Target | MHC | Clinical trial Number | Status | Year | Notes |
|---|---|---|---|---|---|---|
| Cervical Intraepithelial | HPV E7 | HLA-A*02:01 | NCT04411134 | Phase I - Not yet recruiting | 2020 | |
| Soft Tissue Sarcoma | NY-ESO-1 | HLA-A*02:01 | NCT04318964 | Phase I - Recruiting | 2020 | Affinity enhanced TCR |
| Unresectable Hepatocellular Carcinoma | AFP | HLA-A 02:01 | NCT04368182 | Phase I - Recruiting | 2020 | |
| Solid tumour | Tumour antigens | N/A | NCT03891706 | Phase I - Recruiting | 2019 | Screen for tumour reactivity and clone TCRs |
| Oropharyngeal squamous cell carcinoma | HPV E7 | HLA-A*02:01 | NCT04044950 | Phase II - Not yet recruiting | 2019 | |
| Oesophagus, Hepatoma, Glioma, Gastric | NY-ESO-1, Mesothelin, EGFRvIII and DR5 | HLAA*0201 | NCT03941626 | Phase I/II - Recruiting | 2019 | CAR-T/TCR-T cells include four different tumour-specific antibodies |
| HPV-Associated Oropharyngeal | HPV E7 | HLA-A 02:01 | NCT04015336 | Phase II - Recruiting | 2019 | |
| Solid Tumour | Autologous tumour antigen | N/A | NCT03970382 | Phase I - Recruiting | 2019 | 2 arms - with and without anti-PD-1 antibody |
| Vulvar High-Grade Squamous Intraepithelial | HPV E7 | HLA-A*0201 | NCT03937791 | Phase II - Recruiting | 2019 | |
| Head and Neck Squamous Cell Carcinoma | HPV-16 E6 | - | NCT04139057 | Phase I/II - Recruiting | 2019 | anti-PD1 auto-secreted element |
| Human Papillomavirus (HPV) 16+ Relapsed/Refractory | HPV E7 | HLA-A*02:01 | NCT03912831 | Phase I - Recruiting | 2019 | |
| Nasopharyngeal Carcinoma | LMP2 | HLA-A2, HLA-A11 or HLA-A24 | NCT03925896 | Phase I - Recruiting | 2019 | |
| Glioma, glioblastoma | - | - | NCT03392545 | Phase I - Recruiting | 2019 | |
| High Grade Squamous Intraepithelial | HPV E6 | HLA-A*02:01 | NCT03197025 | Phase I - Completed | 2019 | A single patient, no response |
| Pancreatic | KRAS G12V Mutant | HLA-C*08:02 | NCT04146298 | Phase I/II - Recruiting | 2019 | Anti-PD1 adjuvant if required |
| Breast | Autologous tumour antigen | - | NCT04194190 | Individual Patient Expanded Access | 2019 | Single patient individual access |
| Prostate | TP53 | - | NCT04135092 | Individual Patient Expanded Access | 2019 | Single Patient Expanded Access |
| Hepatocellular Carcinoma | AFP | HLA-A 02:01 | NCT03971747 | Phase I - Recruiting | 2019 | |
| Non-small cell lung | Tumour antigens | N/A | NCT03778814 | Phase I - Recruiting | 2018 | Screen for tumour reactivity and clone TCRs |
| Cervical Cancer, Head and Neck Squamous Cell Carcinoma | HPV-E6 | Not provided | NCT03578406 | Phase I - Recruiting | 2018 | TCR-T secretion of anti-PD1 |
| Wide range of solid and liquid cancers | CD19, CD22, CD33, CD38, BCMA, NY-ESO-1, c-met, Mesothelin, CEGFRvIII and DR5 | HLA-A*0201 | NCT03638206 | Phase I/II - Recruiting | 2018 | |
| Colorectal | TGFβRII frameshift antigen | HLA-A*0201 | NCT03431311 | Phase I/II - Terminated | 2018 | Only 1 patient enrolled, no results given |
| Fallopian Tube Carcinoma, Ovarian carcinoma, peritoneal carcinoma | NY-ESO-1 | HLA- A*02.1 and HLA-DP*04 | NCT03691376 | Phase I - Recruiting | 2018 | Melphalan pre-conditioning and TCR+ HSC transfer in addition to CD8+ TCR+ cells |
| Nasopharyngeal Carcinoma | LMP1, LMP2 and EBNA1 | HLA-A*0201/2402/1101 | NCT03648697 | Phase II - Not yet recruiting | 2018 | |
| Bone Sarcoma, Soft Tissue Sarcoma | NY-ESO-1 | HLA-A*02:01 | NCT03462316 | Phase I - Recruiting | 2018 | |
| Solid cancers | Patient-specific mutations | N/A | NCT03412877 | Phase II - Recruiting | 2018 | |
| Gastrointestinal, Pancreatic, Gastric, Colon, Rectal | G12D Variant of Mutated RAS | HLA-A*11:01 | NCT03745326 | Phase I/II - Recruiting | 2018 | Murine TCR. |
| Myeloid and Lymphoid Neoplasms | Preferentially Expressed Antigen in Melanoma (PRAME) | HLA-A*0201 | NCT03503968 | Phase I/II - Recruiting | 2018 | |
| Broad | NY-ESO-1/ LAGE-1a | HLA-A*02:01, HLA-A*02:05, and/or HLA-A*02:06 | NCT03709706 | Phase II - Recruiting | 2018 | Includes a pembrolizumab combination treatment arm |
| Merkel Cell Cancer | Merkel cell polyomavirus | HLA-A*02:01 | NCT03747484 | Phase I/II - Recruiting | 2018 | Inclusion requires previous anti-PD1 treatment |
| Lung Cancer, Non-small Cell, Recurrent | NY-ESO-1 | HLA-A*0201 | NCT03029273 | Phase I - Recruiting | 2017 | Affinity enhanced TCR |
| Acute Leukaemia | HA-1 | HLA-A*0201+ | NCT03326921 | Phase I - Recruiting | 2017 | Relapsed or refractory patients after stem cell transplant |
| Vaginal, Cervical, Anal, Penile, Oropharyngeal | HPV-16 E6 | HLA-A*02:01 | NCT02280811 | Phase I/II - Completed | 2017 | Partial response in 2/12 treated patients |
| Clear Cell Renal Cell Carcinoma | HERV-E | HLA-A*11:01 | NCT03354390 | Phase I - Recruiting | 2017 | HERV-E is an endogenous retrovirus |
| Solid tumour | MAGE-A3/A6 | HLA-DPB1*04:01 | NCT03139370 | Phase I - Recruiting | 2017 | |
| Gastrointestinal, Pancreatic, Gastric, Colon, Rectal | G12V Variant of Mutated RAS | HLA-A*11:01 | NCT03190941 | Phase I/II - Recruiting | 2017 | Murine TCR. |
| Synovial Sarcoma | NY-ESO-1 | (HLA)-A*02:01 or HLA-A*02:06 | NCT03250325 | Phase I/II - Active, not recruiting | 2017 | |
| Cervical Intraepithelial Neoplasia, Carcinoma in Situ, Vulvar | HPV E7 | HLA-A*02:01 | NCT02858310 | Phase I/II - Recruiting | 2016 | |
| Recurrent Hepatocellular Carcinoma | Hepatitis B | Not given | NCT02719782 | Phase I - Recruiting | 2016 | |
| Hepatocellular Carcinoma | Hepatitis B | Not given | NCT02686372 | Phase I - Recruiting | 2016 | |
| Haematological | Cytomegalovirus (CMV) | HLA-A*0201 | NCT02988258 | Phase I - Suspended | 2016 | Suspended (Protocol being re-written to allow inclusion of more patients) |
| Multiple | NY-ESO-1 | HLA-A*0201 | NCT02775292 | Phase I - Completed | 2016 | Also included NY-ESO-1 pulsed Dendritic cells adoptively transferred |
| Multiple | NY-ESO-1 | HLA-A*0201 | NCT02774291 | Phase I - Recruiting | 2016 | Uses murine TCR |
| Melanoma | MART-1 | HLA-A*0201 | NCT02654821 | Phase I/II - Active, not recruiting | 2016 | |
| Multiple | NY-ESO-1 | HLA-A*0201 | NCT02650986 | Phase I/II - Recruiting | 2016 | Also included TGFbDNRII gene. Two treatment arms, one included chemotherapy drug decitabine |
| Metastatic | NY-ESO-1 | HLA-A*0201 | NCT02062359 | Phase II - Terminated | 2016 | Study was closed due to poor accrual. TCR transduced into CD62L+ cells. |
| Melanoma | tyrosinase | HLA-A2 | NCT02870244 | Phase I - Recruiting | 2016 | |
| Acute Myeloid Leukaemia | Wilms tumour [WT]1 | HLA-A*0201 | NCT02770820 | Phase I/II - Active, not recruiting | 2016 | |
| Myelodysplastic Syndromes (MDS)Acute Myeloid Leukaemia (AML) | WT1 | HLA-A*02:01 | NCT02550535 | Phase I/II - Completed | 2015 | No results posted |
| Multiple | NY ESO-1 | HLA-A*02:01 | NCT02457650 | Phase I - Unknown | 2015 | Verified August 2016 by Shenzhen Second People’s Hospital. Recruitment status was: Recruiting |
| Thyroid Cancer | Thyroglobulin | HLA-A*0201 | NCT02390739 | Phase I/II - Withdrawn | 2015 | No results posted |
| Solid | NY-ESO-1 | HLA-A*0201 | NCT02366546 | Phase I - Active, not recruiting | 2015 | |
| Non-small Cell Lung Cancer or Mesothelioma | WT1 | HLA-A*0201 | NCT02408016 | Phase I/II - Active, not recruiting | 2015 | |
| Solid | MAGE-A4 | HLA-A*24:02 | NCT02096614 | Phase I - Unknown | 2014 | Verified November 2017 by Shinichi Kageyama, Mie University. Recruitment status was: Recruiting |
| Breast, Cervical, Renal, Melanoma, Bladder | MAGE-A3 | HLA-A 01 | NCT02153905 | Phase I/II - Terminated | 2014 | Terminated due to slow, insufficient accrual. 1/3 Patients had PR |
| Unidentified solid tumour | NY-ESO-1 | HLA-A*0201 | NCT02070406 | Phase I - Terminated | 2014 | Including DC vaccine and ipilumab treatment. Terminated due to low accrual. |
| Melanoma, Meningioma, Breast, Non-Small Cell Lung, Hepatocellular | NY-ESO-1 | HLA-A*0201 | NCT01967823 | Phase II - Completed | 2013 | No results posted |
| Metastatic | MAGE-A3/12 | HLA-A*0201 | NCT01273181 | Phase I/II - Terminated | 2013 | 4/9 Patients had CR or PR |
| AML and CML | WT1 | HLA-A*0201 | NCT01621724 | Phase I/II - Completed | 2012 | Completed, no results posted |
| Multiple | NY-ESO-1 | HLA-A*0201 | NCT01697527 | Phase II - Active, not recruiting | 2012 | |
| Ovarian | NYESO-1c259 | HLA A*0201, HLA-A*0205, and/or HLA-A*0206 | NCT01567891 | Phase I/II - Completed | 2012 | 0/6 responses |
| Melanoma | tyrosinase | HLA-A2 | NCT01586403 | Phase I - Active, not recruiting | 2012 | |
| Melanoma | gp 100:154, MART-1 F5 | HLA-A*0201 | NCT00923195 | Phase II - Completed | 2012 | Also, peptide vaccines. Progressive disease in 4/4 patients. |
| Leukaemia | WT1 | HLA-A*0201 | NCT01640301 | Phase I/II - Active, not recruiting | 2012 | After allogeneic HCT |
| Melanoma | NY-ESO-1ᶜ2⁵⁹ | HLA-A*0201 | NCT01350401 | Phase I/II - Terminated | 2011 | Terminated due to lack of enrolment. |
| Melanoma | TP53 | HLA-A*0201 | NCT00393029 | Phase II - Completed | 2011 | 1/9 Patients had tumour regression |
| Multiple Myeloma | NY-ESO-1c259 | HLA-A*0201 | NCT01352286 | Phase II - Completed | 2011 | OS of 35.1. |
| Renal, Kidney | TNF-related apoptosis inducing ligand (TRAIL) | HLA-DR4 | NCT00923390 | Phase I/II - Terminated | 2009 | Terminated after 10 years, no results posted |
| Kidney, Melanoma, Unspecified Adult Solid Tumour | TP53 | HLA-A*0201 | NCT00704938 | Phase II - Terminated | 2008 | Includes adenovirus p53 dendritic cell (DC) vaccine. Terminated due to withdrawal of support from collaborator. No CR or PR. |
| Melanoma | MART-1 F5 | HLA-A*0201 | NCT00706992 | Phase II - Terminated | 2008 | <11 subjects were enrolled to each Arm. No immune responses observed. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crowther, M.D.; Svane, I.M.; Met, Ö. T-Cell Gene Therapy in Cancer Immunotherapy: Why It Is No Longer Just CARs on The Road. Cells 2020, 9, 1588. https://doi.org/10.3390/cells9071588
Crowther MD, Svane IM, Met Ö. T-Cell Gene Therapy in Cancer Immunotherapy: Why It Is No Longer Just CARs on The Road. Cells. 2020; 9(7):1588. https://doi.org/10.3390/cells9071588
Chicago/Turabian StyleCrowther, Michael D., Inge Marie Svane, and Özcan Met. 2020. "T-Cell Gene Therapy in Cancer Immunotherapy: Why It Is No Longer Just CARs on The Road" Cells 9, no. 7: 1588. https://doi.org/10.3390/cells9071588
APA StyleCrowther, M. D., Svane, I. M., & Met, Ö. (2020). T-Cell Gene Therapy in Cancer Immunotherapy: Why It Is No Longer Just CARs on The Road. Cells, 9(7), 1588. https://doi.org/10.3390/cells9071588






