Closo-Carboranyl- and Metallacarboranyl [1,2,3]triazolyl-Decorated Lapatinib-Scaffold for Cancer Therapy Combining Tyrosine Kinase Inhibition and Boron Neutron Capture Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemistry
2.2. Instrumentation
2.3. Synthesis of Lapatinib Derivative
2.4. General Procedure for Hybrids 18–23 Preparation
2.4.1. Hybrid 18
2.4.2. Hybrid 19
2.4.3. Hybrid 20
2.4.4. Hybrid 21
2.4.5. Hybrid 22
2.4.6. Bioisoster 23
2.5. Biology
2.5.1. Tumor Cells
2.5.2. Glia Primary Cell Culture
2.6. Study Approval
2.7. Cytotoxicity Assays
2.8. Kinase Enzymatic Assays
2.9. Flow Cytometry Analysis
2.10. Statistical Analysis
2.11. Fluorescence Microscopy Analysis
2.12. In Vitro BNCT Experiments
2.12.1. Determination of Intracellular Boron by Inductively Coupled Plasma Optical Emission Spectroscopy (ICP−OES).
2.12.2. Neutron-Irradiation Procedures
2.12.3. Irradiation of Hybrid 19-, 22- or BPA-Treated HT-29 Cells Assays
2.12.4. Cell Surviving Assay
3. Results and Discussion
3.1. Design and Synthesis of Hybrids Carboranyl-Decorated Lapatinib-Scaffold
3.2. Biological Studies
3.2.1. In Vitro Cytotoxicity Studies
3.2.2. In Vitro Inhibition of EGRF by Hybrid 19
3.2.3. Effect of Hybrid 19 on Simulated Tumor Environment
3.3. In Vitro BNCT Studies
3.3.1. Boron Cellular Accumulation
3.3.2. Neutron Irradiation Treatments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef]
- Ritter, C.A.; Artega, C.L. The epidermal growth factor receptor tyrosine kinase: A promising therapeutic target in solid tumors. Semin. Oncol. 2003, 30, 993–1011. [Google Scholar] [CrossRef]
- Steins, M.; Thomas, M.; Geißler, M. Erlotinib. Recent results. Cancer Res. 2018, 211, 1–17. [Google Scholar] [CrossRef]
- Medina, P.J.; Goodin, S. Lapatinib: A dual inhibitor of human epidermal growth factor receptor tyrosine kinases. Clin. Ther. 2008, 30, 1426–1447. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.Q.; Eckhardt, S.G. Sunitinib: From rational design to clinical efficacy. J. Clin. Oncol. 2007, 25, 884–896. [Google Scholar] [CrossRef]
- Suzuki, M. Boron neutron capture therapy (BNCT): A unique role in radiotherapy with a view to entering the accelerator-based BNCT era. Int. J. Clin. Oncol. 2020, 25, 43–50. [Google Scholar] [CrossRef]
- Sauerwein, W.; Wittig, A.; Moss, R.; Nakagawa, Y. Neutron Capture Therapy. Principles and Applications; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Hopewell, J.W.; Gorlia, T.; Pellettieri, L.; Giusti, V.; H-Stenstam, B.; Sköld, K. Boron neutron capture therapy for newly diagnosed glioblastoma multiforme: An assessment of clinical potential. Appl. Radiat. Isot. 2011, 12, 1737–1740. [Google Scholar] [CrossRef]
- Schwint, A.E.; Monti Hughes, A.; Garabalino, M.A.; Pozzi, E.C.C.; Heber, E.M.; Trivillin, V.A. Chapter 3.6. Optimizing the therapeutic efficacy of boron neutron capture therapy (BNCT) for different pathologies: Research in animal models employing different boron compounds and administration strategies. In Boron-Based Compounds. Potential and Emerging applications in Medicine; Hey-Hawkins, E., Viñas, C., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2018. [Google Scholar]
- Imamichi, S.; Masutani, M. Investigation of biological effect of BNCT system in NCC. Cancer Sci. 2018, 109, 753. [Google Scholar] [CrossRef]
- Wang, L.-W.; Hsueh Liu, Y.-W.; Chou, F.-I.; Jiang, S.-H. Clinical trials for treating recurrent head and neck cancer with boron neutron capture therapy using the Tsing-Hua open pool reactor. Cancer Commun. 2018, 38, 37. [Google Scholar] [CrossRef] [PubMed]
- Sander, A.; Wosniok, W.; Gabel, D. Case numbers for a randomized clinical trial of boron neutron capture therapy for glioblastoma multiforme. Appl. Radiat. Isot. 2014, 88, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Kageji, T.; Nakagawa, Y.; Kitamura, K.; Matsumoto, K.; Hatanaka, H. Pharmacokinetics and boron uptake of BSH (Na2B12H11SH) in patients with intracranial tumors. J. Neuro-Oncol. 1997, 33, 117–130. [Google Scholar] [CrossRef]
- Isono, A.; Tsuji, M.; Sanada, Y.; Matsushita, A.; Masunaga, S.; Hirayama, T.; Nagasawa, H. Design, synthesis, and evaluation of lipopeptide conjugates of mercaptoundecahydrododecaborate for Boron neutron capture therapy. ChemMedChem 2019, 14, 823–832. [Google Scholar] [CrossRef]
- Futamura, G.; Kawabata, S.; Nonoguchi, N.; Hiramatsu, R.; Toho, T.; Tanaka, H.; Masunaga, S.-I.; Hattori, Y.; Kirihata, M.; Ono, K.; et al. Evaluation of a novel sodium borocaptate containing unnatural amino acid as a Boron delivery agent for neutron capture therapy of the F98 rat glioma. Radiat. Oncol. 2017, 12, 26. [Google Scholar] [CrossRef]
- Masunaga, S.; Nagasawa, H.; Gotoh, K.; Uto, Y.; Hori, H.; Sakurai, Y.; Nagata, K.; Suzuki, M.; Maruhashi, A.; Kinashi, Y.; et al. Evaluation of hypoxia-specific cytotoxic bioreductive agent sodium borocaptate-10B conjugates, as 10B-carriers in Boron neutron capture therapy. Radiat. Med. 2006, 24, 98–107. [Google Scholar] [CrossRef] [PubMed]
- STELLA PHARMA. Available online: https://stella-pharma.co.jp/en/news/ (accessed on 28 April 2020).
- Couto, M.; Mastandrea, I.; Cabrera, M.; Cabral, P.; Teixidor, F.; Cerecetto, H.; Viñas, C. Small-molecule kinase-inhibitors-loaded boron cluster as hybrid agents for glioma-cell-targeting therapy. Chem. Eur. J. 2017, 23, 9233–9238. [Google Scholar] [CrossRef] [PubMed]
- Couto, M.; García, M.F.; Alamón, C.; Cabrera, M.; Cabral, P.; Merlino, A.; Teixidor, F.; Cerecetto, H.; Viñas, C. Discovery of potent EGFR inhibitors through the incorporation of a 3D-aromatic-boron-rich-cluster into the 4-anilinoquinazoline scaffold: Potential drugs for glioma treatment. Chem. Eur. J. 2018, 24, 3122–3126. [Google Scholar] [CrossRef] [PubMed]
- Couto, M.; Alamón, C.; Sánchez, C.; Dávila, B.; Fernández, M.; Lecot, N.; Cabral, P.; Teixidor, F.; Viñas, C.; Cerecetto, H. Carboranylanilinoquinazoline EGFR-inhibitors: Toward “lead-to-candidate” stage in the drug-development pipeline. Future Med. Chem. 2019, 11, 2273–2285. [Google Scholar] [CrossRef]
- Couto, M. Design, Synthesis and Biological Evaluation of New Organoboranes with Use in Anti-tumor Activity against Glioblastoma by 10B Neutron Capture Therapy. Ph. D. Thesis, Universidad de la República, Montevideo, Uruguay, 2019. [Google Scholar]
- Fein, M.M.; Grafstein, D.; Paustian, J.E.; Bobinski, J.; Lischtein, B.M.; Mayes, N.; Schwartz, N.N.; Cohen, M.S. Carboranes. II. The preparation of 1- and 1,2-substituted carboranes. Inorg. Chem. 1963, 2, 1115–1119. [Google Scholar] [CrossRef]
- Papetti, S.; Obenland, C.; Heying, T.L. Vapor phase isomerization of o-carborane. Ind. Eng. Chem. Prod. Res. Dev. 1966, 5, 334–337. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Young, D.C.; Andrews, T.D.; Howe, D.V.; Pilling, R.L.; Pitts, A.D.; Reintjes, M.; Warren, L.F.; Wegner, P.A. β-Dicarbollyl derivatives of the transition metals. Metallocene analogs. J. Am. Chem Soc. 1968, 90, 879–896. [Google Scholar] [CrossRef]
- Teixidor, F.; Pedrajas, J.; Rojo, I.; Viñas, C.; Kivekäs, R.; Sillanpää, R.; Sivaev, I.; Bregadze, V.; Sjöberg, S. Chameleonic capacity of [3,3‘-Co(1,2-C2B9H11)2]− in coordination. Generation of the highly uncommon S(thioether)-Na bond. Organometallics 2003, 22, 3414–3423. [Google Scholar] [CrossRef]
- Plešek, J.; Heřmánek, S.; Franken, A.; Císařová, I.; Nachtigan, C. Dimethyl sulfate induced nucleophilic substitution of the [bis(1,2-dicarbollido)-3-cobalt(1-)]ate ion. Syntheses, properties and structures of its 8,8’-μ-sulfato, 8-phenyland 8-dioxane derivatives. Collect Czech Chem. Commun. 1997, 62, 47–56. [Google Scholar] [CrossRef]
- Wojtczak, B.A.; Andrysiak, A.; Gruner, B.; Lesnikowski, Z.J. “Chemical ligation”: A versatile method for nucleoside modification with boron clusters. Chem. Eur. J. 2008, 14, 10675–10682. [Google Scholar] [CrossRef] [PubMed]
- Teixidor, F.; Gómez, S.; Lamrani, M.; Viñas, C.; Sillanpää, R.; Kivakäs, R. Mixed cobaltacarboranes incorporating η5-pyrrolyl and dicarbollide ligands. Synthetic routes, structures, and mechanistic implications. Organometallics 1997, 16, 1278–1283. [Google Scholar] [CrossRef]
- Schildge, S.; Bohrer, C.; Beck, K.; Schachtrup, C. Isolation and culture of mouse cortical astrocytes. J. Vis. Exp. 2013, 71, e50079. [Google Scholar] [CrossRef]
- Guide for the Care and Use of Laboratory Animals. National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Rossini, A.E.; Dagrosa, M.A.; Portu, A.; Saint Martin, G.; Thorp, S.; Casal, M.; Navarro, A.; Juvenal, G.J.; Pisarev, M.A. Assessment of biological effectiveness of boron neutron capture therapy in primary and metastatic melanoma cell lines. Int. J. Radiat. Biol. 2015, 91, 81–89. [Google Scholar] [CrossRef]
- Dagrosa, A.; Carpano, M.; Perona, M.; Thomasz, L.; Nievas, S.; Cabrini, R.; Juvenal, G.; Pisarev, M. Studies for the application of boron neutron capture therapy to the treatment of differentiated thyroid cancer. Appl. Radiat. Isotopes 2011, 69, 1752–1755. [Google Scholar] [CrossRef]
- Dagrosa, M.A.; Viaggi, M.; Jimenez Rebagliati, R.; Castillo, V.A.; Batistoni, D.; Cabrini, R.L.; Castiglia, S.; Juvenal, G.J.; Pisarev, M.A. Biodistribution of p-borophenylalanine (BPA) in dogs with spontaneous undifferentiated thyroid carcinoma (UTC). Appl. Radiat. Isotopes 2004, 61, 911–915. [Google Scholar] [CrossRef]
- Dagrosa, M.A.; Viaggi, M.; Kreimann, E.; Farías, S.; Garavaglia, R.; Agote, M.; Cabrini, R.L.; Dadino, J.L.; Juvenal, G.J.; Pisarev, M.A. Selective uptake of p-borophenylalanine by undifferentiated thyroid carcinoma for boron neutron capture therapy. Thyroid 2002, 12, 7–12. [Google Scholar] [CrossRef]
- Perona, M.; Rodríguez, C.; Carpano, M.; Thomasz, L.; Nievas, S.; Olivera, M.; Thorp, S.; Curotto, P.; Pozzi, E.; Kahl, S.; et al. Improvement of the boron neutron capture therapy (BNCT) by the previous administration of the histone deacetylase inhibitor sodium butyrate for the treatment of thyroid carcinoma. Radiat. Environ. Biophys. 2013, 52, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Wood, E.R.; Truesdale, A.T.; McDonald, O.B.; Yuan, D.; Hassell, A.; Dickerson, S.H.; Ellis, B.; Pennisi, C.; Horne, E.; Lackey, K.; et al. A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): Relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res. 2004, 64, 6652–6659. [Google Scholar] [CrossRef] [PubMed]
- Donati, A.; Ristori, S.; Bonechi, C.; Panza, L.; Martini, G.; Rossi, C. Evidences of Strong C-H···O Bond in an o-Carboranyl β-Lactoside in Solution. J. Am. Chem. Soc. 2002, 124, 8778–8779. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, H.; Yan, H.; Zou, W.; Cremer, D. B–H···π interaction: A new type of nonclassical hydrogen bonding. J. Am. Chem. Soc. 2016, 138, 4334–4337. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.A.; Hughes, A.K. Cage C-H…X interactions in solid-state structures of icosahedral carboranes. Coord. Chem. Rev. 2004, 248, 457–476. [Google Scholar] [CrossRef]
- Lee, H.; Knobler, C.B.; Hawthorne, M.F. Supramolecular self-assembly directed by carborane C–H…F interactions. Chem Commun. 2000, 24, 2485–2486. [Google Scholar] [CrossRef]
- Barberà, G.; Viñas, C.; Teixidor, F.; Rosair, G.M.; Welch, A.J. Self-assembly of carborane molecules via C–H · I hydrogen bonding: The molecular and crystal structures of 3-I-1,2-closo-C2B10H11. J. Chem. Soc. Dalton Trans. 2002, 3647–3648. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Pushechnikov, A. Polyhedral borane derivatives: Unique and versatile structural motifs. Pure Appl. Chem. 2012, 84, 2279–2288. [Google Scholar] [CrossRef]
- Armstrong, A.F.; Valliant, J.F. The bioinorganic and medicinal chemistry of carboranes: From new drug discovery to molecular imaging and therapy. Dalton Trans. 2007, 4240–4251. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, D.; Li, G. The role of microglia in viral encephalitis: A review. J. Neuroinflamm. 2019, 16. [Google Scholar] [CrossRef]
- Brandao, M.; Simon, T.; Critchley, G.; Giamas, G. Astrocytes, the rising stars of the glioblastoma microenvironment. Glia 2019, 67, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Crossley, E.L.; Issa, F.; Scarf, A.M.; Kassiou, M.; Rendina, L.M. Synthesis and cellular uptake of boron-rich pyrazolopyrimidines: Exploitation of the translocator protein for the efficient delivery of boron into human glioma cells. Chem. Commun. 2011, 47, 12179–12181. [Google Scholar] [CrossRef] [PubMed]
- Alberti, D.; Toppino, A.; Geninatti Crich, S.; Meraldi, C.; Prandi, C.; Protti, N.; Bortolussi, S.; Altieri, S.; Aime, S.; Deagostino, A. Synthesis of a carborane-containing cholesterol derivative and evaluation as a potential dual agent for MRI/BNCT applications. Org. Biomol. Chem. 2014, 12, 2457–2467. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, T.; Inoue, Y.; Yao, Y.; Suzuki, M.; Kanamori, K.; Takemoto, H.; Matsui, M.; Tomoda, K.; Nishiyama, N. Poly(vinyl alcohol) boosting therapeutic potential of p-boronophenylalanine in neutron capture therapy by modulating metabolism. Sci. Adv. 2020, 6, eaaz1722. [Google Scholar] [CrossRef] [PubMed]
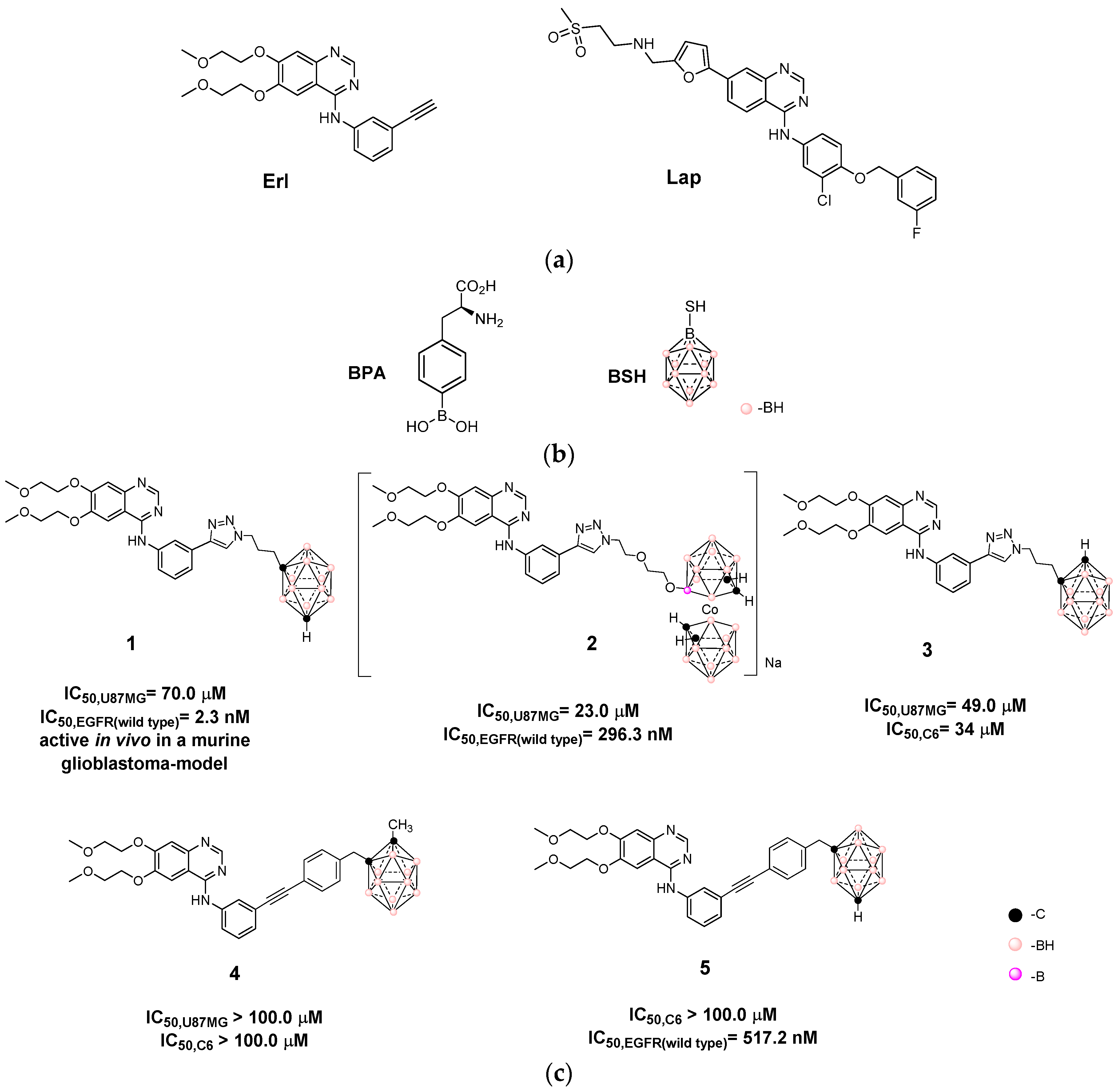
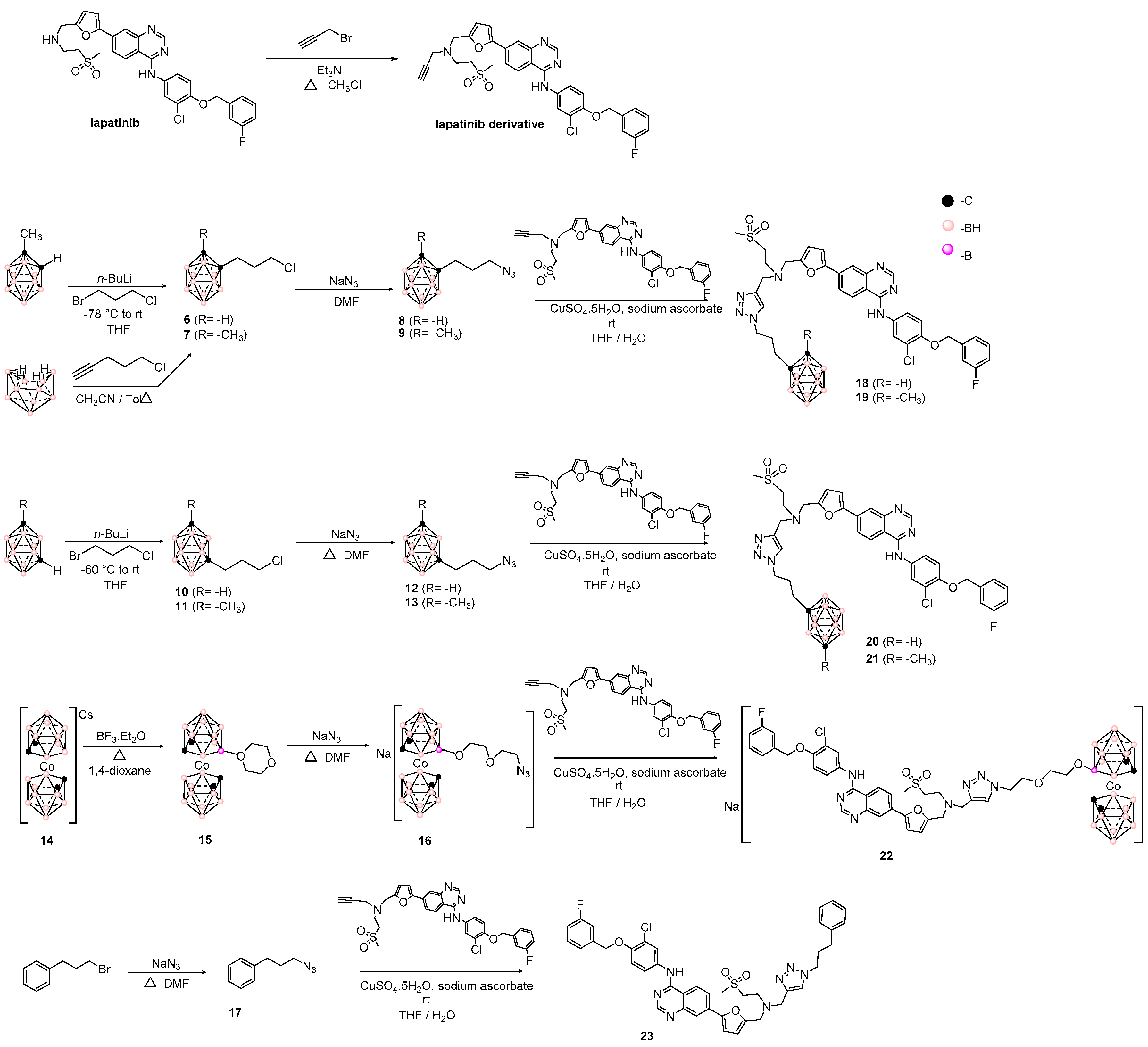
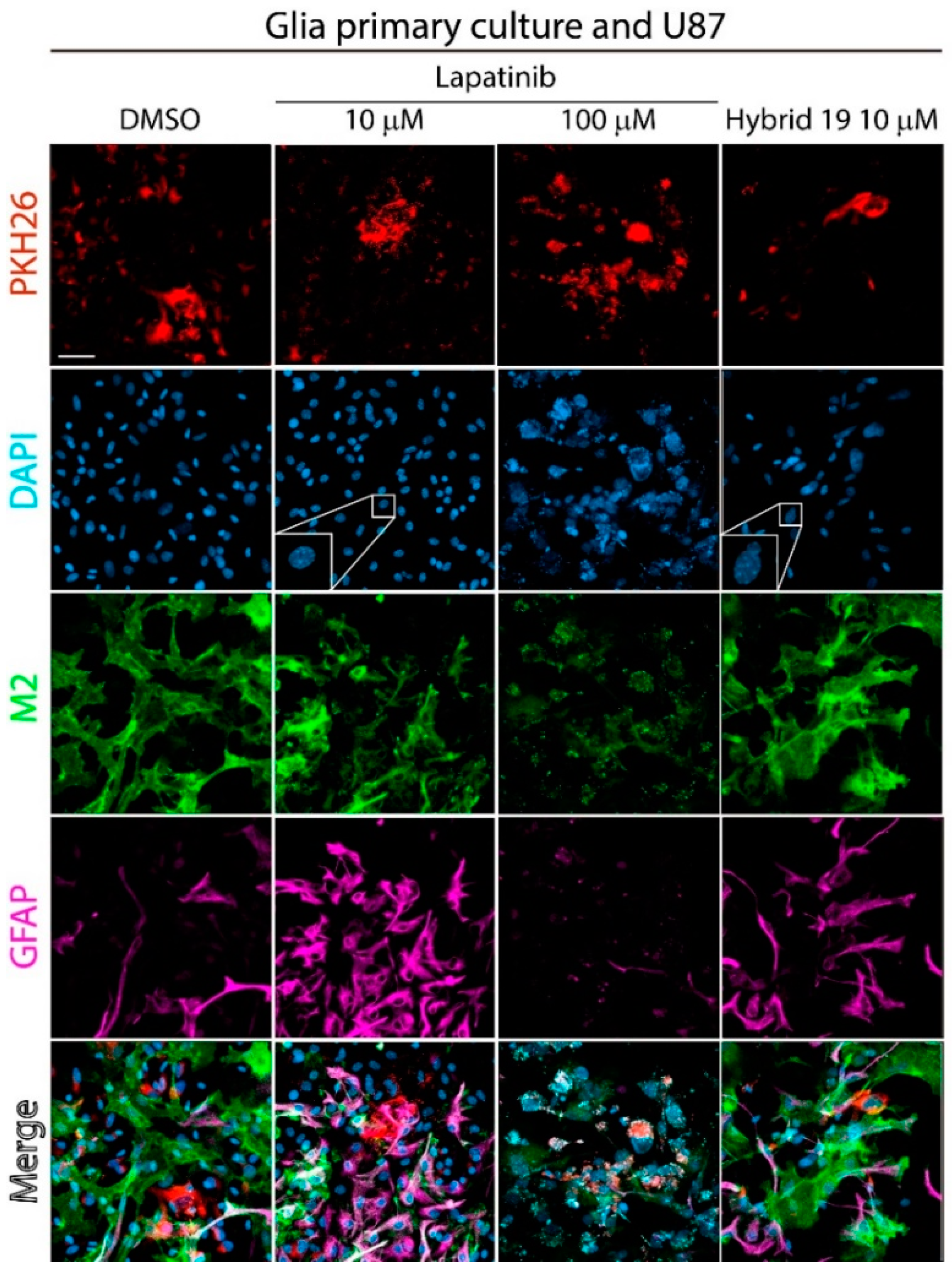
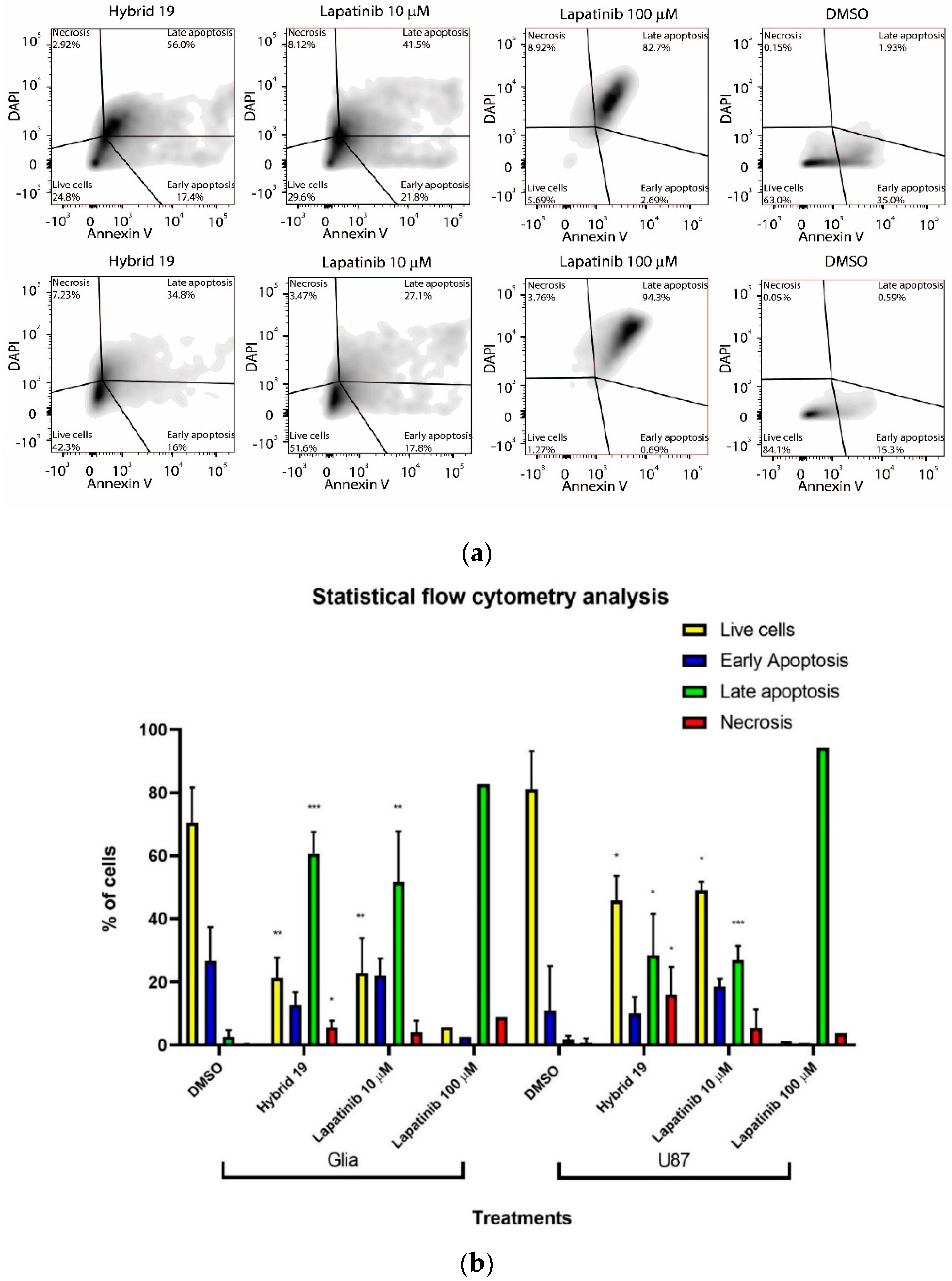
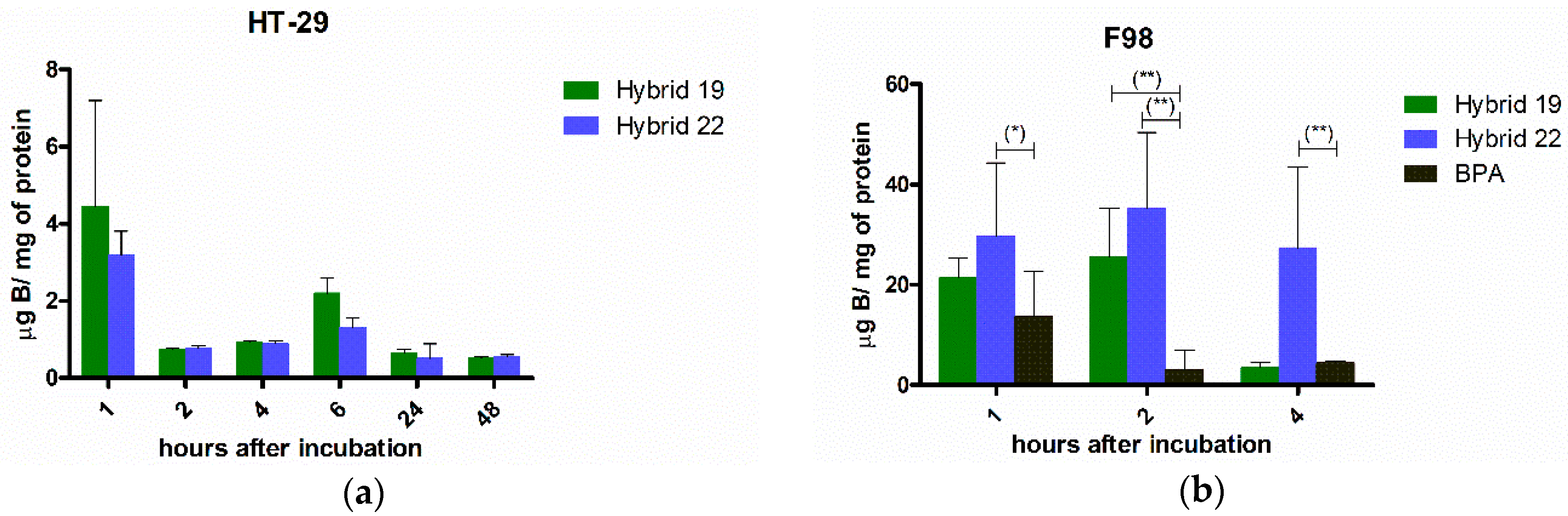
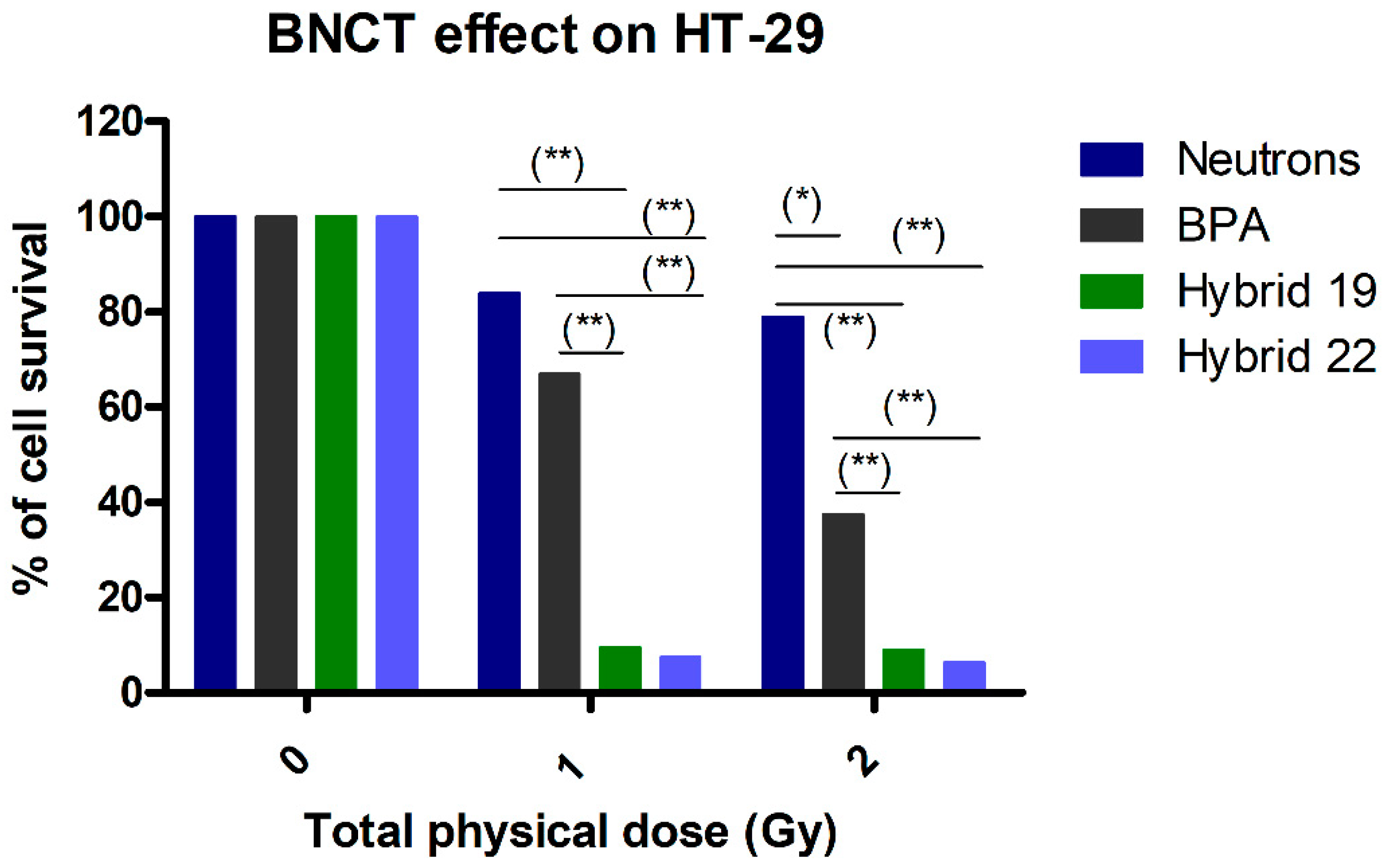
| Hybrid | IC50,HT-29 1 | IC50,U87 MG 1 | IC50,C6 1 | IC50,astrocytes | SI 2 | |
|---|---|---|---|---|---|---|
| U87 MG | C6 | |||||
| 18 | 80 ± 6 | >100 (65 ± 4) | >100 (100) | |||
| 19 | >100 (73 ± 6) | 10.0 ± 0.2 3 | 11.8 ± 0.4 4 | >100 | >10 | >8.5 |
| 20 | 100 ± 8 | >100 (85 ± 2) | >100 (100) | |||
| 21 | 100 ± 8 | >100 (77 ± 4) | >100 (100) | |||
| 22 | 50 ± 5 5 | >100 (66 ± 6) | >100 (100) | |||
| 23 | >100 (64 ± 4) | >100 (91 ± 8) | >100 (100) | |||
| Lap | 6.25 ± 0.05 | >100 (89 ± 5) | 54 ± 14 | 8 ± 3 | <0.08 | 0.15 |
| Irradiation Time (min) | Fluence (×1012, n/cm2) | Dose γ (Gy) | Dose 14N (Gy) | Dose 10B (Gy) | Total Absorbed Dose (Gy) | Relative Error Dose |
|---|---|---|---|---|---|---|
| 5.55 | 1.94 | 0.46 | 0.54 | 0 | 1.0 | ±7% |
| 11.12 | 3.88 | 0.92 | 1.08 | 0 | 2.0 | ±7% |
| 2.07 | 0.722 | 0.17 | 0.20 | 0.63 | 1.0 | ±8% |
| 4.15 | 1.45 | 0.34 | 0.40 | 1.26 | 2.0 | ±8% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Couto, M.; Alamón, C.; García, M.F.; Kovacs, M.; Trias, E.; Nievas, S.; Pozzi, E.; Curotto, P.; Thorp, S.; Dagrosa, M.A.; et al. Closo-Carboranyl- and Metallacarboranyl [1,2,3]triazolyl-Decorated Lapatinib-Scaffold for Cancer Therapy Combining Tyrosine Kinase Inhibition and Boron Neutron Capture Therapy. Cells 2020, 9, 1408. https://doi.org/10.3390/cells9061408
Couto M, Alamón C, García MF, Kovacs M, Trias E, Nievas S, Pozzi E, Curotto P, Thorp S, Dagrosa MA, et al. Closo-Carboranyl- and Metallacarboranyl [1,2,3]triazolyl-Decorated Lapatinib-Scaffold for Cancer Therapy Combining Tyrosine Kinase Inhibition and Boron Neutron Capture Therapy. Cells. 2020; 9(6):1408. https://doi.org/10.3390/cells9061408
Chicago/Turabian StyleCouto, Marcos, Catalina Alamón, María Fernanda García, Mariángeles Kovacs, Emiliano Trias, Susana Nievas, Emiliano Pozzi, Paula Curotto, Silvia Thorp, María Alejandra Dagrosa, and et al. 2020. "Closo-Carboranyl- and Metallacarboranyl [1,2,3]triazolyl-Decorated Lapatinib-Scaffold for Cancer Therapy Combining Tyrosine Kinase Inhibition and Boron Neutron Capture Therapy" Cells 9, no. 6: 1408. https://doi.org/10.3390/cells9061408
APA StyleCouto, M., Alamón, C., García, M. F., Kovacs, M., Trias, E., Nievas, S., Pozzi, E., Curotto, P., Thorp, S., Dagrosa, M. A., Teixidor, F., Viñas, C., & Cerecetto, H. (2020). Closo-Carboranyl- and Metallacarboranyl [1,2,3]triazolyl-Decorated Lapatinib-Scaffold for Cancer Therapy Combining Tyrosine Kinase Inhibition and Boron Neutron Capture Therapy. Cells, 9(6), 1408. https://doi.org/10.3390/cells9061408








