The Quest for the Best: How TCR Affinity, Avidity, and Functional Avidity Affect TCR-Engineered T-Cell Antitumor Responses
Abstract
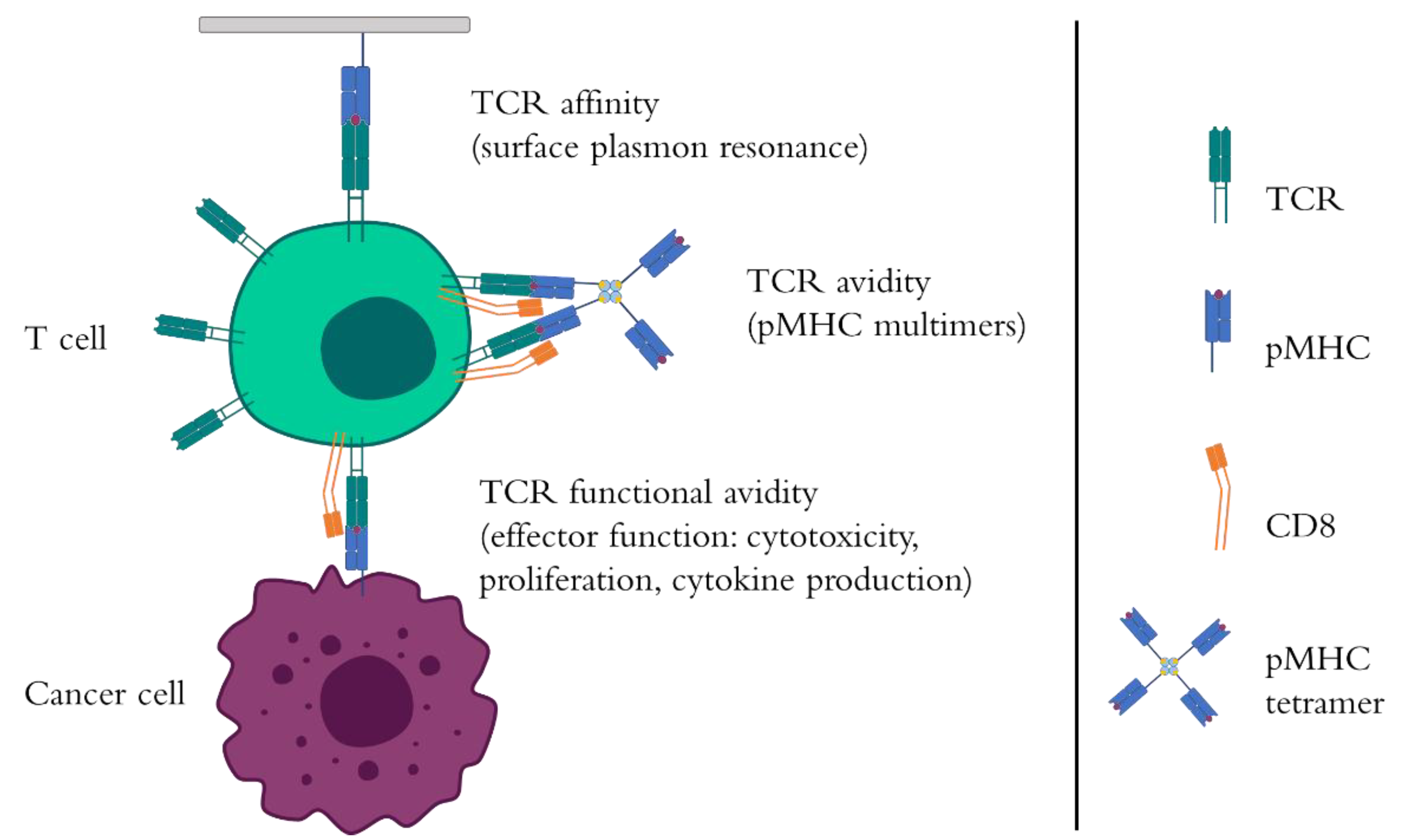
1. Introduction to TCR Affinity, Avidity, and Functional Avidity
2. The Role of Epitope Density
3. The Role of TCR Co-Receptors
4. Selection of Cancer-Specific TCRs
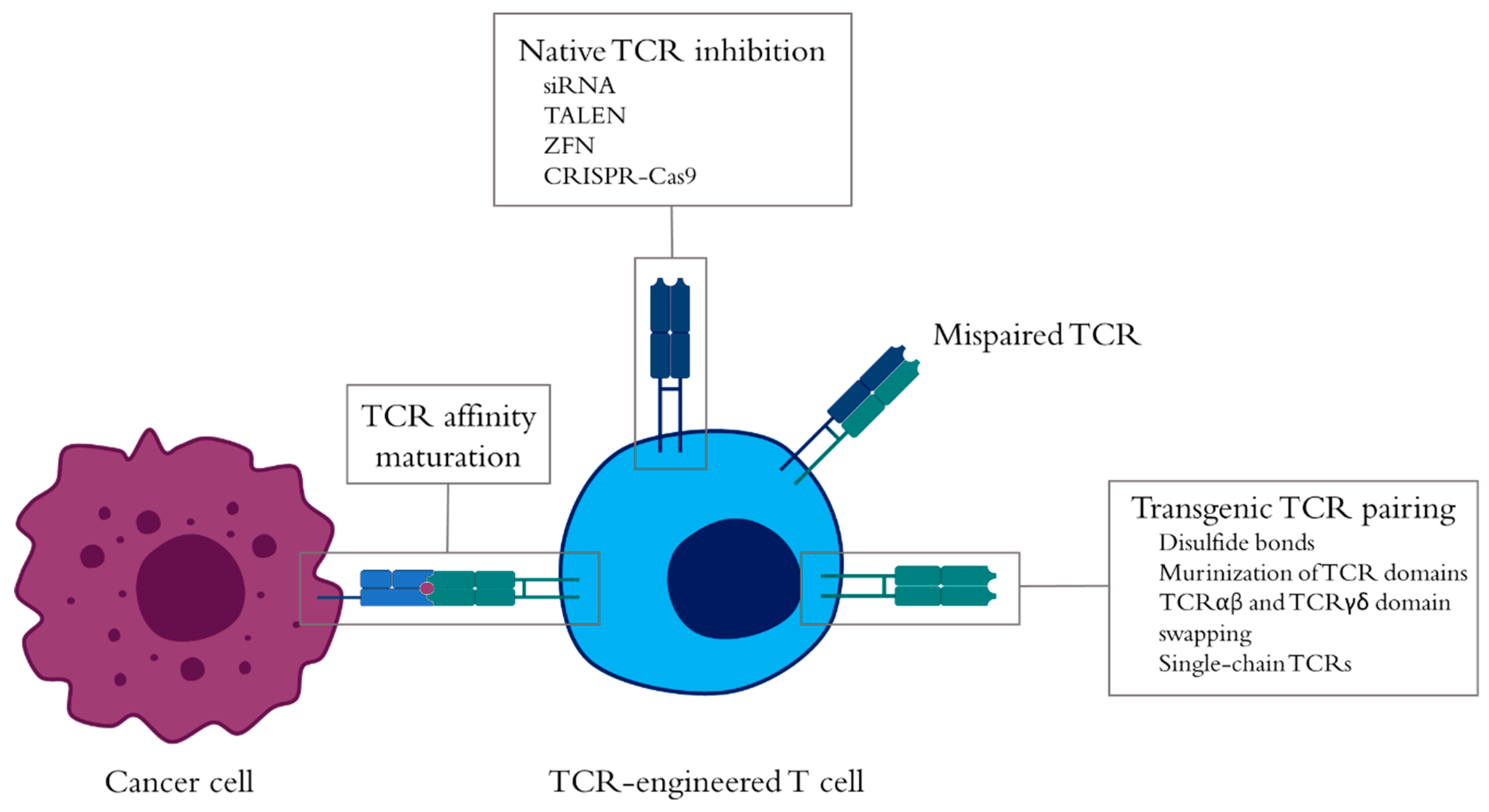
5. Improvement of TCR-Engineered T-Cell Antitumor Responses
6. Clinical Impact of TCR Affinity and Avidity in Cancer-Specific TCR-Engineered T Cells
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Jiang, X.; Xu, J.; Liu, M.; Xing, H.; Wang, Z.; Huang, L.; Mellor, A.L.; Wang, W.; Wu, S. Adoptive CD8(+) T cell therapy against cancer:Challenges and opportunities. Cancer Lett. 2019, 462, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Sasmal, D.K.; Feng, W.; Roy, S.; Leung, P.; He, Y.; Cai, C.; Cao, G.; Lian, H.; Qin, J.; Hui, E.; et al. TCR-pMHC bond conformation controls TCR ligand discrimination. Cell Mol. Immunol. 2020, 17, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.P.; Gerry, A.B.; Brewer, J.E.; Melchiori, L.; Bridgeman, J.S.; Bennett, A.D.; Pumphrey, N.J.; Jakobsen, B.K.; Price, D.A.; Ladell, K.; et al. T cell receptor binding affinity governs the functional profile of cancer-specific CD8+ T cells. Clin. Exp. Immunol. 2015, 180, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Campillo-Davo, D.; Versteven, M.; Roex, G.; Reu, H.; Heijden, S.V.; Anguille, S.; Berneman, Z.N.; Tendeloo, V.; Lion, E. Rapid Assessment of Functional Avidity of Tumor-Specific T Cell Receptors Using an Antigen-Presenting Tumor Cell Line Electroporated with Full-Length Tumor Antigen mRNA. Cancers (Basel) 2020, 12, 256. [Google Scholar] [CrossRef]
- Kammertoens, T.; Blankenstein, T. It’s the peptide-MHC affinity, stupid. Cancer Cell 2013, 23, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Malecek, K.; Johnson, L.A.; Yu, Z.; Vega-Saenz de Miera, E.; Darvishian, F.; McGary, K.; Huang, K.; Boyer, J.; Corse, E.; et al. T-cell receptor affinity and avidity defines antitumor response and autoimmunity in T-cell immunotherapy. Proc. Natl. Acad. Sci. USA 2013, 110, 6973–6978. [Google Scholar] [CrossRef]
- Oren, R.; Hod-Marco, M.; Haus-Cohen, M.; Thomas, S.; Blat, D.; Duvshani, N.; Denkberg, G.; Elbaz, Y.; Benchetrit, F.; Eshhar, Z.; et al. Functional comparison of engineered T cells carrying a native TCR versus TCR-like antibody-based chimeric antigen receptors indicates affinity/avidity thresholds. J. Immunol. 2014, 193, 5733–5743. [Google Scholar] [CrossRef]
- Schmid, D.A.; Irving, M.B.; Posevitz, V.; Hebeisen, M.; Posevitz-Fejfar, A.; Sarria, J.C.; Gomez-Eerland, R.; Thome, M.; Schumacher, T.N.; Romero, P.; et al. Evidence for a TCR affinity threshold delimiting maximal CD8 T cell function. J. Immunol. 2010, 184, 4936–4946. [Google Scholar] [CrossRef]
- Harris, D.T.; Hager, M.V.; Smith, S.N.; Cai, Q.; Stone, J.D.; Kruger, P.; Lever, M.; Dushek, O.; Schmitt, T.M.; Greenberg, P.D.; et al. Comparison of T Cell Activities Mediated by Human TCRs and CARs That Use the Same Recognition Domains. J. Immunol. 2018, 200, 1088–1100. [Google Scholar] [CrossRef]
- Galvez, J.; Galvez, J.J.; Garcia-Penarrubia, P. Is TCR/pMHC Affinity a Good Estimate of the T-cell Response? An Answer Based on Predictions from 12 Phenotypic Models. Front. Immunol. 2019, 10, 349. [Google Scholar] [CrossRef]
- Mahe, E.; Pugh, T.; Kamel-Reid, S. T cell clonality assessment: Past, present and future. J. Clin. Pathol. 2018, 71, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Aleksic, M.; Liddy, N.; Molloy, P.E.; Pumphrey, N.; Vuidepot, A.; Chang, K.M.; Jakobsen, B.K. Different affinity windows for virus and cancer-specific T-cell receptors: Implications for therapeutic strategies. Eur. J. Immunol. 2012, 42, 3174–3179. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Xue, S.A.; Bangham, C.R.; Jakobsen, B.K.; Morris, E.C.; Stauss, H.J. Human T cells expressing affinity-matured TCR display accelerated responses but fail to recognize low density of MHC-peptide antigen. Blood 2011, 118, 319–329. [Google Scholar] [CrossRef]
- Gannon, P.O.; Wieckowski, S.; Baumgaertner, P.; Hebeisen, M.; Allard, M.; Speiser, D.E.; Rufer, N. Quantitative TCR:pMHC Dissociation Rate Assessment by NTAmers Reveals Antimelanoma T Cell Repertoires Enriched for High Functional Competence. J. Immunol. 2015, 195, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, P.A.; Carreno, L.J.; Coombs, D.; Mora, J.E.; Palmieri, E.; Goldstein, B.; Nathenson, S.G.; Kalergis, A.M. T cell receptor binding kinetics required for T cell activation depend on the density of cognate ligand on the antigen-presenting cell. Proc. Natl. Acad. Sci. USA 2005, 102, 4824–4829. [Google Scholar] [CrossRef] [PubMed]
- Engels, B.; Engelhard, V.H.; Sidney, J.; Sette, A.; Binder, D.C.; Liu, R.B.; Kranz, D.M.; Meredith, S.C.; Rowley, D.A.; Schreiber, H. Relapse or eradication of cancer is predicted by peptide-major histocompatibility complex affinity. Cancer Cell 2013, 23, 516–526. [Google Scholar] [CrossRef]
- Reeves, E.; James, E. Antigen processing and immune regulation in the response to tumours. Immunology 2017, 150, 16–24. [Google Scholar] [CrossRef]
- Weinzierl, A.O.; Lemmel, C.; Schoor, O.; Muller, M.; Kruger, T.; Wernet, D.; Hennenlotter, J.; Stenzl, A.; Klingel, K.; Rammensee, H.G.; et al. Distorted relation between mRNA copy number and corresponding major histocompatibility complex ligand density on the cell surface. Mol. Cell Proteom. 2007, 6, 102–113. [Google Scholar] [CrossRef]
- Stranzl, T.; Larsen, M.V.; Lund, O.; Nielsen, M.; Brunak, S. The cancer exome generated by alternative mRNA splicing dilutes predicted HLA class I epitope density. PLoS ONE 2012, 7, e38670. [Google Scholar] [CrossRef]
- Purbhoo, M.A.; Sutton, D.H.; Brewer, J.E.; Mullings, R.E.; Hill, M.E.; Mahon, T.M.; Karbach, J.; Jager, E.; Cameron, B.J.; Lissin, N.; et al. Quantifying and imaging NY-ESO-1/LAGE-1-derived epitopes on tumor cells using high affinity T cell receptors. J. Immunol. 2006, 176, 7308–7316. [Google Scholar] [CrossRef]
- Bossi, G.; Gerry, A.B.; Paston, S.J.; Sutton, D.H.; Hassan, N.J.; Jakobsen, B.K. Examining the presentation of tumor-associated antigens on peptide-pulsed T2 cells. Oncoimmunology 2013, 2, e26840. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Tsukahara, T.; Toji, S.; Saitoh, S.; Hirohashi, Y.; Nakatsugawa, M.; Kubo, T.; Kanaseki, T.; Kameshima, H.; Terui, T.; et al. Development of a T-cell receptor multimer with high avidity for detecting a naturally presented tumor-associated antigen on osteosarcoma cells. Cancer Sci. 2019, 110, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Brameshuber, M.; Zeng, X.; Xie, J.; Li, Q.J.; Chien, Y.H.; Valitutti, S.; Davis, M.M. A single peptide-major histocompatibility complex ligand triggers digital cytokine secretion in CD4(+) T cells. Immunity 2013, 39, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Valitutti, S.; Muller, S.; Cella, M.; Padovan, E.; Lanzavecchia, A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature 1995, 375, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Purbhoo, M.A.; Irvine, D.J.; Huppa, J.B.; Davis, M.M. T cell killing does not require the formation of a stable mature immunological synapse. Nat. Immunol. 2004, 5, 524–530. [Google Scholar] [CrossRef]
- Deeg, J.; Axmann, M.; Matic, J.; Liapis, A.; Depoil, D.; Afrose, J.; Curado, S.; Dustin, M.L.; Spatz, J.P. T cell activation is determined by the number of presented antigens. Nano. Lett. 2013, 13, 5619–5626. [Google Scholar] [CrossRef]
- Segal, G.; Prato, S.; Zehn, D.; Mintern, J.D.; Villadangos, J.A. Target Density, Not Affinity or Avidity of Antigen Recognition, Determines Adoptive T Cell Therapy Outcomes in a Mouse Lymphoma Model. J. Immunol. 2016, 196, 3935–3942. [Google Scholar] [CrossRef]
- Dougan, S.K.; Dougan, M.; Kim, J.; Turner, J.A.; Ogata, S.; Cho, H.I.; Jaenisch, R.; Celis, E.; Ploegh, H.L. Transnuclear TRP1-specific CD8 T cells with high or low affinity TCRs show equivalent antitumor activity. Cancer Immunol. Res. 2013, 1, 99–111. [Google Scholar] [CrossRef]
- Vincent, K.; Hardy, M.P.; Trofimov, A.; Laumont, C.M.; Sriranganadane, D.; Hadj-Mimoune, S.; Salem Fourati, I.; Soudeyns, H.; Thibault, P.; Perreault, C. Rejection of leukemic cells requires antigen-specific T cells with high functional avidity. Biol. Blood Marrow Transplant. 2014, 20, 37–45. [Google Scholar] [CrossRef][Green Version]
- Jaigirdar, A.; Rosenberg, S.A.; Parkhurst, M. A High-avidity WT1-reactive T-Cell Receptor Mediates Recognition of Peptide and Processed Antigen but not Naturally Occurring WT1-positive Tumor Cells. J. Immunother. 2016, 39, 105–116. [Google Scholar] [CrossRef]
- Killeen, N.; Davis, C.B.; Chu, K.; Crooks, M.E.; Sawada, S.; Scarborough, J.D.; Boyd, K.A.; Stuart, S.G.; Xu, H.; Littman, D.R. CD4 function in thymocyte differentiation and T cell activation. Phil. Trans. R. Soc. Lond. B 1993, 342, 25–34. [Google Scholar] [CrossRef]
- Luescher, I.F.; Vivier, E.; Layer, A.; Mahiou, J.; Godeau, F.; Malissen, B.; Romero, P. CD8 modulation of T-cell antigen receptor-ligand interactions on living cytotoxic T lymphocytes. Nature 1995, 373, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Wooldridge, L.; van den Berg, H.A.; Glick, M.; Gostick, E.; Laugel, B.; Hutchinson, S.L.; Milicic, A.; Brenchley, J.M.; Douek, D.C.; Price, D.A.; et al. Interaction between the CD8 coreceptor and major histocompatibility complex class I stabilizes T cell receptor-antigen complexes at the cell surface. J. Biol. Chem. 2005, 280, 27491–27501. [Google Scholar] [CrossRef] [PubMed]
- Barber, E.K.; Dasgupta, J.D.; Schlossman, S.F.; Trevillyan, J.M.; Rudd, C.E. The CD4 and CD8 antigens are coupled to a protein-tyrosine kinase (p56lck) that phosphorylates the CD3 complex. Proc. Natl. Acad. Sci. USA 1989, 86, 3277–3281. [Google Scholar] [CrossRef]
- Veillette, A.; Bookman, M.A.; Horak, E.M.; Bolen, J.B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell 1988, 55, 301–308. [Google Scholar] [CrossRef]
- Spear, T.T.; Wang, Y.; Foley, K.C.; Murray, D.C.; Scurti, G.M.; Simms, P.E.; Garrett-Mayer, E.; Hellman, L.M.; Baker, B.M.; Nishimura, M.I. Critical biological parameters modulate affinity as a determinant of function in T-cell receptor gene-modified T-cells. Cancer Immunol. Immunother. 2017, 66, 1411–1424. [Google Scholar] [CrossRef]
- Hamad, A.R.; O’Herrin, S.M.; Lebowitz, M.S.; Srikrishnan, A.; Bieler, J.; Schneck, J.; Pardoll, D. Potent T cell activation with dimeric peptide-major histocompatibility complex class II ligand: The role of CD4 coreceptor. J. Exp. Med. 1998, 188, 1633–1640. [Google Scholar] [CrossRef]
- Huppa, J.B.; Axmann, M.; Mortelmaier, M.A.; Lillemeier, B.F.; Newell, E.W.; Brameshuber, M.; Klein, L.O.; Schutz, G.J.; Davis, M.M. TCR-peptide-MHC interactions in situ show accelerated kinetics and increased affinity. Nature 2010, 463, 963–967. [Google Scholar] [CrossRef]
- Artyomov, M.N.; Lis, M.; Devadas, S.; Davis, M.M.; Chakraborty, A.K. CD4 and CD8 binding to MHC molecules primarily acts to enhance Lck delivery. Proc. Natl. Acad. Sci. USA 2010, 107, 16916–16921. [Google Scholar] [CrossRef]
- Harding, S.; Lipp, P.; Alexander, D.R. A therapeutic CD4 monoclonal antibody inhibits TCR-zeta chain phosphorylation, zeta-associated protein of 70-kDa Tyr319 phosphorylation, and TCR internalization in primary human T cells. J. Immunol. 2002, 169, 230–238. [Google Scholar] [CrossRef]
- Daniels, M.A.; Jameson, S.C. Critical role for CD8 in T cell receptor binding and activation by peptide/major histocompatibility complex multimers. J. Exp. Med. 2000, 191, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Lyons, G.E.; Moore, T.; Brasic, N.; Li, M.; Roszkowski, J.J.; Nishimura, M.I. Influence of human CD8 on antigen recognition by T-cell receptor-transduced cells. Cancer Res. 2006, 66, 11455–11461. [Google Scholar] [CrossRef] [PubMed]
- Laugel, B.; van den Berg, H.A.; Gostick, E.; Cole, D.K.; Wooldridge, L.; Boulter, J.; Milicic, A.; Price, D.A.; Sewell, A.K. Different T cell receptor affinity thresholds and CD8 coreceptor dependence govern cytotoxic T lymphocyte activation and tetramer binding properties. J. Biol. Chem. 2007, 282, 23799–23810. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, H.A.; Wooldridge, L.; Laugel, B.; Sewell, A.K. Coreceptor CD8-driven modulation of T cell antigen receptor specificity. J. Theor. Biol. 2007, 249, 395–408. [Google Scholar] [CrossRef]
- Irie, H.Y.; Ravichandran, K.S.; Burakoff, S.J. CD8 beta chain influences CD8 alpha chain-associated Lck kinase activity. J. Exp. Med. 1995, 181, 1267–1273. [Google Scholar] [CrossRef]
- Cawthon, A.G.; Lu, H.; Alexander-Miller, M.A. Peptide requirement for CTL activation reflects the sensitivity to CD3 engagement: Correlation with CD8alphabeta versus CD8alphaalpha expression. J. Immunol. 2001, 167, 2577–2584. [Google Scholar] [CrossRef]
- Vigano, S.; Utzschneider, D.T.; Perreau, M.; Pantaleo, G.; Zehn, D.; Harari, A. Functional avidity: A measure to predict the efficacy of effector T cells? Clin. Dev. Immunol. 2012, 2012, 153863. [Google Scholar] [CrossRef]
- Choi, E.M.; Chen, J.L.; Wooldridge, L.; Salio, M.; Lissina, A.; Lissin, N.; Hermans, I.F.; Silk, J.D.; Mirza, F.; Palmowski, M.J.; et al. High avidity antigen-specific CTL identified by CD8-independent tetramer staining. J. Immunol. 2003, 171, 5116–5123. [Google Scholar] [CrossRef]
- Kerry, S.E.; Buslepp, J.; Cramer, L.A.; Maile, R.; Hensley, L.L.; Nielsen, A.I.; Kavathas, P.; Vilen, B.J.; Collins, E.J.; Frelinger, J.A. Interplay between TCR affinity and necessity of coreceptor ligation: High-affinity peptide-MHC/TCR interaction overcomes lack of CD8 engagement. J. Immunol. 2003, 171, 4493–4503. [Google Scholar] [CrossRef]
- Purbhoo, M.A.; Boulter, J.M.; Price, D.A.; Vuidepot, A.L.; Hourigan, C.S.; Dunbar, P.R.; Olson, K.; Dawson, S.J.; Phillips, R.E.; Jakobsen, B.K.; et al. The human CD8 coreceptor effects cytotoxic T cell activation and antigen sensitivity primarily by mediating complete phosphorylation of the T cell receptor zeta chain. J. Biol. Chem. 2001, 276, 32786–32792. [Google Scholar] [CrossRef]
- Wooldridge, L.; Lissina, A.; Vernazza, J.; Gostick, E.; Laugel, B.; Hutchinson, S.L.; Mirza, F.; Dunbar, P.R.; Boulter, J.M.; Glick, M.; et al. Enhanced immunogenicity of CTL antigens through mutation of the CD8 binding MHC class I invariant region. Eur. J. Immunol. 2007, 37, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- Schonrich, G.; Kalinke, U.; Momburg, F.; Malissen, M.; Schmitt-Verhulst, A.M.; Malissen, B.; Hammerling, G.J.; Arnold, B. Down-regulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell 1991, 65, 293–304. [Google Scholar] [CrossRef]
- Stone, J.D.; Kranz, D.M. Role of T cell receptor affinity in the efficacy and specificity of adoptive T cell therapies. Front. Immunol. 2013, 4, 244. [Google Scholar] [CrossRef] [PubMed]
- Inderberg, E.M.; Walchli, S. Long-term surviving cancer patients as a source of therapeutic TCR. Cancer Immunol. Immunother. 2020, 69, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Bezu, L.; Kepp, O.; Cerrato, G.; Pol, J.; Fucikova, J.; Spisek, R.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial watch: Peptide-based vaccines in anticancer therapy. Oncoimmunology 2018, 7, e1511506. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, K.; Lazzaro, S.; Lutz, J.; Rauch, S.; Heidenreich, R. mRNA Cancer Vaccines. Recent Results Cancer Res. 2016, 209, 61–85. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.Y.; Nguyen, H.N.; Wolfl, M.; Kuball, J.; Greenberg, P.D. In vitro methods for generating CD8+ T-cell clones for immunotherapy from the naive repertoire. J Immunol. Methods 2006, 310, 40–52. [Google Scholar] [CrossRef]
- Falkenburg, W.J.; Melenhorst, J.J.; van de Meent, M.; Kester, M.G.; Hombrink, P.; Heemskerk, M.H.; Hagedoorn, R.S.; Gostick, E.; Price, D.A.; Falkenburg, J.H.; et al. Allogeneic HLA-A*02-restricted WT1-specific T cells from mismatched donors are highly reactive but show off-target promiscuity. J. Immunol. 2011, 187, 2824–2833. [Google Scholar] [CrossRef]
- Amir, A.L.; van der Steen, D.M.; van Loenen, M.M.; Hagedoorn, R.S.; de Boer, R.; Kester, M.D.; de Ru, A.H.; Lugthart, G.J.; van Kooten, C.; Hiemstra, P.S.; et al. PRAME-specific Allo-HLA-restricted T cells with potent antitumor reactivity useful for therapeutic T-cell receptor gene transfer. Clin. Cancer Res. 2011, 17, 5615–5625. [Google Scholar] [CrossRef]
- Herr, W.; Eichinger, Y.; Beshay, J.; Bloetz, A.; Vatter, S.; Mirbeth, C.; Distler, E.; Hartwig, U.F.; Thomas, S. HLA-DPB1 mismatch alleles represent powerful leukemia rejection antigens in CD4 T-cell immunotherapy after allogeneic stem-cell transplantation. Leukemia 2017, 31, 434–445. [Google Scholar] [CrossRef]
- Schmitt, T.M.; Aggen, D.H.; Stromnes, I.M.; Dossett, M.L.; Richman, S.A.; Kranz, D.M.; Greenberg, P.D. Enhanced-affinity murine T-cell receptors for tumor/self-antigens can be safe in gene therapy despite surpassing the threshold for thymic selection. Blood 2013, 122, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Rosati, S.F.; Parkhurst, M.R.; Hong, Y.; Zheng, Z.; Feldman, S.A.; Rao, M.; Abate-Daga, D.; Beard, R.E.; Xu, H.; Black, M.A.; et al. A novel murine T-cell receptor targeting NY-ESO-1. J. Immunother. 2014, 37, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Stanislawski, T.; Voss, R.H.; Lotz, C.; Sadovnikova, E.; Willemsen, R.A.; Kuball, J.; Ruppert, T.; Bolhuis, R.L.; Melief, C.J.; Huber, C.; et al. Circumventing tolerance to a human MDM2-derived tumor antigen by TCR gene transfer. Nat. Immunol. 2001, 2, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, H.; Brenner, M.K. Immunotherapy against cancer-related viruses. Cell Res. 2017, 27, 59–73. [Google Scholar] [CrossRef]
- Jiang, T.; Shi, T.; Zhang, H.; Hu, J.; Song, Y.; Wei, J.; Ren, S.; Zhou, C. Tumor neoantigens: From basic research to clinical applications. J. Hematol. Oncol. 2019, 12, 93. [Google Scholar] [CrossRef]
- Liu, S.; Matsuzaki, J.; Wei, L.; Tsuji, T.; Battaglia, S.; Hu, Q.; Cortes, E.; Wong, L.; Yan, L.; Long, M.; et al. Efficient identification of neoantigen-specific T-cell responses in advanced human ovarian cancer. J. Immunother. Cancer 2019, 7, 156. [Google Scholar] [CrossRef]
- Ren, L.; Leisegang, M.; Deng, B.; Matsuda, T.; Kiyotani, K.; Kato, T.; Harada, M.; Park, J.H.; Saloura, V.; Seiwert, T.; et al. Identification of neoantigen-specific T cells and their targets: Implications for immunotherapy of head and neck squamous cell carcinoma. Oncoimmunology 2019, 8, e1568813. [Google Scholar] [CrossRef]
- Yamamoto, T.N.; Kishton, R.J.; Restifo, N.P. Developing neoantigen-targeted T cell-based treatments for solid tumors. Nat. Med. 2019, 25, 1488–1499. [Google Scholar] [CrossRef]
- Hillerdal, V.; Boura, V.F.; Bjorkelund, H.; Andersson, K.; Essand, M. Avidity characterization of genetically engineered T-cells with novel and established approaches. BMC Immunol. 2016, 17, 23. [Google Scholar] [CrossRef]
- Bentzen, A.K.; Hadrup, S.R. Evolution of MHC-based technologies used for detection of antigen-responsive T cells. Cancer Immunol. Immunother. 2017, 66, 657–666. [Google Scholar] [CrossRef]
- Holland, C.J.; Dolton, G.; Scurr, M.; Ladell, K.; Schauenburg, A.J.; Miners, K.; Madura, F.; Sewell, A.K.; Price, D.A.; Cole, D.K.; et al. Enhanced Detection of Antigen-Specific CD4+ T Cells Using Altered Peptide Flanking Residue Peptide-MHC Class II Multimers. J. Immunol. 2015, 195, 5827–5836. [Google Scholar] [CrossRef] [PubMed]
- Rius, C.; Attaf, M.; Tungatt, K.; Bianchi, V.; Legut, M.; Bovay, A.; Donia, M.; Thor Straten, P.; Peakman, M.; Svane, I.M.; et al. Peptide-MHC Class I Tetramers Can Fail To Detect Relevant Functional T Cell Clonotypes and Underestimate Antigen-Reactive T Cell Populations. J. Immunol. 2018, 200, 2263–2279. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, S.; Fujiki, F.; Kondo, K.; Nakajima, H.; Kobayashi, Y.; Inatome, M.; Aoyama, N.; Nishida, Y.; Tsuboi, A.; Oka, Y.; et al. Establishment of a novel platform cell line for efficient and precise evaluation of T cell receptor functional avidity. Oncotarget 2018, 9, 34132–34141. [Google Scholar] [CrossRef] [PubMed]
- Campillo-Davo, D.; Fujiki, F.; Van den Bergh, J.M.J.; De Reu, H.; Smits, E.; Goossens, H.; Sugiyama, H.; Lion, E.; Berneman, Z.N.; Van Tendeloo, V. Efficient and Non-genotoxic RNA-Based Engineering of Human T Cells Using Tumor-Specific T Cell Receptors With Minimal TCR Mispairing. Front. Immunol. 2018, 9, 2503. [Google Scholar] [CrossRef]
- Rosskopf, S.; Leitner, J.; Paster, W.; Morton, L.T.; Hagedoorn, R.S.; Steinberger, P.; Heemskerk, M.H.M. A Jurkat 76 based triple parameter reporter system to evaluate TCR functions and adoptive T cell strategies. Oncotarget 2018, 9, 17608–17619. [Google Scholar] [CrossRef]
- Wolfl, M.; Kuball, J.; Ho, W.Y.; Nguyen, H.; Manley, T.J.; Bleakley, M.; Greenberg, P.D. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood 2007, 110, 201–210. [Google Scholar] [CrossRef]
- Border, E.C.; Sanderson, J.P.; Weissensteiner, T.; Gerry, A.B.; Pumphrey, N.J. Affinity-enhanced T-cell receptors for adoptive T-cell therapy targeting MAGE-A10: Strategy for selection of an optimal candidate. Oncoimmunology 2019, 8, e1532759. [Google Scholar] [CrossRef]
- Mahnke, Y.D.; Devevre, E.; Baumgaertner, P.; Matter, M.; Rufer, N.; Romero, P.; Speiser, D.E. Human melanoma-specific CD8(+) T-cells from metastases are capable of antigen-specific degranulation and cytolysis directly ex vivo. Oncoimmunology 2012, 1, 467–530. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Moysey, R.; Molloy, P.E.; Vuidepot, A.L.; Mahon, T.; Baston, E.; Dunn, S.; Liddy, N.; Jacob, J.; Jakobsen, B.K.; et al. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat. Biotechnol. 2005, 23, 349–354. [Google Scholar] [CrossRef]
- Zhao, Y.; Bennett, A.D.; Zheng, Z.; Wang, Q.J.; Robbins, P.F.; Yu, L.Y.; Li, Y.; Molloy, P.E.; Dunn, S.M.; Jakobsen, B.K.; et al. High-affinity TCRs generated by phage display provide CD4+ T cells with the ability to recognize and kill tumor cell lines. J. Immunol. 2007, 179, 5845–5854. [Google Scholar] [CrossRef]
- Holler, P.D.; Holman, P.O.; Shusta, E.V.; O’Herrin, S.; Wittrup, K.D.; Kranz, D.M. In vitro evolution of a T cell receptor with high affinity for peptide/MHC. Proc. Natl. Acad. Sci. USA 2000, 97, 5387–5392. [Google Scholar] [CrossRef]
- Malecek, K.; Zhong, S.; McGary, K.; Yu, C.; Huang, K.; Johnson, L.A.; Rosenberg, S.A.; Krogsgaard, M. Engineering improved T cell receptors using an alanine-scan guided T cell display selection system. J. Immunol. Methods 2013, 392, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Robbins, P.F.; Li, Y.F.; El-Gamil, M.; Zhao, Y.; Wargo, J.A.; Zheng, Z.; Xu, H.; Morgan, R.A.; Feldman, S.A.; Johnson, L.A.; et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J. Immunol. 2008, 180, 6116–6131. [Google Scholar] [CrossRef] [PubMed]
- Malecek, K.; Grigoryan, A.; Zhong, S.; Gu, W.J.; Johnson, L.A.; Rosenberg, S.A.; Cardozo, T.; Krogsgaard, M. Specific increase in potency via structure-based design of a TCR. J. Immunol. 2014, 193, 2587–2599. [Google Scholar] [CrossRef] [PubMed]
- Cole, D.K.; Sami, M.; Scott, D.R.; Rizkallah, P.J.; Borbulevych, O.Y.; Todorov, P.T.; Moysey, R.K.; Jakobsen, B.K.; Boulter, J.M.; Baker, B.M.; et al. Increased Peptide Contacts Govern High Affinity Binding of a Modified TCR Whilst Maintaining a Native pMHC Docking Mode. Front. Immunol. 2013, 4, 168. [Google Scholar] [CrossRef] [PubMed]
- Bassan, D.; Gozlan, Y.M.; Sharbi-Yunger, A.; Tzehoval, E.; Eisenbach, L. Optimizing T-cell receptor avidity with somatic hypermutation. Int. J. Cancer 2019, 145, 2816–2826. [Google Scholar] [CrossRef]
- Shao, H.; Zhang, W.; Hu, Q.; Wu, F.; Shen, H.; Huang, S. TCR mispairing in genetically modified T cells was detected by fluorescence resonance energy transfer. Mol. Biol. Rep. 2010, 37, 3951–3956. [Google Scholar] [CrossRef] [PubMed]
- van Loenen, M.M.; de Boer, R.; Amir, A.L.; Hagedoorn, R.S.; Volbeda, G.L.; Willemze, R.; van Rood, J.J.; Falkenburg, J.H.; Heemskerk, M.H. Mixed T cell receptor dimers harbor potentially harmful neoreactivity. Proc. Natl. Acad. Sci. USA 2010, 107, 10972–10977. [Google Scholar] [CrossRef]
- Bendle, G.M.; Linnemann, C.; Hooijkaas, A.I.; Bies, L.; de Witte, M.A.; Jorritsma, A.; Kaiser, A.D.; Pouw, N.; Debets, R.; Kieback, E.; et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat. Med. 2010, 16, 565–570. [Google Scholar] [CrossRef]
- Ahmadi, M.; King, J.W.; Xue, S.A.; Voisine, C.; Holler, A.; Wright, G.P.; Waxman, J.; Morris, E.; Stauss, H.J. CD3 limits the efficacy of TCR gene therapy in vivo. Blood 2011, 118, 3528–3537. [Google Scholar] [CrossRef]
- Okamoto, S.; Mineno, J.; Ikeda, H.; Fujiwara, H.; Yasukawa, M.; Shiku, H.; Kato, I. Improved expression and reactivity of transduced tumor-specific TCRs in human lymphocytes by specific silencing of endogenous TCR. Cancer Res. 2009, 69, 9003–9011. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Amaishi, Y.; Goto, Y.; Ikeda, H.; Fujiwara, H.; Kuzushima, K.; Yasukawa, M.; Shiku, H.; Mineno, J. A Promising Vector for TCR Gene Therapy: Differential Effect of siRNA, 2A Peptide, and Disulfide Bond on the Introduced TCR Expression. Mol. Ther. Nucleic Acids 2012, 1, e63. [Google Scholar] [CrossRef]
- Ochi, T.; Fujiwara, H.; Okamoto, S.; An, J.; Nagai, K.; Shirakata, T.; Mineno, J.; Kuzushima, K.; Shiku, H.; Yasukawa, M. Novel adoptive T-cell immunotherapy using a WT1-specific TCR vector encoding silencers for endogenous TCRs shows marked antileukemia reactivity and safety. Blood 2011, 118, 1495–1503. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, X.; Wang, L.; Gao, X.; Xiong, Y.; Liu, L.; Wei, F.; Yang, L.; Ren, X. T-cell receptor gene therapy targeting melanoma-associated antigen-A4 by silencing of endogenous TCR inhibits tumor growth in mice and human. Cell Death Dis. 2019, 10, 475. [Google Scholar] [CrossRef] [PubMed]
- Provasi, E.; Genovese, P.; Lombardo, A.; Magnani, Z.; Liu, P.Q.; Reik, A.; Chu, V.; Paschon, D.E.; Zhang, L.; Kuball, J.; et al. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat. Med. 2012, 18, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Berdien, B.; Mock, U.; Atanackovic, D.; Fehse, B. TALEN-mediated editing of endogenous T-cell receptors facilitates efficient reprogramming of T lymphocytes by lentiviral gene transfer. Gene Ther. 2014, 21, 539–548. [Google Scholar] [CrossRef]
- Osborn, M.J.; Webber, B.R.; Knipping, F.; Lonetree, C.L.; Tennis, N.; DeFeo, A.P.; McElroy, A.N.; Starker, C.G.; Lee, C.; Merkel, S.; et al. Evaluation of TCR Gene Editing Achieved by TALENs, CRISPR/Cas9, and megaTAL Nucleases. Mol. Ther. 2016, 24, 570–581. [Google Scholar] [CrossRef]
- Knipping, F.; Osborn, M.J.; Petri, K.; Tolar, J.; Glimm, H.; von Kalle, C.; Schmidt, M.; Gabriel, R. Genome-wide Specificity of Highly Efficient TALENs and CRISPR/Cas9 for T Cell Receptor Modification. Mol. Ther. Methods Clin. Dev. 2017, 4, 213–224. [Google Scholar] [CrossRef]
- Legut, M.; Dolton, G.; Mian, A.A.; Ottmann, O.G.; Sewell, A.K. CRISPR-mediated TCR replacement generates superior anticancer transgenic T cells. Blood 2018, 131, 311–322. [Google Scholar] [CrossRef]
- Roth, T.L.; Puig-Saus, C.; Yu, R.; Shifrut, E.; Carnevale, J.; Li, P.J.; Hiatt, J.; Saco, J.; Krystofinski, P.; Li, H.; et al. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature 2018, 559, 405–409. [Google Scholar] [CrossRef]
- Cohen, C.J.; Li, Y.F.; El-Gamil, M.; Robbins, P.F.; Rosenberg, S.A.; Morgan, R.A. Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Res. 2007, 67, 3898–3903. [Google Scholar] [CrossRef]
- Frankel, T.L.; Burns, W.R.; Peng, P.D.; Yu, Z.; Chinnasamy, D.; Wargo, J.A.; Zheng, Z.; Restifo, N.P.; Rosenberg, S.A.; Morgan, R.A. Both CD4 and CD8 T cells mediate equally effective in vivo tumor treatment when engineered with a highly avid TCR targeting tyrosinase. J. Immunol. 2010, 184, 5988–5998. [Google Scholar] [CrossRef] [PubMed]
- Kuball, J.; Dossett, M.L.; Wolfl, M.; Ho, W.Y.; Voss, R.H.; Fowler, C.; Greenberg, P.D. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood 2007, 109, 2331–2338. [Google Scholar] [CrossRef] [PubMed]
- Sadio, F.; Stadlmayr, G.; Stadlbauer, K.; Graf, M.; Scharrer, A.; Ruker, F.; Wozniak-Knopp, G. Stabilization of soluble high-affinity T-cell receptor with de novo disulfide bonds. Febs Lett 2020, 594, 477–490. [Google Scholar] [CrossRef]
- Bethune, M.T.; Gee, M.H.; Bunse, M.; Lee, M.S.; Gschweng, E.H.; Pagadala, M.S.; Zhou, J.; Cheng, D.; Heath, J.R.; Kohn, D.B.; et al. Domain-swapped T cell receptors improve the safety of TCR gene therapy. Elife 2016, 5, e19095. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Shao, H.; Zhang, W.; Bo, H.; Wu, F.; Shen, H.; Huang, S. gammadeltaTCR immunoglobulin constant region domain exchange in human alphabetaTCRs improves TCR pairing without altering TCR gene-modified T cell function. Mol. Med. Rep. 2017, 15, 1555–1564. [Google Scholar] [CrossRef][Green Version]
- Goff, S.L.; Johnson, L.A.; Black, M.A.; Xu, H.; Zheng, Z.; Cohen, C.J.; Morgan, R.A.; Rosenberg, S.A.; Feldman, S.A. Enhanced receptor expression and in vitro effector function of a murine-human hybrid MART-1-reactive T cell receptor following a rapid expansion. Cancer Immunol. Immunother. 2010, 59, 1551–1560. [Google Scholar] [CrossRef]
- Cohen, C.J.; Zhao, Y.; Zheng, Z.; Rosenberg, S.A.; Morgan, R.A. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 2006, 66, 8878–8886. [Google Scholar] [CrossRef]
- Spear, T.T.; Foley, K.C.; Garrett-Mayer, E.; Nishimura, M.I. TCR modifications that enhance chain pairing in gene-modified T cells can augment cross-reactivity and alleviate CD8 dependence. J. Leukoc. Biol. 2018, 103, 973–983. [Google Scholar] [CrossRef]
- Bialer, G.; Horovitz-Fried, M.; Ya’acobi, S.; Morgan, R.A.; Cohen, C.J. Selected murine residues endow human TCR with enhanced tumor recognition. J. Immunol. 2010, 184, 6232–6241. [Google Scholar] [CrossRef]
- Knies, D.; Klobuch, S.; Xue, S.A.; Birtel, M.; Echchannaoui, H.; Yildiz, O.; Omokoko, T.; Guillaume, P.; Romero, P.; Stauss, H.; et al. An optimized single chain TCR scaffold relying on the assembly with the native CD3-complex prevents residual mispairing with endogenous TCRs in human T-cells. Oncotarget 2016, 7, 21199–21221. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.A.; Dudley, M.E.; Wunderlich, J.R.; Hughes, M.S.; Yang, J.C.; Sherry, R.M.; Royal, R.E.; Topalian, S.L.; Kammula, U.S.; Restifo, N.P.; et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006, 314, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A.; Heemskerk, B.; Powell, D.J., Jr.; Cohen, C.J.; Morgan, R.A.; Dudley, M.E.; Robbins, P.F.; Rosenberg, S.A. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J. Immunol. 2006, 177, 6548–6559. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A.; Morgan, R.A.; Dudley, M.E.; Cassard, L.; Yang, J.C.; Hughes, M.S.; Kammula, U.S.; Royal, R.E.; Sherry, R.M.; Wunderlich, J.R.; et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 2009, 114, 535–546. [Google Scholar] [CrossRef]
- Chodon, T.; Comin-Anduix, B.; Chmielowski, B.; Koya, R.C.; Wu, Z.; Auerbach, M.; Ng, C.; Avramis, E.; Seja, E.; Villanueva, A.; et al. Adoptive transfer of MART-1 T-cell receptor transgenic lymphocytes and dendritic cell vaccination in patients with metastatic melanoma. Clin. Cancer Res. 2014, 20, 2457–2465. [Google Scholar] [CrossRef]
- Ma, C.; Cheung, A.F.; Chodon, T.; Koya, R.C.; Wu, Z.; Ng, C.; Avramis, E.; Cochran, A.J.; Witte, O.N.; Baltimore, D.; et al. Multifunctional T-cell analyses to study response and progression in adoptive cell transfer immunotherapy. Cancer Discov. 2013, 3, 418–429. [Google Scholar] [CrossRef]
- Tawara, I.; Kageyama, S.; Miyahara, Y.; Fujiwara, H.; Nishida, T.; Akatsuka, Y.; Ikeda, H.; Tanimoto, K.; Terakura, S.; Murata, M.; et al. Safety and persistence of WT1-specific T-cell receptor gene-transduced lymphocytes in patients with AML and MDS. Blood 2017, 130, 1985–1994. [Google Scholar] [CrossRef]
- Parkhurst, M.R.; Yang, J.C.; Langan, R.C.; Dudley, M.E.; Nathan, D.A.; Feldman, S.A.; Davis, J.L.; Morgan, R.A.; Merino, M.J.; Sherry, R.M.; et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol. Ther. 2011, 19, 620–626. [Google Scholar] [CrossRef]
- Robbins, P.F.; Morgan, R.A.; Feldman, S.A.; Yang, J.C.; Sherry, R.M.; Dudley, M.E.; Wunderlich, J.R.; Nahvi, A.V.; Helman, L.J.; Mackall, C.L.; et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 2011, 29, 917–924. [Google Scholar] [CrossRef]
- Robbins, P.F.; Kassim, S.H.; Tran, T.L.; Crystal, J.S.; Morgan, R.A.; Feldman, S.A.; Yang, J.C.; Dudley, M.E.; Wunderlich, J.R.; Sherry, R.M.; et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: Long-term follow-up and correlates with response. Clin. Cancer Res. 2015, 21, 1019–1027. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Melchiori, L.; Merchant, M.S.; Bernstein, D.; Glod, J.; Kaplan, R.; Grupp, S.; Tap, W.D.; Chagin, K.; Binder, G.K.; et al. Antitumor Activity Associated with Prolonged Persistence of Adoptively Transferred NY-ESO-1 (c259)T Cells in Synovial Sarcoma. Cancer Discov. 2018, 8, 944–957. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, I.; Lowther, D.E.; Dryer-Minnerly, R.; Wang, R.; Fayngerts, S.; Nunez, D.; Betts, G.; Bath, N.; Tipping, A.J.; Melchiori, L.; et al. Systemic and local immunity following adoptive transfer of NY-ESO-1 SPEAR T cells in synovial sarcoma. J. Immunother. Cancer 2019, 7, 276. [Google Scholar] [CrossRef]
- Stadtmauer, E.A.; Faitg, T.H.; Lowther, D.E.; Badros, A.Z.; Chagin, K.; Dengel, K.; Iyengar, M.; Melchiori, L.; Navenot, J.M.; Norry, E.; et al. Long-term safety and activity of NY-ESO-1 SPEAR T cells after autologous stem cell transplant for myeloma. Blood Adv. 2019, 3, 2022–2034. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, A.P.; Stadtmauer, E.A.; Binder-Scholl, G.K.; Goloubeva, O.; Vogl, D.T.; Lacey, S.F.; Badros, A.Z.; Garfall, A.; Weiss, B.; Finklestein, J.; et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat. Med. 2015, 21, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.A.; Chinnasamy, N.; Abate-Daga, D.; Gros, A.; Robbins, P.F.; Zheng, Z.; Dudley, M.E.; Feldman, S.A.; Yang, J.C.; Sherry, R.M.; et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 2013, 36, 133–151. [Google Scholar] [CrossRef]
- Kageyama, S.; Ikeda, H.; Miyahara, Y.; Imai, N.; Ishihara, M.; Saito, K.; Sugino, S.; Ueda, S.; Ishikawa, T.; Kokura, S.; et al. Adoptive Transfer of MAGE-A4 T-cell Receptor Gene-Transduced Lymphocytes in Patients with Recurrent Esophageal Cancer. Clin. Cancer Res. 2015, 21, 2268–2277. [Google Scholar] [CrossRef]
- Linette, G.P.; Stadtmauer, E.A.; Maus, M.V.; Rapoport, A.P.; Levine, B.L.; Emery, L.; Litzky, L.; Bagg, A.; Carreno, B.M.; Cimino, P.J.; et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 2013, 122, 863–871. [Google Scholar] [CrossRef]
- Cameron, B.J.; Gerry, A.B.; Dukes, J.; Harper, J.V.; Kannan, V.; Bianchi, F.C.; Grand, F.; Brewer, J.E.; Gupta, M.; Plesa, G.; et al. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med. 2013, 5, 197ra103. [Google Scholar] [CrossRef]
- van den Berg, J.H.; Gomez-Eerland, R.; van de Wiel, B.; Hulshoff, L.; van den Broek, D.; Bins, A.; Tan, H.L.; Harper, J.V.; Hassan, N.J.; Jakobsen, B.K.; et al. Case Report of a Fatal Serious Adverse Event Upon Administration of T Cells Transduced With a MART-1-specific T-cell Receptor. Mol. Ther. 2015, 23, 1541–1550. [Google Scholar] [CrossRef]
- Sanderson, J.P.; Crowley, D.J.; Wiedermann, G.E.; Quinn, L.L.; Crossland, K.L.; Tunbridge, H.M.; Cornforth, T.V.; Barnes, C.S.; Ahmed, T.; Howe, K.; et al. Preclinical evaluation of an affinity-enhanced MAGE-A4-specific T-cell receptor for adoptive T-cell therapy. Oncoimmunology 2020, 9, 1682381. [Google Scholar] [CrossRef]
- Duong, M.N.; Erdes, E.; Hebeisen, M.; Rufer, N. Chronic TCR-MHC (self)-interactions limit the functional potential of TCR affinity-increased CD8 T lymphocytes. J. Immunother. Cancer 2019, 7, 284. [Google Scholar] [CrossRef] [PubMed]
- Stadtmauer, E.A.; Fraietta, J.A.; Davis, M.M.; Cohen, A.D.; Weber, K.L.; Lancaster, E.; Mangan, P.A.; Kulikovskaya, I.; Gupta, M.; Chen, F.; et al. CRISPR-engineered T cells in patients with refractory cancer. Science 2020, 367, eaba7365. [Google Scholar] [CrossRef]
- Qasim, W.; Zhan, H.; Samarasinghe, S.; Adams, S.; Amrolia, P.; Stafford, S.; Butler, K.; Rivat, C.; Wright, G.; Somana, K.; et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Xia, A.L.; He, Q.F.; Wang, J.C.; Zhu, J.; Sha, Y.Q.; Sun, B.; Lu, X.J. Applications and advances of CRISPR-Cas9 in cancer immunotherapy. J. Med. Genet. 2019, 56, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Chapuis, A.G.; Egan, D.N.; Bar, M.; Schmitt, T.M.; McAfee, M.S.; Paulson, K.G.; Voillet, V.; Gottardo, R.; Ragnarsson, G.B.; Bleakley, M.; et al. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat. Med. 2019, 25, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Lu, Y.C.; Parker, L.L.; Li, Y.F.; El-Gamil, M.; Black, M.A.; Xu, H.; Feldman, S.A.; van der Bruggen, P.; Rosenberg, S.A.; et al. Isolation and Characterization of an HLA-DPB1*04: 01-restricted MAGE-A3 T-Cell Receptor for Cancer Immunotherapy. J. Immunother. 2016, 39, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Hunder, N.N.; Wallen, H.; Cao, J.; Hendricks, D.W.; Reilly, J.Z.; Rodmyre, R.; Jungbluth, A.; Gnjatic, S.; Thompson, J.A.; Yee, C. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N. Engl. J. Med. 2008, 358, 2698–2703. [Google Scholar] [CrossRef] [PubMed]
- Klobuch, S.; Hammon, K.; Vatter-Leising, S.; Neidlinger, E.; Zwerger, M.; Wandel, A.; Neuber, L.M.; Heilmeier, B.; Fichtner, R.; Mirbeth, C.; et al. HLA-DPB1 Reactive T Cell Receptors for Adoptive Immunotherapy in Allogeneic Stem Cell Transplantation. Cells 2020, 9, 1264. [Google Scholar] [CrossRef]
- Lu, Y.C.; Parker, L.L.; Lu, T.; Zheng, Z.; Toomey, M.A.; White, D.E.; Yao, X.; Li, Y.F.; Robbins, P.F.; Feldman, S.A.; et al. Treatment of Patients With Metastatic Cancer Using a Major Histocompatibility Complex Class II-Restricted T-Cell Receptor Targeting the Cancer Germline Antigen MAGE-A3. J. Clin. Oncol. 2017, 35, 3322–3329. [Google Scholar] [CrossRef]
- Schmitt, T.M.; Aggen, D.H.; Ishida-Tsubota, K.; Ochsenreither, S.; Kranz, D.M.; Greenberg, P.D. Generation of higher affinity T cell receptors by antigen-driven differentiation of progenitor T cells in vitro. Nat. Biotechnol. 2017, 35, 1188–1195. [Google Scholar] [CrossRef]
- Sharma, P.; Harris, D.T.; Stone, J.D.; Kranz, D.M. T-cell Receptors Engineered De Novo for Peptide Specificity Can Mediate Optimal T-cell Activity without Self Cross-Reactivity. Cancer Immunol. Res. 2019, 7, 2025–2035. [Google Scholar] [CrossRef]
- Matsuda, T.; Leisegang, M.; Park, J.H.; Ren, L.; Kato, T.; Ikeda, Y.; Harada, M.; Kiyotani, K.; Lengyel, E.; Fleming, G.F.; et al. Induction of Neoantigen-Specific Cytotoxic T Cells and Construction of T-cell Receptor-Engineered T Cells for Ovarian Cancer. Clin. Cancer Res. 2018, 24, 5357–5367. [Google Scholar] [CrossRef] [PubMed]
- Marijt, K.A.; Blijleven, L.; Verdegaal, E.M.E.; Kester, M.G.; Kowalewski, D.J.; Rammensee, H.G.; Stevanovic, S.; Heemskerk, M.H.M.; van der Burg, S.H.; van Hall, T. Identification of non-mutated neoantigens presented by TAP-deficient tumors. J. Exp. Med. 2018, 215, 2325–2337. [Google Scholar] [CrossRef] [PubMed]
- Doorduijn, E.M.; Sluijter, M.; Marijt, K.A.; Querido, B.J.; van der Burg, S.H.; van Hall, T. T cells specific for a TAP-independent self-peptide remain naive in tumor-bearing mice and are fully exploitable for therapy. Oncoimmunology 2018, 7, e1382793. [Google Scholar] [CrossRef] [PubMed]
- Garber, K. gammadelta T cells bring unconventional cancer-targeting to the clinic-again. Nat. Biotechnol. 2020, 38, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.; Anderson, J. Engineering Approaches in Human Gamma Delta T Cells for Cancer Immunotherapy. Front. Immunol. 2018, 9, 1409. [Google Scholar] [CrossRef]
- Mensali, N.; Dillard, P.; Hebeisen, M.; Lorenz, S.; Theodossiou, T.; Myhre, M.R.; Fane, A.; Gaudernack, G.; Kvalheim, G.; Myklebust, J.H.; et al. NK cells specifically TCR-dressed to kill cancer cells. EBio. Medicine 2019, 40, 106–117. [Google Scholar] [CrossRef]
- Kierkels, G.J.J.; Scheper, W.; Meringa, A.D.; Johanna, I.; Beringer, D.X.; Janssen, A.; Schiffler, M.; Aarts-Riemens, T.; Kramer, L.; Straetemans, T.; et al. Identification of a tumor-specific allo-HLA-restricted gammadeltaTCR. Blood Adv. 2019, 3, 2870–2882. [Google Scholar] [CrossRef]
- Rubinstein, M.P.; Su, E.W.; Suriano, S.; Cloud, C.A.; Andrijauskaite, K.; Kesarwani, P.; Schwartz, K.M.; Williams, K.M.; Johnson, C.B.; Li, M.; et al. Interleukin-12 enhances the function and anti-tumor activity in murine and human CD8(+) T cells. Cancer Immunol. Immunother. 2015, 64, 539–549. [Google Scholar] [CrossRef]
- Abate-Daga, D.; Hanada, K.; Davis, J.L.; Yang, J.C.; Rosenberg, S.A.; Morgan, R.A. Expression profiling of TCR-engineered T cells demonstrates overexpression of multiple inhibitory receptors in persisting lymphocytes. Blood 2013, 122, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Stadtmauer, E.A.; Cohen, A.D.; Weber, K.; Lacey, S.F.; Gonzalez, V.E.; Melenhorst, J.J.; Fraietta, J.A.; Plesa, G.; Shea, J.; Matlawski, T.; et al. First-in-Human Assessment of Feasibility and Safety of Multiplexed Genetic Engineering of Autologous T Cells Expressing NY-ESO -1 TCR and CRISPR/Cas9 Gene Edited to Eliminate Endogenous TCR and PD-1 (NYCE T cells) in Advanced Multiple Myeloma (MM) and Sarcoma. Blood 2019, 134. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campillo-Davo, D.; Flumens, D.; Lion, E. The Quest for the Best: How TCR Affinity, Avidity, and Functional Avidity Affect TCR-Engineered T-Cell Antitumor Responses. Cells 2020, 9, 1720. https://doi.org/10.3390/cells9071720
Campillo-Davo D, Flumens D, Lion E. The Quest for the Best: How TCR Affinity, Avidity, and Functional Avidity Affect TCR-Engineered T-Cell Antitumor Responses. Cells. 2020; 9(7):1720. https://doi.org/10.3390/cells9071720
Chicago/Turabian StyleCampillo-Davo, Diana, Donovan Flumens, and Eva Lion. 2020. "The Quest for the Best: How TCR Affinity, Avidity, and Functional Avidity Affect TCR-Engineered T-Cell Antitumor Responses" Cells 9, no. 7: 1720. https://doi.org/10.3390/cells9071720
APA StyleCampillo-Davo, D., Flumens, D., & Lion, E. (2020). The Quest for the Best: How TCR Affinity, Avidity, and Functional Avidity Affect TCR-Engineered T-Cell Antitumor Responses. Cells, 9(7), 1720. https://doi.org/10.3390/cells9071720




