Cytokines Differently Define the Immunomodulation of Mesenchymal Stem Cells from the Periodontal Ligament
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. hPDLSCs Culture and Verification of MSC Surface Marker Expression
2.3. CD4+ T Lymphocyte Isolation
2.4. Experimental Protocols
2.4.1. hPDLSCs Treatment
2.4.2. hPDLSCs/CD4+ T Cell Indirect Co-Culture
2.4.3. Immunomediators Expression
2.5. Analysis of Co-Culture Experiments
2.5.1. CD4+ T Cell Proliferation and Apoptosis
2.5.2. Immunomediators Expression Analysis in hPDLSCs
Quantitative Polymerase Chain Reaction (qPCR)
IDO-1 Immunostaining
Measuring L-Kynurenine Levels
PD-L1 and PD-L2 Immunostaining
Prostaglandin E2 ELISA
2.6. Statistical Analysis
3. Results
3.1. Mesenchymal and Hematopoietic Surface Marker Expression in hPDLSCs
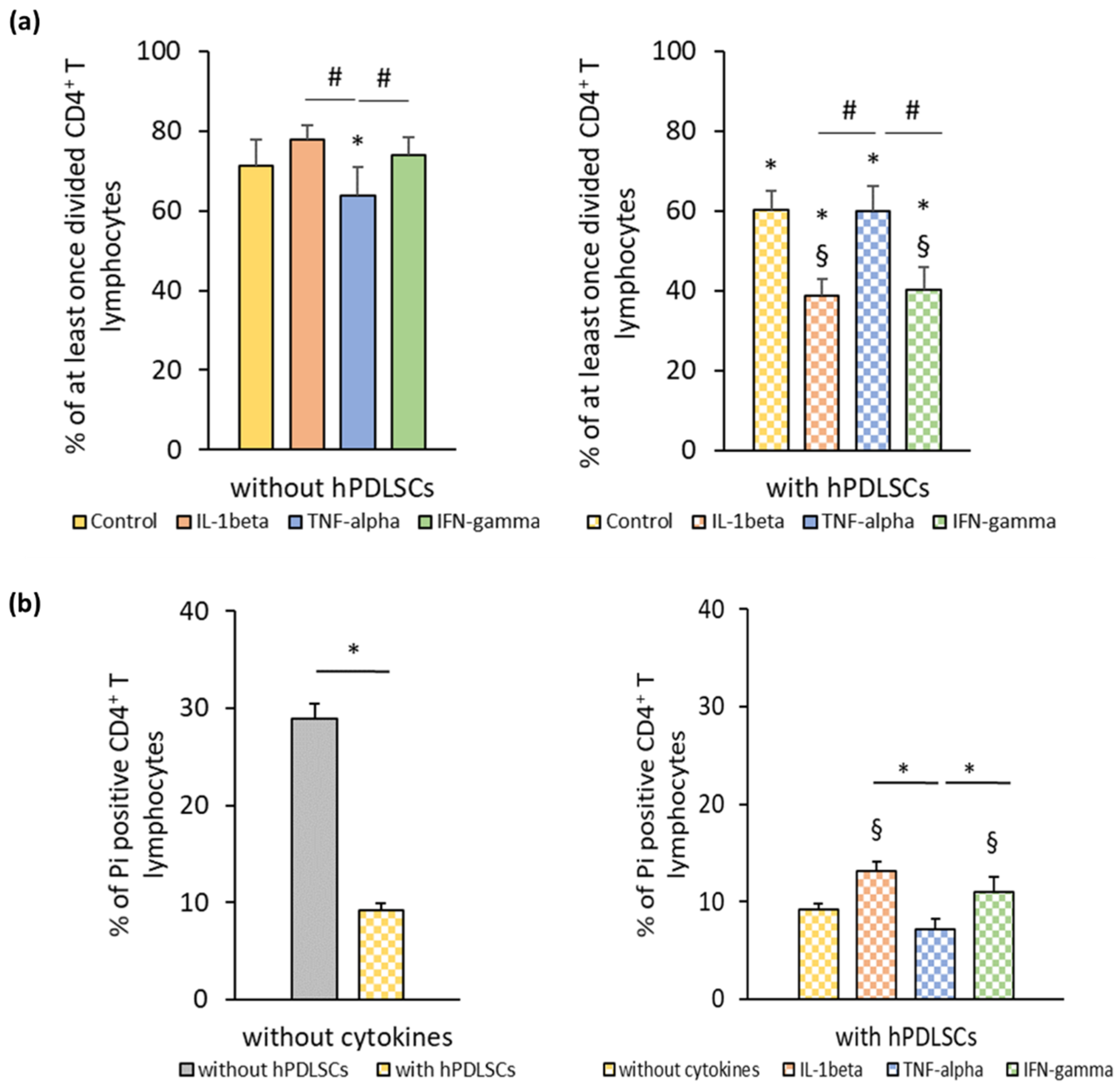
3.2. hPDLSC Mediated Effect of Different Inflammatory Stimuli on CD4+ T Lymphocytes
3.3. Effect of Different Inflammatory Cytokines on the Expression of Immunomodulatory Proteins in hPDLSCs
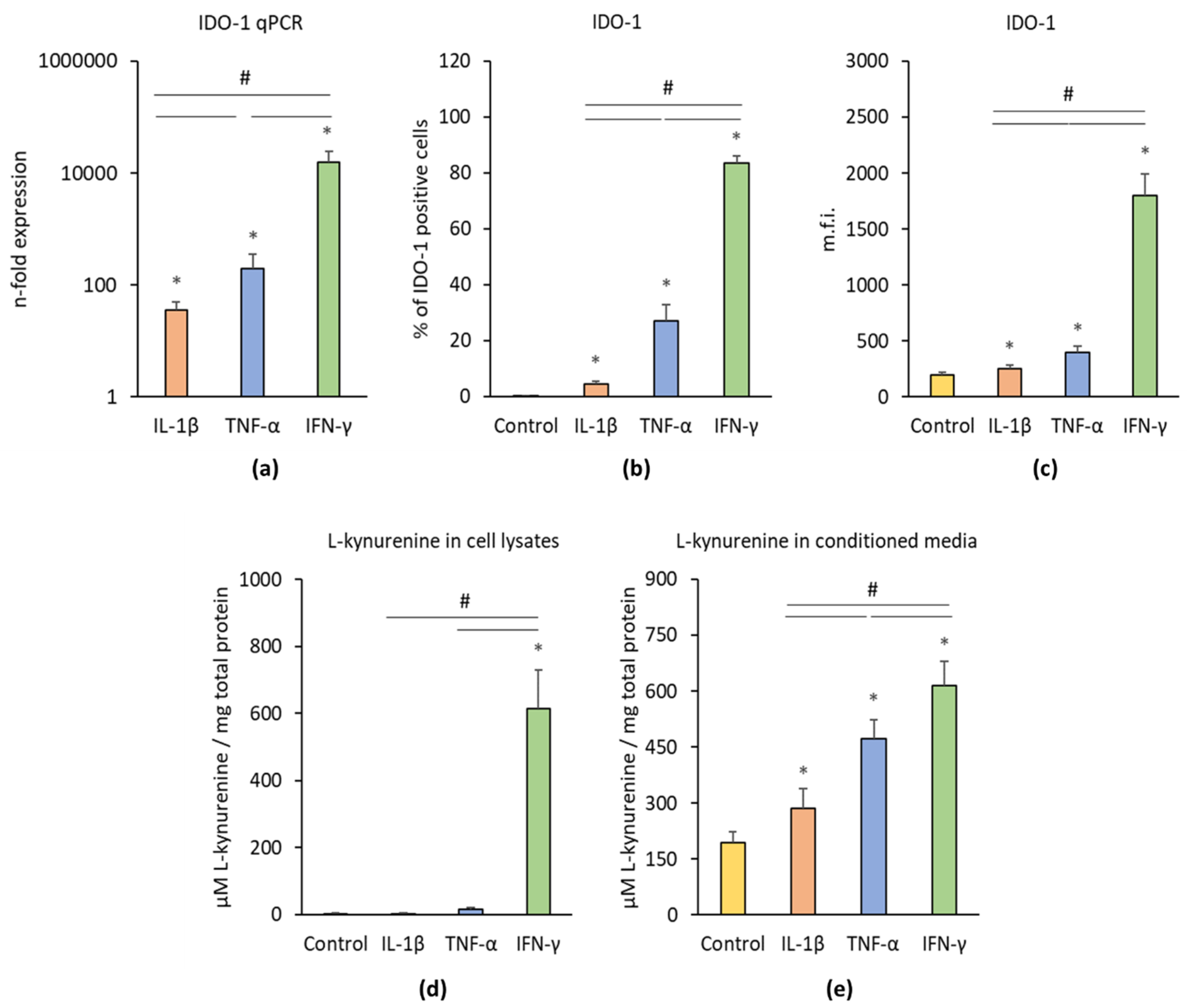
3.3.1. IDO-1 Expression and Activity
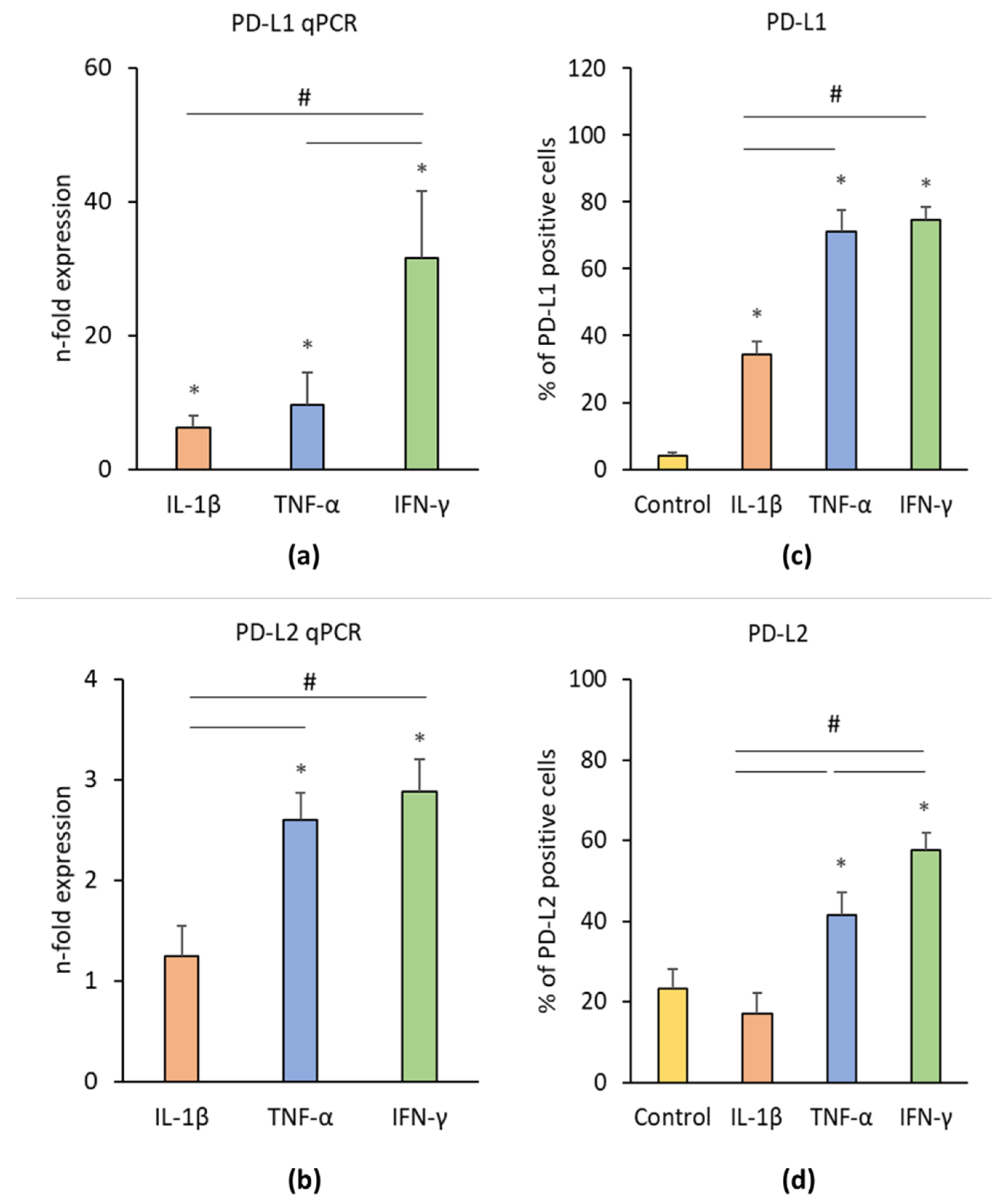
3.3.2. PD-L1/2 Expression
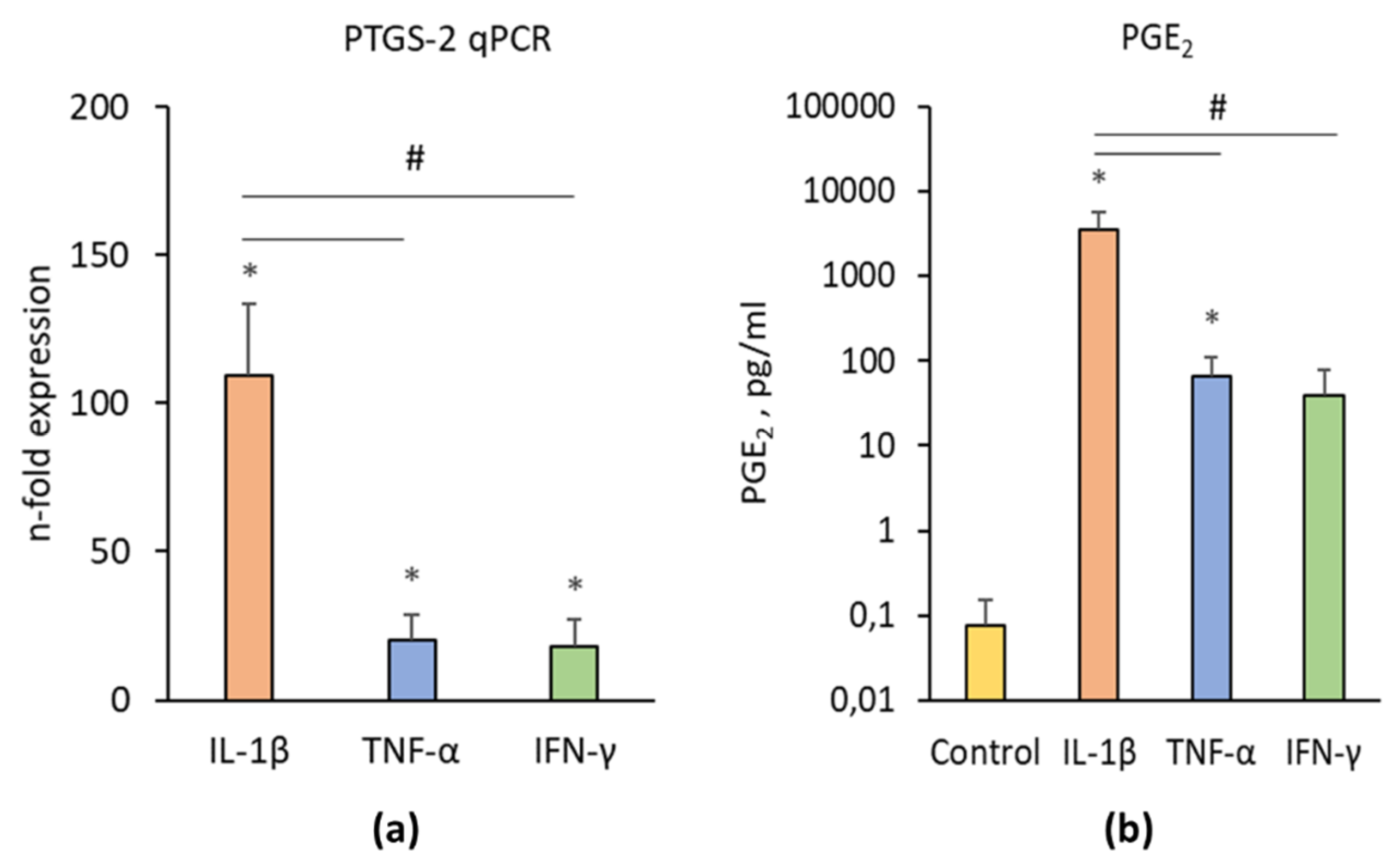
3.3.3. PTGS-2/PGE2 Expression
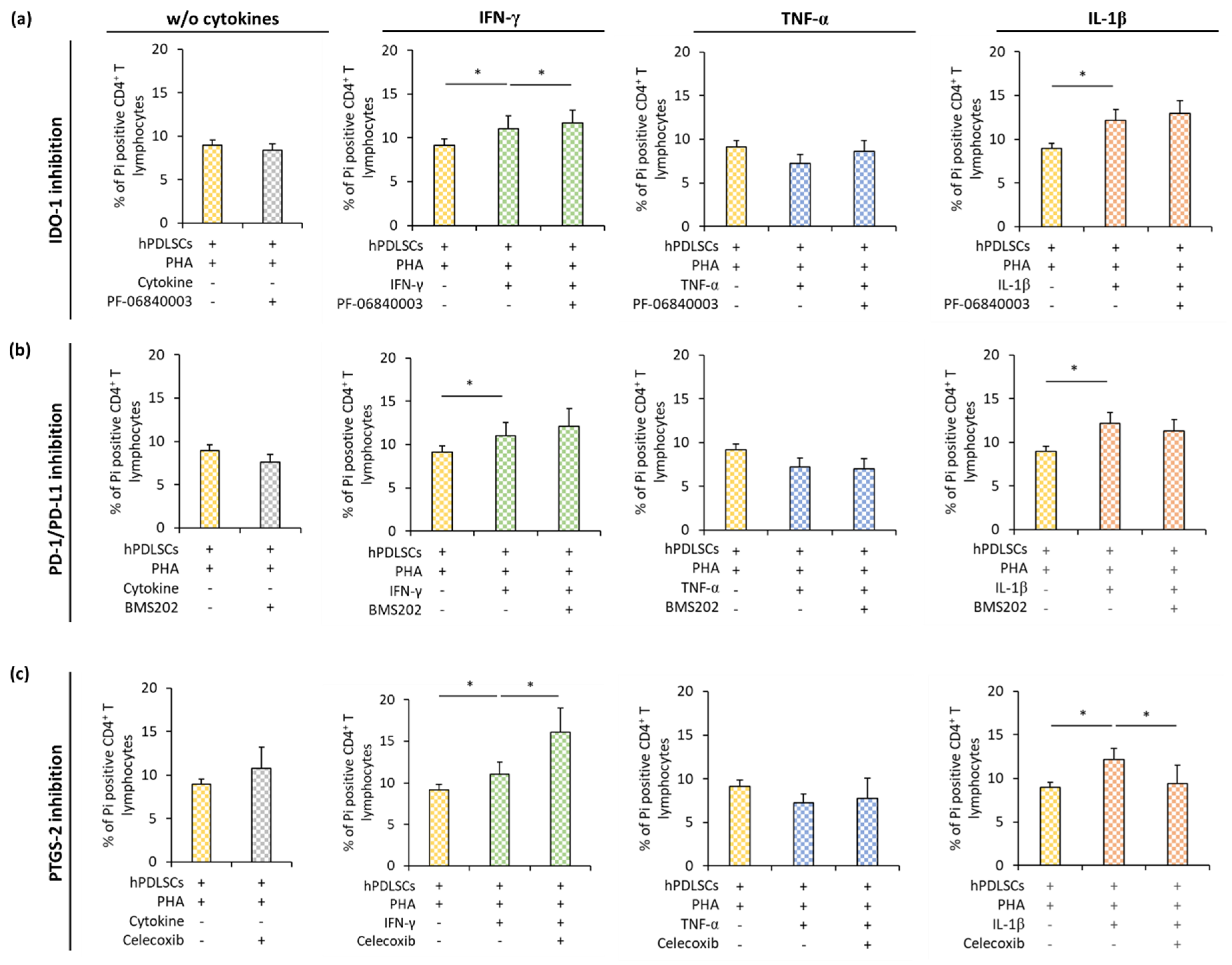
3.4. Effect of IDO-1, PD-L1, and PTGS-2 Inhibition in hPDLSCs on CD4+ T Lymphocyte Proliferation in the Presence of Different Inflammatory Stimuli
3.5. Effect of IDO-1, PD-L1, and PTGS-2 Inhibition in hPDLSCs on CD4+ T Lymphocyte Apoptosis in the Presence of Different Inflammatory Stimuli
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prockop, D.J. Marrow Stromal Cells as Stem Cells for Nonhematopoietic Tissues. J. Sci. 1997, 276, 71–74. [Google Scholar] [CrossRef] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent MesenChymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise Review: Mesenchymal Stem Cells: Their Phenotype, Differentiation Capacity, Immunological Features, and Potential for Homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, N.; Gronthos, S.; Bartold, P.M. Immunomodulatory Effects of Stem cells. Periodontol. 2000 2013, 63, 198–216. [Google Scholar] [CrossRef] [PubMed]
- Andrukhov, O.; Behm, C.; Blufstein, A.; Rausch-Fan, X. Immunomodulatory Properties of Dental Tissue-Derived Mesenchymal Stem Cells: Implication in Disease and Tissue Regeneration. World J. Stem Cells 2019, 11, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of Multipotent Postnatal Stem Cells from Human Periodontal Ligament. Lancet 2004, 149–155. [Google Scholar] [CrossRef]
- Beertsen, W.; McCulloch, C.A.G.; Sodek, J. The Periodontal Ligament: A Unique, Multifunctional Connective Tissue. Periodontol. 2000 1997, 13, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Gronthos, S. Perivascular Niche of Postnatal Mesenchymal Stem Cells in Human Bone Marrow and Dental Pulp. J. Bone Miner. Res. 2003, 18, 696–704. [Google Scholar] [CrossRef] [Green Version]
- Sharpe, P.T.; Yianni, V. Perivascular-Derived Mesenchymal Stem Cells. Crit. Rev. Oral Biol. Med. 2019, 98, 1066–1072. [Google Scholar]
- Racz, G.Z.; Kadar, K.; Foldes, A.; Kallo, K.; Perczel-Kovach, K.; Keremi, B.; Nagy, A.; Varga, G. Immunomodulatory and Potential Therapeutic Role of Mesenchymal Stem Cells in Periodontitis. J. Physiol. Pharmacol. 2014, 65, 327–339. [Google Scholar]
- Xiao, L.; Nasu, M. From Regenerative Dentistry to Regenerative Mmedicine: Progress, Challenges, and Potential Applications of Oral Stem Cells. Stem Cells Cloning Adv. Appl. 2014, 7, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Tipnis, S.; Viswanathan, C.; Majumdar, A.S. Immunosuppressive Properties of Human Umbilical Cord-derived Mesenchymal Stem Cells: Role of B7-H1 and IDO. Immunol. Cell Biol. 2010, 88, 795–806. [Google Scholar] [CrossRef]
- Krampera, M. Mesenchymal Stromal Cell Licensing: A Multistep Process. J. Leukemia 2011, 25, 1408–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal Stromal Cells: Sensors and Switchers of Inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fawzy El-Sayed, K.M.; Elahmady, M.; Adawi, Z.; Aboushadi, N.; Elnaggar, A.; Eid, M.; Hamdy, N.; Sanaa, D.; Dörfer, C.E. The Periodontal Stem/Progenitor Cell Inflammatory-Regenerative Cross Talk: A new Perspective. J. Periodontal Res. 2019, 54, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Menicanin, D.; Shi, S.; Bartold, P.M.; Gronthos, S. Immunomodulatory Properties of Human Periodontal Ligament Stem cells. J. Cell. Physiol. 2009, 219, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Takegawa, D.; Nakanishi, T.; Hirao, K.; Yumoto, H.; Takahashi, K.; Matsuo, T. Modulatory Roles of Interferon-γ Through Indoleamine 2,3-Dioxygenase Induction in Innate Immune Response of Dental Pulp Cells. J. Endod. 2014, 40, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Nisapakultorn, K.; Makrudthong, J.; Sa-Ard-Iam, N.; Rerkyen, P.; Mahanonda, R.; Takikawa, O. Indoleamine 2,3-Dioxygenase Expression and Regulation in Chronic Periodontitis. J. Periodontol. 2009, 80, 114–121. [Google Scholar] [CrossRef]
- Andrukhov, O.; Andrukhova, O.; Özdemir, B.; Haririan, H.; Müller-Kern, M.; Moritz, A.; Rausch-Fan, X. Soluble CD14 Enhances the Response of Periodontal Ligament Stem Cells to P. Gingivalis Lipopolysaccharide. PLoS ONE 2016, 11, e0160848. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.M.; Zhang, P.; Wang, X.; Chen, J.; Yang, J.; Lu, W.; Zhou, W.; Yuan, W.; Feng, Y. Expression of Programmed Death 1 Ligand 1 on Periodontal Tissue Cells as a Possible Protective Feedback Mechanism Against Periodontal Tissue Destruction. Mol. Med. Rep. 2016, 13, 2423–2430. [Google Scholar] [CrossRef] [Green Version]
- Hegyi, B.; Kudlik, G.; Monostori, É.; Uher, F. Activated T-cells and Pro-inflammatory Cytokines Differentially Regulate Prostaglandin E2 Secretion by Mesenchymal Stem Cells. J. Biochem. Biophys. Res. Commun. 2012, 419, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Guzik, K.; Zak, K.M.; Grudnik, P.; Magiera, K.; Musielak, B.; Törner, R.; Skalniak, L.; Dömling, A.; Dubin, G.; Holak, T.A. Small-Molecule Inhibitors of the Programmed Cell Death-1/Programmed Death-Ligand 1 (PD-1/PD-L1) Interaction via Transiently Induced Protein States and Dimerization of PD-L1. Med. Chem. 2017, 60, 5857–5867. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, M.; Ogura, N.; Akutsu, M.; Ito, K.; Kondoh, T. The Anti-Inflammatory Effect of Cyclooxygenase Inhibitors in Fibroblast-like Synoviocytes from The Human Temporomandibular Joint Results from The Suppression of PGE2 Production. J. Oral Pathol. Med. 2013, 42, 499–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrukhov, O.; Hong, J.S.-A.; Andrukhova, O.; Blufstein, A.; Moritz, A.; Rausch-Fan, X. Response of Human Periodontal Ligament Stem Cells to IFN-gamma and TLR-agonists. Sci. Rep. 2017, 7, 12856. [Google Scholar] [CrossRef] [Green Version]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal Stem Versus Stromal Cells: International Society for Cellular Therapy Mesenchymal Stromal Cell Committee Position Statement on Nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [CrossRef]
- Klinker, M.W. Mesenchymal Stem Cells in The Treatment of Inflammatory and Autoimmune Diseases in Experimental Animal Models. World J. Stem Cells 2015, 419, 215–220. [Google Scholar] [CrossRef]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials with Mesenchymal Stem Cells: An Update. Cell Transplant. 2016, 25, 829–848. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of Mesenchymal Stem Cells in Immunomodulation: Pathological and Therapeutic Implications. Nat. Immunol. 2014, 15, 1009–1016. [Google Scholar] [CrossRef]
- García, J.R.; Quirós, M.; Han, W.M.; O’Leary, M.N.; Cox, G.N.; Nusrat, A.; García, A.J. IFN-γ-Tethered Hydrogels Enhance Mesenchymal Stem Cell-based Immunomodulation and Promote Tissue Repair. Biomaterials 2019, 220, 119403. [Google Scholar]
- Engebretson, S.P.; Hey-Hadavi, J.; Ehrhardt, F.J.; Hsu, D.; Celenti, R.S.; Grbic, J.T.; Lamster, I.B. Gingival Crevicular Fluid Levels of Interleukin-1β and Glycemic Control in Patients with Chronic Periodontitis and Type 2 Diabetes. J. Periodontol. 2004, 75, 1203–1208. [Google Scholar] [CrossRef] [Green Version]
- Dutzan, N.; Vernal, R.; Hernandez, M.; Dezerega, A.; Rivera, O.; Silva, N.; Aguillon, J.C.; Puente, J.; Pozo, P.; Gamonal, J. Levels of Interferon-Gamma and Transcription Factor T-Bet in Progressive Periodontal Lesions in Patients With Chronic Periodontitis. J. Periodontol. 2009, 80, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Daĝ, A.; Firat, E.T.; Kadiroĝlu, A.K.; Kale, E.; Yilmaz, M.E. Significance of Elevated Gingival Crevicular Fluid Tumor Necrosis Factor-α and Interleukin-8 Levels in Chronic Hemodialysis Patients with Periodontal Disease. J. Periodontal Res. 2010, 45, 445–450. [Google Scholar] [PubMed]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal Stem Cell-Mediated Immunosuppression Occurs via Concerted Action of Chemokines and Nitric Oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemeda, H.; Jakob, M.; Ludwig, A.-K.; Giebel, B.; Lang, S.; Brandau, S. Interferon-γ and Tumor Necrosis Factor-α Differentially Affect Cytokine Expression and Migration Properties of Mesenchymal Stem Cells. Stem Cells Dev. 2010, 19, 693–706. [Google Scholar] [CrossRef]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human Bone Marrow Stromal Cells Suppress T-lymphocyte Proliferation Induced by Cellular or Nonspecific Mitogenic Stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef]
- Glennie, S.; Soeiro, I.; Dyson, P.J.; Lam, E.W.F.; Dazzi, F. Bone Marrow Mesenchymal Stem Cells Induce Division Arrest Anergy of Activated T Cells. Blood 2005, 105, 2821–2827. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Jin, Y.; Shi, S. Fas ligand Regulates the Immunomodulatory Properties of Dental Pulp Stem Cells. J. Dent. Res. 2012, 91, 948–954. [Google Scholar] [CrossRef] [Green Version]
- Su, W.R.; Zhang, Q.Z.; Shi, S.H.; Nguyen, A.L.; Le, A.D. Human Gingiva-Derived Mesenchymal Stromal Cells Attenuate Contact Hypersensitivity via Prostaglandin E2-dependent Mechanisms. J. Stem Cells 2011, 29, 1849–1860. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, C.M.; An, S.; Cheng, Q.; Huang, Y.F.; Wang, Y.T.; Gou, Y.C.; Xiao, L.; Yu, W.J.; Wang, J. Immunomodulatory Properties of Dental Tissue-derived Mesenchymal Stem Cells. J. Oral Dis. 2014, 20, 25–34. [Google Scholar] [CrossRef]
- Laing, A.G.; Fanelli, G.; Ramirez-Valdez, A.; Lechler, R.I.; Lombardi, G.; Sharpe, P.T. Mesenchymal Stem Cells Inhibit T-Cell Function through Conserved Induction of Cellular Stress. PLoS ONE 2019, 14, e0213170. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Guo, S.; Tong, S.; Sun, Q.; Li, F.; Zhang, X.; Qiao, Y.; Liang, G. Immunosuppression of Human Adipose-Derived Stem Cells on T Cell Subsets via the Reduction of NF-kappaB Activation Mediated by PD-L1/PD-1 and Gal-9/TIM-3 Pathways. Stem Cells Dev. 2018, 27, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.; Monasterio, G.; Cavalla, F.; Córdova, L.A.; Hernández, M.; Heymann, D.; Garlet, G.P.; Sorsa, T.; Pärnänen, P.; Lee, H.-M.; et al. Osteoimmunology of Oral and Maxillofacial Diseases: Translational Applications Based on Biological Mechanisms. Front. Immunol. 2019, 10, 1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garlet, G.P. Critical Reviews in Oral Biology & Medicine: Destructive and Protective Roles of Cytokines in Periodontitis: A Re-appraisal from Host Defense and Tissue Destruction Viewpoints. J. Dent. Res. 2010, 89, 1349–1363. [Google Scholar] [PubMed]
- Graves, D.T.; Cochran, D. The Contribution of Interleukin-1 and Tumor Necrosis Factor to Periodontal Tissue Destruction. J. Periodontol. 2003, 74, 391–401. [Google Scholar] [CrossRef]
- Graves, D. Cytokines That Promote Periodontal Tissue Destruction. J. Periodontol. 2008, 79, 1585–1591. [Google Scholar] [CrossRef] [Green Version]

| MSC Marker | Hematopoietic Marker | |
|---|---|---|
| CD29 | 97.7 ± 0.2 | - |
| CD73 | 96.1 ± 0.2 | - |
| CD90 | 97.9 ± 0.2 | - |
| CD105 | 97.1 ± 0.6 | - |
| CD146 | 61.3 ± 5.7 | - |
| CD31 | - | 0.5 ± 0.1 |
| CD34 | - | 0.6 ± 0.2 |
| CD45 | - | 2.7 ± 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behm, C.; Blufstein, A.; Gahn, J.; Nemec, M.; Moritz, A.; Rausch-Fan, X.; Andrukhov, O. Cytokines Differently Define the Immunomodulation of Mesenchymal Stem Cells from the Periodontal Ligament. Cells 2020, 9, 1222. https://doi.org/10.3390/cells9051222
Behm C, Blufstein A, Gahn J, Nemec M, Moritz A, Rausch-Fan X, Andrukhov O. Cytokines Differently Define the Immunomodulation of Mesenchymal Stem Cells from the Periodontal Ligament. Cells. 2020; 9(5):1222. https://doi.org/10.3390/cells9051222
Chicago/Turabian StyleBehm, Christian, Alice Blufstein, Johannes Gahn, Michael Nemec, Andreas Moritz, Xiaohui Rausch-Fan, and Oleh Andrukhov. 2020. "Cytokines Differently Define the Immunomodulation of Mesenchymal Stem Cells from the Periodontal Ligament" Cells 9, no. 5: 1222. https://doi.org/10.3390/cells9051222





