Fibroblasts Colonizing Nerve Conduits Express High Levels of Soluble Neuregulin1, a Factor Promoting Schwann Cell Dedifferentiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Procedure
2.2. Ethics Approval and Consent to Participate
2.3. Schwann Cell Primary Culture
2.4. Nerve Fibroblast Primary Culture
2.5. RNA Isolation, cDNA Preparation, and Quantitative Real-Time PCR
2.6. Protein Extraction and Western Blot
2.7. Immunohistochemistry
2.8. Statistical Methods
3. Results
3.1. Soluble NRG1 Is Strongly Expressed in the Conduit after Nerve Repair, While ErbB2 and ErbB3 Are Missing
3.2. The PI3K/AKT and ERK/MAPK Pathways Are Activated in the Autograft, Not in the Conduit
3.3. Fibroblast Markers Are Highly Expressed in the Chitosan Conduit
3.4. Primary Cultures of Nerve Fibroblasts Express High Levels of NRG1 Isoforms, While NRG1 Receptors Are Not Expressed
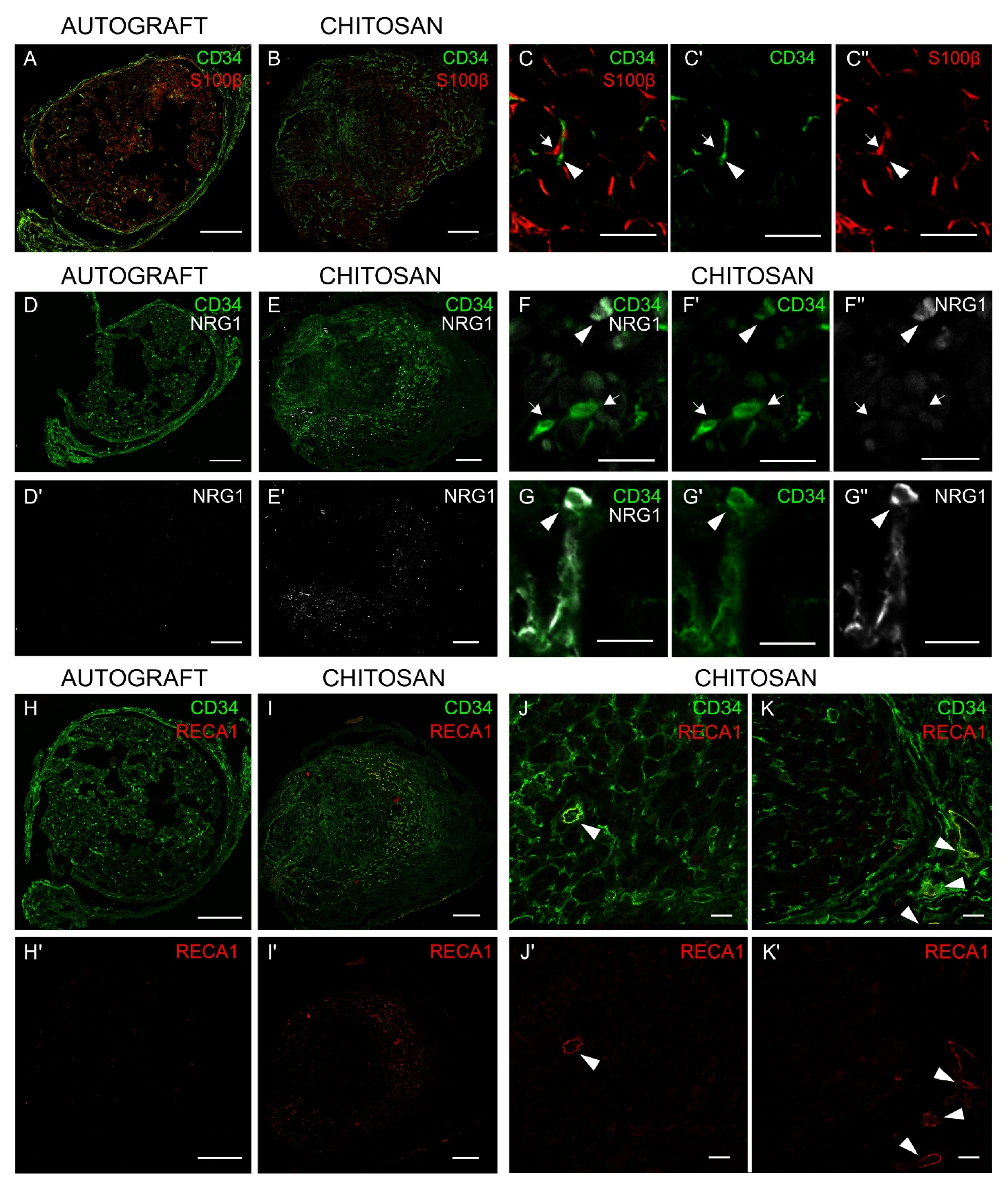
3.5. Immunohistochemistry Analysis Shows that Nerve Fibroblasts Express NRG1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANKRD27 | Ankyrin repeat domain 27 |
| ANOVA | Analysis of variance |
| CI | confidence interval |
| DAAO | D-amino acid oxidase |
| DMEM | Dulbecco Modified Eagle Medium |
| GFAP | glial fibrillary acidic protein |
| ηp2 | partial eta-squared |
| ERK | extracellular signal-regulated kinase |
| FBS | fetal bovine serum |
| IHC | immunohistochemistry |
| MAPK | mitogen-activated protein kinase |
| MBP | Myelin basic protein |
| NDS | Normal Donkey Serum |
| NRG1 | Neuregulin1 |
| OCT | Optimal Cutting Temperature |
| PBS | Phosphate Buffered Saline |
| PI3K | Phosphoinositide 3-Kinase |
| PLL | poly-L-lysine |
| RICTOR | RPTOR Independent Companion Of MTOR, Complex 2 |
| SEM | standard error of the mean |
| VEGF | Vascular-Endothelial Growth Factor. |
References
- Modrak, M.; Talukder, M.A.H.; Gurgenashvili, K.; Noble, M.; Elfar, J.C. Peripheral nerve injury and myelination: Potential therapeutic strategies. J. Neurosci. Res. 2020, 98, 780–795. [Google Scholar] [CrossRef]
- Cattin, A.L.; Burden, J.J.; Van Emmenis, L.; MacKenzie, F.E.; Hoving, J.J.A.; Garcia Calavia, N.; Guo, Y.; McLaughlin, M.; Rosenberg, L.H.; Quereda, V.; et al. Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell 2015, 162, 1127–1139. [Google Scholar] [CrossRef]
- Parrinello, S.; Napoli, I.; Ribeiro, S.; Wingfield Digby, P.; Fedorova, M.; Parkinson, D.B.; Doddrell, R.D.S.; Nakayama, M.; Adams, R.H.; Lloyd, A.C. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell 2010, 143, 145–155. [Google Scholar] [CrossRef]
- Duffy, P.; McMahon, S.; Wang, X.; Keaveney, S.; O’Cearbhaill, E.D.; Quintana, I.; Rodríguez, F.J.; Wang, W. Synthetic bioresorbable poly-α-hydroxyesters as peripheral nerve guidance conduits; a review of material properties, design strategies and their efficacy to date. Biomater. Sci. 2019, 7, 4912–4943. [Google Scholar] [CrossRef]
- Bahm, J.; Esser, T.; Sellhaus, B.; El-kazzi, W.; Schuind, F.T. Tension in peripheral nerve suture. In Treatment of Brachial Plexus Injuries; Vanaclocha, V., Sáiz-Sapena, N., Eds.; IntechOpen: London, UK, 2018; pp. 2–9. [Google Scholar]
- Deumens, R.; Bozkurt, A.; Meek, M.F.; Marcus, M.A.E.; Joosten, E.A.J.; Weis, J.; Brook, G.A. Repairing injured peripheral nerves: Bridging the gap. Prog. Neurobiol. 2010, 92, 245–276. [Google Scholar] [CrossRef]
- Belanger, K.; Dinis, T.M.; Taourirt, S.; Vidal, G.; Kaplan, D.L.; Egles, C. Recent Strategies in Tissue Engineering for Guided Peripheral Nerve Regeneration. Macromol. Biosci. 2016, 16, 472–481. [Google Scholar] [CrossRef]
- Kornfeld, T.; Vogt, P.M.; Radtke, C. Nerve grafting for peripheral nerve injuries with extended defect sizes. Wiener Medizinische Wochenschrift 2018, 169, 240–251. [Google Scholar] [CrossRef]
- Moore, A.M.; Kasukurthi, R.; Magill, C.K.; Farhadi, F.H.; Borschel, G.H.; Mackinnon, S.E. Limitations of conduits in peripheral nerve repairs. Hand 2009, 4, 180–186. [Google Scholar] [CrossRef]
- Haastert-Talini, K.; Geuna, S.; Dahlin, L.B.; Meyer, C.; Stenberg, L.; Freier, T.; Heimann, C.; Barwig, C.; Pinto, L.F.V.; Raimondo, S.; et al. Chitosan tubes of varying degrees of acetylation for bridging peripheral nerve defects. Biomaterials 2013, 34, 9886–9904. [Google Scholar] [CrossRef]
- Freier, T.; Montenegro, R.; Koh, H.S.; Shoichet, M.S. Chitin-based tubes for tissue engineering in the nervous system. Biomaterials 2005, 26, 4624–4632. [Google Scholar] [CrossRef]
- Bąk, M.; Gutkowska, O.N.; Wagner, E.; Gosk, J. The role of chitin and chitosan in peripheral nerve reconstruction. Polym. Med. 2017, 47, 43–47. [Google Scholar] [CrossRef]
- Boecker, A.; Daeschler, S.C.; Kneser, U.; Harhaus, L. Relevance and recent developments of chitosan in peripheral nerve surgery. Front. Cell. Neurosci. 2019, 13, 104. [Google Scholar] [CrossRef]
- Cattin, A.L.; Lloyd, A.C. The multicellular complexity of peripheral nerve regeneration. Curr. Opin. Neurobiol. 2016, 39, 38–46. [Google Scholar] [CrossRef]
- Jessen, K.R.; Arthur-Farraj, P. Repair Schwann cell update: Adaptive reprogramming, EMT, and stemness in regenerating nerves. Glia 2019, 67, 421–437. [Google Scholar] [CrossRef]
- Fricker, F.R.; Bennett, D.L. The role of neuregulin-1 in the response to nerve injury. Future Neurol. 2011, 6, 809–822. [Google Scholar] [CrossRef]
- Ronchi, G.; Haastert-Talini, K.; Fornasari, B.E.; Perroteau, I.; Geuna, S.; Gambarotta, G. The Neuregulin1/ErbB system is selectively regulated during peripheral nerve degeneration and regeneration. Eur. J. Neurosci. 2016, 43, 351–364. [Google Scholar] [CrossRef]
- Stassart, R.M.; Fledrich, R.; Velanac, V.; Brinkmann, B.G.; Schwab, M.H.; Meijer, D.; Sereda, M.W.; Nave, K.A. A role for Schwann cell-derived neuregulin-1 in remyelination. Nat. Neurosci. 2013, 16, 48–54. [Google Scholar] [CrossRef]
- Mei, L.; Xiong, W.C. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat. Rev. Neurosci. 2008, 9, 437–452. [Google Scholar] [CrossRef]
- Falls, D.L. Neuregulins: Functions, forms, and signaling strategies. Exp. Cell Res. 2003, 284, 14–30. [Google Scholar] [CrossRef]
- Wen, D.; Suggs, S.V.; Karunagaran, D.; Liu, N.; Cupples, R.L.; Luo, Y.; Janssen, A.M.; Ben-Baruch, N.; Trollinger, D.B.; Jacobsen, V.L. Structural and functional aspects of the multiplicity of Neu differentiation factors. Mol. Cell. Biol. 1994, 14, 1909–1919. [Google Scholar] [CrossRef]
- Newbern, J.; Birchmeier, C. Nrg1/ErbB signaling networks in Schwann cell development and myelination. Semin. Cell Dev. Biol. 2010, 21, 922–928. [Google Scholar] [CrossRef]
- Gonzalez-Perez, F.; Cobianchi, S.; Geuna, S.; Barwig, C.; Freier, T.; Udina, E.; Navarro, X. Tubulization with chitosan guides for the repair of long gap peripheral nerve injury in the rat. Microsurgery 2015, 35, 300–308. [Google Scholar] [CrossRef]
- Meyer, C.; Stenberg, L.; Gonzalez-Perez, F.; Wrobel, S.; Ronchi, G.; Udina, E.; Suganuma, S.; Geuna, S.; Navarro, X.; Dahlin, L.B.; et al. Chitosan-film enhanced chitosan nerve guides for long-distance regeneration of peripheral nerves. Biomaterials 2016, 76, 33–51. [Google Scholar] [CrossRef]
- Shapira, Y.; Tolmasov, M.; Nissan, M.; Reider, E.; Koren, A.; Biron, T.; Bitan, Y.; Livnat, M.; Ronchi, G.; Geuna, S.; et al. Comparison of results between chitosan hollow tube and autologous nerve graft in reconstruction of peripheral nerve defect: An experimental study. Microsurgery 2016, 36, 664–671. [Google Scholar] [CrossRef]
- Boni, R.; Ali, A.; Shavandi, A.; Clarkson, A.N. Current and novel polymeric biomaterials for neural tissue engineering. J. Biomed. Sci. 2018, 25, 90. [Google Scholar] [CrossRef]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Development of biomaterial scaffold for nerve tissue engineering: Biomaterial mediated neural regeneration. J. Biomed. Sci. 2009, 16, 108. [Google Scholar] [CrossRef]
- Williams, L.R.; Longo, F.M.; Powell, H.C.; Lundborg, G.; Varon, S. Spatial-Temporal progress of peripheral nerve regeneration within a silicone chamber: Parameters for a bioassay. J. Comp. Neurol. 1983, 218, 460–470. [Google Scholar] [CrossRef]
- Carvalho, C.R.; Oliveira, J.M.; Reis, R.L. Modern Trends for Peripheral Nerve Repair and Regeneration: Beyond the Hollow Nerve Guidance Conduit. Front. Bioeng. Biotechnol. 2019, 7, 337. [Google Scholar] [CrossRef]
- Ronchi, G.; Fornasari, B.E.; Crosio, A.; Budau, C.A.; Tos, P.; Perroteau, I.; Battiston, B.; Geuna, S.; Raimondo, S.; Gambarotta, G. Chitosan Tubes Enriched with Fresh Skeletal Muscle Fibers for Primary Nerve Repair. Biomed. Res. Int. 2018, 2018, 9175248. [Google Scholar] [CrossRef]
- Kaewkhaw, R.; Scutt, A.M.; Haycock, J.W. Integrated culture and purification of rat Schwann cells from freshly isolated adult tissue. Nat. Protoc. 2012, 7, 1996–2004. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Gambarotta, G.; Ronchi, G.; Friard, O.; Galletta, P.; Perroteau, I.; Geuna, S. Identification and validation of suitable housekeeping genes for normalizing quantitative real-time PCR assays in injured peripheral nerves. PLoS ONE 2014, 9, e105601. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, G.; Cillino, M.; Gambarotta, G.; Fornasari, B.E.; Raimondo, S.; Pugliese, P.; Tos, P.; Cordova, A.; Moschella, F.; Geuna, S. Irreversible changes occurring in long-term denervated Schwann cells affect delayed nerve repair. J. Neurosurg. 2017, 127, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Gambarotta, G.; Garzotto, D.; Destro, E.; Mautino, B.; Giampietro, C.; Cutrupi, S.; Dati, C.; Cattaneo, E.; Fasolo, A.; Perroteau, I. ErbB4 expression in neural progenitor cells (ST14A) is necessary to mediate neuregulin-1beta1-induced migration. J. Biol. Chem. 2004, 279, 48808–48816. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.N.; Cui, X.J.; Su, K.X.; Wang, X.H.; Guo, J.H. Beneficial reciprocal effects of bone marrow stromal cells and Schwann cells from adult rats in a dynamic co-culture system in vitro without intercellular contact. Mol. Med. Rep. 2015, 12, 4931–4938. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Richard, L.; Védrenne, N.; Vallat, J.-M.; Funalot, B. Characterization of Endoneurial Fibroblast-like Cells from Human and Rat Peripheral Nerves. J. Histochem. Cytochem. 2014, 62, 424–435. [Google Scholar] [CrossRef]
- Van Slooten, A.R.; Sun, Y.; Clarkson, A.N.; Connor, B.J. L-NIO as a novel mechanism for inducing focal cerebral ischemia in the adult rat brain. J. Neurosci. Methods 2015, 245, 44–57. [Google Scholar] [CrossRef]
- Duijvestijn, A.; van Goor, H.; Klatter, F.; Majoor, G.; van Bussel, E.; van Breda Vriesman, P. Antibodies Defining Rat Endothelial Cells: RECA-1, a Pan-Endothelial Cell-Specific Monoclonal Antibody. Lab. Investig. 2012, 66, 459–466. [Google Scholar]
- Fledrich, R.; Akkermann, D.; Schütza, V.; Abdelaal, T.A.; Hermes, D.; Schäffner, E.; Soto-Bernardini, M.C.; Götze, T.; Klink, A.; Kusch, K.; et al. NRG1 type I dependent autoparacrine stimulation of Schwann cells in onion bulbs of peripheral neuropathies. Nat. Commun. 2019, 10, 1467. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Richardson, J.T.E. Eta squared and partial eta squared as measures of effect size in educational research. Educ. Res. Rev. 2011, 6, 135–147. [Google Scholar] [CrossRef]
- Fornasari, B.E.; El Soury, M.; De Marchis, S.; Perroteau, I.; Geuna, S.; Gambarotta, G. Neuregulin1 alpha activates migration of neuronal progenitors expressing ErbB4. Mol. Cell. Neurosci. 2016, 77, 87–94. [Google Scholar] [CrossRef]
- Meek, M.F.; Coert, J.H. US Food and Drug Administration /Conformit Europe- approved absorbable nerve conduits for clinical repair of peripheral and cranial nerves. Ann. Plast. Surg. 2008, 60, 466–472. [Google Scholar] [CrossRef]
- Stenberg, L.; Kodama, A.; Lindwall-Blom, C.; Dahlin, L.B. Nerve regeneration in chitosan conduits and in autologous nerve grafts in healthy and in type 2 diabetic Goto-Kakizaki rats. Eur. J. Neurosci. 2016, 43, 463–473. [Google Scholar] [CrossRef]
- Carroll, S.L.; Miller, M.L.; Frohnert, P.W.; Kim, S.S.; Corbett, J.A. Expression of neuregulins and their putative receptors, ErbB2 and ErbB3, is induced during Wallerian degeneration. J. Neurosci. 1997, 17, 1642–1659. [Google Scholar] [CrossRef]
- Monje, P.V.; Bartlett Bunge, M.; Wood, P.M. Cyclic AMP synergistically enhances neuregulin-dependent ERK and Akt activation and cell cycle progression in Schwann cells. Glia 2006, 53, 649–659. [Google Scholar] [CrossRef]
- Parkinson, D.B.; Bhaskaran, A.; Droggiti, A.; Dickinson, S.; D’Antonio, M.; Mirsky, R.; Jessen, K.R. Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death. J. Cell Biol. 2004, 164, 385–394. [Google Scholar] [CrossRef]
- Syed, N.; Reddy, K.; Yang, D.P.; Taveggia, C.; Salzer, J.L.; Maurel, P.; Kim, H.A. Soluble neuregulin-1 has bifunctional, concentration-dependent effects on Schwann cell myelination. J. Neurosci. 2010, 30, 6122–6131. [Google Scholar] [CrossRef]
- de Souza, F.I.; Zumiotti, A.V.; da Silva, C.F. Neuregulins 1-alpha and 1-beta on the regeneration the peripheral nerves. Acta Ortop. Bras. 2010, 18, 250–254. [Google Scholar] [CrossRef]
- Pascal, D.; Giovannelli, A.; Gnavi, S.; Hoyng, S.A.; de Winter, F.; Morano, M.; Fregnan, F.; Dell’Albani, P.; Zaccheo, D.; Perroteau, I.; et al. Characterization of glial cell models and in vitro manipulation of the neuregulin1/ErbB system. Biomed. Res. Int. 2014, 2014, 310215. [Google Scholar] [CrossRef][Green Version]
- Dreesmann, L.; Mittnacht, U.; Lietz, M.; Schlosshauer, B. Nerve fibroblast impact on Schwann cell behavior. Eur. J. Cell Biol. 2009, 88, 285–300. [Google Scholar] [CrossRef]
- van Neerven, S.G.A.; Pannaye, P.; Bozkurt, A.; Van Nieuwenhoven, F.; Joosten, E.; Hermans, E.; Taccola, G.; Deumens, R. Schwann cell migration and neurite outgrowth are influenced by media conditioned by epineurial fibroblasts. Neuroscience 2013, 252, 144–153. [Google Scholar] [CrossRef]
- Caroni, P. Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J. Neurosci. Methods 1997, 71, 3–9. [Google Scholar] [CrossRef]
- Richard, L.; Topilko, P.; Magy, L.; Decouvelaere, A.V.; Charnay, P.; Funalot, B.; Vallat, J.M. Endoneurial fibroblast-like cells. J. Neuropathol. Exp. Neurol. 2012, 71, 938–947. [Google Scholar] [CrossRef]
- Sorrell, J.M.; Caplan, A.I. Fibroblasts-a diverse population at the center of it all. Int. Rev. Cell Mol. Biol. 2009, 276, 161–214. [Google Scholar]
- Muangsanit, P.; Shipley, R.J.; Phillips, J.B. Vascularization Strategies for Peripheral Nerve Tissue Engineering. Anat. Rec. (Hoboken) 2018, 301, 1657–1667. [Google Scholar] [CrossRef]
- Atkins, S.; Smith, K.G.; Loescher, A.R.; Boissonade, F.M.; O’Kane, S.; Ferguson, M.W.J.; Robinson, P.P. Scarring impedes regeneration at sites of peripheral nerve repair. Neuroreport 2006, 17, 1245–1249. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Wang, G.; Chen, L.; Chen, J.; Liu, Z.; Zhang, Z.; Shen, H.; Jin, Y.; Shen, Z. The effect of co-transplantation of nerve fibroblasts and Schwann cells on peripheral nerve repair. Int. J. Biol. Sci. 2017, 13, 1507–1519. [Google Scholar] [CrossRef]
- Pabari, A.; Yang, S.Y.; Mosahebi, A.; Seifalian, A.M. Recent advances in artificial nerve conduit design: Strategies for the delivery of luminal fillers. J. Control. Release 2011, 156, 2–10. [Google Scholar] [CrossRef]
- Zhao, Q.; Dahlin, L.B.; Kanje, M.; Lundborg, G. Repair of the transected rat sciatic nerve: Matrix formation within implanted silicone tubes. Restor. Neurol. Neurosci. 1993, 5, 197–204. [Google Scholar] [CrossRef]
- El Soury, M.; Fornasari, B.E.; Morano, M.; Grazio, E.; Ronchi, G.; Incarnato, D.; Giacobini, M.; Geuna, S.; Provero, P.; Gambarotta, G. Soluble Neuregulin1 Down-Regulates Myelination Genes in Schwann Cells. Front. Mol. Neurosci. 2018, 11, 157. [Google Scholar] [CrossRef]
- Fricker, F.R.; Antunes-Martins, A.; Galino, J.; Paramsothy, R.; La Russa, F.; Perkins, J.; Goldberg, R.; Brelstaff, J.; Zhu, N.; McMahon, S.B.; et al. Axonal neuregulin 1 is a rate limiting but not essential factor for nerve remyelination. Brain 2013, 136, 2279–2297. [Google Scholar] [CrossRef]
- Moore, A.M.; Borschel, G.H.; Santosa, K.B.; Flagg, E.R.; Tong, A.Y.; Kasukurthi, R.; Newton, P.; Yan, Y.; Hunter, D.A.; Johnson, P.J.; et al. A transgenic rat expressing green fluorescent protein (GFP) in peripheral nerves provides a new hindlimb model for the study of nerve injury and regeneration. J. Neurosci. Methods 2012, 204, 19–27. [Google Scholar] [CrossRef]



| Autograft vs. Chitosan | |||||||||
| 7 Days | 14 Days | 28 Days | |||||||
| ηp2 | p | CI 95% | ηp2 | p | CI 95% | ηp2 | p | CI 95% | |
| S100β | 0.85 | 0.001 | 0.1–0.24 | 0.87 | 0.001 | 0.11–0.25 | 0.63 | 0.02 | 0.35–0.26 |
| p75 | 0.97 | 0.000 | 64.99–93.51 | 0.81 | 0.002 | 24.70–71.92 | 0.67 | 0.01 | 4.21–23.61 |
| Thy1 | 0.05 | 0.59 | (−5.67)–9.11 | 0.87 | 0.001 | (−14.38)–(−6.27) | 0.87 | 0.001 | (−15.04)–(−6.70) |
| sNRG1 | 0.00 | 0.907 | (−1.82)–2.02 | 0.93 | 0.000 | (−6.16)–(−3.71) | 0.85 | 0.000 | (−1.43)–(−0.65) |
| NRG1α | 0.11 | 0.42 | (−10.58)–22.33 | 0.69 | 0.01 | (−51.10)–(−10.24) | 0.94 | 0.000 | (−19.71)–(−11.59) |
| NRG1β | 0.20 | 0.738 | (−0.46)–0.61 | 0.78 | 0.004 | (−1.20)–(−0.37) | 0.47 | 0.06 | (−0.51)–0.47 |
| Control vs. Autograft | |||||||||
| 7 Days | 14 Days | 28 Days | |||||||
| ηp2 | p | CI 95% | ηp2 | p | CI 95% | ηp2 | p | CI 95% | |
| S100β | 0.99 | 0.000 | 0.92–1.11 | 0.99 | 0.000 | 0.92–1.06 | 0.99 | 0.000 | 0.82–1.04 |
| p75 | 0.97 | 0.000 | 72.65–101.16 | 0.83 | 0.002 | 28.37–75.59 | 0.87 | 0.001 | 14.70–34.11 |
| Thy1 | 0.4 | 0.09 | (−13.5)–1.28 | 0.94 | 0.000 | (−20.22)–(−12.12) | 0.91 | 0.000 | (−17.46)–(−9.12) |
| sNRG1 | 0.08 | 0.45 | (−1.18)–2.41 | 0.10 | 0.4 | (−1.59)–(−0.72) | 0.50 | 0.033 | (−0.77)–(−0.05) |
| NRG1α | 0.48 | 0.06 | (−0.71)–32.21 | 0.06 | 0.57 | (–15.39)–25.47 | 0.06 | 0.57 | (−3.07)–5.05 |
| NRG1β | 0.55 | 0.03 | (−1.14)–(−0.06) | 0.80 | 0.003 | (−1.26)–(−0.42) | 0.87 | 0.01 | (−0.90)–( −0.41) |
| Control vs. Chitosan | |||||||||
| 7 Days | 14 Days | 28 Days | |||||||
| ηp2 | p | CI 95% | ηp2 | p | CI 95% | ηp2 | p | CI 95% | |
| S100β | 0.99 | 0.000 | (−1.05)–(−0.91) | 0.99 | 0.000 | (−1.06)–(−0.92) | 0.99 | 0.000 | (−1.04)–(−0.82) |
| p75 | 0.22 | 0.237 | (−21.91)–6.6 | 0.024 | 0.717 | (−27.28)–19.94 | 0.54 | 0.04 | (−20.21)–(−0.79) |
| Thy1 | 0.4 | 0.09 | (−1.28)–13.5 | 0.94 | 0.000 | 12.12–20.22 | 0.91 | 0.000 | 17.46–0.91 |
| sNRG1 | 0.06 | 0.52 | (−2.31)–1.28 | 0.92 | 0.000 | (−5.64)–(−3.34) | 0.97 | 0.000 | (−0.95)–(−0.27) |
| NRG1α | 0.26 | 0.19 | (−26.34)–6.57 | 0.75 | 0.005 | (−56.14)–(−15.28) | 0.94 | 0.000 | (−20.71)–(−12.57) |
| NRG1β | 0.61 | 0.022 | 0.14–1.22 | 0.02 | 0.75 | 0.36–0.48 | 0.73 | 0.007 | 0.16–0.66 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fornasari, B.E.; El Soury, M.; Nato, G.; Fucini, A.; Carta, G.; Ronchi, G.; Crosio, A.; Perroteau, I.; Geuna, S.; Raimondo, S.; et al. Fibroblasts Colonizing Nerve Conduits Express High Levels of Soluble Neuregulin1, a Factor Promoting Schwann Cell Dedifferentiation. Cells 2020, 9, 1366. https://doi.org/10.3390/cells9061366
Fornasari BE, El Soury M, Nato G, Fucini A, Carta G, Ronchi G, Crosio A, Perroteau I, Geuna S, Raimondo S, et al. Fibroblasts Colonizing Nerve Conduits Express High Levels of Soluble Neuregulin1, a Factor Promoting Schwann Cell Dedifferentiation. Cells. 2020; 9(6):1366. https://doi.org/10.3390/cells9061366
Chicago/Turabian StyleFornasari, Benedetta E., Marwa El Soury, Giulia Nato, Alessia Fucini, Giacomo Carta, Giulia Ronchi, Alessandro Crosio, Isabelle Perroteau, Stefano Geuna, Stefania Raimondo, and et al. 2020. "Fibroblasts Colonizing Nerve Conduits Express High Levels of Soluble Neuregulin1, a Factor Promoting Schwann Cell Dedifferentiation" Cells 9, no. 6: 1366. https://doi.org/10.3390/cells9061366
APA StyleFornasari, B. E., El Soury, M., Nato, G., Fucini, A., Carta, G., Ronchi, G., Crosio, A., Perroteau, I., Geuna, S., Raimondo, S., & Gambarotta, G. (2020). Fibroblasts Colonizing Nerve Conduits Express High Levels of Soluble Neuregulin1, a Factor Promoting Schwann Cell Dedifferentiation. Cells, 9(6), 1366. https://doi.org/10.3390/cells9061366









