Mesenchymal Stem Cells in Embryo-Maternal Communication under Healthy Conditions or Viral Infections: Lessons from a Bovine Model
Abstract
1. Introduction
2. The Role of Maternal MSC in Embryo Implantation
2.1. Evidence for the Existence of MSC in the Maternal Reproductive Tract
2.2. Relevance of Immunoregulation during Implantation
2.3. MSC Plasticity
3. The Other Side of the Line: The Embryo Trophoblast
4. Interaction between Embryonic and Maternal MSC in Homeostasis—The Role of the Secretome: Soluble Mediators and Extracellular Vesicles
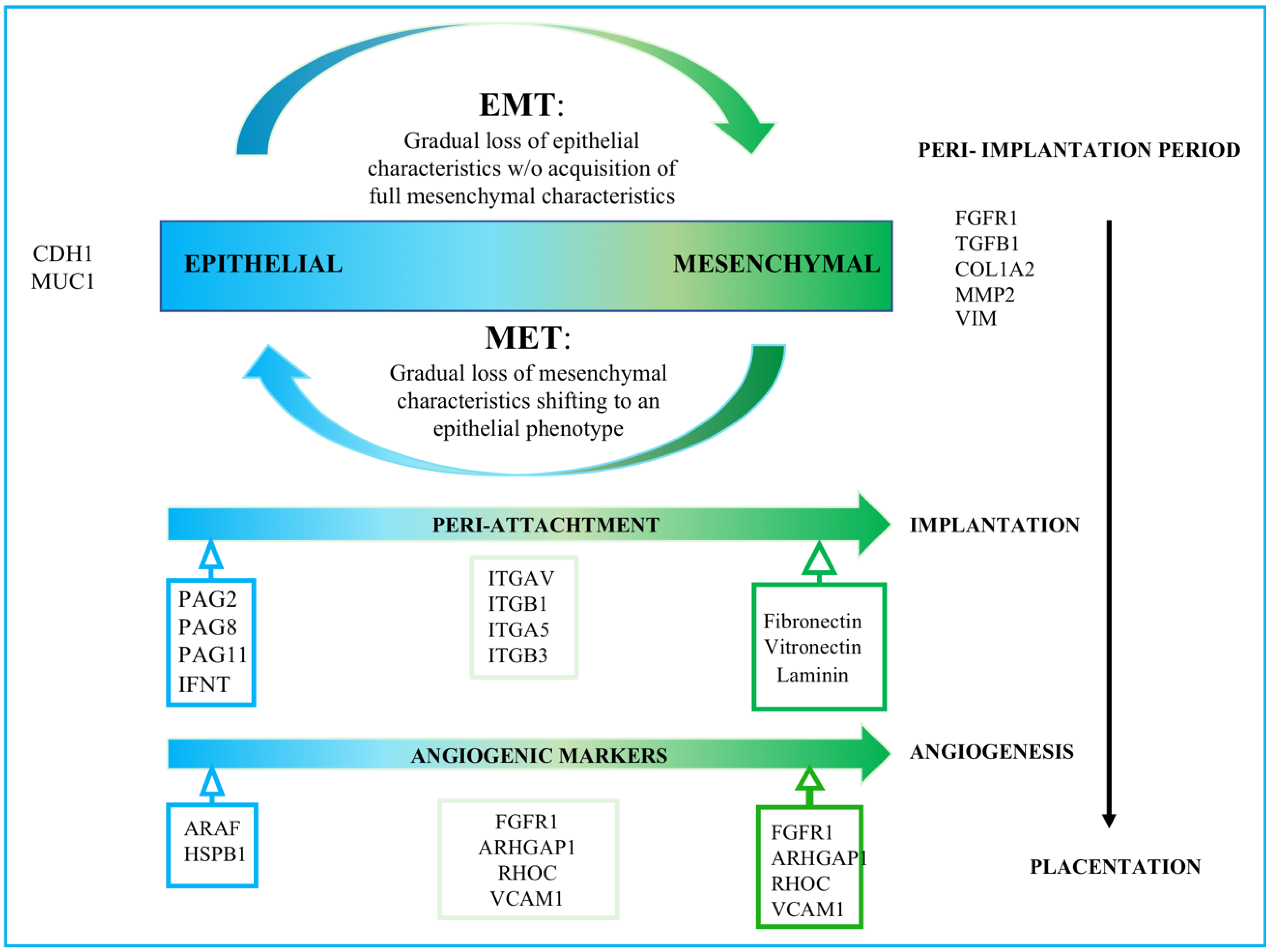
4.1. Trophoblastic-Derived Secretome and EMT
4.2. Embryonic Secretome and Inflammatory Cytokines Induce Maternal MSC Chemotaxis towards the Implantation Niche
4.3. Tissular Rearrangements for Implantation
4.4. Maternal MSC-EV-Cargo Modulate Embryonic EV-Cargo
5. Challenges and Obstacles in Bovine Embryo-Maternal Communication Study When a Third Viral Actor Breaks in
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Westhusin, M.E.; Pryor, J.H.; Bondioli, K.R. Nuclear transfer in the bovine embryo: A comparison of 5-day, 6-day, frozen-thawed, and nuclear transfer donor embryos. Mol. Reprod. Dev. 1991, 28, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Lopera-Vasquez, R.; Hamdi, M.; Maillo, V.; Gutierrez-Adan, A.; Bermejo-Alvarez, P.; Ramirez, M.A.; Yañez-Mo, M.; Rizos, D. Effect of bovine oviductal extracellular vesicles on embryo development and quality in vitro. Reproduction 2017, 153, 461–470. [Google Scholar] [CrossRef]
- Lopera-Vasquez, R.; Hamdi, M.; Fernandez-Fuertes, B.; Maillo, V.; Beltrán-Breña, P.; Calle, A.; Redruello, A.; López-Martín, S.; Gutierrez-Adan, A.; Yáñez-Mó, M.; et al. Extracellular Vesicles from BOEC in In Vitro Embryo Development and Quality. PLoS ONE 2016, 11, e0148083. [Google Scholar] [CrossRef]
- Alminana-Brines, C.; Corbin, E.; Tsikis, G.; Neto, A.S.D.A.; Labas, V.; Reynaud, K.; Galio, L.; Uzbekov, R.; Garanina, A.; Druart, X.; et al. Oviduct extracellular vesicles protein content and their role during oviduct–embryo crosstalk. Reproduction 2017, 154, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Mazzarella, R.; Bastos, N.M.; Bridi, A.; del Collado, M.; Andrade, G.M.; Pinzon, J.; Prado, C.M.; Silva, L.A.; Meirelles, F.V.; Pugliesi, G.; et al. Changes in Oviductal Cells and Small Extracellular Vesicles miRNAs in Pregnant Cows. Front. Vet. Sci. 2021, 8, 639752. [Google Scholar] [CrossRef] [PubMed]
- González-Brusi, L.; Algarra, B.; Moros-Nicolás, C.; Izquierdo-Rico, M.J.; Avilés, M.; Jiménez-Movilla, M. A Comparative View on the Oviductal Environment during the Periconception Period. Biomolecules 2020, 10, 1690. [Google Scholar] [CrossRef]
- Talukder, A.K.; Marey, M.A.; Shirasuna, K.; Kusama, K.; Shimada, M.; Imakawa, K.; Miyamoto, A. Roadmap to pregnancy in the first 7 days post-insemination in the cow: Immune crosstalk in the corpus luteum, oviduct, and uterus. Theriogenology 2020, 150, 313–320. [Google Scholar] [CrossRef]
- Wolf, E.; Arnold, G.J.; Bauersachs, S.; Beier, H.M.; Blum, H.; Einspanier, R.; Frohlich, T.; Herrler, A.; Hiendleder, S.; Kölle, S.; et al. Embryo-Maternal Communication in Bovine—Strategies for Deciphering a Complex Cross-Talk. Reprod. Domest. Anim. 2003, 38, 276–289. [Google Scholar] [CrossRef]
- Salek Farrokhi, A.; Zarnani, A.H.; Moazzeni, S.M. Mesenchymal stem cells therapy protects fetuses from resorption and induces Th2 type cytokines profile in abortion prone mouse model. Transpl. Immunol. 2018, 47, 26–31. [Google Scholar] [CrossRef]
- Ott, T.L. Symposium review: Immunological detection of the bovine conceptus during early pregnancy. J. Dairy Sci. 2019, 102, 3766–3777. [Google Scholar] [CrossRef]
- Fair, T. The contribution of the maternal immune system to the establishment of pregnancy in cattle. Front. Immunol. 2015, 6, 7. [Google Scholar] [CrossRef]
- Szekeres-Bartho, J.; Wegmann, T.G. A progesterone-dependent immunomodulatory protein alters the Th1/Th2 balance. J. Reprod. Immunol. 1996, 31, 81–95. [Google Scholar] [CrossRef]
- Saito, S.; Nakashima, A.; Shima, T.; Ito, M. Th1/Th2/Th17 and Regulatory T-Cell Paradigm in Pregnancy. Am. J. Reprod. Immunol. 2010, 63, 601–610. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Lindner, U.; Kramer, J.; Rohwedel, J.; Schlenke, P. Mesenchymal Stem or Stromal Cells: Toward a Better Understanding of Their Biology? Transfus. Med. Hemotherapy 2010, 37, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Trohatou, O.; Roubelakis, M.G. Mesenchymal Stem/Stromal Cells in Regenerative Medicine: Past, Present, and Future. Cell. Reprogramming 2017, 19, 217–224. [Google Scholar] [CrossRef]
- Prianishnikov, V.A. On the concept of stem cell and a model of functional-morphological structure of the endometrium. Contraception 1978, 18, 213–223. [Google Scholar] [CrossRef]
- Chan, R.W.; Schwab, K.E.; Gargett, C.E. Clonogenicity of human endometrial epithelial and stromal cells. Biol. Reprod. 2004, 70, 1738–1750. [Google Scholar] [CrossRef]
- Lara, E.; Rivera, N.; Rojas, D.; Rodríguez-Alvarez, L.L.; Castro, F.O. Characterization of mesenchymal stem cells in bovine endometrium during follicular phase of oestrous cycle. Reprod. Domest. Anim. 2017, 52, 707–714. [Google Scholar] [CrossRef]
- Łupicka, M.; Bodek, G.; Shpigel, N.; Elnekave, E.; Korzekwa, A.J. Identification of pluripotent cells in bovine uterus: In situ and in vitro studies. Reproduction 2015, 149, 317–327. [Google Scholar] [CrossRef]
- De Moraes, C.N.; Maia, L.; Dias, M.C.; Dell’Aqua, C.P.; da Mota, L.S.; Chapwanya, A.; Landim-Alvarenga, F.D.; Oba, E. Bovine endometrial cells: A source of mesenchymal stem/progenitor cells. Cell Biol. Int. 2016, 40, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Calle, A.; López-Martín, S.; Monguió-Tortajada, M.; Borràs, F.E.; Yáñez-Mó, M.; Ramírez, M. Bovine endometrial MSC: Mesenchymal to epithelial transition during luteolysis and tropism to implantation niche for immunomodulation. Stem Cell Res. Ther. 2019, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Schwab, K.E.; Chan, R.W.; Gargett, C.E. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil. Steril. 2005, 84, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Taylor, H.S. Contribution of Bone Marrow-Derived Stem Cells to Endometrium and Endometriosis. Stem Cells 2007, 25, 2082–2086. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.C.R.; Lima, F.D.S.; Vinolo, M.A.R.; Hastreiter, A.; Curi, R.; Borelli, P.; Fock, R.A. Protein Malnutrition Induces Bone Marrow Mesenchymal Stem Cells Commitment to Adipogenic Differentiation Leading to Hematopoietic Failure. PLoS ONE 2013, 8, e58872. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA 2004, 292, 81–85. [Google Scholar] [CrossRef]
- Mints, M.; Jansson, M.; Sadeghi, B.; Westgren, M.; Uzunel, M.; Hassan, M.; Palmblad, J. Endometrial endothelial cells are derived from donor stem cells in a bone marrow transplant recipient. Hum. Reprod. 2008, 23, 139–143. [Google Scholar] [CrossRef]
- Gargett, C.E.; Masuda, H. Adult stem cells in the endometrium. Mol. Hum. Reprod. 2010, 16, 818–834. [Google Scholar] [CrossRef]
- Calle, A.; Gutiérrez-Reinoso, M.; Re, M.; Blanco, J.; De La Fuente, J.; Monguió-Tortajada, M.; Borràs, F.E.; Yáñez-Mó, M.; Ramírez, M. Bovine peripheral blood MSCs chemotax towards inflammation and embryo implantation stimuli. J. Cell. Physiol. 2021, 236, 1054–1067. [Google Scholar] [CrossRef]
- Calle, A.; Toribio, V.; Yáñez-Mó, M.; Ramírez, M.Á. Embryonic Trophectoderm Secretomics Reveals Chemotactic Migration and Intercellular Communication of Endometrial and Circulating MSCs in Embryonic Implantation. Int. J. Mol. Sci. 2021, 22, 5638. [Google Scholar] [CrossRef]
- Gallardo, D.; De La Camara, R.; Nieto, J.B.; Espigado, I.; Iriondo, A.; Jiménez-Velasco, A.; Vallejo, C.; Martín, C.; Caballero, L.; Brunet, S.; et al. Is mobilized peripheral blood comparable with bone marrow as a source of hematopoietic stem cells for allogeneic transplantation from HLA-identical sibling donors? A case-control study. Haematologica 2009, 94, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Ock, S.A.; Baregundi Subbarao, R.; Lee, Y.M.; Lee, J.H.; Jeon, R.H.; Lee, S.L.; Park, J.K.; Hwang, S.C.; Rho, G.J. Comparison of Immunomodulation Properties of Porcine Mesenchymal Stromal/Stem Cells Derived from the Bone Marrow, Adipose Tissue, and Dermal Skin Tissue. Stem Cells Int. 2016, 2016, 9581350. [Google Scholar] [CrossRef]
- Perez-Sepulveda, A.; Torres, M.J.; Khoury, M.; Illanes, S.E. Innate Immune System and Preeclampsia. Front. Immunol. 2014, 5, 244. [Google Scholar] [CrossRef] [PubMed]
- Magatti, M.; Stefani, F.R.; Papait, A.; Cargnoni, A.; Masserdotti, A.; Silini, A.R.; Parolini, O. Perinatal Mesenchymal Stromal Cells and Their Possible Contribution to Fetal-Maternal Tolerance. Cells 2019, 8, 1401. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.-H.; Zhu, X.-H.; Yan, L.-Y.; Zhang, Y.; Jin, H.-Y.; Xia, X.; Li, R.; Qiao, J. Bone mesenchymal stem cells improve pregnancy outcome by inducing maternal tolerance to the allogeneic fetus in abortion-prone matings in mouse. Placenta 2016, 47, 29–36. [Google Scholar] [CrossRef]
- Hansen, P.J. Medawar Redux—An Overview on the Use of Farm Animal Models to Elucidate Principles of Reproductive Immunology. Am. J. Reprod. Immunol. 2010, 64, 225–230. [Google Scholar] [CrossRef]
- Rocha, C.C.; da Silveira, J.C.; Forde, N.; Binelli, M.; Pugliesi, G. Conceptus-modulated innate immune function during early pregnancy in ruminants: A review. Anim. Reprod. 2021, 18, e20200048. [Google Scholar] [CrossRef]
- Oliveira, L.J.; Barreto, R.S.; Perecin, F.; Mansouri-Attia, N.; Pereira, F.T.; Meirelles, F.V. Modulation of maternal immune system during pregnancy in the cow. Reprod. Domest. Anim. 2012, 47, 384–393. [Google Scholar] [CrossRef]
- Dunn, C.L.; Kelly, R.W.; Critchley, H.O. Decidualization of the human endometrial stromal cell: An enigmatic transformation. Reprod. Biomed. Online 2003, 7, 151–161. [Google Scholar] [CrossRef]
- Mossman, H.W. Comparative morphogenesis of the fetal membranes and accessory uterine structures. Placenta 1991, 12, 1–5. [Google Scholar] [CrossRef]
- Alam, S.M.K.; Konno, T.; Dai, G.; Lu, L.; Wang, D.; Dunmore, J.H.; Godwin, A.R.; Soares, M.J. A uterine decidual cell cytokine ensures pregnancy-dependent adaptations to a physiological stressor. Development 2007, 134, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kannan, A.; Wang, W.; DeMayo, F.J.; Taylor, R.N.; Bagchi, M.K.; Bagchi, I.C. Bone Morphogenetic Protein 2 Functions via a Conserved Signaling Pathway Involving Wnt4 to Regulate Uterine Decidualization in the Mouse and the Human. J. Biol. Chem. 2007, 282, 31725–31732. [Google Scholar] [CrossRef] [PubMed]
- Björkman, N. Fine structure of the fetal-maternal area of exchange in the epitheliochorial and endotheliochorial types of placentation. Acta Anat. Suppl. 1973, 61, 1–22. [Google Scholar] [CrossRef]
- Johnson, G.A.; Burghardt, R.C.; Joyce, M.M.; Spencer, T.E.; Bazer, F.W.; Pfarrer, C.; Gray, C.A. Osteopontin Expression in Uterine Stroma Indicates a Decidualization-Like Differentiation During Ovine Pregnancy. Biol. Reprod. 2003, 68, 1951–1958. [Google Scholar] [CrossRef]
- Hugo, H.; Ackland, M.L.; Blick, T.; Lawrence, M.G.; Clements, J.A.; Williams, E.D.; Thompson, E.W. Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression. J. Cell. Physiol. 2007, 213, 374–383. [Google Scholar] [CrossRef]
- Imakawa, K.; Bai, R.; Kusama, K. Integration of molecules to construct the processes of conceptus implantation to the maternal endometrium. J. Anim. Sci. 2018, 96, 3009–3021. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Maruyama, T.; Nishikawa-Uchida, S.; Oda, H.; Miyazaki, K.; Yamasaki, A.; Yoshimura, Y. Studies Using an in Vitro Model Show Evidence of Involvement of Epithelial-Mesenchymal Transition of Human Endometrial Epithelial Cells in Human Embryo Implantation. J. Biol. Chem. 2012, 287, 4441–4450. [Google Scholar] [CrossRef]
- Zhang, X.H.; Liang, X.; Liang, X.H.; Wang, T.S.; Qi, Q.R.; Deng, W.B.; Sha, A.G.; Yang, Z.M. The mesenchymal-epithelial transition during in vitro decidualization. Reprod. Sci. 2013, 20, 354–360. [Google Scholar] [CrossRef]
- Patterson, A.L.; Zhang, L.; Arango, N.A.; Teixeira, J.; Pru, J.K. Mesenchymal-to-Epithelial Transition Contributes to Endometrial Regeneration Following Natural and Artificial Decidualization. Stem Cells Dev. 2013, 22, 964–974. [Google Scholar] [CrossRef]
- Cousins, F.L.; Murray, A.; Esnal, A.; Gibson, D.A.; Critchley, H.O.; Saunders, P.T. Evidence from a mouse model that epithelial cell migration and mesenchymal-epithelial transition contribute to rapid restoration of uterine tissue integrity during menstruation. PLoS ONE 2014, 9, e86378. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Berga, S.L.; Johnston-MacAnanny, E.B.; Sidell, N.; Bagchi, I.C.; Bagchi, M.K.; Taylor, R.N. Endometrial Stromal Decidualization Responds Reversibly to Hormone Stimulation and Withdrawal. Endocrinology 2016, 157, 2432–2446. [Google Scholar] [CrossRef]
- Wallingford, G.I. (Ed.) Laboratory Production of Cattle Embryos; Cabi: Wallingford, UK, 2003. [Google Scholar]
- Kimmins, S.; Maclaren, L.A. Oestrous Cycle and Pregnancy Effects on the Distribution of Oestrogen and Progesterone Receptors in Bovine Endometrium. Placenta 2001, 22, 742–748. [Google Scholar] [CrossRef]
- Talbot, N.C.; Caperna, T.J.; Edwards, J.L.; Garrett, W.; Wells, K.D.; Ealy, A.D. Bovine Blastocyst-Derived Trophectoderm and Endoderm Cell Cultures: Interferon Tau and Transferrin Expression as Respective In Vitro Markers. Biol. Reprod. 2000, 62, 235–247. [Google Scholar] [CrossRef]
- Ramos-Ibeas, P.; Calle, A.; Pericuesta, E.; Laguna-Barraza, R.; Moros-Mora, R.; Lopera-Vásquez, R.; Maillo, V.; Yáñez-Mó, M.; Gutierrez-Adan, A.; Rizos, D.; et al. An Efficient System to Establish Biopsy-Derived Trophoblastic Cell Lines from Bovine Embryos. Biol. Reprod. 2014, 91, 15. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, S.; Bai, R.; Chaen, T.; Ideta, A.; Aoyagi, Y.; Sakurai, T.; Konno, T.; Imakawa, K. Expression of mesenchymal-related genes by the bovine trophectoderm following conceptus attachment to the endometrial epithelium. Reproduction 2012, 143, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Villarreal-Ponce, A.; Fallahi, M.; Ovadia, J.; Sun, P.; Yu, Q.-C.; Ito, S.; Sinha, S.; Nie, Q.; Dai, X. Transcriptional Mechanisms Link Epithelial Plasticity to Adhesion and Differentiation of Epidermal Progenitor Cells. Dev. Cell 2014, 29, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Pfarrer, C.D. Characterization of the bovine placenta by cytoskeleton, integrin receptors, and extracellular matrix. Methods Mol. Med. 2006, 121, 323–335. [Google Scholar] [CrossRef]
- Acloque, H.; Adams, M.S.; Fishwick, K.; Bronner-Fraser, M.; Nieto, M.A. Epithelial-mesenchymal transitions: The importance of changing cell state in development and disease. J. Clin. Investig. 2009, 119, 1438–1449. [Google Scholar] [CrossRef]
- Kusama, K.; Bai, R.; Ideta, A.; Aoyagi, Y.; Okuda, K.; Imakawa, K. Regulation of epithelial to mesenchymal transition in bovine conceptuses through the interaction between follistatin and activin A. Mol. Cell. Endocrinol. 2016, 434, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Shimada, A.; Imai, K.; Takahashi, T.; Hashizume, K. The cytoplasmic expression of E-cadherin and β-catenin in bovine trophoblasts during binucleate cell differentiation. Placenta 2005, 26, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Kusama, K.; Nakamura, K.; Sakurai, T.; Kimura, K.; Ideta, A.; Aoyagi, Y.; Imakawa, K. Down-regulation of transcription factor OVOL2 contributes to epithelial–mesenchymal transition in a non-invasive type of trophoblast implantation to the maternal endometrium. FASEB J. 2018, 32, 3371–3384. [Google Scholar] [CrossRef]
- Škovierová, H.; Okajčeková, T.; Strnádel, J.; Vidomanová, E.; Halašová, E. Molecular regulation of epithelial-to-mesenchymal transition in tumorigenesis (Review). Int. J. Mol. Med. 2018, 41, 1187–1200. [Google Scholar] [CrossRef]
- Pang, M.F.; Georgoudaki, A.-M.; Lambut, L.; Johansson, J.E.; Tabor, V.; Hagikura, K.; Jin, Y.; Jansson, M.; Alexander, J.S.; Nelson, C.M.; et al. TGF-β1-induced EMT promotes targeted migration of breast cancer cells through the lymphatic system by the activation of CCR7/CCL21-mediated chemotaxis. Oncogene 2015, 35, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, D.; Rosato, B.; Nanni, M.; Magenta, A.; Belleudi, F.; Torrisi, M.R. Expression of the FGFR2 mesenchymal splicing variant in epithelial cells drives epithelial-mesenchymal transition. Oncotarget 2016, 7, 5440–5460. [Google Scholar] [CrossRef]
- Billottet, C.; Tuefferd, M.; Gentien, D.; Rapinat, A.; Thiery, J.-P.; Broët, P.; Jouanneau, J. Modulation of several waves of gene expression during FGF-1 induced epithelial-mesenchymal transition of carcinoma cells. J. Cell. Biochem. 2008, 104, 826–839. [Google Scholar] [CrossRef]
- King, G.J.; Atkinson, B.A.; Robertson, H.A. Development of the intercaruncular areas during early gestation and establishment of the bovine placenta. J. Reprod. Fertil. 1981, 61, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Granot, I.; Gnainsky, Y.; Dekel, N. Endometrial inflammation and effect on implantation improvement and pregnancy outcome. Reproduction 2012, 144, 661–668. [Google Scholar] [CrossRef]
- Roberts, R.M.; Chen, Y.; Ezashi, T.; Walker, A.M. Interferons and the maternal–conceptus dialog in mammals. Semin. Cell Dev. Biol. 2008, 19, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; Brennan, J.P.; Slavin, J.L.; Blick, T.; Thompson, E.W.; Williams, E.D. Mesenchymal-to-Epithelial Transition Facilitates Bladder Cancer Metastasis: Role of Fibroblast Growth Factor Receptor-2. Cancer Res. 2006, 66, 11271–11278. [Google Scholar] [CrossRef]
- Mendez, M.G.; Kojima, S.; Goldman, R.D. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 2010, 24, 1838–1851. [Google Scholar] [CrossRef]
- Spencer, T.E.; Sandra, O.; Wolf, E. Genes involved in conceptus–endometrial interactions in ruminants: Insights from reductionism and thoughts on holistic approaches. Reproduction 2008, 135, 165–179. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wan, C.; Li, G. Concise Review: Multipotent Mesenchymal Stromal Cells in Blood. Stem Cells 2007, 25, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, G. Circulating mesenchymal stem cells and their clinical implications. J. Orthop. Transl. 2014, 2, 1–7. [Google Scholar] [CrossRef]
- Lin, W.; Xu, L.; Zwingenberger, S.; Gibon, E.; Goodman, S.B.; Li, G. Mesenchymal stem cells homing to improve bone healing. J. Orthop. Transl. 2017, 9, 19–27. [Google Scholar] [CrossRef]
- Du, H.; Naqvi, H.; Taylor, H.S. Ischemia/Reperfusion Injury Promotes and Granulocyte-Colony Stimulating Factor Inhibits Migration of Bone Marrow-Derived Stem Cells to Endometrium. Stem Cells Dev. 2012, 21, 3324–3331. [Google Scholar] [CrossRef] [PubMed]
- Zhukareva, V.; Obrocka, M.; Houle, J.D.; Fischer, I.; Neuhuber, B. Secretion profile of human bone marrow stromal cells: Donor variability and response to inflammatory stimuli. Cytokine 2010, 50, 317–321. [Google Scholar] [CrossRef]
- Paradisi, M.; Alviano, F.; Pirondi, S.; Lanzoni, G.; Fernandez, M.; Lizzo, G.; Giardino, L.; Giuliani, A.; Costa, R.; Marchionni, C.; et al. Human Mesenchymal Stem Cells Produce Bioactive Neurotrophic Factors: Source, Individual Variability and Differentiation Issues. Int. J. Immunopathol. Pharmacol. 2014, 27, 391–402. [Google Scholar] [CrossRef]
- Vakhrushev, I.V.; Vdovin, A.S.; Strukova, L.A.; Yarygin, K.N. Variability of the Phenotype and Proliferation and Migration Characteristics of Human Mesenchymal Stromal Cells Derived from the Deciduous Teeth Pulp of Different Donors. Bull. Exp. Biol. Med. 2016, 160, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.R.; Creskey, M.M.; Muradia, G.; Bell, G.I.; Sherman, S.E.; Gao, J.; Stewart, D.J.; Cyr, T.D.; Hess, D.A.; Rosu-Myles, M. Brief Report: Elastin Microfibril Interface 1 and Integrin-Linked Protein Kinase Are Novel Markers of Islet Regenerative Function in Human Multipotent Mesenchymal Stromal Cells. Stem Cells 2016, 34, 2249–2255. [Google Scholar] [CrossRef][Green Version]
- Paladino, F.V.; Sardinha, L.R.; Piccinato, C.A.; Goldberg, A.C. Intrinsic Variability Present in Wharton’s Jelly Mesenchymal Stem Cells and T Cell Responses May Impact Cell Therapy. Stem Cells Int. 2017, 2017, 8492797. [Google Scholar] [CrossRef] [PubMed]
- Calle, A.; Barrajón-Masa, C.; Gómez-Fidalgo, E.; Martín-Lluch, M.; Cruz-Vigo, P.; Sánchez-Sánchez, R.; Ramírez, M. Iberian pig mesenchymal stem/stromal cells from dermal skin, abdominal and subcutaneous adipose tissues, and peripheral blood: In vitro characterization and migratory properties in inflammation. Stem Cell Res. Ther. 2018, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.-G.; Hosoe, M.; Fujii, S.; Kanahara, H.; Sakumoto, R. Temporal expression and localization of vascular endothelial growth factor family members in the bovine uterus during peri-implantation period. Theriogenology 2019, 133, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Hoffert-Goeres, K.A.; Batchelder, C.A.; Bertolini, M.; Moyer, A.L.; Famula, T.R.; Anderson, G.B. Angiogenesis in Day-30 Bovine Pregnancies Derived from Nuclear Transfer. Cloning Stem Cells 2007, 9, 595–607. [Google Scholar] [CrossRef]
- Clemente, M.; Lopez-Vidriero, I.; O’Gaora, P.; Mehta, J.P.; Forde, N.; Gutierrez-Adan, A.; Lonergan, P.; Rizos, D. Transcriptome Changes at the Initiation of Elongation in the Bovine Conceptus. Biol. Reprod. 2011, 85, 285–295. [Google Scholar] [CrossRef]
- Pfarrer, C.; Hirsch, P.; Guillomot, M.; Leiser, R. Interaction of Integrin Receptors with Extracellular Matrix is Involved in Trophoblast Giant Cell Migration in Bovine Placentomes. Placenta 2003, 24, 588–597. [Google Scholar] [CrossRef]
- Caltabiano, S.; Hum, W.-T.; Attwell, G.J.; Gralnick, D.N.; Budman, L.J.; Cannistraci, A.M.; Bex, F.J. The integrin specificity of human recombinant osteopontin. Biochem. Pharmacol. 1999, 58, 1567–1578. [Google Scholar] [CrossRef]
- Bai, R.; Bai, H.; Kuse, M.; Ideta, A.; Aoyagi, Y.; Fujiwara, H.; Okuda, K.; Imakawa, K.; Sakurai, T. Involvement of VCAM1 in the bovine conceptus adhesion to the uterine endometrium. Reproduction 2014, 148, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Schnapp, L.M.; Hatch, N.; Ramos, D.M.; Klimanskaya, I.V.; Sheppard, D.; Pytela, R. The Human Integrin α8β1 Functions as a Receptor for Tenascin, Fibronectin, and Vitronectin. J. Biol. Chem. 1995, 270, 23196–23202. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, O.; Yanez-Mo, M.; Serrador, J.M.; Montoya, M.C.; Vicente-Manzanares, M.; Tejedor, R.; Furthmayr, H.; Sanchez-Madrid, F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J. Cell Biol. 2002, 157, 1233–1245. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Huergo, S.P.; Hockl, P.F.; Stupirski, J.C.; Maller, S.M.; Morosi, L.G.; Pinto, N.A.; Berón, A.M.; Musuruana, J.L.; Nasswetter, G.G.; Cavallasca, J.A.; et al. Clinical Relevance of Galectin-1 and Galectin-3 in Rheumatoid Arthritis Patients: Differential Regulation and Correlation with Disease Activity. Front. Immunol. 2018, 9, 3057. [Google Scholar] [CrossRef]
- Colnot, C.; Fowlis, D.; Ripoche, M.A.; Bouchaert, I.; Poirier, F. Embryonic implantation in galectin 1/galectin 3 double mutant mice. Dev. Dyn. 1998, 211, 306–313. [Google Scholar] [CrossRef]
- Murray, M.J.; Lessey, B.A. Embryo Implantation and Tumor Metastasis: Common Pathways of Invasion and Angiogenesis. Semin. Reprod. Med. 1999, 17, 275–290. [Google Scholar] [CrossRef]
- Fukuda, M.N.; Sugihara, K.; Nakayama, J. Trophinin: What embryo implantation teaches us about human cancer. Cancer Biol. Ther. 2008, 7, 1165–1170. [Google Scholar] [CrossRef][Green Version]
- Holtan, S.G.; Creedon, D.J.; Haluska, P.; Markovic, S.N. Cancer and Pregnancy: Parallels in Growth, Invasion, and Immune Modulation and Implications for Cancer Therapeutic Agents. Mayo Clin. Proc. 2009, 84, 985–1000. [Google Scholar] [CrossRef]
- Jones, R.L.; Salamonsen, L.A.; Findlay, J.K. Potential roles for endometrial inhibins, activins and follistatin during human embryo implantation and early pregnancy. Trends Endocrinol. Metab. 2002, 13, 144–150. [Google Scholar] [CrossRef]
- Mitko, K.; Ulbrich, S.E.; Wenigerkind, H.; Sinowatz, F.; Blum, H.; Wolf, E.; Bauersachs, S. Dynamic changes in messenger RNA profiles of bovine endometrium during the oestrous cycle. Reproduction 2008, 135, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Hatayama, T.; Takigawa, T.; Takeuchi, S.; Shiota, K. Characteristic Expression of High Molecular Mass Heat Shock Protein HSP105 during Mouse Embryo Development. Cell Struct. Funct. 1997, 22, 517–525. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuan, J.-X.; Xiao, L.-J.; Lu, C.-L.; Zhang, X.-S.; Liu, T.; Chen, M.; Hu, Z.-Y.; Gao, F.; Liu, Y.-X. Increased expression of heat shock protein 105 in rat uterus of early pregnancy and its significance in embryo implantation. Reprod. Biol. Endocrinol. 2009, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.L. Infectious causes of bovine abortion during mid- to late-gestation. Theriogenology 2007, 68, 474–486. [Google Scholar] [CrossRef]
- Romeo, F.; Louge Uriarte, E.; Delgado, S.G.; González-Altamiranda, E.; Pereyra, S.; Morán, P.; Odeón, A.; Pérez, S.; Verna, A. Effect of bovine viral diarrhea virus on subsequent infectivity of bovine gammaherpesvirus 4 in endometrial cells in primary culture: An in vitro model of viral co-infection. J. Virol. Methods 2021, 291, 114097. [Google Scholar] [CrossRef]
- Ranjan, R.; Biswal, J.K.; Subramaniam, S.; Singh, K.P.; Stenfeldt, C.; Rodriguez, L.L.; Pattnaik, B.; Arzt, J. Foot-and-Mouth Disease Virus-Associated Abortion and Vertical Transmission following Acute Infection in Cattle under Natural Conditions. PLoS ONE 2016, 11, e0167163. [Google Scholar] [CrossRef]
- Esposito, C.; Fiorito, F.; Miletti, G.; Serra, F.; Balestrieri, A.; Cioffi, B.; Cerracchio, C.; Galiero, G.; De Carlo, E.; Amoroso, M.G.; et al. Involvement of herpesviruses in cases of abortion among water buffaloes in southern Italy. Vet. Res. Commun. 2022. [Google Scholar] [CrossRef]
- Lanyon, S.R.; Hill, F.I.; Reichel, M.P.; Brownlie, J. Bovine viral diarrhoea: Pathogenesis and diagnosis. Vet. J. 2014, 199, 201–209. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Yang, G.-H.; Zhang, L.-L.; Wang, J.; Wang, J.-F. Melatonin as Immune Potentiator for Enhancing Subunit Vaccine Efficacy against Bovine Viral Diarrhea Virus. Vaccines 2021, 9, 1039. [Google Scholar] [CrossRef]
- Toker, E.B.; Yeşilbağ, K. In vitro antiviral activity of Thymbra spicata L. extract on bovine respiratory viruses (BCoV, BPIV-3, BRSV, BVDV and BoHV-1). J. Appl. Microbiol. 2021, 132, 2625–2632. [Google Scholar] [CrossRef]
- Tan, B.; Giangaspero, M.; Sun, N.; Jin, Y.; Liu, K.; Wang, Q.; Cheng, S.; Wang, Y.; Zhang, S. Antiviral Effect of Ginsenoside Rb2 and Rb3 Against Bovine Viral Diarrhea Virus and Classical Swine Fever Virus in vitro. Front. Veter. Sci. 2021, 8, 764909. [Google Scholar] [CrossRef]
- Grabowska, K.; Wąchalska, M.; Graul, M.; Rychłowski, M.; Bieńkowska-Szewczyk, K.; Lipińska, A.D. Alphaherpesvirus gB Homologs Are Targeted to Extracellular Vesicles, but They Differentially Affect MHC Class II Molecules. Viruses 2020, 12, 429. [Google Scholar] [CrossRef]
- Florencia, R.; Julieta, M.; Sandra, P.; Enrique, L.U.; Maia, M.; German, C.; Leunda, M.R.; Erika, G.A.; Susana, P.; Maximiliano, S.; et al. Characterization of the first bovine gammaherpesvirus 4 strain isolated from an aborted bovine fetus in Argentina. Arch. Virol. 2020, 165, 719–723. [Google Scholar] [CrossRef]
- González Altamiranda, E.; Manrique, J.M.; Pérez, S.E.; Ríos, G.L.; Odeón, A.C.; Leunda, M.R.; Jones, L.R.; Verna, A. Molecular Characterization of the First Bovine Herpesvirus 4 (BoHV-4) Strains Isolated from In Vitro Bovine Embryos production in Argentina. PLoS ONE 2015, 10, e0132212. [Google Scholar] [CrossRef]
- Franceschi, V.; Capocefalo, A.; Jacca, S.; Rosamilia, A.; Cavirani, S.; Xu, F.; Qiao, W.; Donofrio, G. BoHV-4 immediate early 1 gene is a dispensable gene and its product is not a bone marrow stromal cell antigen 2 counteracting factor. BMC Vet. Res. 2015, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Thomas, C.; Zhang, S.; Wathes, D.C.; Cheng, Z. Comparison of the Ability of High and Low Virulence Strains of Non-cytopathic Bovine Viral Diarrhea Virus-1 to Modulate Expression of Interferon Tau Stimulated Genes in Bovine Endometrium. Front. Vet. Sci. 2021, 8, 659330. [Google Scholar] [CrossRef]
- Chanrot, M.; Blomqvist, G.; Guo, Y.; Ullman, K.; Juremalm, M.; Bage, R.; Donofrio, G.; Valarcher, J.-F.; Humblot, P. Bovine herpes virus type 4 alters TNF-α and IL-8 profiles and impairs the survival of bovine endometrial epithelial cells. Reprod. Biol. 2017, 17, 225–232. [Google Scholar] [CrossRef]
- Donofrio, G.; Herath, S.; Sartori, C.; Cavirani, S.; Flammini, C.F.; Sheldon, I.M. Bovine herpesvirus 4 is tropic for bovine endometrial cells and modulates endocrine function. Reproduction 2007, 134, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Jacca, S.; Franceschi, V.; Agosti, M.; Cavirani, S.; Mistretta, F.; Donofrio, G. Interferon gamma-mediated BoHV-4 replication restriction in bovine endometrial stromal cells is host IDO1 gene expression independent and BoHV-4 IE2 gene expression dependent. Biol. Reprod. 2014, 91, 112. [Google Scholar] [CrossRef]
- Bielanski, A.; Loewen, K.S.; Del Campo, M.R.; Sirard, M.A.; Willadsen, S. Isolation of bovine herpesvirus-1 (BHV-1) and bovine viral diarrhea virus (BVDV) in association with the in vitro production of bovine embryos. Theriogenology 1993, 40, 531–538. [Google Scholar] [CrossRef]
- Brenner, M.P.; Silva-Frade, C.; Ferrarezi, M.C.; Garcia, A.F.; Flores, E.F.; Cardoso, T.C. Evaluation of developmental changes in bovine in vitro produced embryos following exposure to bovine Herpesvirus type 5. Reprod. Biol. Endocrinol. 2012, 10, 53. [Google Scholar] [CrossRef]
- Silva-Frade, C.; Gameiro, R.; Martins, A.; Cardoso, T.C. Apoptotic and developmental effects of bovine Herpesvirus type-5 infection on in vitro-produced bovine embryos. Theriogenology 2010, 74, 1296–1303. [Google Scholar] [CrossRef]
- Ryan, E.; Horsington, J.; Durand, S.; Brooks, H.; Alexandersen, S.; Brownlie, J.; Zhang, Z. Foot-and-mouth disease virus infection in young lambs: Pathogenesis and tissue tropism. Vet. Microbiol. 2008, 127, 258–274. [Google Scholar] [CrossRef]
- Ryan, E.; Horsington, J.; Brownlie, J.; Zhang, Z. Foot-and-mouth disease virus infection in fetal lambs: Tissue tropism and cytokine response. J. Comp. Pathol. 2008, 138, 108–120. [Google Scholar] [CrossRef]
- Mao, R.; Sun, D.; Yang, F.; Tian, H.; Zhu, Z.; Zheng, H.; Liu, X. Establishment and Evaluation of a Stable Bovine Thyroid Cell Line for Investigating Foot-and-Mouth Disease Virus. Front. Microbiol. 2018, 9, 2149. [Google Scholar] [CrossRef] [PubMed]
- Brehm, K.E.; Ferris, N.P.; Lenk, M.; Riebe, R.; Haas, B. Highly sensitive fetal goat tongue cell line for detection and isolation of foot-and-mouth disease virus. J. Clin. Microbiol. 2009, 47, 3156–3160. [Google Scholar] [CrossRef][Green Version]
- Snowdon, W.A. Growth of foot-and mouth disease virus in monolayer cultures of calf thyroid cells. Nature 1966, 210, 1079–1080. [Google Scholar] [CrossRef]
- Li, K.; Wang, C.; Yang, F.; Cao, W.; Zhu, Z.; Zheng, H. Virus-Host Interactions in Foot-and-Mouth Disease Virus Infection. Front. Immunol. 2021, 12, 571509. [Google Scholar] [CrossRef]
- Bai, X.-W.; Bao, H.-F.; Li, P.-H.; Ma, X.-Q.; Sun, P.; Bai, Q.-F.; Zhang, M.; Yuan, H.; Chen, D.-D.; Li, K.; et al. Engineering Responses to Amino Acid Substitutions in the VP0- and VP3-Coding Regions of PanAsia-1 Strains of Foot-and-Mouth Disease Virus Serotype O. J. Virol. 2019, 93, e02278-18. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, J.; Li, S.; Dang, W.; Xin, S.; Long, S.; Zhang, W.; Cao, P.; Lu, J. Extracellular Vesicles Regulated by Viruses and Antiviral Strategies. Front. Cell Dev. Biol. 2021, 9, 722020. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Yin, Y.; Chen, Y.; Mao, L. The Role of Viral Proteins in the Regulation of Exosomes Biogenesis. Front. Cell. Infect. Microbiol. 2021, 11, 671625. [Google Scholar] [CrossRef]
- Heilingloh, C.S.; Kummer, M.; Mühl-Zürbes, P.; Drassner, C.; Daniel, C.; Klewer, M.; Steinkasserer, A. L Particles Transmit Viral Proteins from Herpes Simplex Virus 1-Infected Mature Dendritic Cells to Uninfected Bystander Cells, Inducing CD83 Downmodulation. J. Virol. 2015, 89, 11046–11055. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Koike, M.; Moriishi, E.; Kawabata, A.; Tang, H.; Oyaizu, H.; Uchiyama, Y.; Yamanishi, K. Human herpesvirus-6 induces MVB formation, and virus egress occurs by an exosomal release pathway. Traffic 2008, 9, 1728–1742. [Google Scholar] [CrossRef] [PubMed]
- Crump, C.M.; Yates, C.; Minson, T. Herpes simplex virus type 1 cytoplasmic envelopment requires functional Vps4. J. Virol. 2007, 81, 7380–7387. [Google Scholar] [CrossRef]
- Kharkwal, H.; Smith, C.G.; Wilson, D.W. Blocking ESCRT-mediated envelopment inhibits microtubule-dependent trafficking of alphaherpesviruses in vitro. J. Virol. 2014, 88, 14467–14478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Xu, S.; Shi, X.; Xu, G.; Shen, C.; Liu, X.; Zheng, H. Exosomes-mediated transmission of foot-and-mouth disease virus in vivo and in vitro. Vet. Microbiol. 2019, 233, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Xu, S.; Shi, X.; Shen, C.; Zhang, D.; Zhang, T.; Hou, J.; Zhang, K.; Zheng, H.; Liu, X. Foot-and-mouth disease virus degrades Rab27a to suppress the exosome-mediated antiviral immune response. Vet. Microbiol. 2020, 251, 108889. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calle, A.; Ramírez, M.Á. Mesenchymal Stem Cells in Embryo-Maternal Communication under Healthy Conditions or Viral Infections: Lessons from a Bovine Model. Cells 2022, 11, 1858. https://doi.org/10.3390/cells11121858
Calle A, Ramírez MÁ. Mesenchymal Stem Cells in Embryo-Maternal Communication under Healthy Conditions or Viral Infections: Lessons from a Bovine Model. Cells. 2022; 11(12):1858. https://doi.org/10.3390/cells11121858
Chicago/Turabian StyleCalle, Alexandra, and Miguel Ángel Ramírez. 2022. "Mesenchymal Stem Cells in Embryo-Maternal Communication under Healthy Conditions or Viral Infections: Lessons from a Bovine Model" Cells 11, no. 12: 1858. https://doi.org/10.3390/cells11121858
APA StyleCalle, A., & Ramírez, M. Á. (2022). Mesenchymal Stem Cells in Embryo-Maternal Communication under Healthy Conditions or Viral Infections: Lessons from a Bovine Model. Cells, 11(12), 1858. https://doi.org/10.3390/cells11121858





