Increasing the Duration of Light Physical Activity Ameliorates Insulin Resistance Syndrome in Metabolically Healthy Obese Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Participants, and Anthropometric Measurements
2.2. PA Measurements
2.3. Measurement of Blood Metabolic Markers
2.4. Flow Cytometry for Immune Cell Markers
2.5. Enzyme-Linked Immunosorbent Assay (ELISA) for Measuring Soluble Proteins and Inflammatory Cytokines/Chemokines
2.6. Statistical Analysis
3. Results
3.1. Demographics and Clinical Characteristics of the Study Population
3.2. Monocyte Subset Markers and Plasma Cytokines
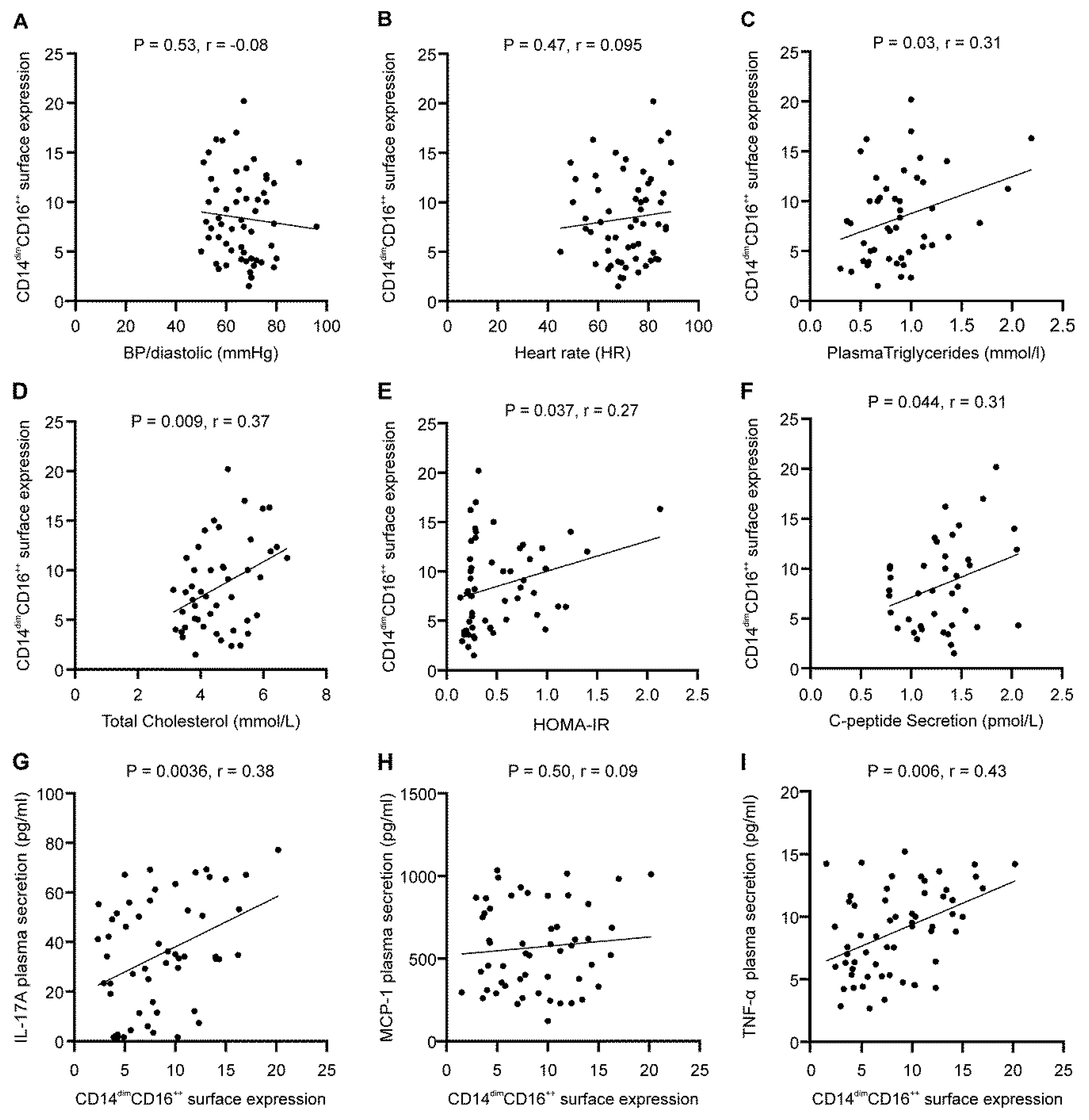
3.3. Association between CD14dimCD16++ Monocyte Markers Expression and Various IRS Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Meldrum, D.R.; Morris, M.A.; Gambone, J.C. Obesity pandemic: Causes, consequences, and solutions-but do we have the will? Fertil. Steril. 2017, 107, 833–839. [Google Scholar] [CrossRef]
- Faeh, D.; Braun, J.; Tarnutzer, S.; Bopp, M. Obesity but not overweight is associated with increased mortality risk. Eur. J. Epidemiol. 2011, 26, 647–655. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Magno, C.P.; Lane, K.T.; Hinojosa, M.W.; Lane, J.S. Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: Findings from the national health and nutrition examination survey, 1999 to 2004. J. Am. Coll. Surg. 2008, 207, 928–934. [Google Scholar] [CrossRef]
- Weiderpass, E.; Botteri, E.; Longenecker, J.C.; Alkandari, A.; Al-Wotayan, R.; Al Duwairi, Q.; Tuomilehto, J. The prevalence of overweight and obesity in an adult kuwaiti population in 2014. Front. Endocrinol. (Lausanne) 2019, 10, 449. [Google Scholar] [CrossRef] [PubMed]
- Mokdad, A.H.; Forouzanfar, M.H.; Daoud, F.; El Bcheraoui, C.; Moradi-Lakeh, M.; Khalil, I.; Afshin, A.; Tuffaha, M.; Charara, R.; Barber, R.M.; et al. Health in times of uncertainty in the eastern mediterranean region, 1990–2013: A systematic analysis for the global burden of disease study 2013. Lancet Glob. Health 2016, 4, e704–e713. [Google Scholar] [CrossRef]
- Badr, H.; Maktabi, M.A.; Al-Kandari, M.; Sibai, A.M. Review of non-communicable disease research activity in kuwait: Where is the evidence for the best practice? Ann. Glob. Health 2019, 85, 45. [Google Scholar] [CrossRef] [PubMed]
- Shuster, A.; Patlas, M.; Pinthus, J.H.; Mourtzakis, M. The clinical importance of visceral adiposity: A critical review of methods for visceral adipose tissue analysis. Br. J. Radiol. 2012, 85, 1–10. [Google Scholar] [CrossRef]
- Stolarczyk, E. Adipose tissue inflammation in obesity: A metabolic or immune response? Curr. Opin. Pharmacol. 2017, 37, 35–40. [Google Scholar] [CrossRef]
- Maury, E.; Brichard, S.M. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol. Cell. Endocrinol. 2010, 314, 1–16. [Google Scholar] [CrossRef]
- Boutens, L.; Stienstra, R. Adipose tissue macrophages: Going off track during obesity. Diabetologia 2016, 59, 879–894. [Google Scholar] [CrossRef]
- Thapa, B.; Lee, K. Metabolic influence on macrophage polarization and pathogenesis. BMB Rep. 2019, 52, 360–372. [Google Scholar] [CrossRef]
- Schmidt, F.M.; Weschenfelder, J.; Sander, C.; Minkwitz, J.; Thormann, J.; Chittka, T.; Mergl, R.; Kirkby, K.C.; Fasshauer, M.; Stumvoll, M.; et al. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS ONE 2015, 10, e0121971. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.; Park, K.S. Responses of inflammatory cytokines following moderate intensity walking exercise in overweight or obese individuals. J. Exerc. Rehabil. 2017, 13, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.G.; Oktedalen, O.; Opstad, P.K.; Lyberg, T. Plasma cytokine profiles in long-term strenuous exercise. J. Sports Med. (Hindawi Publ Corp) 2016, 2016, 7186137. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Nakaji, S.; Yamada, M.; Totsuka, M.; Sato, K.; Sugawara, K. Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc. Immunol. Rev. 2002, 8, 6–48. [Google Scholar]
- Suzuki, K.; Nakaji, S.; Kurakake, S.; Totsuka, M.; Sato, K.; Kuriyama, T.; Fujimoto, H.; Shibusawa, K.; Machida, K.; Sugawara, K. Exhaustive exercise and type-1/type-2 cytokine balance with special focus on interleukin-12 p40/p70. Exerc. Immunol. Rev. 2003, 9, 48–57. [Google Scholar]
- Jakicic, J.M.; Otto, A.D. Physical activity considerations for the treatment and prevention of obesity. Am. J. Clin. Nutr. 2005, 82, 226S–229S. [Google Scholar] [CrossRef]
- Freedson, P.S.; Melanson, E.; Sirard, J. Calibration of the computer science and applications, inc. Accelerometer. Med. Sci. Sports Exerc. 1998, 30, 777–781. [Google Scholar] [CrossRef]
- Barter, P.; McPherson, Y.R.; Song, K.; Kesäniemi, Y.A.; Mahley, R.; Waeber, G.; Bersot, T.; Mooser, V.; Waterworth, D.; Grundy, S.M. Serum insulin and inflammatory markers in overweight individuals with and without dyslipidemia. J. Clin. Endocrinol. Metab. 2007, 92, 2041–2045. [Google Scholar] [CrossRef][Green Version]
- Eshtiaghi, R.; Keihani, S.; Hosseinpanah, F.; Barzin, M.; Azizi, F. Natural course of metabolically healthy abdominal obese adults after 10 years of follow-up: The tehran lipid and glucose study. Int. J. Obes. (Lond) 2015, 39, 514–519. [Google Scholar] [CrossRef]
- De Vries, S.I.; Van Hirtum, H.W.; Bakker, I.; Hopman-Rock, M.; Hirasing, R.A.; Van Mechelen, W. Validity and reproducibility of motion sensors in youth: A systematic update. Med. Sci. Sports Exerc. 2009, 41, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Corder, K.; Ekelund, U.; Steele, R.M.; Wareham, N.J.; Brage, S. Assessment of physical activity in youth. J. Appl. Physiol. (1985) 2008, 105, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Ayabe, M.; Kumahara, H.; Morimura, K.; Sakane, N.; Ishii, K.; Tanaka, H. Accumulation of short bouts of non-exercise daily physical activity is associated with lower visceral fat in japanese female adults. Int. J. Sports Med. 2013, 34, 62–67. [Google Scholar] [CrossRef] [PubMed]
- DeBusk, R.F.; Stenestrand, U.; Sheehan, M.; Haskell, W.L. Training effects of long versus short bouts of exercise in healthy subjects. Am. J. Cardiol. 1990, 65, 1010–1013. [Google Scholar] [CrossRef]
- Woolf-May, K.; Kearney, E.M.; Owen, A.; Jones, D.W.; Davison, R.C.; Bird, S.R. The efficacy of accumulated short bouts versus single daily bouts of brisk walking in improving aerobic fitness and blood lipid profiles. Health Educ. Res. 1999, 14, 803–815. [Google Scholar] [CrossRef]
- Janssen, I.; Roberts, K.C.; Thompson, W. Is adherence to the canadian 24-hour movement behaviour guidelines for children and youth associated with improved indicators of physical, mental, and social health? Appl. Physiol. Nutr. Metab. 2017, 42, 725–731. [Google Scholar] [CrossRef]
- Tremblay, M.S.; Carson, V.; Chaput, J.P.; Connor Gorber, S.; Dinh, T.; Duggan, M.; Faulkner, G.; Gray, C.E.; Gruber, R.; Janson, K.; et al. Canadian 24-hour movement guidelines for children and youth: An integration of physical activity, sedentary behaviour, and sleep. Appl. Physiol. Nutr. Metab. 2016, 41, S311–S327. [Google Scholar] [CrossRef]
- Makki, K.; Froguel, P.; Wolowczuk, I. Adipose tissue in obesity-related inflammation and insulin resistance: Cells, cytokines, and chemokines. ISRN Inflamm. 2013, 2013, 139239. [Google Scholar] [CrossRef]
- Devevre, E.F.; Renovato-Martins, M.; Clement, K.; Sautes-Fridman, C.; Cremer, I.; Poitou, C. Profiling of the three circulating monocyte subpopulations in human obesity. J. Immunol. 2015, 194, 3917–3923. [Google Scholar] [CrossRef]
- Laredo-Aguilera, J.A.; Cobo-Cuenca, A.I.; Santacruz-Salas, E.; Martins, M.M.; Rodríguez-Borrego, M.A.; López-Soto, P.J.; Carmona-Torres, J.M. Levels of physical activity, obesity and related factors in young adults aged 18–30 during 2009–2017. Int. J. Environ. Res. Public Health 2019, 16, 4033. [Google Scholar] [CrossRef]
- Miller, G.D.; Jakicic, J.M.; Rejeski, W.J.; Whit-Glover, M.C.; Lang, W.; Walkup, M.P.; Hodges, M.L. Effect of varying accelerometry criteria on physical activity: The look ahead study. Obesity (Silver Spring) 2013, 21, 32–44. [Google Scholar] [CrossRef]
- Gando, Y.; Yamamoto, K.; Murakami, H.; Ohmori, Y.; Kawakami, R.; Sanada, K.; Higuchi, M.; Tabata, I.; Miyachi, M. Longer time spent in light physical activity is associated with reduced arterial stiffness in older adults. Hypertension 2010, 56, 540–546. [Google Scholar] [CrossRef]
- Sattelmair, J.; Pertman, J.; Ding, E.L.; Kohl, H.W.; Haskell, W.; Lee, I.M. Dose response between physical activity and risk of coronary heart disease: A meta-analysis. Circulation 2011, 124, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Fishman, E.I.; Steeves, J.A.; Zipunnikov, V.; Koster, A.; Berrigan, D.; Harris, T.A.; Murphy, R. Association between objectively measured physical activity and mortality in nhanes. Med. Sci. Sports Exerc. 2016, 48, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Matthews, C.E.; Keadle, S.K.; Troiano, R.P.; Kahle, L.; Koster, A.; Brychta, R.; Van Domelen, D.; Caserotti, P.; Chen, K.Y.; Harris, T.B.; et al. Accelerometer-measured dose-response for physical activity, sedentary time, and mortality in us adults. Am. J. Clin. Nutr. 2016, 104, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Farrell, S.W.; Kampert, J.B.; Kohl, H.W.; Barlow, C.E.; Macera, C.A.; Paffenbarger, R.S.; Gibbons, L.W.; Blair, S.N. Influences of cardiorespiratory fitness levels and other predictors on cardiovascular disease mortality in men. Med. Sci. Sports Exerc. 1998, 30, 899–905. [Google Scholar]
- Mentz, R.J.; Kelly, J.P.; von Lueder, T.G.; Voors, A.A.; Lam, C.S.; Cowie, M.R.; Kjeldsen, K.; Jankowska, E.A.; Atar, D.; Butler, J.; et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J. Am. Coll. Cardiol. 2014, 64, 2281–2293. [Google Scholar] [CrossRef]
- Hanley, A.J.; Williams, K.; Stern, M.P.; Haffner, S.M. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: The san antonio heart study. Diabetes Care 2002, 25, 1177–1184. [Google Scholar] [CrossRef]
- Patel, N.; Taveira, T.H.; Choudhary, G.; Whitlatch, H.; Wu, W.C. Fasting serum c-peptide levels predict cardiovascular and overall death in nondiabetic adults. J. Am. Heart Assoc. 2012, 1, e003152. [Google Scholar] [CrossRef]
- Herzig, K.H.; Ahola, R.; Leppäluoto, J.; Jokelainen, J.; Jämsä, T.; Keinänen-Kiukaanniemi, S. Light physical activity determined by a motion sensor decreases insulin resistance, improves lipid homeostasis and reduces visceral fat in high-risk subjects: Prediabex study rct. Int. J. Obes. (Lond.) 2014, 38, 1089–1096. [Google Scholar] [CrossRef]
- Cockcroft, E.J.; Moudiotis, C.; Kitchen, J.; Bond, B.; Williams, C.A.; Barker, A.R. High-intensity interval exercise and glycemic control in adolescents with type one diabetes mellitus: A case study. Physiol. Rep. 2017, 5, e13339. [Google Scholar] [CrossRef] [PubMed]
- Mohebbi, H.; Campbell, I.T.; Keegan, M.A.; Malone, J.J.; Hulton, A.T.; MacLaren, D.P.M. Hyperinsulinaemia and hyperglycaemia promote glucose utilization and storage during low- and high-intensity exercise. Eur. J. Appl. Physiol. 2019, 120, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Devlin, J.T.; Horton, E.S. Effects of prior high-intensity exercise on glucose metabolism in normal and insulin-resistant men. Diabetes 1985, 34, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, E.J.; Williams, C.A.; Jackman, S.R.; Bassi, S.; Armstrong, N.; Barker, A.R. A single bout of high-intensity interval exercise and work-matched moderate-intensity exercise has minimal effect on glucose tolerance and insulin sensitivity in 7- to 10-year-old boys. J. Sports Sci. 2018, 36, 149–155. [Google Scholar] [CrossRef]
- Broderick, T.L.; Sennott, J.M.; Gutkowska, J.; Jankowski, M. Anti-inflammatory and angiogenic effects of exercise training in cardiac muscle of diabetic mice. Diabetes Metab. Syndr. Obes. 2019, 12, 565–573. [Google Scholar] [CrossRef]
- Skou, S.T.; Pedersen, B.K.; Abbott, J.H.; Patterson, B.; Barton, C. Physical activity and exercise therapy benefit more than just symptoms and impairments in people with hip and knee osteoarthritis. J. Orthop. Sports Phys. Ther. 2018, 48, 439–447. [Google Scholar] [CrossRef]
- Pedersen, B.K. Anti-inflammatory effects of exercise: Role in diabetes and cardiovascular disease. Eur. J. Clin. Investig. 2017, 47, 600–611. [Google Scholar] [CrossRef]
- Ziegler-Heitbrock, L. The cd14+ cd16+ blood monocytes: Their role in infection and inflammation. J. Leukoc. Biol. 2007, 81, 584–592. [Google Scholar] [CrossRef]
- Cottam, D.R.; Schaefer, P.A.; Shaftan, G.W.; Velcu, L.; Angus, L.D. Effect of surgically-induced weight loss on leukocyte indicators of chronic inflammation in morbid obesity. Obes. Surg. 2002, 12, 335–342. [Google Scholar] [CrossRef]
- Rogacev, K.S.; Ulrich, C.; Blömer, L.; Hornof, F.; Oster, K.; Ziegelin, M.; Cremers, B.; Grenner, Y.; Geisel, J.; Schlitt, A.; et al. Monocyte heterogeneity in obesity and subclinical atherosclerosis. Eur. Heart J. 2010, 31, 369–376. [Google Scholar] [CrossRef]
- Ip, B.; Cilfone, N.A.; Belkina, A.C.; DeFuria, J.; Jagannathan-Bogdan, M.; Zhu, M.; Kuchibhatla, R.; McDonnell, M.E.; Xiao, Q.; Kepler, T.B.; et al. Th17 cytokines differentiate obesity from obesity-associated type 2 diabetes and promote tnfα production. Obesity (Silver Spring) 2016, 24, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Sun, Q. Macrophage recruitment in obese adipose tissue. Obes. Rev. 2015, 16, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, K.L.; Flynn, M.G.; Coen, P.M.; Markofski, M.M.; Pence, B.D. Exercise training-induced lowering of inflammatory (cd14+cd16+) monocytes: A role in the anti-inflammatory influence of exercise? J. Leukoc. Biol. 2008, 84, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- de Matos, M.A.; Garcia, B.C.C.; Vieira, D.V.; de Oliveira, M.F.A.; Costa, K.B.; Aguiar, P.F.; Magalhães, F.C.; Brito-Melo, G.A.; Amorim, F.T.; Rocha-Vieira, E. High-intensity interval training reduces monocyte activation in obese adults. Brain Behav. Immun. 2019, 80, 818–824. [Google Scholar] [CrossRef]
- de Matos, M.A.; Duarte, T.C.; Ottone Vde, O.; Sampaio, P.F.; Costa, K.B.; de Oliveira, M.F.; Moseley, P.L.; Schneider, S.M.; Coimbra, C.C.; Brito-Melo, G.E.; et al. The effect of insulin resistance and exercise on the percentage of cd16(+) monocyte subset in obese individuals. Cell Biochem. Funct. 2016, 34, 209–216. [Google Scholar] [CrossRef]
| Standardized Regression Coefficients (95% CI) | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Overall Activity (%) | Light Intensity (%) | Moderate Intensity (%) | High Intensity (%) | |||||
| Age (years) | 33.2 ± 3.4 | 0.06 (−0.18 to 0.31) | 0.59 | 0.11 (−0.14 to 0.36) | 0.37 | −0.09 (−0.34 to 0.16) | 0.45 | 0.02 (−0.23 to 0.27) | 0.84 |
| Weight (kg) | 93.2 ± 11.8 | −0.02 (−0.28 to 0.22) | 0.83 | −0.07 (−0.32 to 0.18) | 0.58 | −0.01 (−0.27 to 0.24) | 0.90 | −0.04 (−0.29 to 0.21) | 0.76 |
| Height (cm) | 166.4 ± 10.3 | 0.21 (−0.04 to 0.44) | 0.10 | 0.08 (−0.18 to 0.32 | 0.54 | 0.28 (0.03 to 0.50) | 0.026 * | 0.15 (−0.10 to 0.39 | 0.23 |
| BMI (kg/m2) | 33.3 ± 2.6 | −0.14 (−0.38 to 0.11) | 0.26 | −0.12 (−0.36 to 0.13) | 0.36 | −0.17 (−0.41 to 0.08) | 0.18 | −0.13 (−0.37 to 0.12) | 0.31 |
| Waist circumference (inch) | 41.5 ± 4.9 | −0.10 (−0.34 to 0.15) | 0.44 | −0.09 (−0.34 to 0.16) | 0.47 | −0.13 (−0.38 to 0.12) | 0.30 | −0.11 (−0.35 to 0.14) | 0.40 |
| Hip circumference (inch) | 46.6 ± 3.4 | −0.08 (−0.32 to 0.17) | 0.53 | −0.00 (−0.26 to 0.24) | 0.96 | −0.17 (−0.41 to 0.08) | 0.18 | −0.20 (−0.43 to 0.05) | 0.12 |
| Fat weight (kg) | 38.6 ± 6.6 | −0.32 (−0.53 to −0.08) | 0.01 ** | −0.20 (−0.44 to 0.05) | 0.113 | −0.26 (−0.48 to −0.01) | 0.04 * | −0.16 (−0.40 to 0.09) | 0.20 |
| Lean weight (kg) | 54.5 ± 9.7 | 0.18 (−0.07 to 0.41) | 0.16 | 0.10 (−0.15 to 0.35) | 0.43 | 0.23 (−0.01 to 0.46) | 0.06 | 0.10 (−0.15 to 0.35) | 0.41 |
| Fat %age | 36.84± 5.0 | −0.29 (−0.51 to −0.04) | 0.02 * | −0.19 (−0.42 to 0.06) | 0.14 | −0.32 (−0.53 to −0.07) | 0.01 * | −0.20 (−0.43 to 0.05) | 0.11 |
| Standardized Regression Coefficients (95% CI) | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Overall Activity (%) | Light Intensity (%) | Moderate Intensity (%) | High Intensity (%) | |||||
| BP/systolic (mmHg) | 118.4 ± 9.3 | −0.17 (−0.41 to 0.09) | 0.2 | −0.10 (−0.35 to 0.15) | 0.42 | −0.21 (−0.44 to 0.04) | 0.10 | −0.05 (−0.30 to 0.20) | 0.70 |
| BP/diastolic (mmHg) | 73.6 ± 9.3 | −0.42 (−0.61 to −0.19) | 0.001 *** | −0.32 (−0.53 to −0.07) | 0.01 ** | −0.40 (−0.59 to −0.15) | 0.002 ** | −0.17 (−0.41 to 0.08) | 0.19 |
| HR | 76.7 ± 6.7 | −0.37 (−0.59 to −0.15) | 0.002 ** | −0.31 (−0.53 to −0.06) | 0.01 ** | −0.31 (−0.52 to −0.05) | 0.017 * | −0.19 (−0.42 to 0.07) | 0.15 |
| Triglycerides (mmol/L) | 0.97 ± 0.1 | −0.35 (−0.60 to −0.03) | 0.03 * | −0.37 (−0.62 to −0.05) | 0.02 * | −0.13 (−0.43 to 0.20) | 0.44 | −0.05 (−0.37 to 0.27) | 0.74 |
| Total cholesterol (mmol/L) | 5.2 ± 0.7 | −0.38 (−0.63 to −0.07) | 0.01 ** | −0.30 (−0.57 to 0.023) | 0.06 | −0.31 (−0.57 to 0.01) | 0.059 | −0.25 (−0.53 to 0.07) | 0.12 |
| HDL cholesterol (mmol/L) | 1.2 ± 0.2 | −0.12 (−0.43 to 0.21) | 0.47 | -0.01 (−0.34 to 0.31) | 0.92 | −0.24 (−0.53 to 0.08) | 0.14 | −0.16 (−0.47 to 0.16) | 0.32 |
| Fasting glucose (mmol/L) | 5.2 ± 0.6 | −0.22 (−0.45 to 0.04) | 0.10 | −0.23 (−0.46 to 0.03) | 0.08 | − 0.11 (−0.36 to 0.1604) | 0.42 | 0.03 (−0.23 to 0.29) | 0.78 |
| Insulin Con. (mu/) | 2 ± 1.3 | 0.08 (−0.18 to 0.33) | 0.54 | −0.05 (−0.31 to 0.21) | 0.69 | 0.24 (−0.02 to 0.47) | 0.07 | 0.30 (0.04 to 0.52) | 0.02 ** |
| HOMA−IR | 0.4 ± 0.3 | −0.33 (−0.55 to −0.08) | 0.01 ** | −0.37 (−0.57 to −0.12) | 0.005 ** | 0.00 (−0.26 to 0.26) | 0.98 | 0.01 (−0.24 to 0.28) | 0.89 |
| C−Peptide (pg/mL) | 1.5 ± 0.4 | −0.34 (−0.59 to −0.03) | 0.02 * | −0.31 (−0.57 to −0.00) | 0.04 * | − 0.10 (−0.39 to 0.21) | 0.53 | − 0.30 (−0.56 to 0.00) | 0.053 |
| Standardized Regression Coefficients (95% CI) | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Overall Activity (%) | Light Intensity (%) | Moderate Intensity (%) | High Intensity (%) | |||||
| Classical (%) (CD14+CD16-) | 64.1 ± 17.6 | −0.14 (−0.51 to 0.27) | 0.50 | −0.12 (−0.50 to 0.29) | 0.56 | −0.11 (−0.49 to 0.30) | 0.59 | −0.00 (−0.40 to 0.39) | 0.97 |
| Intermediate (%) (CD14+CD16+) | 7.8 ± 2.4 | 0.02 (−0.38 to 0.41) | 0.92 | 0.08 (−0.32 to 0.47) | 0.69 | −0.06 (−0.45 to 0.34) | 0.75 | −0.15 (−0.52 to 0.26) | 0.46 |
| Non-classical (%) (CD14dimCD16++) | 13.9 ± 16.9 | −0.53 (−0.77 to −0.16) | 0.007 ** | −0.48 (−0.74 to −0.10) | 0.01 ** | −0.28 (−0.62 to 0.12) | 0.17 | −0.34 (−0.65 to 0.07) | 0.10 |
| CD14+CD163+CD206+ (%) (M2 subset expression) | 8.1 ± 9.4 | 0.85 (0.60 to 0.95) | <0.0001 **** | 0.69 (0.25 to 0.89) | 0.006 ** | 0.67 (0.22 to 0.88) | 0.008 ** | 0.62 (0.03 to 0.83) | 0.04 * |
| CD14+CD11c+HLA-DR+ (%) (M1 subset expression) | 93.8 ± 3.8 | 0.19 (−0.28 to 0.59) | 0.41 | 0.33 (−0.14 to 0.68) | 0.16 | −0.16 (−0.57 to 0.31) | 0.51 | −0.00 (−0.45 to 0.45) | 0.99 |
| Standardized Regression Coefficients (95% CI) | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Overall Activity (%) | Light Intensity (%) | Moderate Intensity (%) | High Intensity (%) | |||||
| TNF-α (pg/mL) | 64.1 ± 17.6 | −0.53 (−0.74 to −0.21) | 0.002 ** | −0.44 (−0.69 to −0.09) | 0.01 ** | −0.37 (−0.65 to −0.02) | 0.039 * | −0.40 (−0.66 to −0.04) | 0.03 * |
| Il−1β (pg/mL) | 7.8 ± 2.4 | 0.04 (−0.32 to 0.39) | 0.82 | −0.02 (−0.38 to 0.33) | 0.88 | 0.21 (−0.15 to 0.53) | 0.25 | 0.08 (−0.28 to 0.42) | 0.66 |
| IL−6 (pg/mL) | 13.9 ± 16.9 | −0.25 (−0.55 to 0.10) | 0.16 | −0.31 (−0.60 to 0.04) | 0.09 | −0.01 (−0.36 to 0.34) | 0.95 | 0.14 (−0.21 to 0.47) | 0.43 |
| IL−17A (pg/mL) | 48.8 ± 18.8 | −0.4 (−0.66 to −0.045) | 0.03 * | −0.38 (−0.65 to −0.02) | 0.04 * | −0.21 (−0.53 to 0.15) | 0.24 | −0.14 (−0.48 to 0.22) | 0.43 |
| MCP−1 (pg/mL) | 456.1 ± 293.1 | −0.42 (−0.67 to −0.07) | 0.02 * | −0.48 (−0.71 to −0.14) | 0.007 ** | −0.01 (−0.36 to 0.35) | 0.95 | −0.08 (−0.43 to 0.28) | 0.65 |
| VEGF (pg/mL) | 652.4 ± 330.9 | 0.02 (−0.33 to 0.38) | 0.90 | 0.00 (−0.35 to 0.36) | 0.99 | 0.13 ( −0.24 to 0.46) | 0.48 | −0.07 (−0.42 to 0.28) | 0.68 |
| Metabolic Parameters | CD14dimCD16++ | CD14+CD206+CD163+ | ||||
|---|---|---|---|---|---|---|
| Standardized Coefficient β | 95% Confidence Interval | p-Value | Standardized Coefficient β | 95% Confidence Interval | p-Value | |
| BMI (kg/m2) | 0.19 | −0.27 to 0.52 | 0.51 | 4.02 | −67.74 to 34.37 | 0.15 |
| BP/diastolic (mmHg) | 0.20 | −0.43 to 0.41 | 0.96 | 6.30 | −58.51 to 101.6 | 0.18 |
| Resting Heart Rate (bpm) | 0.11 | 0.03 to 0.50 | 0.03 * | 7.59 | −91.12 to 101.7 | 0.61 |
| Triglycerides (mmol/l) | 3.07 | −6.41 to 6.40 | 0.99 | 479.50 | −6525 to 5661 | 0.53 |
| Total cholesterol (mmol/l) | 1.16 | −0.90 to 3.94 | 0.21 | 144.70 | −1706 to 1971 | 0.53 |
| HOMA-IR | 4.02 | 0.64 to 17.42 | 0.04 * | 157.40 | −2001 to 1998 | 0.99 |
| C-Peptide (pg/mL) | 3.45 | 2.14 to 16.54 | 0.01 * | 290.30 | −3584 to 3793 | 0.78 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Rashed, F.; Alghaith, A.; Azim, R.; AlMekhled, D.; Thomas, R.; Sindhu, S.; Ahmad, R. Increasing the Duration of Light Physical Activity Ameliorates Insulin Resistance Syndrome in Metabolically Healthy Obese Adults. Cells 2020, 9, 1189. https://doi.org/10.3390/cells9051189
Al-Rashed F, Alghaith A, Azim R, AlMekhled D, Thomas R, Sindhu S, Ahmad R. Increasing the Duration of Light Physical Activity Ameliorates Insulin Resistance Syndrome in Metabolically Healthy Obese Adults. Cells. 2020; 9(5):1189. https://doi.org/10.3390/cells9051189
Chicago/Turabian StyleAl-Rashed, Fatema, Abdulwahab Alghaith, Rafaat Azim, Dawood AlMekhled, Reeby Thomas, Sardar Sindhu, and Rasheed Ahmad. 2020. "Increasing the Duration of Light Physical Activity Ameliorates Insulin Resistance Syndrome in Metabolically Healthy Obese Adults" Cells 9, no. 5: 1189. https://doi.org/10.3390/cells9051189
APA StyleAl-Rashed, F., Alghaith, A., Azim, R., AlMekhled, D., Thomas, R., Sindhu, S., & Ahmad, R. (2020). Increasing the Duration of Light Physical Activity Ameliorates Insulin Resistance Syndrome in Metabolically Healthy Obese Adults. Cells, 9(5), 1189. https://doi.org/10.3390/cells9051189








