Cetuximab and IL-15 Promote NK and Dendritic Cell Activation In Vitro in Triple Negative Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Tumor Cell Lines
2.2. Abs and Reagents
2.3. Preparation of Monocyte-Derived DCs and NK Cells from Peripheral Blood Mononuclear Cells (PBMCs)
2.4. Co-Culture of NK Cells, DCs and TNBC Cells
2.5. Flow Cytometry
2.6. Evaluation of NK Cell-Mediated Cytotoxicity and DC Phagocytosis by Flow Cytometry
2.7. Interleukin-12p70 Production
2.8. Statistical Analysis
3. Results
3.1. NK Cells Promoted DC Uptake of Antigen Material When TNBC Cells Were Coated with Cetuximab
3.2. IFN-γ and TNF-α Production Increased When NK Cells Were Activated by Cetuximab-Coated TNBC Cells
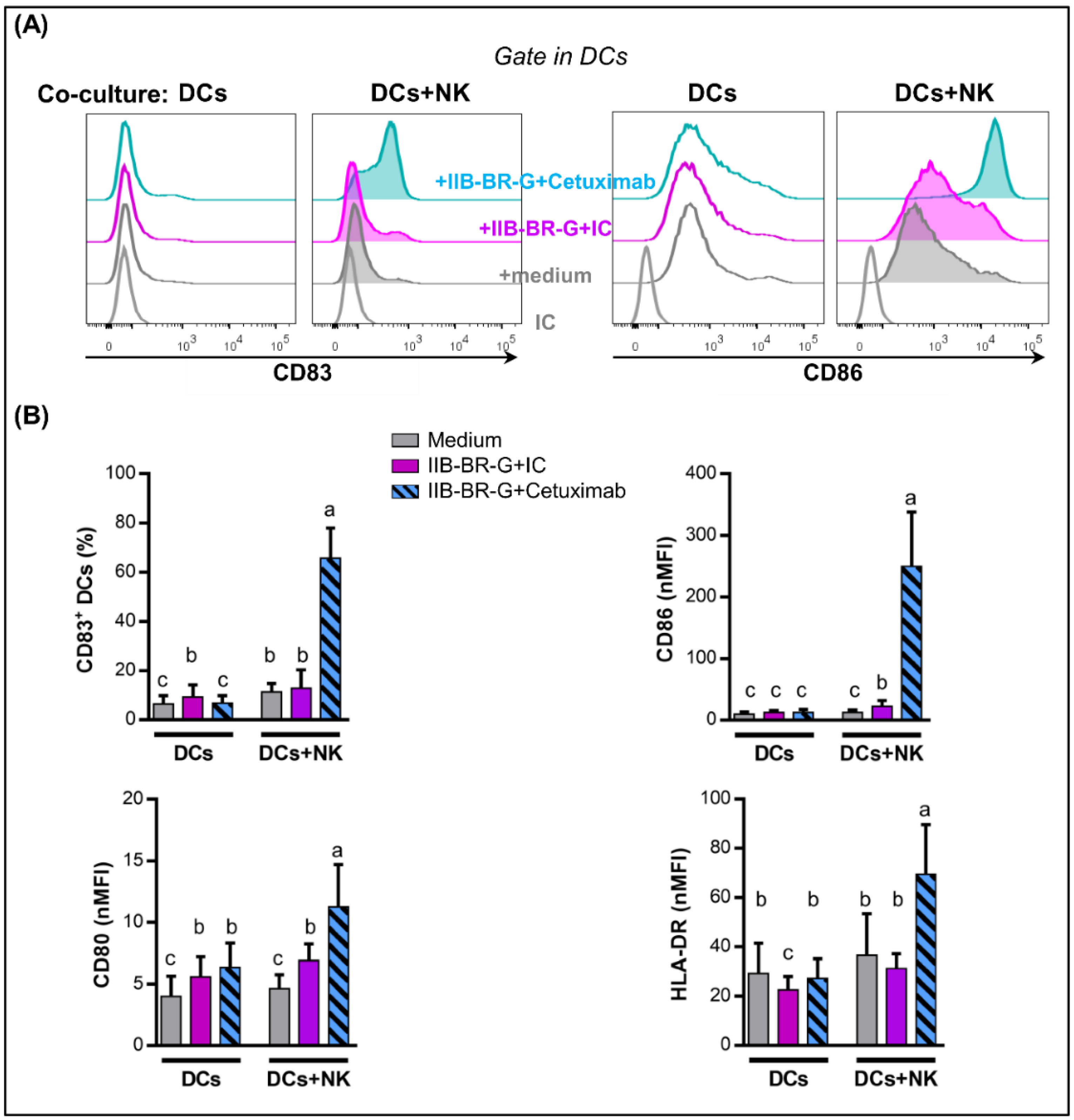
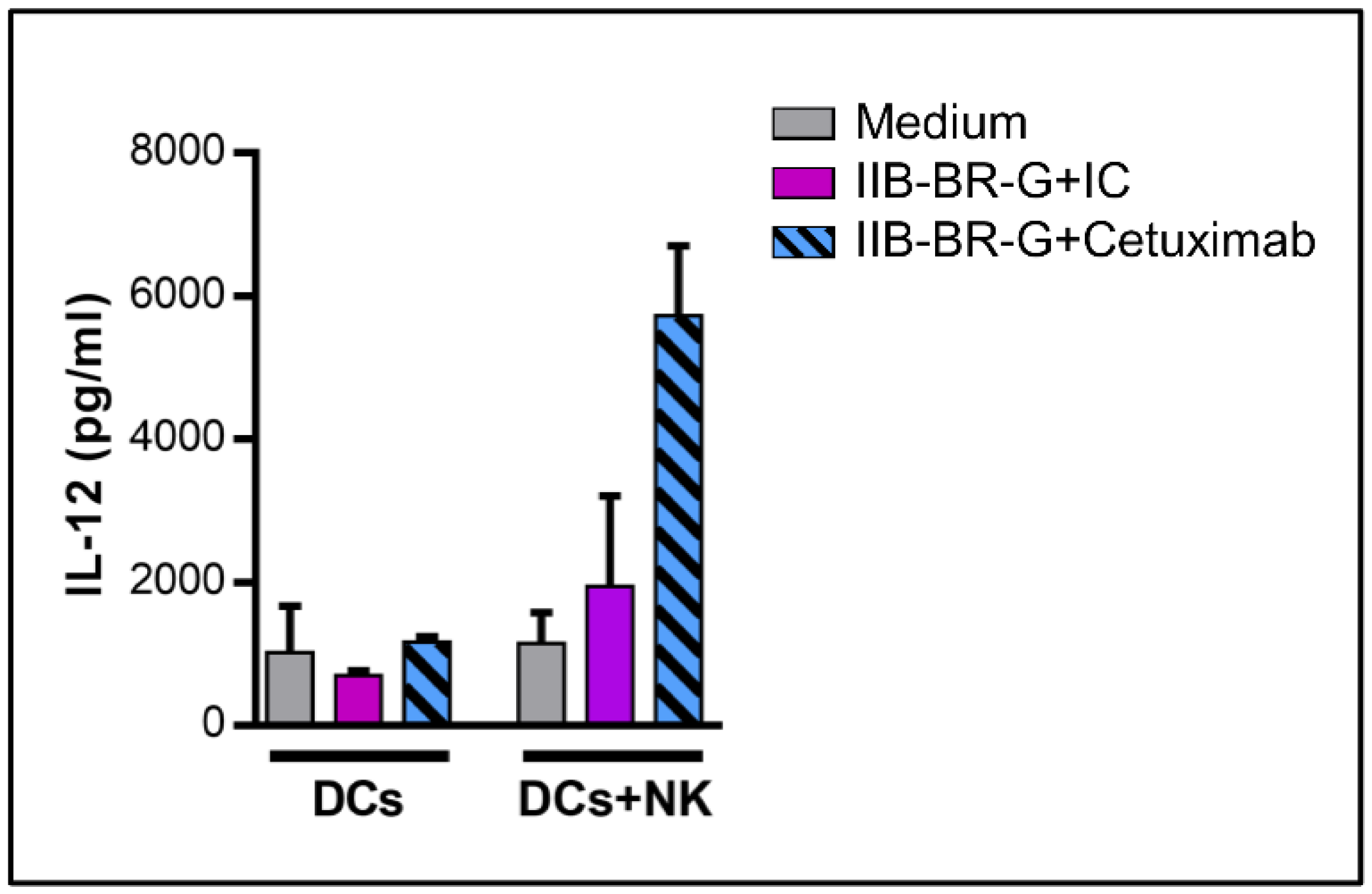
3.3. NK Cells Promoted DC Maturation and IL-12 Production When TNBC Cells Were Coated with Cetuximab
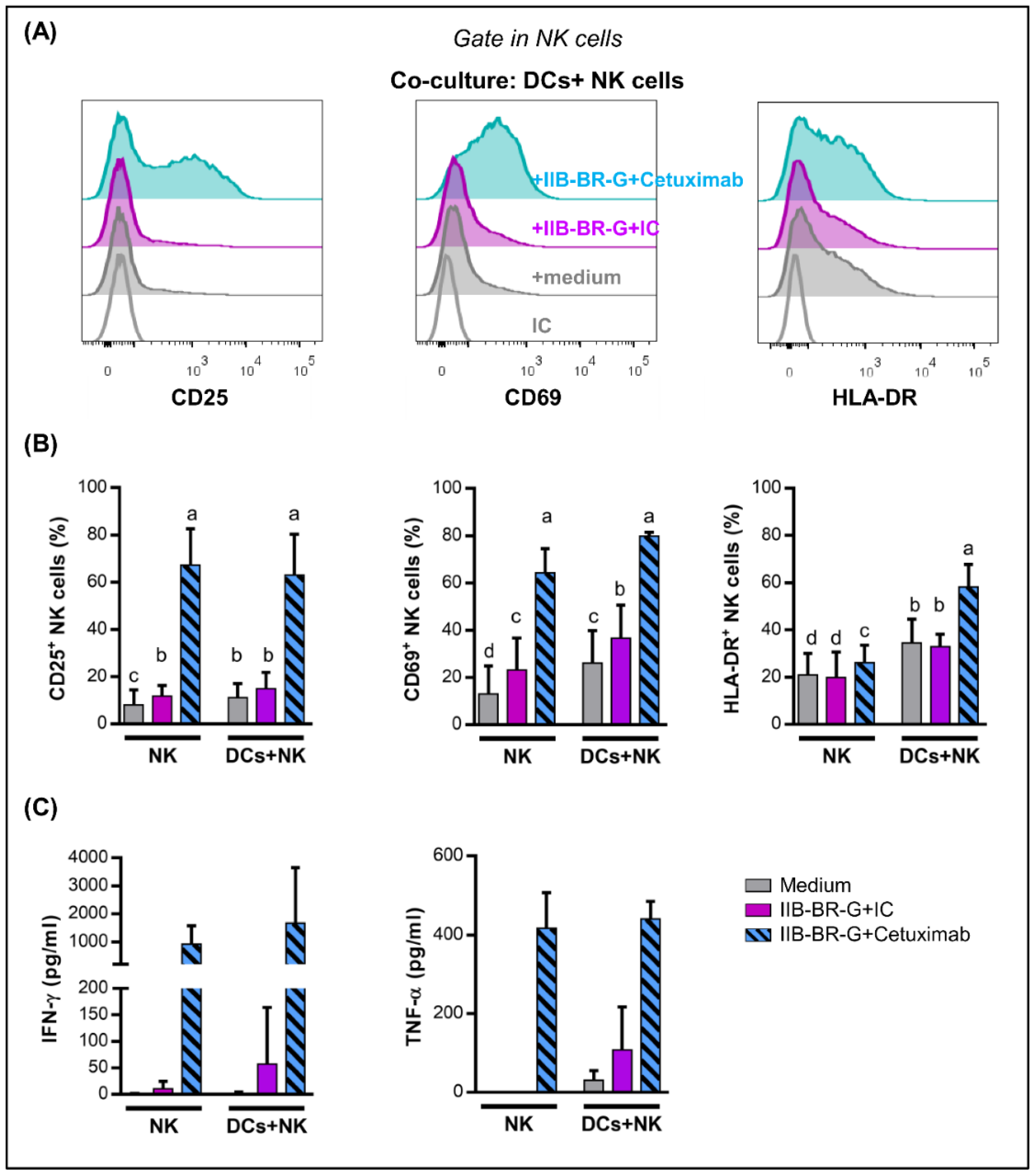
3.4. IL-15 Increased NK Cell Activation and DC Maturation Triggered by Cetuximab
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, C.; Mazouni, C.; Hess, K.R.; André, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M.; et al. Response to Neoadjuvant Therapy and Long-Term Survival in Patients With Triple-Negative Breast Cancer. J. Clin. Oncol. 2008, 26, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Drubay, D.; Adams, S.; Pruneri, G.; Francis, P.A.; Lacroix-Triki, M.; Joensuu, H.; Dieci, M.V.; Badve, S.; Demaria, S.; et al. Tumor-infiltrating lymphocytes and prognosis: A pooled individual patient analysis of early-stage triple-negative breast cancers. J. Clin. Oncol. 2019, 37, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; von Minckwitz, G.; Brase, J.C.; Sinn, B.V.; Gade, S.; Kronenwett, R.; Pfitzner, B.M.; Salat, C.; Loi, S.; Schmitt, W.D.; et al. Tumor-Infiltrating Lymphocytes and Response to Neoadjuvant Chemotherapy With or Without Carboplatin in Human Epidermal Growth Factor Receptor 2–Positive and Triple-Negative Primary Breast Cancers. J. Clin. Oncol. 2015, 33, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Sanchez, K.; McArthur, H.L.; Page, D. Immunotherapy in Triple-Negative Breast Cancer: Present and Future. Curr. Breast Cancer Rep. 2019, 11, 259–271. [Google Scholar] [CrossRef]
- Mandal, A.; Viswanathan, C. Natural killer cells: In health and disease. Hematol. Oncol. Stem Cell Ther. 2015, 8, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Marcus, A.; Gowen, B.G.; Thompson, T.W.; Iannello, A.; Ardolino, M.; Deng, W.; Wang, L.; Shifrin, N.; Raulet, D.H. Recognition of tumors by the innate immune system and natural killer cells. In Advances in Immunology; Elsevier Inc.: New York, NY, USA, 2014; Volume 122, pp. 91–128. ISBN 9780128002674. [Google Scholar]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef] [PubMed]
- Pampena, M.B.; Levy, E.M. Natural killer cells as helper cells in dendritic cell cancer vaccines. Front. Immunol. 2015, 6, 13. [Google Scholar] [CrossRef]
- Van Elssen, C.H.M.J.; Oth, T.; Germeraad, W.T.V.; Bos, G.M.J.; Vanderlocht, J. Natural killer cells: The secret weapon in dendritic cell vaccination strategies. Clin. Cancer Res. 2014, 20, 1095–1103. [Google Scholar] [CrossRef]
- Celli, S.; Breart, B.; Bousso, P. Intravital two-photon imaging of natural killer cells and dendritic cells in lymph nodes. Methods Mol. Biol. 2008, 415, 119–126. [Google Scholar]
- Bajénoff, M.; Breart, B.; Huang, A.Y.C.; Qi, H.; Cazareth, J.; Braud, V.M.; Germain, R.N.; Glaichenhaus, N. Natural killer cell behavior in lymph nodes revealed by static and real-time imaging. J. Exp. Med. 2006, 203, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Buentke, E.; Heffler, L.C.; Wilson, J.L.; Wallin, R.P.A.; Loöfman, C.; Chambers, B.J.; Ljunggren, H.G.; Scheynius, A. Natural killer and dendritic cell contact in lesional atopic dermatitis skin-Malassezia-influenced cell interaction. J. Investig. Dermatol. 2002, 119, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Mocikat, R.; Braumüller, H.; Gumy, A.; Egeter, O.; Ziegler, H.; Reusch, U.; Bubeck, A.; Louis, J.; Mailhammer, R.; Riethmüller, G.; et al. Natural killer cells activated by MHC class ILow targets prime dendritic cells to induce protective CD8 T cell responses. Immunity 2003, 19, 561–569. [Google Scholar] [CrossRef]
- Roberti, M.P.; Rocca, Y.S.; Amat, M.; Pampena, M.B.; Loza, J.; Coló, F.; Fabiano, V.; Loza, C.M.; Arriaga, J.M.; Bianchini, M.; et al. IL-2- or IL-15-activated NK cells enhance Cetuximab-mediated activity against triple-negative breast cancer in xenografts and in breast cancer patients. Breast Cancer Res. Treat. 2012, 136, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Juliá, E.P.; Amante, A.; Pampena, M.B.; Mordoh, J.; Levy, E.M. Avelumab, an IgG1 anti-PD-L1 immune checkpoint inhibitor, triggers NK cell-mediated cytotoxicity and cytokine production against triple negative breast cancer cells. Front. Immunol. 2018, 9, 2140. [Google Scholar]
- Roberti, M.P.; Barrio, M.M.; Bravo, A.I.; Rocca, Y.S.; Arriaga, J.M.; Bianchini, M.; Mordoh, J.; Levy, E.M. IL-15 and IL-2 increase Cetuximab-mediated cellular cytotoxicity against triple negative breast cancer cell lines expressing EGFR. Breast Cancer Res. Treat. 2011, 130, 465–475. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.; Zhao, S.; Wang, Y.; Di, W.; Zhao, G.; Yang, M.; Zhang, Q. Prognostic value of survivin and EGFR protein expression in triple-negative breast cancer (TNBC) patients. Target. Oncol. 2014, 9, 349–357. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Cai, X.; Pan, Z.; Liu, J.; Yin, W.; Chen, H.; Xie, Z.; Liang, H.; Wang, W.; et al. Efficacy and safety of first line treatments for patients with advanced epidermal growth factor receptor mutated, non-small cell lung cancer: Systematic review and network meta-analysis. BMJ 2019, 367, l5460. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, E.; Cardone, C.; Troiani, T.; Normanno, N.; Pisconti, S.; Sforza, V.; Bordonaro, A.R.; Rachiglio, A.M.; Lambiase, M.; Latiano, T.P.; et al. Clinical activity and tolerability of FOLFIRI and cetuximab in elderly patients with metastatic colorectal cancer in the CAPRI-GOIM first-line trial. ESMO Open 2016, 1, e000086. [Google Scholar] [CrossRef] [PubMed]
- Agulnik, M. New approaches to EGFR inhibition for locally advanced or metastatic squamous cell carcinoma of the head and neck (SCCHN). Med. Oncol. 2012, 29, 2481–2491. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Gómez, P.; Greil, R.; Braga, S.; Climent, M.A.; Wardley, A.M.; Kaufman, B.; Stemmer, S.M.; Pego, A.; Chan, A.; et al. Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J. Clin. Oncol. 2013, 31, 2586–2592. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, T.A. The shared and contrasting roles of IL2 and IL15 in the life and death of normal and neoplastic lymphocytes: Implications for cancer therapy. Cancer Immunol. Res. 2015, 3, 219–227. [Google Scholar] [CrossRef]
- Cornish, G.H.; Sinclair, L.V.; Cantrell, D.A. Differential regulation of T-cell growth by IL-2 and IL-15. Blood 2006, 108, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, G.; Pack, M.; Thomas, D.; Paludan, C.; Schmid, D.; Strowig, T.; Bougras, G.; Muller, W.A.; Moretta, L.; Münz, C. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc. Natl. Acad. Sci. USA 2004, 101, 16606–16611. [Google Scholar] [CrossRef] [PubMed]
- Anguille, S.; Van Acker, H.H.; Van Den Bergh, J.; Willemen, Y.; Goossens, H.; Van Tendeloo, V.F.; Smits, E.L.; Berneman, Z.N.; Lion, E. Interleukin-15 dendritic cells harness NK cell cytotoxic effector function in a contact- and IL-15-dependent manner. PLoS ONE 2015, 10, e0123340. [Google Scholar] [CrossRef]
- Bover, L.; Barrio, M.; Slavutsky, I.; Bravo, A.I.; Quintans, C.; Bagnati, A.; Lema, B.; Schiaffi, J.; Yomha, R.; Mordoh, J. Description of a new human breast cancer cell line, IIB-BR-G, established from a primary undifferentiated tumor. Breast Cancer Res. Treat. 1991, 19, 47–56. [Google Scholar] [CrossRef]
- El Guerrab, A.; Bamdad, M.; Kwiatkowski, F.; Bignon, Y.J.; Penault-Llorca, F.; Aubel, C. Anti-EGFR monoclonal antibodies and EGFR tyrosine kinase inhibitors as combination therapy for triple-negative breast cancer. Oncotarget 2016, 7, 73618–73637. [Google Scholar] [CrossRef]
- Remes Lenicov, F.; Rodriguez Rodrigues, C.; Sabatté, J.; Cabrini, M.; Jancic, C.; Ostrowski, M.; Merlotti, A.; Gonzalez, H.; Alonso, A.; Pasqualini, R.A.; et al. Semen Promotes the Differentiation of Tolerogenic Dendritic Cells. J. Immunol. 2012, 189, 4777–4786. [Google Scholar] [CrossRef]
- Mailliard, R.B.; Wankowicz-Kalinska, A.; Cai, Q.; Wesa, A.; Hilkens, C.M.; Kapsenberg, M.L.; Kirkwood, J.M.; Storkus, W.J.; Kalinski, P. α-type-1 polarized dendritic cells: A novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004, 64, 5934–5937. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C. InfoStat Versión 2017; Grupo InfoStat, FCA, Universidad Nacional de Córdoba: Córdoba, Argentina, 2017. [Google Scholar]
- José, C.; Pinheiro, D.M.B. Mixed-Effects Models in S and S-PLUS; Springer Statistics and Computing: New York, NY, USA, 2000; ISBN 0-387-98957-9. [Google Scholar]
- Holmes, T.D.; Wilson, E.B.; Black, E.V.I.; Benest, A.V.; Vaz, C.; Tan, B.; Tanavde, V.M.; Cook, G.P. Licensed human natural killer cells aid dendritic cell maturation via TNFSF14/LIGHT. Proc. Natl. Acad. Sci. USA 2014, 111, E5688–E5696. [Google Scholar] [CrossRef]
- Harizi, H. Reciprocal crosstalk between dendritic cells and natural killer cells under the effects of PGE2 in immunity and immunopathology. Cell. Mol. Immunol. 2013, 10, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Hung, M.C.; Yamaguchi, H. A perspective on anti-EGFR therapies targeting triple-negative breast cancer. Am. J. Cancer Res. 2016, 6, 1609–1623. [Google Scholar] [PubMed]
- Markov, O.V.; Mironova, N.L.; Vlasov, V.V.; Zenkova, M.A. Molecular and cellular mechanisms of antitumor immune response activation by dendritic cells. Acta Nat. 2016, 8, 17–30. [Google Scholar] [CrossRef]
- Wittrup, K.D. Antitumor Antibodies Can Drive Therapeutic T Cell Responses. Trends Cancer 2017, 3, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, X.; Mortenson, E.D.; Radkevich-Brown, O.; Wang, Y.; Fu, Y.X. Cetuximab-mediated tumor regression depends on innate and adaptive immune responses. Mol. Ther. 2013, 21, 91–100. [Google Scholar] [CrossRef]
- Sánchez-Muñoz, A.; Gallego, E.; de Luque, V.; Pérez-Rivas, L.G.; Vicioso, L.; Ribelles, N.; Lozano, J.; Alba, E. Lack of evidence for KRAS oncogenic mutations in triple-negative breast cancer. BMC Cancer 2010, 10, 136. [Google Scholar] [CrossRef]
- Grob, T.J.; Heilenkötter, U.; Geist, S.; Paluchowski, P.; Wilke, C.; Jaenicke, F.; Quaas, A.; Wilczak, W.; Choschzick, M.; Sauter, G.; et al. Rare oncogenic mutations of predictive markers for targeted therapy in triple-negative breast cancer. Breast Cancer Res. Treat. 2012, 134, 561–567. [Google Scholar] [CrossRef]
- Bournazos, S.; Wang, T.T.; Ravetch, J.V. The Role and Function of Fcγ Receptors on Myeloid Cells. Microbiol. Spectr. 2016, 4, 1–19. [Google Scholar]
- Banerjee, D.; Matthews, P.; Matayeva, E.; Kaufman, J.L.; Steinman, R.M.; Dhodapkar, K.M. Enhanced T-cell responses to glioma cells coated with the anti-EGF receptor antibody and targeted to activating FcgRs on human dendritic cells. J. Immunother. 2008, 31, 113–120. [Google Scholar] [CrossRef]
- Srivastava, R.M.; Lee, S.C.; Andrade Filho, P.A.; Lord, C.A.; Jie, H.-B.; Davidson, H.C.; Lopez-Albaitero, A.; Gibson, S.P.; Gooding, W.E.; Ferrone, S.; et al. Cetuximab-Activated Natural Killer and Dendritic Cells Collaborate to Trigger Tumor Antigen-Specific T-cell Immunity in Head and Neck Cancer Patients. Clin. Cancer Res. 2013, 19, 1858–1872. [Google Scholar] [CrossRef] [PubMed]
- Skak, K.; Frederiksen, K.S.; Lundsgaard, D. Interleukin-21 activates human natural killer cells and modulates their surface receptor expression. Immunology 2008, 123, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Costa-Garcia, M.; Vera, A.; Moraru, M.; Vilches, C.; López-Botet, M.; Muntasell, A. Antibody-Mediated Response of NKG2C bright NK Cells against Human Cytomegalovirus. J. Immunol. 2015, 194, 2715–2724. [Google Scholar] [CrossRef] [PubMed]
- Vieira, P.L.; de Jong, E.C.; Wierenga, E.A.; Kapsenberg, M.L.; Kaliński, P. Development of Th1-Inducing Capacity in Myeloid Dendritic Cells Requires Environmental Instruction. J. Immunol. 2000, 164, 4507–4512. [Google Scholar] [CrossRef] [PubMed]
- Deauvieau, F.; Ollion, V.; Doffin, A.C.; Achard, C.; Fonteneau, J.F.; Verronese, E.; Durand, I.; Ghittoni, R.; Marvel, J.; Dezutter-Dambuyant, C.; et al. Human natural killer cells promote cross-presentation of tumor cell-derived antigens by dendritic cells. Int. J. Cancer 2015, 136, 1085–1094. [Google Scholar] [CrossRef]
- Trédan, O.; Campone, M.; Jassem, J.; Vyzula, R.; Coudert, B.; Pacilio, C.; Prausova, J.; Hardy-Bessard, A.C.; Arance, A.; Mukhopadhyay, P.; et al. Ixabepilone alone or with cetuximab as first-line treatment for advanced/metastatic triple-negative breast cancer. Clin. Breast Cancer 2015, 15, 8–15. [Google Scholar] [CrossRef]
- Nabholtz, J.M.; Chalabi, N.; Radosevic-Robin, N.; Dauplat, M.M.; Mouret-Reynier, M.A.; Van Praagh, I.; Servent, V.; Jacquin, J.; Benmammar, K.E.; Kullab, S.; et al. Multicentric neoadjuvant pilot Phase II study of cetuximab combined with docetaxel in operable triple negative breast cancer. Int. J. Cancer 2016, 138, 2274–2280. [Google Scholar] [CrossRef]
- Crozier, J.A.; Advani, P.P.; Laplant, B.; Hobday, T.; Jaslowski, A.J.; Moreno-Aspitia, A.; Perez, E.A. N0436 (Alliance): A Phase II Trial of Irinotecan with Cetuximab in Patients with Metastatic Breast Cancer Previously Exposed to Anthracycline and/or Taxane-Containing Therapy. Clin. Breast Cancer 2016, 16, 23–30. [Google Scholar] [CrossRef]
- Conlon, K.C.; Lugli, E.; Welles, H.C.; Rosenberg, S.A.; Fojo, A.T.; Morris, J.C.; Fleisher, T.A.; Dubois, S.P.; Perera, L.P.; Stewart, D.M.; et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J. Clin. Oncol. 2015, 33, 74–82. [Google Scholar] [CrossRef]
- Miller, J.S.; Morishima, C.; McNeel, D.G.; Patel, M.R.; Kohrt, H.E.K.; Thompson, J.A.; Sondel, P.M.; Wakelee, H.A.; Disis, M.L.; Kaiser, J.C.; et al. A first-in-human phase I study of subcutaneous outpatient recombinant human IL15 (rhIL15) in adults with advanced solid tumors. Clin. Cancer Res. 2018, 24, 1525–1535. [Google Scholar] [CrossRef]
- Conlon, K.C.; Lake Potter, E.; Pittaluga, S.; Lee, C.C.R.; Miljkovic, M.D.; Fleisher, T.A.; Dubois, S.; Bryant, B.R.; Petrus, M.; Perera, L.P.; et al. IL15 by continuous intravenous infusion to adult patients with solid tumors in a phase I trial induced dramatic NK-cell subset expansion. Clin. Cancer Res. 2019, 25, 4945–4954. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.O.; Schluns, K.S. The potential and promise of IL-15 in immuno-oncogenic therapies. Immunol. Lett. 2017, 190, 159–168. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juliá, E.P.; Mordoh, J.; Levy, E.M. Cetuximab and IL-15 Promote NK and Dendritic Cell Activation In Vitro in Triple Negative Breast Cancer. Cells 2020, 9, 1573. https://doi.org/10.3390/cells9071573
Juliá EP, Mordoh J, Levy EM. Cetuximab and IL-15 Promote NK and Dendritic Cell Activation In Vitro in Triple Negative Breast Cancer. Cells. 2020; 9(7):1573. https://doi.org/10.3390/cells9071573
Chicago/Turabian StyleJuliá, Estefanía Paula, José Mordoh, and Estrella Mariel Levy. 2020. "Cetuximab and IL-15 Promote NK and Dendritic Cell Activation In Vitro in Triple Negative Breast Cancer" Cells 9, no. 7: 1573. https://doi.org/10.3390/cells9071573
APA StyleJuliá, E. P., Mordoh, J., & Levy, E. M. (2020). Cetuximab and IL-15 Promote NK and Dendritic Cell Activation In Vitro in Triple Negative Breast Cancer. Cells, 9(7), 1573. https://doi.org/10.3390/cells9071573



