Integrating Biophysics in Toxicology
Abstract
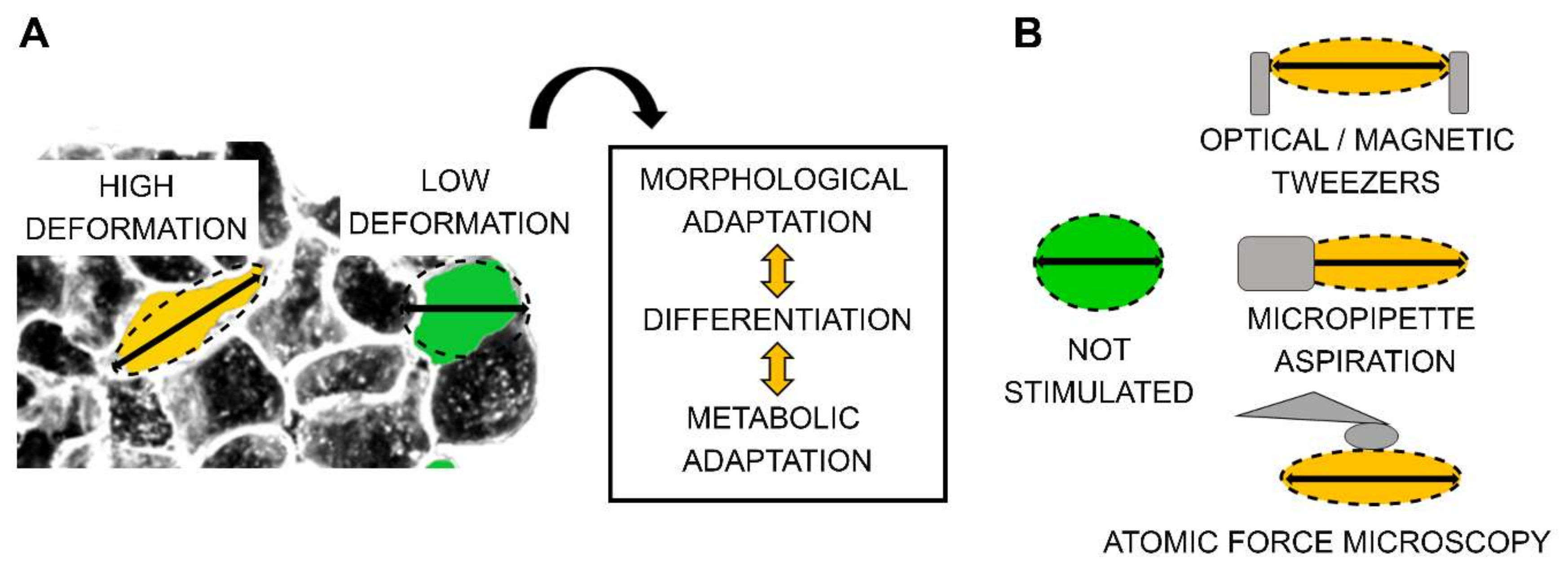
1. Introduction
- i)
- ii)
- iii)
- iv)
- v)
2. Substrate Stiffness
Modulation of ECM / Substrate Stiffness in Toxicological Context
3. Shear Stress
3.1. Modulation of Shear Stress in Toxicological Context
3.1.1. Endothelial Cells
Interaction of Endothelial Cells and NPs
Interaction of Endothelial Cells with Pharmaceuticals
3.1.2. Non-Endothelial Cells
4. Mechanical Substrate Deformation/Strain
Modulation of Mechanical Strain in the Toxicological Context
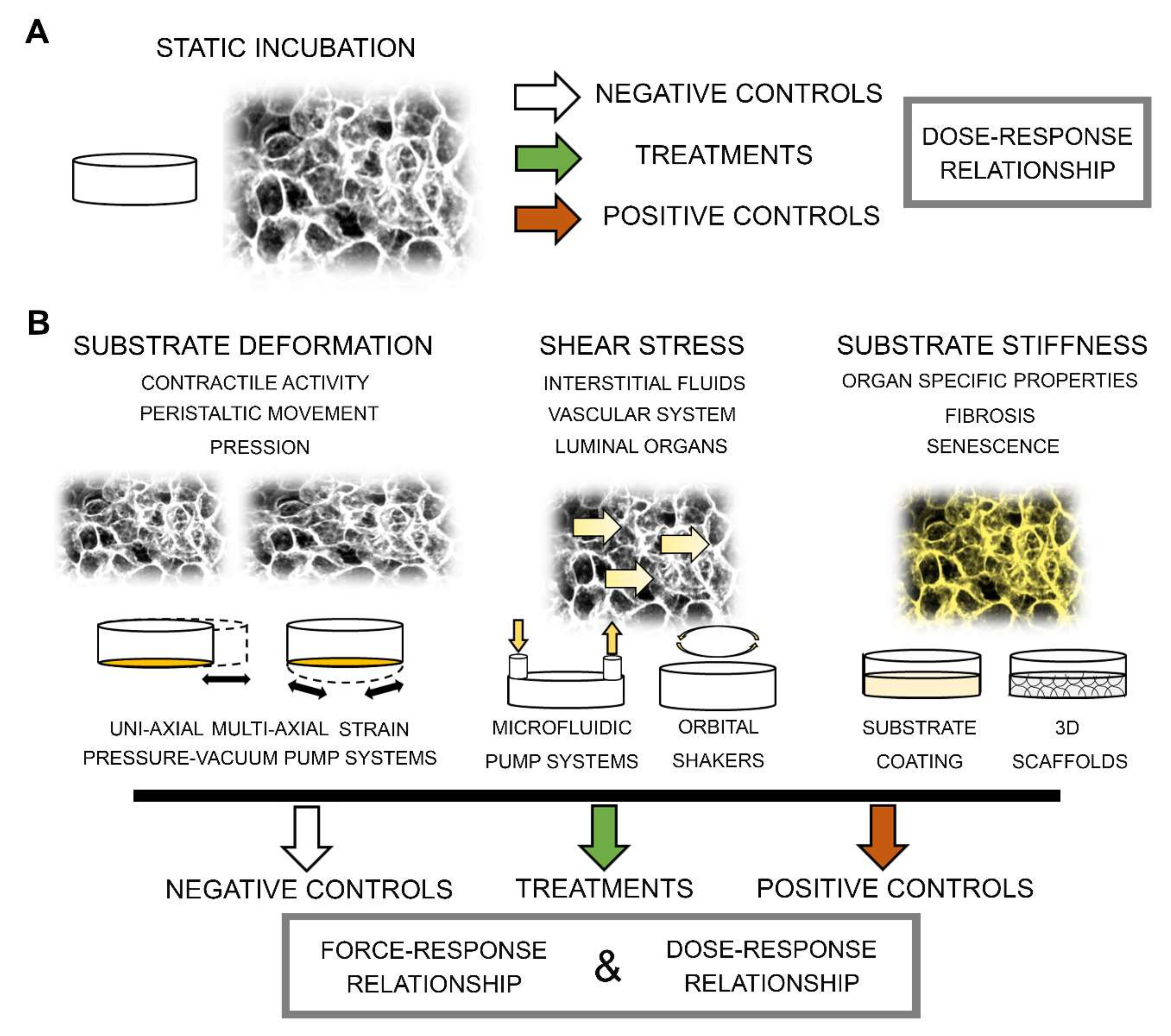
5. Integrating Biophysical Stimulation in Experimental Layout and Data Analysis
6. Integrating Biophysical Stimulation in Toxicokinetics Models
6.1. Absorption
6.2. Distribution
6.3. Metabolism
6.4. Excretion
7. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, B.; Radisic, M. Organ-on-a-chip devices advance to market. Lab a Chip 2017, 17, 2395–2420. [Google Scholar] [CrossRef]
- Wnorowski, A.; Yang, H.; Wu, J.C. Progress, obstacles, and limitations in the use of stem cells in organ-on-a-chip models. Adv. Drug Deliv. Rev. 2019, 140, 3–11. [Google Scholar] [CrossRef]
- Wilmer, M.J.; Ng, C.P.; Vulto, P.; Suter-Dick, L.; Masereeuw, R.; Information, P.E.K.F.C.; Lanz, H. Kidney-on-a-Chip Technology for Drug-Induced Nephrotoxicity Screening. Trends Biotechnol. 2016, 34, 156–170. [Google Scholar] [CrossRef]
- Weinhart, M.; Hocke, A.; Hippenstiel, S.; Kurreck, J.; Hedtrich, S. 3D organ models—Revolution in pharmacological research? Pharmacol. Res. 2019, 139, 446–451. [Google Scholar] [CrossRef]
- Tetsuka, K.; Ohbuchi, M.; Tabata, K. Recent Progress in Hepatocyte Culture Models and Their Application to the Assessment of Drug Metabolism, Transport, and Toxicity in Drug Discovery: The Value of Tissue Engineering for the Successful Development of a Microphysiological System. J. Pharm. Sci. 2017, 106, 2302–2311. [Google Scholar] [CrossRef]
- Osaki, T.; Shin, Y.; Sivathanu, V.; Campisi, M.; Kamm, R.D. In Vitro Microfluidic Models for Neurodegenerative Disorders. Adv. Heal. Mater. 2017, 7, 1700489. [Google Scholar] [CrossRef]
- Nikolic, M.; Sustersic, T.; Filipovic, N. In vitro Models and On-Chip Systems: Biomaterial Interaction Studies With Tissues Generated Using Lung Epithelial and Liver Metabolic Cell Lines. Front. Bioeng. Biotechnol. 2018, 6, 120. [Google Scholar] [CrossRef]
- Lelièvre, S.A.; Kwok, T.; Chittiboyina, S. Architecture in 3D cell culture: An essential feature for in vitro toxicology. Toxicol. Vitr. 2017, 45, 287–295. [Google Scholar] [CrossRef]
- Kim, S.; Takayama, S. Organ-on-a-chip and the kidney. Kidney Res. Clin. Pr. 2015, 34, 165–169. [Google Scholar] [CrossRef]
- Jang, K.-J.; Mehr, A.P.; Hamilton, G.A.; McPartlin, L.A.; Chung, S.; Suh, K.-Y.; Ingber, D.E. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr. Boil. 2013, 5, 1119–1129. [Google Scholar] [CrossRef]
- Hiemstra, P.S.; Grootaers, G.; Van Der Does, A.; Krul, C.A.; Kooter, I.M. Human lung epithelial cell cultures for analysis of inhaled toxicants: Lessons learned and future directions. Toxicol. Vitr. 2018, 47, 137–146. [Google Scholar] [CrossRef]
- Cho, S.; Yoon, J.-Y. Organ-on-a-chip for assessing environmental toxicants. Curr. Opin. Biotechnol. 2017, 45, 34–42. [Google Scholar] [CrossRef]
- Neuži, P.; Giselbrecht, S.; Länge, K.; Huang, T.J.; Manz, A.; Neužil, P. Revisiting lab-on-a-chip technology for drug discovery. Nat. Rev. Drug Discov. 2012, 11, 620–632. [Google Scholar] [CrossRef]
- Hoffman, B.D.; Grashoff, C.; Schwartz, M. Dynamic molecular processes mediate cellular mechanotransduction. Nature 2011, 475, 316–323. [Google Scholar] [CrossRef]
- Chen, C.S. Mechanotransduction—A field pulling together? J. Cell Sci. 2008, 121, 3285–3292. [Google Scholar] [CrossRef]
- Charras, G.; Yap, A.S. Tensile Forces and Mechanotransduction at Cell–Cell Junctions. Curr. Boil. 2018, 28, R445–R457. [Google Scholar] [CrossRef]
- Morris, C.; Homann, U. Cell surface area regulation and membrane tension. J. Membr. Boil. 2001, 179, 79–102. [Google Scholar] [CrossRef]
- Huveneers, S.; Danen, E.H. Adhesion signaling—Crosstalk between integrins, Src and Rho. J. Cell Sci. 2009, 122, 1059–1069. [Google Scholar] [CrossRef]
- Tamiello, C.; Buskermolen, A.B.C.; Baaijens, F.P.T.; Broers, J.L.V.; Bouten, C.V.C. Heading in the Right Direction: Understanding Cellular Orientation Responses to Complex Biophysical Environments. Cell. Mol. Bioeng. 2015, 9, 12–37. [Google Scholar] [CrossRef]
- Anishkin, A.; Loukin, S.H.; Teng, J.; Kung, C. Feeling the hidden mechanical forces in lipid bilayer is an original sense. Proc. Natl. Acad. Sci. USA 2014, 111, 7898–7905. [Google Scholar] [CrossRef]
- Vogel, V.; Sheetz, M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Boil. 2006, 7, 265–275. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Ling, J.-Y.; Chen, W.-C.; Lin, H.-H.; Tang, M. Mechanotransduction of matrix stiffness in regulation of focal adhesion size and number: Reciprocal regulation of caveolin-1 and β1 integrin. Sci. Rep. 2017, 7, 15008. [Google Scholar] [CrossRef]
- He, Z.; Zhang, W.; Mao, S.; Li, N.; Li, H.; Lin, J.-M. Shear Stress-Enhanced Internalization of Cell Membrane Proteins Indicated by a Hairpin-Type DNA Probe. Anal. Chem. 2018, 90, 5540–5545. [Google Scholar] [CrossRef]
- Suchyna, T.M. Piezo channels and GsMTx4: Two milestones in our understanding of excitatory mechanosensitive channels and their role in pathology. Prog. Biophys. Mol. Boil. 2017, 130, 244–253. [Google Scholar] [CrossRef]
- Coste, B.; Xiao, B.; Santos, J.S.; Syeda, R.; Grandl, J.; Spencer, K.S.; Kim, S.E.; Schmidt, M.; Mathur, J.; Dubin, A.E.; et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 2012, 483, 176–181. [Google Scholar] [CrossRef]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 Are Essential Components of Distinct Mechanically Activated Cation Channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef]
- Chubinskiy-Nadezhdin, V.I.; Negulyaev, Y.A.; Morachevskaya, E.A. Functional coupling of ion channels in cellular mechanotransduction. Biochem. Biophys. Res. Commun. 2014, 451, 421–424. [Google Scholar] [CrossRef]
- Isermann, P.; Lammerding, J. Nuclear mechanics and mechanotransduction in health and disease. Curr. Boil. 2013, 23, R1113–R1121. [Google Scholar] [CrossRef]
- Shao, Y.; Mann, J.M.; Chen, W.; Fu, J. Global architecture of the F-actin cytoskeleton regulates cell shape-dependent endothelial mechanotransduction. Integr. Boil. 2014, 6, 300–311. [Google Scholar] [CrossRef]
- Chicurel, M.E.; Singer, R.H.; Meyer, C.J.; Ingber, N.E. Integrin binding and mechanical tension induce movement of mRNA and ribosomes to focal adhesions. Nature 1998, 392, 730–733. [Google Scholar] [CrossRef]
- Rog-Zielinska, E.A.; O’Toole, E.T.; Hoenger, A.; Kohl, P. Mitochondrial Deformation During the Cardiac Mechanical Cycle. Anat. Rec. Adv. Integr. Anat. Evol. Boil. 2018, 302, 146–152. [Google Scholar] [CrossRef]
- Del Favero, G.; Woelflingseder, L.; Janker, L.; Neuditschko, B.; Seriani, S.; Gallina, P.; Sbaizero, O.; Gerner, C.; Marko, D. Deoxynivalenol induces structural alterations in epidermoid carcinoma cells A431 and impairs the response to biomechanical stimulation. Sci. Rep. 2018, 8, 11351. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Dobrowolski, P.; Winiarska-Mieczan, A.; Kwiecień, M.; Muszyński, S.; Tomczyk-Warunek, T. The effect of tannic acid on bone mechanical and geometric properties, bone density, and trabecular histomorphometry as well as the morphology of articular and growth cartilages in rats co-exposed to cadmium and lead is dose dependent. Toxicol. Ind. Heal. 2017, 33, 855–866. [Google Scholar] [CrossRef]
- Kazantzis, G. Cadmium, osteoporosis and calcium metabolism. Biometals Int. J. Role Metal Ions Biol. Biochem. Med. 2004, 17, 493–498. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, G.; Shi, Y.; Weng, S.; Jin, T.; Kong, Q.; Nordberg, G. Influence of Environmental Cadmium Exposure on Forearm Bone Density. J. Bone Miner. Res. 2003, 18, 553–560. [Google Scholar] [CrossRef]
- Sbaizero, O.; DelFavero, G.; Martinelli, V.; Long, C.; Mestroni, L. Analysis of long- and short-range contribution to adhesion work in cardiac fibroblasts: An atomic force microscopy study. Mater. Sci. Eng. C 2015, 49, 217–224. [Google Scholar] [CrossRef]
- Codan, B.; Del Favero, G.; Martinelli, V.; Long, C.; Mestroni, L.; Sbaizero, O. Exploring the elasticity and adhesion behavior of cardiac fibroblasts by atomic force microscopy indentation. Mater. Sci. Eng. C 2014, 40, 427–434. [Google Scholar] [CrossRef]
- Wakatsuki, T.; Schwab, B.; Thompson, N.C.; Elson, E.L. Effects of cytochalasin D and latrunculin B on mechanical properties of cells. J. Cell Sci. 2001, 114, 1025–1036. [Google Scholar]
- Spedden, E.; White, J.D.; Naumova, E.N.; Kaplan, D.L.; Staii, C. Elasticity Maps of Living Neurons Measured by Combined Fluorescence and Atomic Force Microscopy. Biophys. J. 2012, 103, 868–877. [Google Scholar] [CrossRef]
- Rotsch, C.; Radmacher, M. Drug-induced changes of cytoskeletal structure and mechanics in fibroblasts: An atomic force microscopy study. Biophys. J. 2000, 78, 520–535. [Google Scholar] [CrossRef]
- Mohammed, D.; Versaevel, M.; Bruyère, C.; Alaimo, L.; Luciano, M.; Vercruysse, E.; Procès, A.; Gabriele, S. Innovative Tools for Mechanobiology: Unraveling Outside-In and Inside-Out Mechanotransduction. Front. Bioeng. Biotechnol. 2019, 7, 162. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Jansen, K.A.; Atherton, P.; Ballestrem, C. Mechanotransduction at the cell-matrix interface. Semin. Cell Dev. Boil. 2017, 71, 75–83. [Google Scholar] [CrossRef]
- Gauthier, N.C.; Roca-Cusachs, P. Mechanosensing at integrin-mediated cell–matrix adhesions: From molecular to integrated mechanisms. Curr. Opin. Cell Boil. 2018, 50, 20–26. [Google Scholar] [CrossRef]
- LaFoya, B.; Munroe, J.A.; Miyamoto, A.; Detweiler, M.A.; Crow, J.J.; Gazdik, T.; Albig, A.R. Beyond the Matrix: The Many Non-ECM Ligands for Integrins. Int. J. Mol. Sci. 2018, 19, 449. [Google Scholar] [CrossRef]
- Lin, H.Y.; Lansing, L.; Merillon, J.M.; Davis, F.B.; Tang, H.Y.; Shih, A.; Vitrac, X.; Krisa, S.; Keating, T.; Cao, H.J.; et al. Integrin alphaVbeta3 contains a receptor site for resveratrol. FASEB J. Offic. Pub. Fed. Am. Soc. Exp. Biol. 2006, 20, 1742–1744. [Google Scholar]
- Jia, B.; Shi, T.; Li, Z.; Shan, S.; Ji, P.; Li, Z. Toxicological effects of bisphenol A exposure-induced cancer cells migration via activating directly integrin beta1. Chemosphere 2019, 220, 783–792. [Google Scholar] [CrossRef]
- Gao, J.; Song, T.; Che, D.; Li, C.; Jiang, J.; Pang, J.; Yang, Y.; Li, P. Goma The effect of bisphenol a exposure onto endothelial and decidualized stromal cells on regulation of the invasion ability of trophoblastic spheroids in in vitro co-culture model. Biochem. Biophys. Res. Commun. 2019, 516, 506–514. [Google Scholar] [CrossRef]
- Qu, T.; Zhang, S.-M.; Yu, L.-L.; Zhang, S.; Yuan, D.-Z.; Xu, Q.; Zhang, J.-H.; He, Y.-P.; Yue, L.-M. Relocalisation and activation of integrins induced rapidly by oestrogen via G-protein-coupled receptor 30 in mouse blastocysts. Reprod. Fertil. Dev. 2016, 28, 1679–1685. [Google Scholar] [CrossRef]
- Plotnikov, S.V.; Pasapera, A.M.; Sabass, B.; Waterman, C.M. Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell 2012, 151, 1513–1527. [Google Scholar] [CrossRef]
- Lv, H.; Li, L.; Sun, M.; Zhang, Y.; Chen, L.; Rong, Y.; Li, Y. Mechanism of regulation of stem cell differentiation by matrix stiffness. Stem Cell Res. Ther. 2015, 6, 103. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, T.; Zhang, Z.; Fu, Y. Matrix Stiffness Differentially Regulates Cellular Uptake Behavior of Nanoparticles in Two Breast Cancer Cell Lines. ACS Appl. Mater. Interfaces 2017, 9, 25915–25928. [Google Scholar] [CrossRef]
- Ali, M.Y.; Chuang, C.-Y.; A Saif, M.T. Reprogramming cellular phenotype by soft collagen gels. Soft Matter. 2014, 10, 8829–8837. [Google Scholar] [CrossRef]
- D’Andrea, P.; Civita, D.; Cok, M.; Severino, L.U.; Vita, F.; Scaini, D.; Casalis, L.; Lorenzon, P.; Donati, I.; Bandiera, A. Myoblast adhesion, proliferation and differentiation on human elastin-like polypeptide (HELP) hydrogels. J. Appl. Biomater. Funct. Mater. 2016, 15, 43. [Google Scholar] [CrossRef]
- Discher, D.; Janmey, P.; Wang, Y.-L. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef]
- Peña, B.; Bosi, S.; Aguado, B.A.; Borin, D.; Farnsworth, N.; Dobrinskikh, E.; Rowland, T.J.; Martinelli, V.; Jeong, M.; Taylor, M.R.G.; et al. Injectable Carbon Nanotube-Functionalized Reverse Thermal Gel Promotes Cardiomyocytes Survival and Maturation. ACS Appl. Mater. Interfaces 2017, 9, 31645–31656. [Google Scholar] [CrossRef]
- Fabbro, A.; Prato, M.; Ballerini, L. Carbon nanotubes in neuroregeneration and repair. Adv. Drug Deliv. Rev. 2013, 65, 2034–2044. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; E Discher, D. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Tay, C.Y.; Yu, H.-Y.; Pal, M.; Leong, W.S.; Tan, N.S.; Ng, K.W.; Leong, D.T.; Tan, L.P. Micropatterned matrix directs differentiation of human mesenchymal stem cells towards myocardial lineage. Exp. Cell Res. 2010, 316, 1159–1168. [Google Scholar] [CrossRef]
- Parandakh, A.; Anbarlou, A.; Tafazzoli-Shadpour, M.; Ardeshirylajimi, A.; Khani, M.-M. Substrate topography interacts with substrate stiffness and culture time to regulate mechanical properties and smooth muscle differentiation of mesenchymal stem cells. Colloids Surfaces B: Biointerfaces 2019, 173, 194–201. [Google Scholar] [CrossRef]
- Friedl, P.; Alexander, S. Cancer Invasion and the Microenvironment: Plasticity and Reciprocity. Cell 2011, 147, 992–1009. [Google Scholar] [CrossRef] [PubMed]
- Emon, A.B.; Bauer, J.; Jain, Y.; Jung, B.; A Saif, M.T. Biophysics of Tumor Microenvironment and Cancer Metastasis—A Mini Review. Comput. Struct. Biotechnol. J. 2018, 16, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Dufort, C.C.; Paszek, M.J.; Weaver, V.M. Balancing forces: Architectural control of mechanotransduction. Nat. Rev. Mol. Cell Boil. 2011, 12, 308–319. [Google Scholar] [CrossRef]
- Weaver, V.M.; Lelievre, S.; Lakins, J.N.; Chrenek, M.A.; Jones, J.C.; Giancotti, F.; Werb, Z.; Bissell, M.J. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell 2002, 2, 205–216. [Google Scholar] [CrossRef]
- Yeldag, G.; Rice, A.; Hernandez, A.D.R. Chemoresistance and the Self-Maintaining Tumor Microenvironment. Cancers 2018, 10, 471. [Google Scholar] [CrossRef] [PubMed]
- Broders-Bondon, F.; Ho-Bouldoires, T.H.N.; Sanchez, M.E.F.; Farge, E. Mechanotransduction in tumor progression: The dark side of the force. J. Cell Boil. 2018, 217, 1571–1587. [Google Scholar] [CrossRef]
- Furuta, S.; Bissell, M.J. Pathways Involved in Formation of Mammary Organoid Architecture Have Keys to Understanding Drug Resistance and to Discovery of Druggable Targets. Cold Spring Harb. Symp. Quant. Boil. 2016, 81, 207–217. [Google Scholar] [CrossRef][Green Version]
- Kuczek, D.E.; Larsen, A.M.H.; Thorseth, M.-L.; Carretta, M.; Kalvisa, A.; Siersbæk, M.S.; Simões, A.M.C.; Roslind, A.; Engelholm, L.H.; Noessner, E.; et al. Collagen density regulates the activity of tumor-infiltrating T cells. J. Immunother. Cancer 2019, 7, 68. [Google Scholar] [CrossRef]
- Sethi, T.; Rintoul, R.C.; Moore, S.M.; MacKinnon, A.C.; Salter, N.; Choo, C.; Chilvers, E.R.; Dransfield, I.; Donnelly, S.C.; Strieter, R.; et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: A mechanism for small cell lung cancer growth and drug resistance in vivo. Nat. Med. 1999, 5, 662–668. [Google Scholar] [CrossRef]
- Corvaisier, M.; Bauzone, M.; Corfiotti, F.; Renaud, F.; El Amrani, M.; Monte, D.; Truant, S.; Leteurtre, E.; Formstecher, P.; Van Seuningen, I.; et al. Regulation of cellular quiescence by YAP/TAZ and Cyclin E1 in colon cancer cells: Implication in chemoresistance and cancer relapse. Oncotarget 2016, 7, 56699–56712. [Google Scholar] [CrossRef]
- Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Boil. 2017, 18, 758–770. [Google Scholar] [CrossRef]
- Zustiak, S.P. The Role of Matrix Compliance on Cell Responses to Drugs and Toxins: Towards Predictive Drug Screening Platforms. Macromol. Biosci. 2015, 15, 589–599. [Google Scholar] [CrossRef]
- Yu, C.K.; Xu, T.; Assoian, R.K.; Rader, D.J. Mining the Stiffness-Sensitive Transcriptome in Human Vascular Smooth Muscle Cells Identifies Long Noncoding RNA Stiffness Regulators. Arter. Thromb. Vasc. Boil. 2017, 38, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-Y.; Kumar, R.A.; Sukumaran, S.M.; Hogg, M.G.; Clark, D.S.; Dordick, J.S. Three-dimensional cellular microarray for high-throughut toxicology assays. Proc. Natl. Acad. Sci. USA 2007, 105, 59–63. [Google Scholar] [CrossRef]
- Loessner, D.; Stok, K.S.; Lutolf, M.P.; Hutmacher, D.W.; Clements, J.A.; Rizzi, S.C. Bioengineered 3D platform to explore cell–ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials 2010, 31, 8494–8506. [Google Scholar] [CrossRef]
- Lan, S.-F.; Starly, B. Alginate based 3D hydrogels as an in vitro co-culture model platform for the toxicity screening of new chemical entities. Toxicol. Appl. Pharmacol. 2011, 256, 62–72. [Google Scholar] [CrossRef]
- Mih, J.D.; Sharif, A.S.; Liu, F.; Marinković, A.; Symer, M.; Tschumperlin, D.J. A Multiwell Platform for Studying Stiffness-Dependent Cell Biology. PLoS ONE 2011, 6, e19929. [Google Scholar] [CrossRef]
- Huang, C.; Butler, P.J.; Tong, S.; Muddana, H.S.; Bao, G.; Zhang, S. Substrate Stiffness Regulates Cellular Uptake of Nanoparticles. Nano Lett. 2013, 13, 1611–1615. [Google Scholar] [CrossRef]
- Mambetsariev, I.; Tian, Y.; Wu, T.; Lavoie, T.; Solway, J.; Birukova, A.A.; Birukova, A.A. Stiffness-Activated GEF-H1 Expression Exacerbates LPS-Induced Lung Inflammation. PLoS ONE 2014, 9, e92670. [Google Scholar] [CrossRef]
- Ramamoorthi, K.; Hara, J.; Ito, C.; Asuri, P. Role of Three-Dimensional Matrix Stiffness in Regulating the Response of Human Neural Cells to Toxins. Cell. Mol. Bioeng. 2014, 7, 278–284. [Google Scholar] [CrossRef]
- Zustiak, S.P.; Dadhwal, S.; Medina, C.; Steczina, S.; Chehreghanianzabi, Y.; Ashraf, A.; Asuri, P. Three-dimensional matrix stiffness and adhesive ligands affect cancer cell response to toxins. Biotechnol. Bioeng. 2015, 113, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.J.; Cortes, E.; Lachowski, D.; Cheung, B.C.H.; A Karim, S.; Morton, J.P.; Hernandez, A.D.R. Matrix stiffness induces epithelial-mesenchymal transition and promotes chemoresistance in pancreatic cancer cells. Oncogenesis 2017, 6, e352. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.F. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat. Clin. Pr. Neurol. 2008, 6, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.; Schwartz, M.A. Mechanotransduction in vascular physiology and atherogenesis. Nat. Rev. Mol. Cell Boil. 2009, 10, 53–62. [Google Scholar] [CrossRef]
- Huang, Q.; Hu, X.; He, W.; Zhao, Y.; Hao, S.; Wu, Q.; Li, S.; Zhang, S.; Shi, M. Fluid shear stress and tumor metastasis. Am. J. Cancer Res. 2018, 8, 763–777. [Google Scholar]
- Mitchell, M.J.; King, M.R. Computational and Experimental Models of Cancer Cell Response to Fluid Shear Stress. Front. Oncol. 2013, 3, 44. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, Y.-S.; Chien, S. Shear stress-initiated signaling and its regulation of endothelial function. Arter. Thromb. Vasc. Boil. 2014, 34, 2191–2198. [Google Scholar] [CrossRef]
- Pethő, Z.; Najder, K.; Bulk, E.; Schwab, A. Mechanosensitive ion channels push cancer progression. Cell Calcium 2019, 80, 79–90. [Google Scholar] [CrossRef]
- Bowden, N.; Bryan, M.T.; Duckles, H.; Feng, S.; Hsiao, S.; Kim, H.R.; Mahmoud, M.; Moers, B.; Serbanovic-Canic, J.; Xanthis, I.; et al. Experimental Approaches to Study Endothelial Responses to Shear Stress. Antioxidants Redox Signal. 2016, 25, 389–400. [Google Scholar] [CrossRef]
- Rochfort, K.; E Collins, L.; McLoughlin, A.; Cummins, P.M. Shear-dependent attenuation of cellular ROS levels can suppress proinflammatory cytokine injury to human brain microvascular endothelial barrier properties. Br. J. Pharmacol. 2015, 35, 1648–1656. [Google Scholar] [CrossRef]
- Bryan, M.T.; Duckles, H.; Feng, S.; Hsiao, S.; Kim, H.R.; Serbanovic-Canic, J.; Evans, P.C. Mechanoresponsive Networks Controlling Vascular Inflammation. Arter. Thromb. Vasc. Boil. 2014, 34, 2199–2205. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, T.; Maruyama, A.; Kang, M.-I.; Kawatani, Y.; Shibata, T.; Uchida, K.; Itoh, K.; Yamamoto, M. Differential Responses of the Nrf2-Keap1 System to Laminar and Oscillatory Shear Stresses in Endothelial Cells. J. Boil. Chem. 2005, 280, 27244–27250. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Wang, J.; Tong, Y.; Hao, Q.; Li, Y.; Yang, H.; Huang, L.; Liao, F. Anti-inflammatory effect of acetylharpagide demonstrated by its influence on leukocyte adhesion and transmigration in endothelial cells under controlled shear stress. Clin. Hemorheol. Microcirc. 2014, 56, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Tzima, E.; Irani-Tehrani, M.; Kiosses, W.B.; Dejana, E.; Schultz, D.A.; Engelhardt, B.; Cao, G.; Delisser, H.; Schwartz, M. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 2005, 437, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Conway, D.E.; Breckenridge, M.T.; Hinde, E.; Gratton, E.; Chen, C.S.; Schwartz, M. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr. Boil. 2013, 23, 1024–1030. [Google Scholar] [CrossRef]
- Marin, T.; Gongol, B.; Chen, Z.; Woo, B.; Subramaniam, S.; Chien, S.; Shyy, J.Y.-J. Mechanosensitive microRNAs-role in endothelial responses to shear stress and redox state. Free. Radic. Boil. Med. 2013, 64, 61–68. [Google Scholar] [CrossRef]
- Morigi, M.; Zoja, C.; Figliuzzi, M.; Foppolo, M.; Micheletti, G.; Bontempelli, M.; Saronni, M.; Remuzzi, G.; Remuzzi, A. Fluid shear stress modulates surface expression of adhesion molecules by endothelial cells. Blood 1995, 85, 1696–1703. [Google Scholar] [CrossRef]
- Chiu, J.-J.; Lee, P.-L.; Chen, C.-N.; Lee, C.-I.; Chang, S.-F.; Chen, L.-J.; Lien, S.-C.; Ko, Y.-C.; Usami, S.; Chien, S. Shear Stress Increases ICAM-1 and Decreases VCAM-1 and E-selectin Expressions Induced by Tumor Necrosis Factor-α in Endothelial Cells. Arter. Thromb. Vasc. Boil. 2004, 24, 73–79. [Google Scholar] [CrossRef]
- Bailey, K.A.; Haj, F.G.; Simon, S.I.; Passerini, A. Atherosusceptible Shear Stress Activates Endoplasmic Reticulum Stress to Promote Endothelial Inflammation. Sci. Rep. 2017, 7, 8196. [Google Scholar] [CrossRef]
- Yamamoto, K.; Imamura, H.; Ando, J. Shear stress augments mitochondrial ATP generation that triggers ATP release and Ca2+ signaling in vascular endothelial cells. Am. J. Physiol. Circ. Physiol. 2018, 315, H1477–H1485. [Google Scholar] [CrossRef]
- Wu, L.-H.; Chang, H.-C.; Ting, P.-C.; Wang, D.L. Laminar shear stress promotes mitochondrial homeostasis in endothelial cells. J. Cell. Physiol. 2018, 233, 5058–5069. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Peng, I.-C.; Cui, X.; Li, Y.-S.; Chien, S.; Shyy, J.Y.-J. Shear stress, SIRT1, and vascular homeostasis. Proc. Natl. Acad. Sci. USA 2010, 107, 10268–10273. [Google Scholar] [CrossRef]
- Wang, X.; Fang, X.; Zhou, J.; Chen, Z.; Zhao, B.; Xiao, L.; Liu, A.; Li, Y.-S.J.; Shyy, J.Y.-J.; Guan, Y.; et al. Shear stress activation of nuclear receptor PXR in endothelial detoxification. Proc. Natl. Acad. Sci. USA 2013, 110, 13174–13179. [Google Scholar] [CrossRef]
- DeVerse, J.S.; Sandhu, A.S.; Mendoza, N.; Edwards, C.M.; Sun, C.; Simon, S.I.; Passerini, A. Shear stress modulates VCAM-1 expression in response to TNF-α and dietary lipids via interferon regulatory factor-1 in cultured endothelium. Am. J. Physiol. Circ. Physiol. 2013, 305, H1149–H1157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, D.; Lin, Y.-S.; Haynes, C.L. On-Chip Evaluation of Shear Stress Effect on Cytotoxicity of Mesoporous Silica Nanoparticles. Anal. Chem. 2011, 83, 8377–8382. [Google Scholar] [CrossRef] [PubMed]
- Fede, C.; Fortunati, I.; Weber, V.; Rossetto, N.; Bertasi, F.; Petrelli, L.; Guidolin, D.; Signorini, R.; De Caro, R.; Albertin, G.; et al. Evaluation of gold nanoparticles toxicity towards human endothelial cells under static and flow conditions. Microvasc. Res. 2015, 97, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Klingberg, H.; Loft, S.; Oddershede, L.B.; Møller, P. The influence of flow, shear stress and adhesion molecule targeting on gold nanoparticle uptake in human endothelial cells. Nanoscale 2015, 7, 11409–11419. [Google Scholar] [CrossRef]
- Fede, C.; Albertin, G.; Petrelli, L.; De Caro, R.; Fortunati, I.; Weber, V.; Ferrante, C. Influence of shear stress and size on viability of endothelial cells exposed to gold nanoparticles. J. Nanoparticle Res. 2017, 19, 316. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Garcia, M.J.; Doiron, A.L.; Steele, R.R.M.; Labouta, H.; Vafadar, B.; Shepherd, R.D.; Gates, I.D.; Cramb, D.T.; Childs, S.J.; Rinker, K. Nanoparticle localization in blood vessels: Dependence on fluid shear stress, flow disturbances, and flow-induced changes in endothelial physiology. Nanoscale 2018, 10, 15249–15261. [Google Scholar] [CrossRef]
- Feng, S.; Mao, S.; Zhang, Q.; Li, W.; Lin, J.-M. Online Analysis of Drug Toxicity to Cells with Shear Stress on an Integrated Microfluidic Chip. ACS Sensors 2019, 4, 521–527. [Google Scholar] [CrossRef]
- Davies, J.E.; LoPresto, D.; Apta, B.H.; Lin, Z.; Ma, W.; Harper, M.T. Using Yoda-1 to mimic laminar flow in vitro: A tool to simplify drug testing. Biochem. Pharmacol. 2019, 168, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, J.; Wang, Z.; Chen, S. Ivabradine Prevents Low Shear Stress Induced Endothelial Inflammation and Oxidative Stress via mTOR/eNOS Pathway. PLoS ONE 2016, 11, e0149694. [Google Scholar] [CrossRef] [PubMed]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, N.-J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, F.; Gui, L.; He, Q.; Yao, Y.; Chen, H. The potential of biomimetic nanoparticles for tumor-targeted drug delivery. Nanomedicine 2018, 13, 2099–2118. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, M.D.L.L.Z.; González-Reza, R.M.; Mendoza-Munoz, N.; Miranda-Linares, V.; Bernal-Couoh, T.F.; Mendoza-Elvira, S.; Quintanar-Guerrero, D. Nanosystems in Edible Coatings: A Novel Strategy for Food Preservation. Int. J. Mol. Sci. 2018, 19, 705. [Google Scholar] [CrossRef]
- Winkler, H.; Suter, M.; Naegeli, H. Critical review of the safety assessment of nano-structured silica additives in food. J. Nanobiotechnology 2016, 14, 44. [Google Scholar] [CrossRef]
- Gromnicova, R.; Kaya, M.; Romero, I.A.; Williams, P.; Satchell, S.; Sharrack, B.; Male, D. Transport of Gold Nanoparticles by Vascular Endothelium from Different Human Tissues. PLoS ONE 2016, 11, e0161610. [Google Scholar] [CrossRef]
- Nassoy, P.; Lamaze, C. Stressing caveolae new role in cell mechanics. Trends Cell Boil. 2012, 22, 381–389. [Google Scholar] [CrossRef]
- Dai, J.; Sheetz, M.P.; Wan, X.; Morris, C. Membrane Tension in Swelling and Shrinking Molluscan Neurons. J. Neurosci. 1998, 18, 6681–6692. [Google Scholar] [CrossRef]
- Kusunose, J.; Zhang, H.; Gagnon, M.K.J.; Pan, T.; Simon, S.I.; Ferrara, K.W. Microfluidic system for facilitated quantification of nanoparticle accumulation to cells under laminar flow. Ann. Biomed. Eng. 2012, 41, 89–99. [Google Scholar] [CrossRef]
- Bagriantsev, S.N.; O Gracheva, E.; Gallagher, P.G. Piezo Proteins: Regulators of Mechanosensation and Other Cellular Processes. J. Boil. Chem. 2014, 289, 31673–31681. [Google Scholar] [CrossRef] [PubMed]
- Van Der Helm, M.W.; Van Der Meer, A.; Eijkel, J.C.T.; Berg, A.V.D.; I Segerink, L. Microfluidic organ-on-chip technology for blood-brain barrier research. Tissue Barriers 2016, 4, e1142493. [Google Scholar] [CrossRef] [PubMed]
- Park, T.-E.; Mustafaoglu, N.; Herland, A.; Hasselkus, R.; Mannix, R.; Fitzgerald, E.A.; Prantil-Baun, R.; Watters, A.; Henry, O.; Benz, M.; et al. Hypoxia-enhanced Blood-Brain Barrier Chip recapitulates human barrier function and shuttling of drugs and antibodies. Nat. Commun. 2019, 10, 2621. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.; Hinojosa, C.D.; Ingber, N.E.; Kim, H.J. Human Intestinal Morphogenesis Controlled by Transepithelial Morphogen Gradient and Flow-Dependent Physical Cues in a Microengineered Gut-on-a-Chip. iScience 2019, 15, 391–406. [Google Scholar] [CrossRef]
- Barrila, J.; Crabbé, A.; Yang, J.; Franco, K.; Nydam, S.D.; Forsyth, R.J.; Davis, R.R.; Gangaraju, S.; Ott, C.M.; Coyne, C.B.; et al. Modeling Host-Pathogen Interactions in the Context of the Microenvironment: Three-Dimensional Cell Culture Comes of Age. Infect. Immun. 2018, 86, e00282-18. [Google Scholar] [CrossRef]
- Kim, H.J.; Huh, N.; Hamilton, G.; Ingber, N.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab a Chip 2012, 12, 2165. [Google Scholar] [CrossRef]
- Maschmeyer, I.; Lorenz, A.; Ramme, A.; Hasenberg, T.; Schimek, K.; Hubner, J.; Lauster, R.; Marx, U. A microfluidic four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Toxicol. Lett. 2015, 238, S176. [Google Scholar] [CrossRef]
- Vriend, J.; Peters, J.G.; Nieskens, T.T.; Škovroňová, R.; Blaimschein, N.; Schmidts, M.; Roepman, R.; Schirris, T.J.; Russel, F.G.; Masereeuw, R.; et al. Flow stimulates drug transport in a human kidney proximal tubule-on-a-chip independent of primary cilia. Biochim. et Biophys. Acta (BBA) - Gen. Subj. 2020, 1864, 129433. [Google Scholar] [CrossRef]
- Lecluyse, E.; Witek, R.P.; Andersen, M.E.; Powers, M.J. Organotypic liver culture models: Meeting current challenges in toxicity testing. Crit. Rev. Toxicol. 2012, 42, 501–548. [Google Scholar] [CrossRef]
- Rashidi, H.; Alhaque, S.; Szkolnicka, D.; Flint, O.; Hay, D.C. Fluid shear stress modulation of hepatocyte-like cell function. Arch. Toxicol. 2016, 90, 1757–1761. [Google Scholar] [CrossRef]
- Xia, L.; Ng, S.; Han, R.; Tuo, X.; Xiao, G.; Leo, H.L.; Cheng, T.-M.; Yu, H. Laminar-flow immediate-overlay hepatocyte sandwich perfusion system for drug hepatotoxicity testing. Biomaterials 2009, 30, 5927–5936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tong, W.; Zheng, B.; Susanto, T.A.; Xia, L.; Zhang, C.; Ananthanarayanan, A.; Tuo, X.; Sakban, R.B.; Jia, R.; et al. A robust high-throughput sandwich cell-based drug screening platform. Biomaterials 2011, 32, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Deng, R.; Tong, W.H.; Huan, L.; Way, N.C.; IslamBadhan, A.; Iliescu, C.; Yu, H. A perfusion incubator liver chip for 3D cell culture with application on chronic hepatotoxicity testing. Sci. Rep. 2017, 7, 14528. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Fluri, D.A.; Marchan, R.; Boonen, K.; Mohanty, S.; Singh, P.; Hammad, S.; Landuyt, B.; Hengstler, J.G.; Kelm, J.M.; et al. 3D spherical microtissues and microfluidic technology for multi-tissue experiments and analysis. J. Biotechnol. 2015, 205, 24–35. [Google Scholar] [CrossRef]
- Luo, C.-W.; Wu, C.-C.; Ch’Ang, H.-J. Radiation sensitization of tumor cells induced by shear stress: The roles of integrins and FAK. Biochim. Biophys. Acta (BBA) Bioenerg. 2014, 1843, 2129–2137. [Google Scholar] [CrossRef]
- Del Favero, G.; Zaharescu, R.; Marko, D. Functional impairment triggered by altertoxin II (ATXII) in intestinal cells in vitro: Cross-talk between cytotoxicity and mechanotransduction. Arch. Toxicol. 2018, 92, 3535–3547. [Google Scholar] [CrossRef]
- Kang, T.; Cho, Y.; Park, C.; Kim, S.-D.; Oh, E.; Cui, J.-H.; Cao, Q.-R.; Lee, B.-J. Effect of biomimetic shear stress on intracellular uptake and cell-killing efficiency of doxorubicin in a free and liposomal formulation. Int. J. Pharm. 2016, 510, 42–47. [Google Scholar] [CrossRef]
- Sung, J.H.; Kam, C.; Shuler, M.L. A microfluidic device for a pharmacokinetic–pharmacodynamic (PK–PD) model on a chip. Lab a Chip 2010, 10, 446. [Google Scholar] [CrossRef]
- Kim, S.; LesherPerez, S.C.; Kim, B.C.C.; Yamanishi, C.; Labuz, J.M.; Leung, B.; Takayama, S. Pharmacokinetic profile that reduces nephrotoxicity of gentamicin in a perfused kidney-on-a-chip. Biofabrication 2016, 8, 015021. [Google Scholar] [CrossRef]
- Spencer, A.; Baker, A.B. High Throughput Label Free Measurement of Cancer Cell Adhesion Kinetics Under Hemodynamic Flow. Sci. Rep. 2016, 6, 19854. [Google Scholar] [CrossRef]
- Hosta-Rigau, L.; Städler, B. Shear Stress and Its Effect on the Interaction of Myoblast Cells with Nanosized Drug Delivery Vehicles. Mol. Pharm. 2013, 10, 2707–2712. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.-W.; Chen, P.-J.; Sun, Y.-S.; Chou, S.-E.; Lin, F.-Y.; Lo, K.-Y. Establishing a quick screening method by using a microfluidic chip to evaluate cytotoxicity of metal contaminants. Sci. Total. Environ. 2019, 651, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Uhler, C.; Shivashankar, G.V. Regulation of genome organization and gene expression by nuclear mechanotransduction. Nat. Rev. Mol. Cell Boil. 2017, 18, 717–727. [Google Scholar] [CrossRef]
- Lan, Q.; O Mercurius, K.; Davies, P.F. Stimulation of transcription factors NF kappa B and AP1 in endothelial cells subjected to shear stress. Biochem. Biophys. Res. Commun. 1994, 201, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Baeriswyl, D.C.; Prionisti, I.; Peach, T.; Tsolkas, G.; Chooi, K.Y.; Vardakis, J.; Morel, S.; Diagbouga, M.R.; Bijlenga, P.; Cuhlmann, S.; et al. Disturbed flow induces a sustained, stochastic NF-kappaB activation which may support intracranial aneurysm growth in vivo. Sci. Rep. 2019, 9, 4738. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.F.; Vaidya, A.B.; Evans, S.M.; Lee, H.J.; Aertker, B.M.; Alexander, A.; Price, K.M.; Ozuna, J.A.; Liao, G.P.; Aroom, K.R.; et al. Biomechanical Forces Promote Immune Regulatory Function of Bone Marrow Mesenchymal Stromal Cells. STEM CELLS 2017, 35, 1259–1272. [Google Scholar] [CrossRef]
- Andrés-Delgado, L.; Mercader, N. Interplay between cardiac function and heart development. Biochim. Biophys. Acta BBA Mol. Cell Res. 2016, 1863, 1707–1716. [Google Scholar]
- Hornberger, T.; Esser, K.A. Mechanotransduction and the regulation of protein synthesis in skeletal muscle. Proc. Nutr. Soc. 2004, 63, 331–335. [Google Scholar] [CrossRef]
- Heher, P.; Maleiner, B.; Prüller, J.; Teuschl, A.H.; Kollmitzer, J.; Monforte, X.; Wolbank, S.; Redl, H.; Rünzler, D.; Fuchs, C. A novel bioreactor for the generation of highly aligned 3D skeletal muscle-like constructs through orientation of fibrin via application of static strain. Acta Biomater. 2015, 24, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-S.; Kim, J.-J.; Kim, H.-W.; Lewis, M.P.; Wall, I.B. Impact of mechanical stretch on the cell behaviors of bone and surrounding tissues. J. Tissue Eng. 2016, 7, 2041731415618342. [Google Scholar] [CrossRef]
- Rosa, N.; Simões, R.; Magalhães, F.D.; Marques, A.T. From mechanical stimulus to bone formation: A review. Med Eng. Phys. 2015, 37, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Bayati, V.; Sadeghi, Y.; Shokrgozar, M.A.; Haghighipour, N.; Azadmanesh, K.; Amanzadeh, A.; Azari, S. The evaluation of cyclic uniaxial strain on myogenic differentiation of adipose-derived stem cells. Tissue Cell 2011, 43, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Egusa, H.; Kobayashi, M.; Matsumoto, T.; Sasaki, J.-I.; Uraguchi, S.; Yatani, H. Application of Cyclic Strain for Accelerated Skeletal Myogenic Differentiation of Mouse Bone Marrow-Derived Mesenchymal Stromal Cells with Cell Alignment. Tissue Eng. Part A 2013, 19, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Seriani, S.; Del Favero, G.; Mahaffey, J.; Marko, D.; Gallina, P.; Long, C.; Mestroni, L.; Sbaizero, O. The cell-stretcher: A novel device for the mechanical stimulation of cell populations. Rev. Sci. Instruments 2016, 87, 084301. [Google Scholar] [CrossRef] [PubMed]
- Matheson, L.A.; Maksym, G.N.; Santerre, J.P.; Labow, R.S. Differential effects of uniaxial and biaxial strain on U937 macrophage-like cell morphology: Influence of extracellular matrix type proteins. J. Biomed. Mater. Res. Part A 2007, 81, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Matheson, L.; Jackfairbank, N.; Maksym, G.; Paulsanterre, J.; Labow, R.S. Characterization of the Flexcell™ Uniflex™ cyclic strain culture system with U937 macrophage-like cells. Biomater. 2006, 27, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Chandorkar, Y.; Nava, A.C.; Schweizerhof, S.; Van Dongen, M.; Haraszti, T.; Köhler, J.; Zhang, H.; Windoffer, R.; Mourran, A.; Moeller, M.; et al. Cellular responses to beating hydrogels to investigate mechanotransduction. Nat. Commun. 2019, 10, 4027. [Google Scholar] [CrossRef]
- Cui, Y.; Hameed, F.M.; Yang, B.; Lee, K.; Pan, C.Q.; Park, S.; Sheetz, M. Cyclic stretching of soft substrates induces spreading and growth. Nat. Commun. 2015, 6, 6333. [Google Scholar] [CrossRef]
- Freese, C.; Schreiner, D.; Anspach, L.; Bantz, C.; Maskos, M.; Unger, R.E.; Kirkpatrick, C.J. In vitro investigation of silica nanoparticle uptake into human endothelial cells under physiological cyclic stretch. Part. Fibre Toxicol. 2014, 11, 68. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Y. Cyclic Strain Enhances Cellular Uptake of Nanoparticles. J. Nanomater. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Rouse, J.G.; Haslauer, C.; Loboa, E.G.; Monteiro-Riviere, N.A. Cyclic tensile strain increases interactions between human epidermal keratinocytes and quantum dot nanoparticles. Toxicol. Vitr. 2008, 22, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Huh, N.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting Organ-Level Lung Functions on a Chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C.; Welck, J.; Tavernaro, I.; Grinberg, M.; Rahnenführer, J.; Kiemer, A.K.; Van Thriel, C.; Hengstler, J.G.; Kraegeloh, A. Mechanical strain mimicking breathing amplifies alterations in gene expression induced by SiO2 NPs in lung epithelial cells. Nanotoxicology 2019, 13, 1227–1243. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Wang, J.-W.; Wang, Y.; Dong, W.-W.; Xu, Z.-F. Propofol Protects Lung Endothelial Barrier Function by Suppression of High-Mobility Group Box 1 (HMGB1) Release and Mitochondrial Oxidative Damage Catalyzed by HMGB1. Med Sci. Monit. 2019, 25, 3199–3211. [Google Scholar] [CrossRef] [PubMed]
- Huh, N.; Kim, H.J.; Fraser, J.P.; E Shea, D.; Khan, M.; Bahinski, A.; A Hamilton, G.; E Ingber, N. Microfabrication of human organs-on-chips. Nat. Protoc. 2013, 8, 2135–2157. [Google Scholar] [CrossRef]
- Evans, N.D.; Oreffo, R.; Healy, E.; Thurner, P.J.; Man, Y.H. Epithelial mechanobiology, skin wound healing, and the stem cell niche. J. Mech. Behav. Biomed. Mater. 2013, 28, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, M.; Telias, I.; Urner, M.; Long, M.; Del Sorbo, L.; Fan, E.; Sinderby, C.; Beck, J.; Liu, L.; Qiu, H.; et al. A novel non-invasive method to detect excessively high respiratory effort and dynamic transpulmonary driving pressure during mechanical ventilation. Crit. Care 2019, 23, 346. [Google Scholar] [CrossRef]
- Sree, V.D.; Rausch, M.K.; Tepole, A.B. Linking microvascular collapse to tissue hypoxia in a multiscale model of pressure ulcer initiation. Biomech. Model. Mechanobiol. 2019, 18, 1947–1964. [Google Scholar] [CrossRef]
- Roell, K.R.; Reif, D.M.; Motsinger-Reif, A.A. An Introduction to Terminology and Methodology of Chemical Synergy—Perspectives from Across Disciplines. Front. Pharmacol. 2017, 8, 458. [Google Scholar] [CrossRef]
- Chou, T.-C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
- Esch, M.B.; Smith, A.S.; Prot, J.-M.; Oleaga, C.; Hickman, J.J.; Shuler, M.L. How multi-organ microdevices can help foster drug development. Adv. Drug Deliv. Rev. 2014, 69, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson-Bouchard, K.; Vunjak-Novakovic, G. Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development. Cell Stem Cell 2018, 22, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Warboys, C.M.; Ghim, M.; Weinberg, P. Understanding mechanobiology in cultured endothelium: A review of the orbital shaker method. Atherosclerosis 2019, 285, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Wall, M.; Butler, D.; El Haj, A.; Bodle, J.C.; Loboa, E.G.; Banes, A.J. Key Developments that Impacted the Field of Mechanobiology and Mechanotransduction. J. Orthop. Res. 2017, 36, 605–619. [Google Scholar] [CrossRef]
- Regnault, C.; Dheeman, D.S.; Hochstetter, A. Microfluidic Devices for Drug Assays. High-Throughput 2018, 7, 18. [Google Scholar] [CrossRef]
- Bein, A.; Shin, W.; Jalili-Firoozinezhad, S.; Park, M.H.; Sontheimer-Phelps, A.; Tovaglieri, A.; Chalkiadaki, A.; Kim, H.J.; Ingber, D.E. Microfluidic Organ-on-a-Chip Models of Human Intestine. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 659–668. [Google Scholar] [CrossRef]
- Lee, S.H.; Choi, N.; Sung, J.H. Pharmacokinetic and pharmacodynamic insights from microfluidic intestine-on-a-chip models. Expert Opin. Drug Metab. Toxicol. 2019, 15, 1005–1019. [Google Scholar] [CrossRef]
- Chang, S.; Weber, E.J.; Van Ness, K.P.; Eaton, D.L.; Kelly, E.J. Liver and Kidney on Chips: Microphysiological Models to Understand Transporter Function. Clin. Pharmacol. Ther. 2016, 100, 464–478. [Google Scholar] [CrossRef]
- Prantil-Baun, R.; Novak, R.; Das, D.; Somayaji, M.R.; Przekwas, A.; Ingber, D.E. Physiologically Based Pharmacokinetic and Pharmacodynamic Analysis Enabled by Microfluidically Linked Organs-on-Chips. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 37–64. [Google Scholar] [CrossRef]
- Kimura, H.; Sakai, Y.; Fujii, T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab. Pharmacokinet. 2018, 33, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Isoherranen, N.; Madabushi, R.; Huang, S.-M. Emerging Role of Organ-on-a-Chip Technologies in Quantitative Clinical Pharmacology Evaluation. Clin. Transl. Sci. 2019, 12, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Ha, S.K.; Choi, I.; Choi, N.; Park, T.H.; Sung, J.H. Microtechnology-based organ systems and whole-body models for drug screening. Biotechnol. J. 2016, 11, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Wang, Y.I.; Shuler, M.L. Strategies for using mathematical modeling approaches to design and interpret multi-organ microphysiological systems (MPS). APL Bioeng. 2019, 3, 021501. [Google Scholar] [CrossRef] [PubMed]
- Hubatsch, I.; E Ragnarsson, E.G.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef]
- Schimpel, C.; Teubl, B.; Absenger, M.; Claudia, M.; Fröhlich, E.; Leitinger, G.; Zimmer, A.; Roblegg, E. Development of an Advanced Intestinal in Vitro Triple Culture Permeability Model To Study Transport of Nanoparticles. Mol. Pharm. 2014, 11, 808–818. [Google Scholar] [CrossRef]
- Pocock, K.; Delon, L.C.; Bala, V.; Rao, S.; Priest, C.; Prestidge, C.A.; Thierry, B. Intestine-on-a-Chip Microfluidic Model for Efficient in Vitro Screening of Oral Chemotherapeutic Uptake. ACS Biomater. Sci. Eng. 2017, 3, 951–959. [Google Scholar] [CrossRef]
- Hinderliter, P.M.; Minard, K.R.; Orr, G.; Chrisler, W.B.; Thrall, B.D.; Pounds, J.G.; Teeguarden, J. ISDD: A computational model of particle sedimentation, diffusion and target cell dosimetry for in vitro toxicity studies. Part. Fibre Toxicol. 2010, 7, 36. [Google Scholar] [CrossRef]
- Toy, R.; Hayden, E.; Shoup, C.; Baskaran, H.; Karathanasis, E. The effects of particle size, density and shape on margination of nanoparticles in microcirculation. Nanotechnology 2011, 22, 115101. [Google Scholar] [CrossRef]
- Yazdimamaghani, M.; Barber, Z.B.; Moghaddam, S.P.H.; Ghandehari, H. Influence of Silica Nanoparticle Density and Flow Conditions on Sedimentation, Cell Uptake, and Cytotoxicity. Mol. Pharm. 2018, 15, 2372–2383. [Google Scholar] [CrossRef]
- Charwat, V.; Calvo, I.O.; Rothbauer, M.; Kratz, S.R.A.; Jungreuthmayer, C.; Zanghellini, J.; Grillari, J.; Ertl, P. Combinatorial in Vitro and in Silico Approach To Describe Shear-Force Dependent Uptake of Nanoparticles in Microfluidic Vascular Models. Anal. Chem. 2018, 90, 3651–3655. [Google Scholar] [CrossRef]
- Kilinc, D.; Schwab, J.; Rampini, S.; Ikpekha, O.W.; Thampi, A.; Blasiak, A.; Li, P.; Schwamborn, R.; Kolch, W.; Matallanas, D.; et al. A microfluidic dual gradient generator for conducting cell-based drug combination assays. Integr. Boil. 2016, 8, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.J.; Lee, J.-H.; Gye, H.G.; Lee, J.M.; Kim, E.-J.; Geum, D.; Sun, W.; Chung, B.G. A microfluidic gradient device for drug screening with human iPSC-derived motoneurons. Anal. 2020, 145, 3081–3089. [Google Scholar] [CrossRef] [PubMed]
- Oleaga, C.; Bernabini, C.; Smith, A.S.; Srinivasan, B.; Jackson, M.; McLamb, W.; Platt, V.; Bridges, R.; Cai, Y.; Santhanam, N.; et al. Multi-Organ toxicity demonstration in a functional human in vitro system composed of four organs. Sci. Rep. 2016, 6, 20030. [Google Scholar] [CrossRef]
- Tang, Y.; Qiu, Q.-F.; Zhang, F.-L.; Xie, M.; Huang, W.-H. Quantifying orientational regeneration of injured neurons by natural product concentration gradients in a 3D microfluidic device. Lab a Chip 2018, 18, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Frey, O.; Misun, P.M.; Fluri, D.A.; Hengstler, J.G.; Hierlemann, A. Reconfigurable microfluidic hanging drop network for multi-tissue interaction and analysis. Nat. Commun. 2014, 5, 4250. [Google Scholar] [CrossRef] [PubMed]
- Soriani, M.; Yi, W.; He, A.; Ml, S. Faculty Opinions recommendation of Microfluidic blood-brain barrier model provides in vivo-like barrier properties for drug permeability screening. Faculty Opinions – Post-Publication Peer Review of the Biomedical Literature 2017, 114, 184–194. [Google Scholar] [CrossRef]
- Partyka, P.P.; Godsey, G.; Galie, J.R.; Kosciuk, M.C.; Acharya, N.K.; Nagele, R.G.; Galie, P.A. Mechanical stress regulates transport in a compliant 3D model of the blood-brain barrier. Biomater. 2017, 115, 30–39. [Google Scholar] [CrossRef]
- Elbakary, B.; Badhan, R.K.S. A dynamic perfusion based blood-brain barrier model for cytotoxicity testing and drug permeation. Sci. Rep. 2020, 10, 3788. [Google Scholar] [CrossRef]
- An, F.; Qu, Y.; Luo, Y.; Fang, N.; Liu, Y.; Gao, Z.; Zhao, W.; Lin, B. A Laminated Microfluidic Device for Comprehensive Preclinical Testing in the Drug ADME Process. Sci. Rep. 2016, 6, 25022. [Google Scholar] [CrossRef]
- Bertero, T.; Gaggioli, C. Mechanical forces rewire metabolism in the tumor niche. Mol. Cell. Oncol. 2019, 6, 1592945. [Google Scholar] [CrossRef]
- Pasqualini, F.S.; Nesmith, A.P.; Horton, R.; Sheehy, S.P.; Parker, K.K. Mechanotransduction and Metabolism in Cardiomyocyte Microdomains. BioMed Res. Int. 2016, 2016, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Bowden, N.; Fragiadaki, M.; Souilhol, C.; Hsiao, S.; Mahmoud, M.; Allen, S.; Pirri, D.; Ayllon, B.T.; Akhtar, S.; et al. Mechanical Activation of Hypoxia-Inducible Factor 1α Drives Endothelial Dysfunction at Atheroprone Sites. Arter. Thromb. Vasc. Boil. 2017, 37, 2087–2101. [Google Scholar] [CrossRef] [PubMed]
- Dupont, N.; Codogno, P. Autophagy transduces physical constraints into biological responses. Int. J. Biochem. Cell Boil. 2016, 79, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Maji, S.; Agarwal, T.; Chakraborty, S.; Maiti, T.K. Hemodynamic shear stress induces protective autophagy in HeLa cells through lipid raft-mediated mechanotransduction. Clin. Exp. Metastasis 2018, 35, 135–148. [Google Scholar] [CrossRef]
- Fleming, I. Cytochrome P450 epoxygenases as EDHF synthase(s). Pharmacol. Res. 2004, 49, 525–533. [Google Scholar] [CrossRef]
- Fisslthaler, B.; Popp, R.; Michaelis, U.R.; Kiss, L.; Fleming, I.; Busse, R. Cyclic stretch enhances the expression and activity of coronary endothelium-derived hyperpolarizing factor synthase. Hypertens. 2001, 38, 1427–1432. [Google Scholar] [CrossRef][Green Version]
- Eskin, S.G.; Turner, N.A.; McIntire, L.V. Endothelial Cell Cytochrome P450 1A1 and 1B1: Up-Regulation by Shear Stress. Endothel. 2004, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.M.; Eskin, S.G.; McIntire, L.V.; Teng, C.L.; Lu, C.-M.; Russell, C.G.; Chittur, K.K. DNA microarray reveals changes in gene expression of shear stressed human umbilical vein endothelial cells. Proc. Natl. Acad. Sci. USA 2001, 98, 8955–8960. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Miwa, Y.; Obikane, H.; Mitsumata, M.; Takahashi-Yanaga, F.; Morimoto, S.; Sasaguri, T. Aryl hydrocarbon receptor mediates laminar fluid shear stress-induced CYP1A1 activation and cell cycle arrest in vascular endothelial cells. Cardiovasc. Res. 2007, 77, 809–818. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamamoto, M. Stress-sensing mechanisms and the physiological roles of the Keap1–Nrf2 system during cellular stress. J. Boil. Chem. 2017, 292, 16817–16824. [Google Scholar] [CrossRef]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genome Res. 1999, 13, 76–86. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.; Itoh, K.; Yamamoto, M.; A Chanas, S.; Henderson, C.J.; I McLellan, L.; Wolf, C.R.; Cavin, C.; Hayes, J. The Cap’n’Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001, 61, 3299–3307. [Google Scholar] [PubMed]
- Chen, X.-L.; Varner, S.E.; Rao, A.S.; Grey, J.Y.; Thomas, S.; Cook, C.K.; A Wasserman, M.; Medford, R.M.; Jaiswal, A.K.; Kunsch, C. Laminar Flow Induction of Antioxidant Response Element-mediated Genes in Endothelial Cells. J. Boil. Chem. 2002, 278, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Healy, Z.R.; Lee, N.H.; Gao, X.; Goldring, M.B.; Talalay, P.; Kensler, T.W.; Konstantopoulos, K. Divergent responses of chondrocytes and endothelial cells to shear stress: Cross-talk among COX-2, the phase 2 response, and apoptosis. Proc. Natl. Acad. Sci. USA 2005, 102, 14010–14015. [Google Scholar] [CrossRef]
- Fledderus, J.; Boon, R.A.; Volger, O.L.; Hurttila, H.; Ylä-Herttuala, S.; Pannekoek, H.; Levonen, A.-L.; Horrevoets, A.J. KLF2 Primes the Antioxidant Transcription Factor Nrf2 for Activation in Endothelial Cells. Arter. Thromb. Vasc. Boil. 2008, 28, 1339–1346. [Google Scholar] [CrossRef]
- Doddaballapur, A.; Michalik, K.M.; Manavski, Y.; Lucas, T.; Houtkooper, R.H.; You, X.; Chen, W.; Zeiher, A.M.; Potente, M.; Dimmeler, S.; et al. Laminar Shear Stress Inhibits Endothelial Cell Metabolism via KLF2-Mediated Repression of PFKFB3. Arter. Thromb. Vasc. Boil. 2015, 35, 137–145. [Google Scholar] [CrossRef]
- Raghavan, V.; Weisz, O.A. Discerning the role of mechanosensors in regulating proximal tubule function. Am. J. Physiol. Physiol. 2015, 310, F1–F5. [Google Scholar] [CrossRef][Green Version]
- Jayagopal, A.; Brakeman, P.R.; Soler, R.; Ferrell, N.; Fissell, W.; Kroetz, D.L.; Roy, S. Apical Shear Stress Enhanced Organic Cation Transport in Human OCT2/MATE1-Transfected Madin-Darby Canine Kidney Cells Involves Ciliary Sensing. J. Pharmacol. Exp. Ther. 2019, 369, 523–530. [Google Scholar] [CrossRef]
- Frohlich, E.M.; Zhang, X.; Charest, J.L. The use of controlled surface topography and flow-induced shear stress to influence renal epithelial cell function. Integr. Boil. 2012, 4, 75–83. [Google Scholar] [CrossRef]
- Musah, S.; Mammoto, A.; Ferrante, T.C.; Jeanty, S.S.F.; Hirano-Kobayashi, M.; Mammoto, T.; Roberts, K.; Chung, S.; Novak, R.; Ingram, M.; et al. Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat. Biomed. Eng. 2017, 1, 0069. [Google Scholar] [CrossRef]
| Physical Stimulation Stiffness | Chemical Stimulation | Cell Model | Response | Reference |
|---|---|---|---|---|
| Collagen Type I or alginate | Doxorubicin, 5-Fluorouracil, Tamoxifen | Hepatocellular carcinoma Hep3B and Breast adenocarcinoma MCF-7 | No major difference between normal 96-well plates and 3D. Tendency toward increased resistance in the 3D structures. | [74] |
| PEG + RGD 241 ± 19 Pa 637 ± 93 Pa 1201 ± 121 Pa | Paclitaxel | human epithelial ovarian cancer cell line OV-MZ-6 and ovarian serous adenocarcinoma cell line SKOV-3 | Shape and size of spheroids dependent on the matrix (> Stiffness > compactness and < size). RDG-enhanced proliferation. 3D culture decreased sensitivity to drug. | [75] |
| Alginate hydrogels | Acetaminophen, Diclofenac Rifampin, Quinidin | Hepatocellular carcinoma HepG2 and Breast adenocarcinoma MCF-7 | Acetaminophen, Diclofenac, 3D cultures are more sensitive than 2D, Rifampin, Quinidine similar toxicity. | [76] |
| Collagen-coated glass Polyacrylamide 1 kPa | Multiple chemicals Including: Cantharidin and Okadaic acid Taxol, Cytochalasin D, PD173074 Blebbistatin | 12 cell types: 16HBE14o- A549; c2c12; hASC HEK293; hMSC L929; MDCKII; MLE12 NHBE; NHDF; NHLF NIH3T3; RLE6TN | Cantharidin and Okadaic acid attenuated cell growth on soft substrates Taxol, Cytochalasin D, PD173074, major effect on rigid substrates. Blebbistatin growth stimuli on soft substrates and inhibitory on rigid substrates. No effect at 20kPa. | [77] |
| Polyacrylamide 1.6-5.7 kPa | NP | bovine aortic endothelial cells (BAECs) | Internalization per cell increases at higher stiffness (100 nm carboxylated polystyrene nanoparticles). | [78] |
| Polyacrylamide 1.5 and 40 kPa | LPS and TNF-α | Human pulmonary artery endothelial cells (HPAEC) and human lung microvascular endothelial cells (HLMVEC) | ↑ stiffness ↑ response (ICAM1/VCAM1 and fibronectin). | [79] |
| Alginate hydrogels & tissue-culture polystyrene Storage modulus (Pa) 343 ± 28/3041 ± 191 | Acetaminophen, Acrylamide, Cadmium chloride, and quinidine | Human U-87 and U-251 glioblastoma, IMR-32 neuroblastoma, and HEK 293 cells | Cells in soft alginate matrices ↑ sensitivity in comparison to cells encapsulated in stiffer matrices or 2D. RhoA activity modulation restores the resistance. | [80] |
| Alginate hydrogels ±RGD Storage modulus 400 and 3500 kPa | Acrylamide and Cadmium | Glioblastoma cells U-87 and U-251 | Soft substrate without RGD ↑ sensitivity | [81] |
| Polyacrylamide 1-4-25 kPa | Gemcitabine and Paclitaxel | Pancreatic cancer cells BxPC-3 and Suit2-007 AsPC-1 cells | Response to nucleoside analogue gemcitabine was unaffected. Stiffness < 4 kPa ↑resistance to paclitaxel. | [82] |
| Polyacrylamide 1–25 kPa | NP | Breast cancer cell lines MCF-7 & MDA-MB-231 | Internalization efficiency increases at higher stiffness’s (pluronic PEG-based micellar nanoparticles). | [52] |
| Physical Stimulation Shear Stress | N/m2 (Pascal) | Chemical Stimulation | Response | Reference |
|---|---|---|---|---|
| 6.6–3.3–0.5 N/m2 | 6.6-3.3-0.5 | Mesoporous Silica NP | Shear stress modulate cytotoxic potential. | [105] |
| 5 µL/min (0.1 dyn/cm2) | 0.01 | Gold Nanoparticles (13±3 nm Ø) | ↑ viability in microfluidic device Live/Dead Assay. | [106] |
| 10 dyn 3 h 10 dyn 24 h pre-inc. | 1 | Gold Nanoparticles (80 nm Ø; 5 µg/mL; 9.67 × 108 particles/mL) TNF-α 10 ng/mL | ↓ AuNPs uptake with shear stress and ↑ anti-ICAM-1 AuNPs uptake with shear stress and TNF-α. | [107] |
| 5 µL/min (0.1 dyn /cm2) | 0.01 | Gold Nanoparticles (13 ± 3 and 24 ± 8 nm Ø) | ↑ viability in microfluidic device Live/Dead Assay. | [108] |
| 0.1-0.2-0.8 Pa 0.1 Pa 24 h pre-inc. | 0.1-0.2-0.8 | Red Fluorophore-loaded carboxylate-capped NP (200 nm Ø) | Uptake dependent on the laminar or disturbed flow. | [109] |
| 0.01-0.09 Pa | 0.01-0.09 | Vandetanib 8 µM (no toxicity static) | Shear stress + Vandetanib induced morphological changes, ROS and apoptosis rate (%). No effect for drugs and shear stress alone. | [110] |
| 5 dyn /cm2 | 0.5 | TNF-α 100 U/mL Doxorubicin 1 µM | Shear stress ↓ICAM-1 and VCAM-1 induced by TNF-α. Shear Stress ↑ toxicity of Doxorubicin. | [111] |
| 2–12 dyn /cm2 | 0.2-1.2 | TNF-α 0.3 ng/mL | 2-4 dyn /cm2 ↑ VCAM-1; 12 dyn /cm2 ↓VCAM-1 expression induced by TNF-α. Triglyceride-rich lipoproteins and shear stress modulate TNF-induced VCAM-1. | [104] |
| 2 dyn /cm2 | 0.2 | Ivabradine 0.04 μM | Ivabradine treatment ↓VCAM-1, IL-6 and ROS induced by shear stress. | [112] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Favero, G.; Kraegeloh, A. Integrating Biophysics in Toxicology. Cells 2020, 9, 1282. https://doi.org/10.3390/cells9051282
Del Favero G, Kraegeloh A. Integrating Biophysics in Toxicology. Cells. 2020; 9(5):1282. https://doi.org/10.3390/cells9051282
Chicago/Turabian StyleDel Favero, Giorgia, and Annette Kraegeloh. 2020. "Integrating Biophysics in Toxicology" Cells 9, no. 5: 1282. https://doi.org/10.3390/cells9051282
APA StyleDel Favero, G., & Kraegeloh, A. (2020). Integrating Biophysics in Toxicology. Cells, 9(5), 1282. https://doi.org/10.3390/cells9051282





