Evaluation of a Novel Boron-Containing α-d-Mannopyranoside for BNCT
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Synthesis
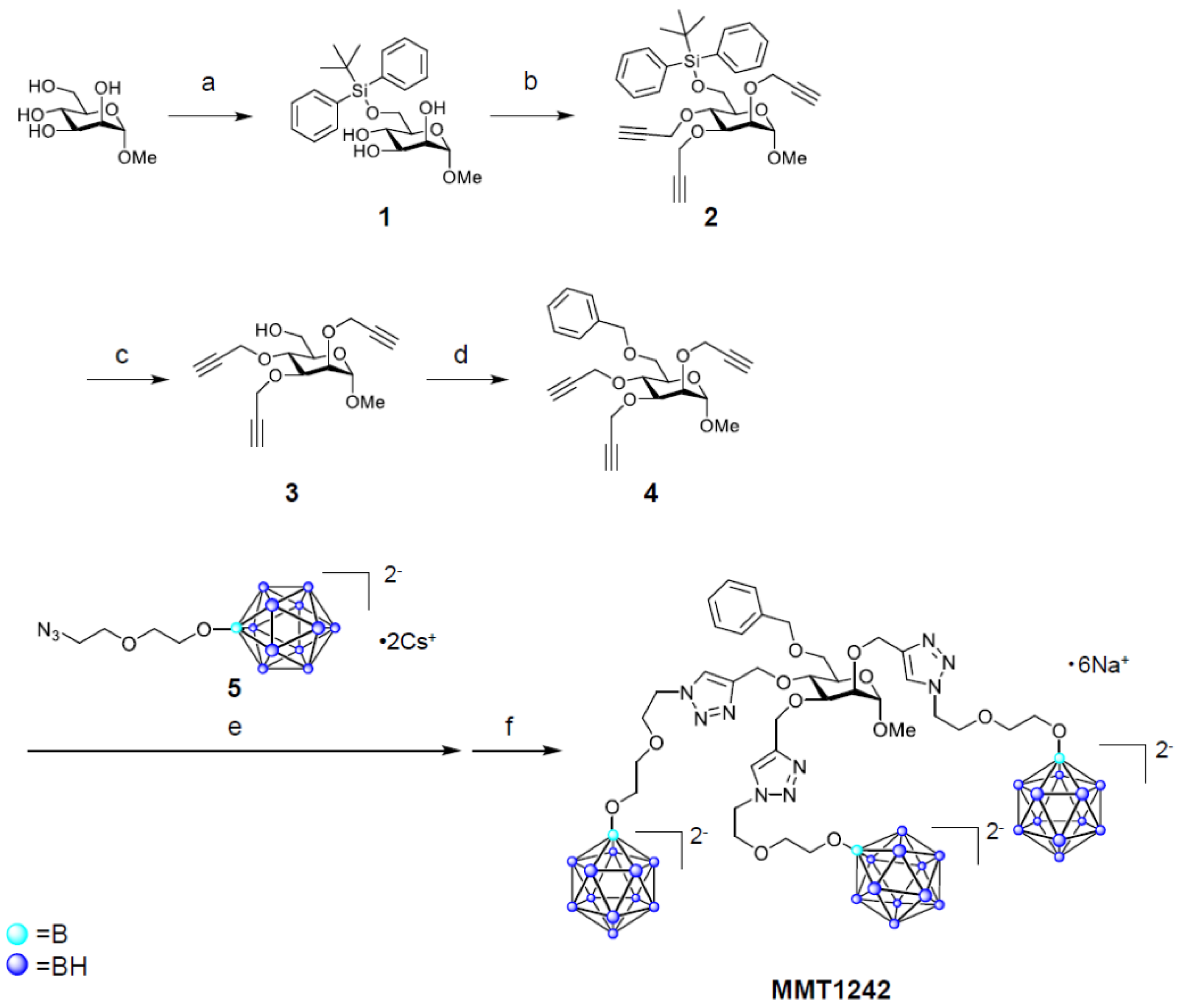
2.1.1. Synthesis of MMT1242
Methyl 6-O-(tert-butyldiphenylsilanyl)-α-d-Mannopyranoside (1) [45,46]
Methyl 6-O-(tert-butyldiphenylsilanyl)-2,3,4-tris-O-(prop-2-ynyl)-α-d-Mannopyranoside (2)
Methyl 2,3,4-tris-O-(prop-2-ynyl)-α-d-Mannopyranoside (3)
Methyl 6-O-benzyl-2,3,4-tris-O-(prop-2-ynyl)-α-d-Mannopyranoside (4)
Methyl 6-O-benzyl-2,3,4-tris-O-[[1-[2-[2-(undecahydro-closo-dodecaboranyloxy)ethoxy]ethyl] triazol-4-yl]methyl]-α-d-Mannopyranoside Hexasodium Salt (MMT1242)
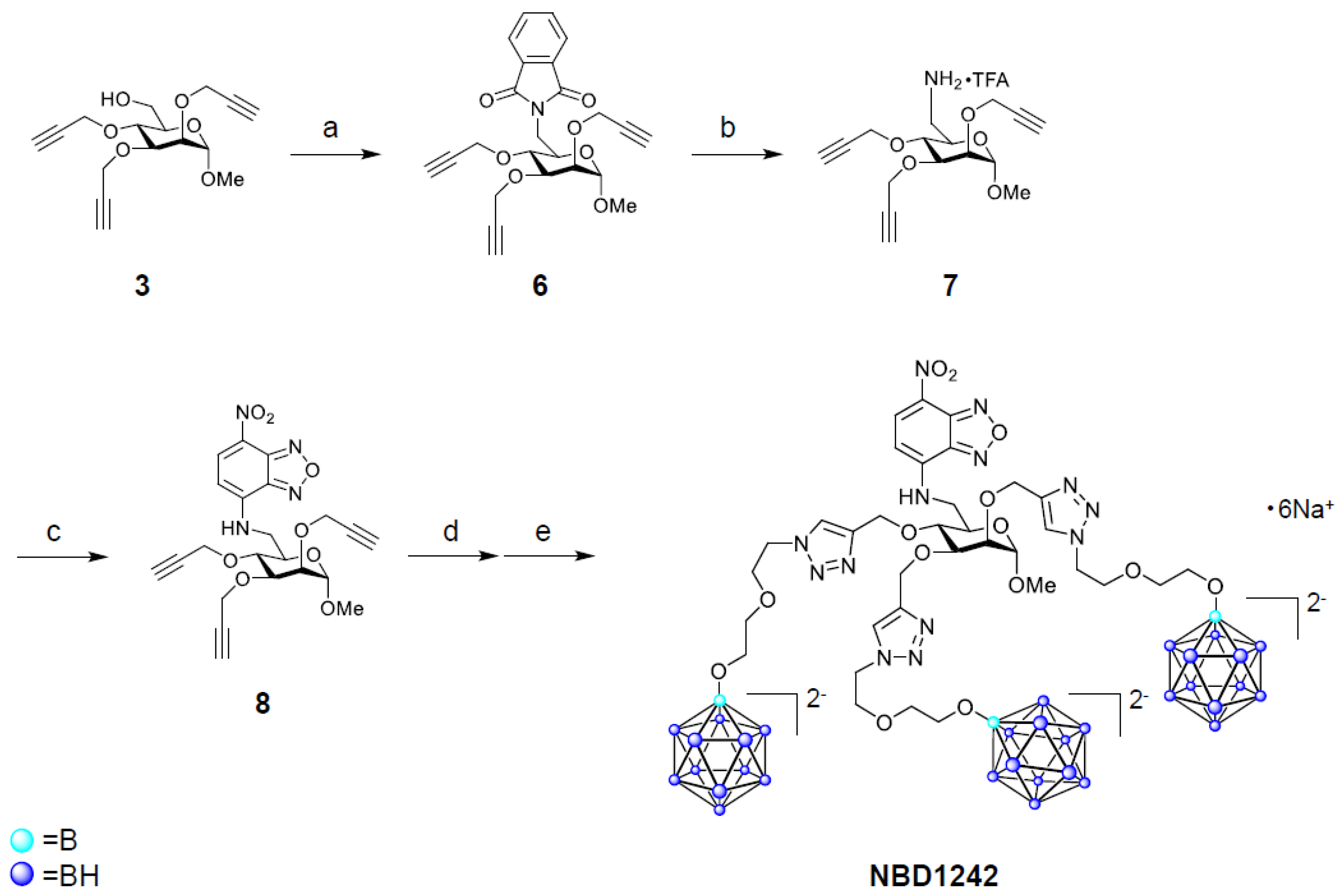
2.1.2. Synthesis of NBD1242
2-[[(3R,4S,6S)-6-methoxy-3,4,5-tris(prop-2-ynoxy)tetrahydropyran-2-yl]methyl]isoindoline-1,3-Dione (6)
[(3R,4S,6S)-6-methoxy-3,4,5-tris(prop-2-ynoxy)tetrahydropyran-2-yl]methanamine trifluoroacetate (7)
N-[[(3R,4S,6S)-6-methoxy-3,4,5-tris(prop-2-ynoxy)tetrahydropyran-2-yl]methyl]-4-nitro-2,1,3-benzoxadiazol-7-amine (8)
N-[[(3R,4S,6S)-6-methoxy-3,4,5-tris-O-[[1-[2-[2-(undecahydro-closo-dodecaboranyloxy)ethoxy]ethyl]triazol-4-yl]methyl]-tetrahydropyran-2-yl]methyl]-4-nitro-2,1,3-benzoxadiazol-7-amine hexasodium salt (NBD1242)
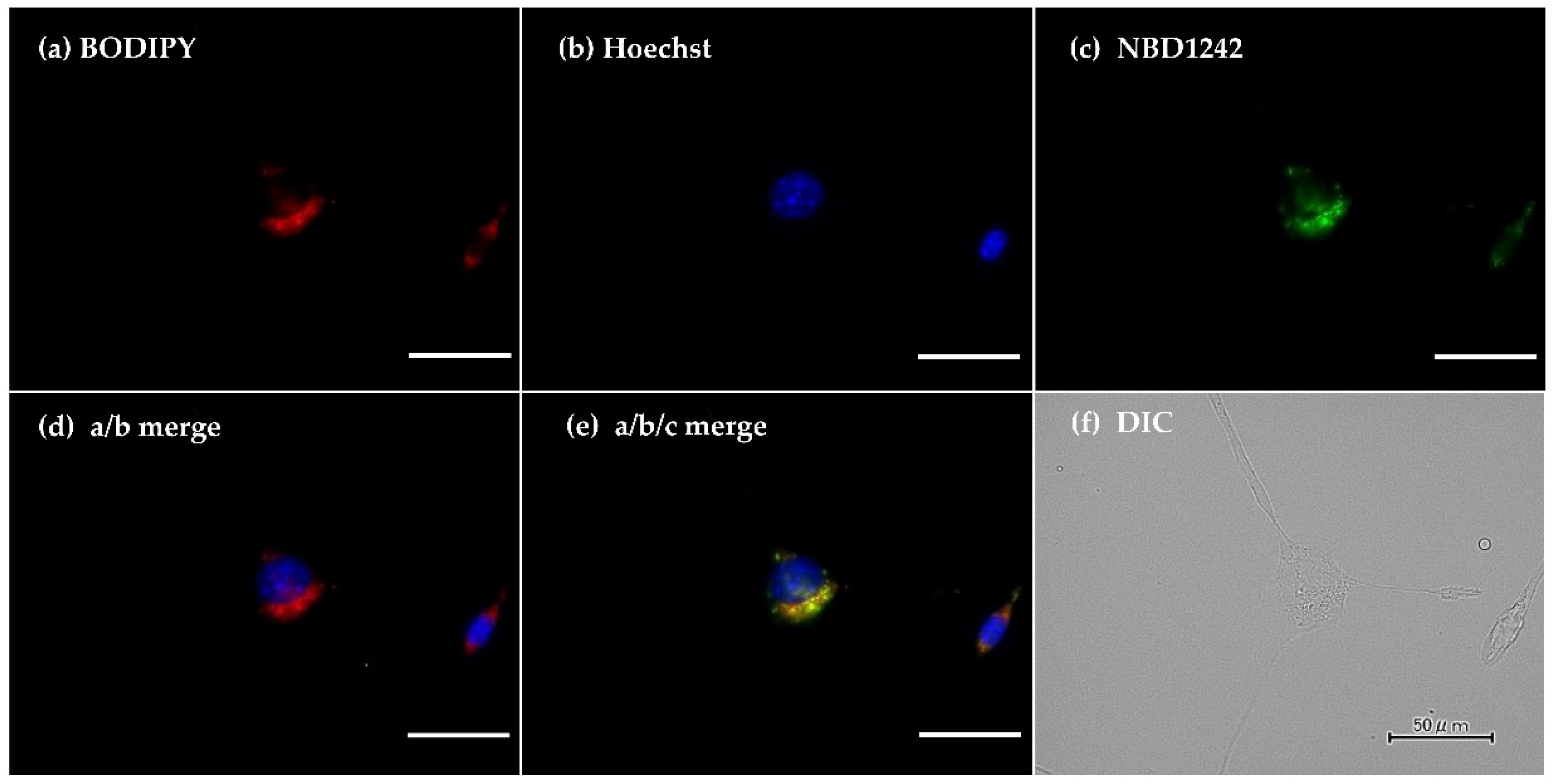
2.2. Intracellular Distribution of the MMT1242 In Vitro
2.3. Intracellular Uptake of MMT1242 In Vitro
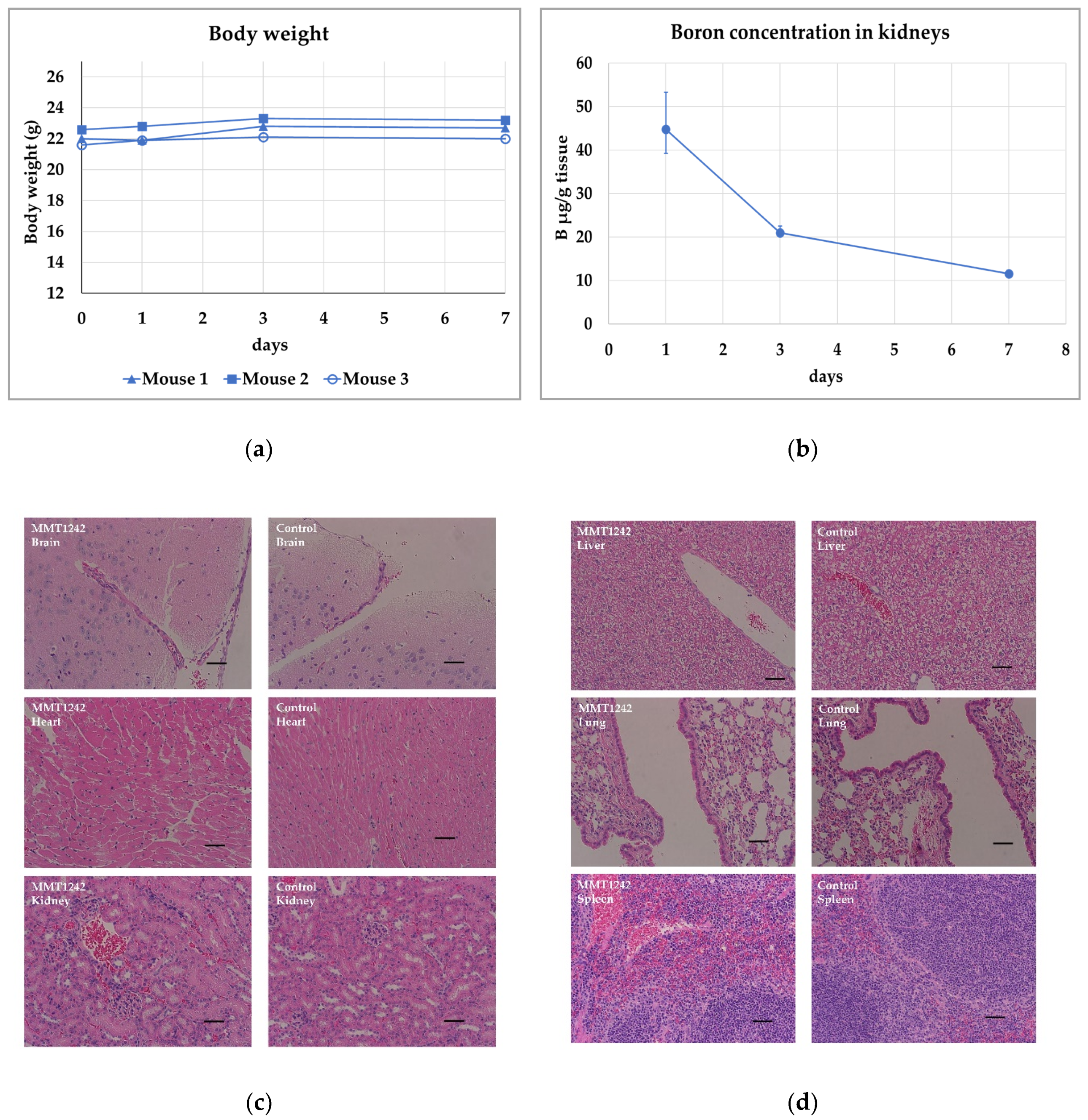
2.4. Toxicity Study Using Healthy Mice
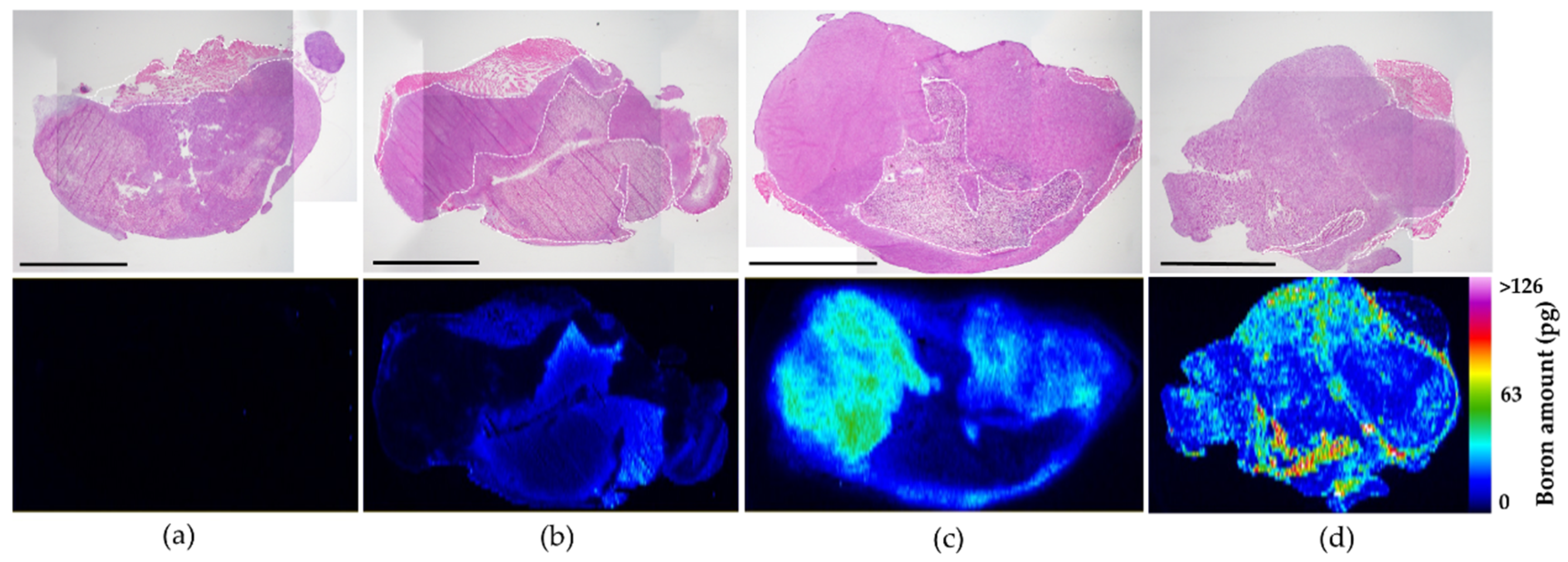
2.5. In Vivo Fluorescence Imaging of 10B Distribution in Tumor Tissues
2.6. Biodistribution of Boron Compounds In Vivo
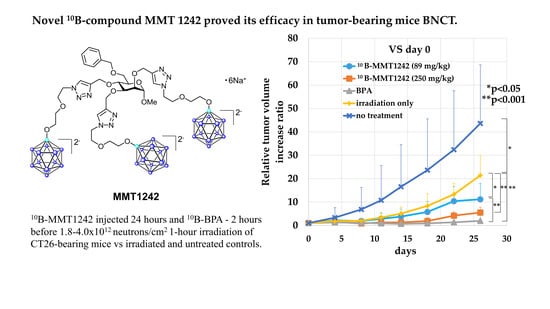
2.7. Irradiation Study Using a Mouse Tumor Model
3. Results
3.1. Intracellular Distribution of MMT1242 In Vitro
3.2. Intracellular Uptake of Boron MMT1242 In Vitro
3.2.1. Intracellular Uptake of Boron in CT26 Mouse Colon Tumor Cells
3.2.2. Intracellular Uptake of Boron in the B16-F10 Mouse Melanoma Cells/C6 Rat Brain Tumor Cells
3.2.3. Retention of Intracellular Boron in CT26 Tumor Cells
3.2.4. Temperature Dependence of Intracellular Uptake of Boron in CT26 Tumor Cells
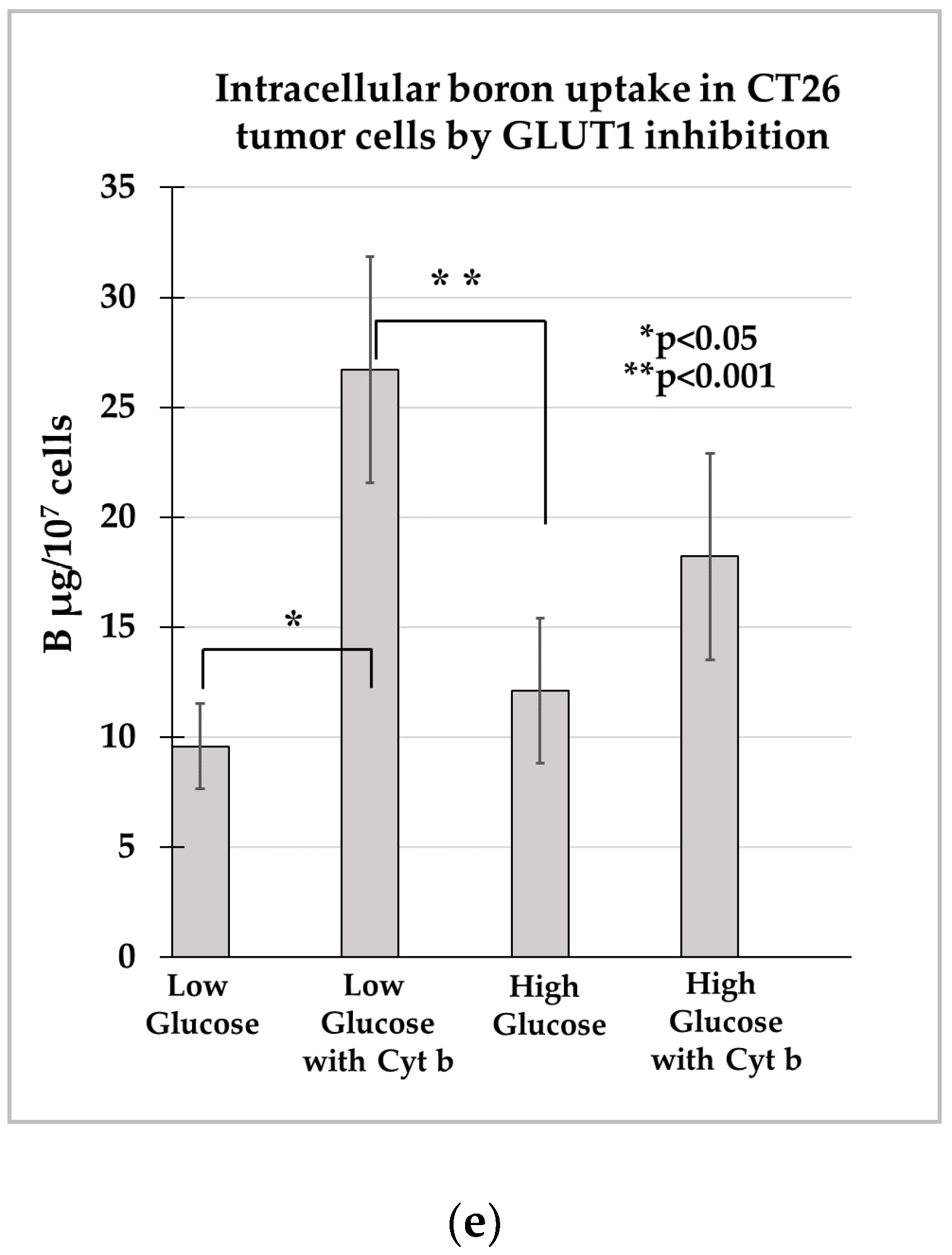
3.2.5. Intracellular Boron Uptake in CT26 Tumor Cells by GLUT1 Inhibition
3.3. Toxicity Study Using Healthy Mice
3.3.1. In Vivo Fluorescence Imaging of 10B Distribution in Tumor Tissues
3.3.2. Biodistribution of Boron Compounds In Vivo
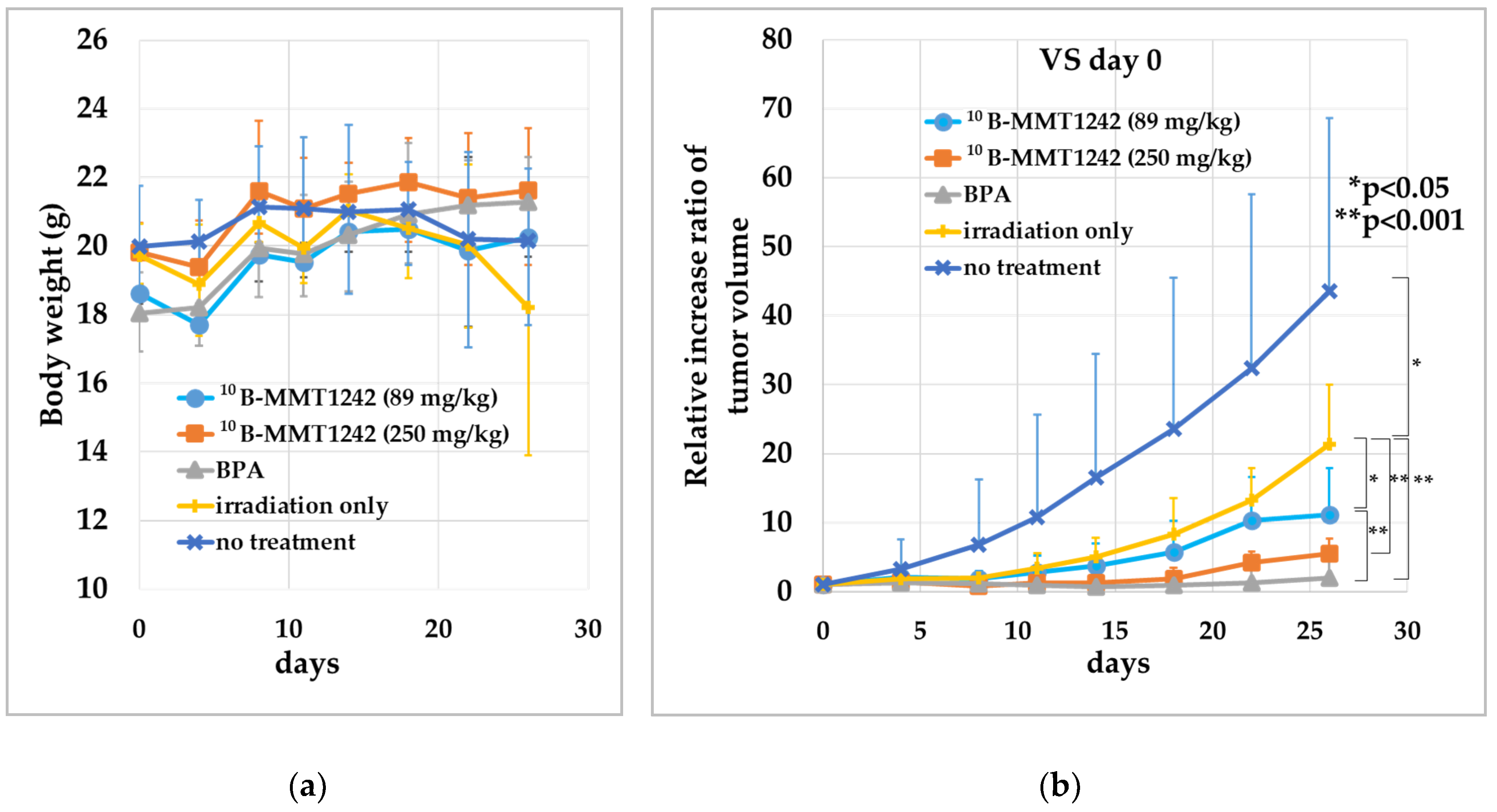
3.3.3. Irradiation Study Using Mouse Subcutaneous Tumor Model
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Locher, G. Biological effects and therapeutic possibilities of neutrons. Am. J. Roentgenol. Radium Ther. 1936, 36, 1–13. [Google Scholar]
- Soloway, A.H.; Tjarks, W.; Barnum, B.A.; Rong, F.G.; Barth, R.F.; Codogni, I.M.; Wilson, J.G. The chemistry of neutron capture therapy. Chem. Rev. 1998, 98, 1515–1562. [Google Scholar] [CrossRef] [PubMed]
- Sauerwein, W.A.G.; Wittig, A.; Moss, R.; Nakagawa, Y. Neutron Capture Therapy. Principles and Applications; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Sivaev, I.B.; Bregadze, V.I. Polyhedral boranes for medical applications: Current status and perspectives. Eur. J. Inorg. Chem. 2009, 11, 1433–1450. [Google Scholar] [CrossRef]
- Barth, R.F.; Zhang, Z.; Liu, T. A realistic appraisal of boron neutron capture therapy as a cancer treatment modality. Cancer Commun. (Lond) 2018, 38, 36. [Google Scholar] [CrossRef]
- Kreiner, A.J.; Bergueiro, J.; Cartelli, D.; Baldo, M.; Castell, W.; Asoia, J.G.; Padulo, J.; Suárez Sandín, J.C.; Igarzabal, M.; Erhardt, J.; et al. Present status of Accelerator-Based BNCT. Rep. Pract. Oncol. Radiother. 2016, 21, 95–101. [Google Scholar] [CrossRef]
- Taskaev, S. Development of an accelerator-based epithermal neutron source for boron neutron capture therapy. Phys. Part. Nucl. 2019, 50, 569–575. [Google Scholar] [CrossRef]
- Barth, R.F.; Mi, P.; Yang, W. Boron delivery agents for neutron capture therapy of cancer. Cancer Commun. (Lond) 2018, 38, 35. [Google Scholar] [CrossRef]
- Sato, E.; Zaboronok, A.; Yamamoto, T.; Nakai, K.; Taskaev, S.; Volkova, O.; Mechetina, L.; Taranin, A.; Kanygin, V.; Isobe, T.; et al. Radiobiological response of U251MG, CHO-K1 and V79 cell lines to accelerator-based boron neutron capture therapy. J. Radiat. Res. 2018, 59, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Nakai, K.; Tsurubuchi, T.; Matsuda, M.; Shirakawa, M.; Zaboronok, A.; Endo, K.; Matsumura, A. Boron neutron capture therapy for newly diagnosed glioblastoma: A pilot study in Tsukuba. Appl. Radiat. Isot. 2009, 67 (Suppl. 7–8), S25–S26. [Google Scholar] [CrossRef]
- Kawabata, S.; Miyatake, S.; Hiramatsu, R.; Hirota, Y.; Miyata, S.; Takekita, Y.; Kuroiwa, T.; Kirihata, M.; Sakurai, Y.; Maruhashi, A.; et al. Phase II clinical study of boron neutron capture therapy combined with X-ray radiotherapy/temozolomide in patients with newly diagnosed glioblastoma multiforme--study design and current status report. Appl. Radiat. Isot. 2011, 69, 1796–1799. [Google Scholar] [CrossRef]
- Miyatake, S.; Kawabata, S.; Hiramatsu, R.; Kuroiwa, T.; Suzuki, M.; Kondo, N.; Ono, K. Boron Neutron Capture Therapy for Malignant Brain Tumors. Neurol. Med. Chir. (Tokyo) 2016, 56, 361–371. [Google Scholar] [CrossRef]
- Wang, L.W.; Chen, Y.W.; Ho, C.Y.; Hsueh Liu, Y.W.; Chou, F.I.; Liu, Y.H.; Liu, H.M.; Peir, J.J.; Jiang, S.H.; Chang, C.W.; et al. Fractionated Boron Neutron Capture Therapy in Locally Recurrent Head and Neck Cancer: A Prospective Phase I/II Trial. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Hattori, Y.; Ohta, Y.; Ishimura, M.; Nakagawa, Y.; Sanada, Y.; Tanaka, H.; Fukutani, S.; Masunaga, S.I.; Hiraoka, M.; et al. Comparison of the pharmacokinetics between L-BPA and L-FBPA using the same administration dose and protocol: A validation study for the theranostic approach using [18F]-L-FBPA positron emission tomography in boron neutron capture therapy. BMC Cancer 2016, 16, 859. [Google Scholar] [CrossRef] [PubMed]
- Moss, L. Critical review, with an optimistic outlook, on boron neutron capture therapy (BNCT). Appl. Radiat. Isot. 2014, 88, 2–11. [Google Scholar] [CrossRef]
- Xuan, S.; Vicente, M.G.H. Recent advances in boron delivery agents for boron neutron capture therapy (BNCT). In Boron-Based Compounds: Potential and Emerging Applications in Medicine; Hey-Hawkins, E., Viñas Teixidor, C., Eds.; John Wiley & Sons Ltd.: Oxford, UK, 2018; pp. 298–342. [Google Scholar]
- Tietze, L.F.; Griesbach, U.; Bothe, U.; Nakamura, H.; Yamamoto, Y. Novel carboranes with a DNA binding unit for the treatment of cancer by boron neutron capture therapy. Chembiochem 2002, 3, 219–225. [Google Scholar] [CrossRef]
- Calabrese, G.; Daou, A.; Barbu, E.; Tsibouklis, J. Towards carborane-functionalised structures for the treatment of brain cancer. Drug Discov. Today 2018, 23, 63–75. [Google Scholar] [CrossRef]
- Wood, F.C., Jr.; Chill, G.F., Jr. Mannose utilization in man. J. Clin. Invest. 1963, 42, 1300–1312. [Google Scholar] [CrossRef]
- Alton, G.; Kjaergaard, S.; Etchison, J.R.; Skovby, F.; Freeze, H.H. Oral ingestion of mannose elevates blood mannose levels: A first step toward a potential therapy for carbohydrate-deficient glycoprotein syndrome type I. Biochem. Mol. Med. 1997, 60, 127–133. [Google Scholar] [CrossRef]
- Hao, Z.F.; Cui, Y.X.; Li, M.H.; Du, D.; Liu, M.F.; Tao, H.Q.; Li, S.; Cao, F.Y.; Chen, Y.L.; Lei, X.H.; et al. Liposomes modified with P-aminophenyl-α-D-mannopyranoside: A carrier for targeting cerebral functional regions in mice. Eur. J. Pharm. Biopharm. 2013, 84, 505–516. [Google Scholar] [CrossRef]
- Sharma, V.; Ichikawa, M.; Freeze, H.H. Mannose metabolism: More than meets the eye. Biochem. Biophys. Res. Commun. 2014, 453, 220–228. [Google Scholar] [CrossRef]
- Du, D.; Chang, N.; Sun, S.; Li, M.; Yu, H.; Liu, M.; Liu, X.; Wang, G.; Li, H.; Liu, X.; et al. The role of glucose transporters in the distribution of p-aminophenyl-α-d-mannopyranoside modified liposomes within mice brain. J. Control. Release 2014, 182, 99–110. [Google Scholar] [CrossRef]
- Nishioka, T.; Oda, Y.; Seino, Y.; Yamamoto, T.; Inagaki, N.; Yano, H.; Imura, H.; Shigemoto, R.; Kikuchi, H. Distribution of the glucose transporters in human brain tumors. Cancer Res. 1992, 52, 3972–3979. [Google Scholar]
- Pardridge, W.M. Transport of small molecules through the blood–brain barrier: Biology and methodology. Adv. Drug Deliv. Rev. 1995, 15, 5–36. [Google Scholar] [CrossRef]
- Vannucci, S.J.; Maher, F.; Simpson, I.A. Glucose transporter proteins in brain: Delivery of glucose to neurons and glia. Glia 1997, 21, 2–21. [Google Scholar] [CrossRef]
- Zeller, K.; Rahner-Welsch, S.; Kuschinsky, W. Distribution of Glut1 glucose transporters in different brain structures compared to glucose utilization and capillary density of adult rat brains. J. Cereb. Blood Flow Metab. 1997, 17, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, F.; Eto, Y. Liposome targeting to mouse brain: Mannose as a recognition marker. Biochem. Biophys. Res. Commun. 1988, 153, 1038–1044. [Google Scholar] [CrossRef]
- Ahadova, A.; Gebert, J.; von Knebel Doeberitz, M.; Kopitz, J.; Kloor, M. Dose-dependent effect of 2-deoxy-D-glucose on glycoprotein mannosylation in cancer cells. IUBMB Life 2015, 67, 218–226. [Google Scholar] [CrossRef]
- Jeong, H.S.; Na, K.S.; Hwang, H.; Oh, P.S.; Kim, D.H.; Lim, S.T.; Sohn, M.H.; Jeong, H.J. Effect of space length of mannose ligand on uptake of mannosylated liposome in RAW 264.7 cells: In vitro and in vivo studies. J. Biomed. Mater. Res. A 2014, 102, 4545–4553. [Google Scholar] [CrossRef]
- Xiong, M.; Lei, Q.; You, X.; Gao, T.; Song, X.; Xia, Y.; Ye, T.; Zhang, L.; Wang, N.; Yu, L. Mannosylated liposomes improve therapeutic effects of paclitaxel in colon cancer models. J. Microencapsul. 2017, 34, 513–521. [Google Scholar] [CrossRef]
- Tran, Q.; Lee, H.; Park, J.; Kim, S.H.; Park, J. Targeting cancer metabolism—Revisiting the Warburg effects. Toxicol. Res. 2016, 32, 177–193. [Google Scholar] [CrossRef]
- Itoh, T.; Tamura, K.; Ueda, H.; Tanaka, T.; Sato, K.; Kuroda, R.; Aoki, S. Design and synthesis of boron containing monosaccharides by the hydroboration of d-glucal for use in boron neutron capture therapy (BNCT). Bioorg. Med. Chem. 2018, 26, 5922–5933. [Google Scholar] [CrossRef] [PubMed]
- Imperio, D.; Del Grosso, E.; Fallarini, S.; Lombardi, G.; Panza, L. Anomeric sugar boronic acid analogues as potential agents for boron neutron capture therapy. Beilstein. J. Org. Chem. 2019, 15, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Lin, Y.C.; Chou, F.I.; Liang, C.F.; Lin, E.W.; Chuang, Y.J.; Lin, C.C. Design of multivalent galactosyl carborane as a targeting specific agent for potential application to boron neutron capture therapy. Chem. Commun. (Camb) 2012, 48, 612–614. [Google Scholar] [CrossRef]
- Orlova, A.V.; Kononov, L.O. Synthesis of conjugates of polyhedral boron compounds with carbohydrates. Russ. Chem. Rev. 2009, 78, 629–648. [Google Scholar] [CrossRef]
- Marepally, R.; Yao, M.-L.; Kabalka, G.W. Boronated carbohydrate derivatives as potential boron neutron capture therapy reagents. Future Med. Chem. 2013, 5, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, R.; Dash, B.P.; Mahanta, C.S.; Swain, B.R.; Jena, B.B.; Hosmane, N.S. Glycoconjugates of polyhedral boron clusters. J. Organomet. Chem. 2015, 798, 13–23. [Google Scholar] [CrossRef]
- Kotora, M. Carboranyl-saccharide Derivatives: Syntheses and Biological Evaluation. In Handbook of Boron Science with Applications in Organometallics, Catalysis, Materials and Medicine; Hosmane, N.S., Eagling, R., Eds.; World Scientific: London, UK, 2018; Volume 4 (Boron in Medicine), pp. 69–99. [Google Scholar]
- Lechtenberg, B.; Gabel, D. Synthesis of a (B12H11S)2− containing glucuronoside as potential prodrug for BNCT. J. Organomet. Chem. 2005, 690, 2780–2782. [Google Scholar] [CrossRef]
- Orlova, A.V.; Kondakov, N.N.; Zinin, A.I.; Kimel’, B.G.; Kononov, L.O.; Sivaev, I.B.; Bregadze, V.I. A uniform approach to the synthesis of carbohydrate conjugates of polyhedral boron compounds as potential agents for boron neutron capture therapy. Russ. J. Bioorg. Chem. 2006, 32, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Imperio, D.; Muz, B.; Azab, A.K.; Fallarini, S.; Lombardi, G.; Panza, L. A Short and Convenient Synthesis of closo-Dodecaborate Sugar Conjugates. Eur. J. Org. Chem. 2019, 7228–7232. [Google Scholar] [CrossRef]
- Orlova, A.V.; Kondakov, N.N.; Kimel’, B.G.; Kononov, L.O.; Kononova, E.G.; Sivaev, I.B.; Bregadze, V.I. Synthesis of novel derivatives of closo-dodecaborate anion with azido group at the terminal position of the spacer. Appl. Organomet. Chem. 2007, 21, 98–100. [Google Scholar] [CrossRef]
- Semioshkin, A.A.; Osipov, S.N.; Grebenyuk, J.N.; Nizhnik, E.A.; Godovikov, I.A.; Shchetnikov, G.T.; Bregadze, V.I. An Effective Approach to 1,2,3-Triazole-Containing 12-Vertex closo-Dodecaborates. Collect. Czech. Chem. Commun. 2007, 72, 1717–1724. [Google Scholar] [CrossRef]
- Khan, S.H.; Abbas, S.A.; Matta, K.L. Synthesis of 4-nitrophenyl O-(2-acetamido-2-deoxy-beta-D-glucopyranosyl)- (1-2)-O-(4-O-methyl-alpha-D-mannopyranosyl)-(1-6)-beta-D-glucopyr Anoside. A Potential Specific Acceptor-Substrate for N-acetylglucosaminyltransferase-V (GnT V). Carbohydr. Res. 1990, 205, 385–397. [Google Scholar] [CrossRef]
- Traboni, S.; Bedini, E.; Iadonisi, A. Orthogonal protection of saccharide polyols through solvent-free one-pot sequences based on regioselective silylations. Beilstein. J. Org. Chem. 2016, 12, 2748–2756. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Sakata, S.; Makino, Y.; Obayashi, H.; Ohara, S.; Hattori, K.; Hirata, T. iQuant2: Software for rapid and quantitative imaging using laser ablation-ICP mass spectrometry. Mass. Spectrom. 2018, 7, A0065. [Google Scholar] [CrossRef] [PubMed]
- Heinsbroek, S.E.; Squadrito, M.L.; Schilderink, R.; Hilbers, F.W.; Verseijden, C.; Hofmann, M.; Helmke, A.; Boon, L.; Wildenberg, M.E.; Roelofs, J.J.; et al. miR-511-3p, embedded in the macrophage mannose receptor gene, contributes to intestinal inflammation. Mucosal. Immunol. 2016, 9, 960–973. [Google Scholar] [CrossRef]
- Kataoka, H.; Nishie, H.; Hayashi, N.; Tanaka, M.; Nomoto, A.; Yano, S.; Joh, T. New photodynamic therapy with next-generation photosensitizers. Ann. Transl. Med. 2017, 5, 183. [Google Scholar] [CrossRef]
- Liu, D.R.; Guan, Q.L.; Gao, M.T.; Jiang, L.; Kang, H.X. Mannose receptor as a potential biomarker for gastric cancer: A pilot study. Int. J. Biol. Mark. 2017, 32, e278–e283. [Google Scholar] [CrossRef]
- Wei, Y.; Shi, D.; Liang, Z.; Liu, Y.; Li, Y.; Xing, Y.; Liu, W.; Ai, Z.; Zhuang, J.; Chen, X.; et al. IL-17A secreted from lymphatic endothelial cells promotes tumorigenesis by upregulation of PD-L1 in hepatoma stem cells. J. Hepatol. 2019, 71, 1206–1215. [Google Scholar] [CrossRef]
- Babicky, M.L.; Harper, M.M.; Chakedis, J.; Cazes, A.; Mose, E.S.; Jaquish, D.V.; French, R.P.; Childers, B.; Alakus, H.; Schmid, M.C.; et al. MST1R kinase accelerates pancreatic cancer progression via effects on both epithelial cells and macrophages. Oncogene 2019, 38, 5599–5611. [Google Scholar] [CrossRef]
- Scatena, R.; Bottoni, P.; Pontoglio, A.; Giardina, B. Revisiting the Warburg effect in cancer cells with proteomics. The emergence of new approaches to diagnosis, prognosis and therapy. Proteom. Clin. Appl. 2010, 4, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Palsson-McDermott, E.M.; Curtis, A.M.; Goel, G.; Lauterbach, M.A.; Sheedy, F.J.; Gleeson, L.E.; van den Bosch, M.W.; Quinn, S.R.; Domingo-Fernandez, R.; Johnston, D.G.; et al. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the Warburg effect in LPS-activated macrophages. Cell Metab. 2015, 21, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Yuen, C.A.; Asuthkar, S.; Guda, M.R.; Tsung, A.J.; Velpula, K.K. Cancer stem cell molecular reprogramming of the Warburg effect in glioblastomas: A new target gleaned from an old concept. CNS Oncol. 2016, 5, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.; Oliveira, T.; Bartels, M.F.; Miyoshi, E.; Pierce, M.; Taniguchi, N.; Carneiro, F.; Seruca, R.; Reis, C.A.; Strahl, S.; et al. O-mannosylation and N-glycosylation: Two coordinated mechanisms regulating the tumour suppressor functions of E-cadherin in cancer. Oncotarget 2016, 7, 65231–65246. [Google Scholar] [CrossRef] [PubMed]
- Westerlund, K.; Honarvar, H.; Norrström, E.; Strand, J.; Mitran, B.; Orlova, A.; Karlström, A.E.; Tolmachev, V. Increasing the Net Negative Charge by Replacement of DOTA Chelator with DOTAGA Improves the Biodistribution of Radiolabeled Second-Generation Synthetic Affibody Molecules. Mol. Pharm. 2016, 13, 1668–1678. [Google Scholar] [CrossRef]
- Rinne, S.S.; Leitao, C.D.; Saleh-nihad, Z.; Mitran, B.; Tolmachev, V.; Ståhl, S.; Löfblom, J.; Orlova, A. Benefit of Later-Time-Point PET Imaging of HER3 Expression Using Optimized Radiocobalt-Labeled Affibody Molecules. Int. J. Mol. Sci. 2020, 21, 1972. [Google Scholar] [CrossRef]
- Fukuda, H.; Honda, C.; Wadabayashi, N.; Kobayashi, T.; Yoshino, K.; Hiratsuka, J.; Takahashi, J.; Akaizawa, T.; Abe, Y.; Ichihashi, M.; et al. Pharmacokinetics of 10B-p-boronophenylalanine in tumours, skin and blood of melanoma patients: A study of boron neutron capture therapy for malignant melanoma. Melanoma Res. 1999, 9, 75–83. [Google Scholar] [CrossRef]
- Kiger, W.S., 3rd; Palmer, M.R.; Riley, K.J.; Zamenhof, R.G.; Busse, P.M. A pharmacokinetic model for the concentration of 10B in blood after boronophenylalanine-fructose administration in humans. Radiat. Res. 2001, 155, 611–618. [Google Scholar] [CrossRef]
- Miura, M.; Micca, P.L.; Fisher, C.D.; Heinrichs, J.C.; Donaldson, J.A.; Finkel, G.C.; Slatkin, D.N. Synthesis of a nickel tetracarboranylphenylporphyrin for boron neutron-capture therapy: Biodistribution and toxicity in tumor-bearing mice. Int. J. Cancer 1996, 68, 114–119. [Google Scholar] [CrossRef]
- Wu, H.; Micca, P.L.; Makar, M.S.; Miura, M. Total syntheses of three copper (II) tetracarboranylphenylporphyrins containing 40 or 80 boron atoms and their biological properties in EMT-6 tumor-bearing mice. Bioorg. Med. Chem. 2006, 14, 5083–5092. [Google Scholar] [CrossRef]
- Zhuo, J.C.; Cai, J.; Soloway, A.H.; Barth, R.F.; Adams, D.M.; Ji, W.; Tjarks, W. Synthesis and biological evaluation of boron-containing polyamines as potential agents for neutron capture therapy of brain tumors. J. Med. Chem. 1999, 42, 1282–1292. [Google Scholar] [CrossRef]
- Carlsson, J.; Kullberg, E.B.; Capala, J.; Sjöberg, S.; Edwards, K.; Gedda, L. Ligand Liposomes and Boron Neutron Capture Therapy. J. Neurooncol. 2003, 62, 47–59. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsurubuchi, T.; Shirakawa, M.; Kurosawa, W.; Matsumoto, K.; Ubagai, R.; Umishio, H.; Suga, Y.; Yamazaki, J.; Arakawa, A.; Maruyama, Y.; et al. Evaluation of a Novel Boron-Containing α-d-Mannopyranoside for BNCT. Cells 2020, 9, 1277. https://doi.org/10.3390/cells9051277
Tsurubuchi T, Shirakawa M, Kurosawa W, Matsumoto K, Ubagai R, Umishio H, Suga Y, Yamazaki J, Arakawa A, Maruyama Y, et al. Evaluation of a Novel Boron-Containing α-d-Mannopyranoside for BNCT. Cells. 2020; 9(5):1277. https://doi.org/10.3390/cells9051277
Chicago/Turabian StyleTsurubuchi, Takao, Makoto Shirakawa, Wataru Kurosawa, Kayo Matsumoto, Risa Ubagai, Hiroshi Umishio, Yasuyo Suga, Junko Yamazaki, Akihiro Arakawa, Yutaka Maruyama, and et al. 2020. "Evaluation of a Novel Boron-Containing α-d-Mannopyranoside for BNCT" Cells 9, no. 5: 1277. https://doi.org/10.3390/cells9051277
APA StyleTsurubuchi, T., Shirakawa, M., Kurosawa, W., Matsumoto, K., Ubagai, R., Umishio, H., Suga, Y., Yamazaki, J., Arakawa, A., Maruyama, Y., Seki, T., Shibui, Y., Yoshida, F., Zaboronok, A., Suzuki, M., Sakurai, Y., Tanaka, H., Nakai, K., Ishikawa, E., & Matsumura, A. (2020). Evaluation of a Novel Boron-Containing α-d-Mannopyranoside for BNCT. Cells, 9(5), 1277. https://doi.org/10.3390/cells9051277