Abstract
Regenerative medicine aims to repair damaged, tissues or organs for the treatment of various diseases, which have been poorly managed with conventional drugs and medical procedures. To date, multimodal regenerative methods include transplant of healthy organs, tissues, or cells, body stimulation to activate a self-healing response in damaged tissues, as well as the combined use of cells and bio-degradable scaffold to obtain functional tissues. Certainly, stem cells are promising tools in regenerative medicine due to their ability to induce de novo tissue formation and/or promote organ repair and regeneration. Currently, several studies have shown that the beneficial stem cell effects, especially for mesenchymal stem cells (MSCs) and induced pluripotent stem cells (iPSCs) in damaged tissue restore are not dependent on their engraftment and differentiation on the injury site, but rather to their paracrine activity. It is now well known that paracrine action of stem cells is due to their ability to release extracellular vesicles (EVs). EVs play a fundamental role in cell-to-cell communication and are directly involved in tissue regeneration. In the present review, we tried to summarize the molecular mechanisms through which MSCs and iPSCs-derived EVs carry out their therapeutic action and their possible application for the treatment of several diseases.
1. Introduction
Differently from lower vertebrates, such as zebrafish and amphibians, humans have a limited ability to regenerate damaged tissues or organs, restoring their original state. To date, the clinical strategies to recover organ or tissue function fall into three main categories: drug therapy, auto or heterotransplants, and cell therapy and tissue engineering. More than 15 years ago, the term “regenerative medicine” entered into our scientific lexicon. It is a new interdisciplinary branch of medicine that develops methods to regrow, repair or replace cells, organs or tissues damaged by age, disease, or trauma, as well as to normalize congenital defects.
Precursors of regenerative medicine can be considered cell or organ transplants. More than half a century ago the first successful organ transplantation was performed in Boston [1], and it has been the cornerstone therapy for replacing diseased or malfunctioning ones. Besides, in the same period, the first bone marrow transplant was performed [2]. The principal downsides with organ transplantation includes the lack of donors, the immunological compatibility and the immune suppression to avoid organ rejection.
A great stimulus to regenerative medicine derives from the discovery of stem cells more than four decades ago, thanks to their ability to self-renew and differentiate into a variety of cell lineages. In fact, the two main components of regenerative medicine are stem cell-based therapy, either endogenous or injected, and tissue engineering regenerative medicine, based on the use of biomaterials alone or seeded with stem cells. Regenerative medicine minimalizes the problem of transplanted organ rejection. In several human diseases, stem cells have been successfully applied, especially in the hematological field [3,4,5], even if stem cell therapy has not yet reached the level of solid organ regeneration.
2. Stem Cells
Stem cells are undifferentiated cells characterized by their self-renewal capability, the ability to divide generating cells equal to themselves, and by their competence to give rise to specialized cells. According to their differentiation potential they could be classified into: totipotent stem cells, which are able to differentiate in all the body cell types plus the extra-embryonic cells [6]; pluripotent cells that are able to give rise to all of cell types of the body [7,8]; multipotent stem cells, which can develop several cell types within one particular lineage [9], and finally, unipotent cells responsible for the differentiation of only one cell type [10,11]. Moreover, depending on their origin, stem cells are classified into embryonic (ESCs), fetal (e.g., umbilical stem cells, amniotic stem cells), adult, and induced pluripotent stem cells (iPSCs) [12,13]. The latter are pluripotent stem cells derived from adult somatic cells, genetically reprogrammed to an embryonic stem cell-like state [14], with self-renewal and differentiation capability, but free from the ethical issues that afflicted ESC use. The first successful reprogramming of human somatic cells was reported in 2007 by Takahashi and co-workers [15]. Initially, the reprogramming of somatic cells was obtained retrovirally introducing four key transcription factors that are responsible for pluripotency maintaining (i.e., Oct3/4, Sox2, Klf4, and c-Myc). Nowadays, different and less dangerous methods to introduce the “Yamanaka factors” within somatic cells have been successful applied [16,17,18,19].
3. Stem Cells and Regenerative Medicine
Over the past 20 years, much attention has been paid to stem cell biology, resulting in an extensive understanding of their characteristics and therapeutic application potential [20].
The application of stem cells in regenerative medicine mainly regards hematological disorders and skin regeneration [21,22]. Already in 1984, Gallico et al. demonstrated that the human epidermal cells isolated from a skin biopsy were able to perform epithelial sheets, which when implanted on burn wounds generated a permanent epidermidis [21]. Today it is well known that the epidermidis undergoes continuous renewal due to the presence of a special population of keratinocytes stem cells, which could be massively expanded in culture [23]. To date, substantial progresses have been made in human keratinocytes culture methods prior to be implanted on extensive third degree burn wound. In particular, Ronfard et al. demonstrated that fibrin matrices make epithelia very easy to handle. Moreover, they maintain their original dimensions when detached, and they are also three times larger than grafts obtained without fibrin matrix [24]. Autologous transgenic keratinocytes were also used to regenerate the entire epidermidis in a patient suffering from junctional epidermolysis bullosa, a genetic defect causing chronic wounds in both skin and mucosa [25].
Today, several multipotent stem cells of different sources and iPSCs have been introduced for tissue repair. Some preclinical studies and clinical trials confirmed the potential of stem cell therapy in treating several diseases, such as diabetes mellitus [26,27], heart failure [28,29], disorders of the nervous system [30,31,32], and so on. Particularly attracting as candidates for regenerative medicine are mesenchymal stromal stem cell (MSCs) for their anti-inflammatory and immunomodulatory features [33,34,35]. MSCs do not express significant histocompatibility complexes and immune stimulating molecules, are not detected by immune surveillance, and do not lead to graft rejection after transplantation. In addition, MSCs display a great homing potential and migration capability to the damaged sites [36]. Their potential in regenerative medicine is also due their ability to enhance angiogenesis and accelerate tissue healing [37]. MSCs can easily undergo multilineage differentiation, such as adipocytes, hepatocytes, osteoblasts, chondrocytes, myoblasts, and neuronal cells. [38]. They can be isolated from almost all adult tissues, such as bone marrow, adipose tissue, synovium, dental pulp, from perinatal tissues, such as umbilical cord blood, placenta, amniotic fluid, and umbilical cord Wharton’s jelly [39,40,41,42,43,44,45,46], as well as from solid organs, e.g., brain, lung, liver, pancreas. Adipose derived mesenchymal stem cells (ADSCs) show several advantages over other MSCs. ADSCs are easily accessible and their extraction is technically easy and safe, with a higher proliferative capacity [47,48,49]. All these advantages make ADSCs optimal for stem cell-based therapies [50,51,52].
Stem cells (i.e., bone marrow derived stem cells) were successfully used for improving wound repair of skin radiation burns, a common radiotherapy complication. Conventional surgical treatment coupled with stem cell therapy (i.e., autologous MSCs). was for the first time applied on a human patient by Lataillade et al. [53]. Following this combined therapy, they observed that the healing of the lesion proceeded easily and that there were no side effects. Moreover, the healing persisted one year after the procedure. The researchers hypothesized that MSC effects on the radiation burn healing could be mediated by the secretion of soluble mediators, cytokines, and trophic factors that may play important roles to counteract the local inflammatory processes [53]. A similar positive result was obtained two years later by Bey et al. [54].
The positive effects of MSCs in severe radiation burns were also demonstrated by Linard et al., as they observed the positive outcomes of stem cell utilization in preclinical pig model of radiation induced proctitis and of severe radiation burn [55,56]. In the former model, MSC injection markedly reduced tissue inflammation by diminishing infiltrates of macrophages and changing their phenotype towards M2 anti-inflammatory macrophages [56].
After severe irradiation, exposure lesions may extend beyond the skin affecting the underlying muscles as well. In this case, MSC therapy stimulates the angiogenic factor VEGF, which is involved in muscle cell regeneration and growth, and a macrophage M1/M2 balance [55].
Differently from bone marrow and cord blood transplantation in leukemia or other hematological disorder treatment, the use of other stem cells, including keratinocytes used for skin regeneration, needs expansion. This process requires stem and progenitor cells to be preserved throughout all the culture steps. To this aim, several studies have been performed to identify the molecular stemness regulators. One of these regulators is the Klf4 gene, which is also one of the four factors responsible for somatic cell reprogramming to obtain IPSCs [15]. In skin therapy by keratinocytes the preservation of both stem and progenitor cells features is particularly important, as it is a necessary condition for successful long-term graft outcome. Fortunel et al. have demonstrated that Klf4 downregulation increased keratinocyte stem cells immaturity and its clonogenic potential, increasing their ex vivo expansion. In addition, Klf4 deficient cells displayed improved graft capacity in an in vivo xenograft model [57].
4. Stem Cells Drawbacks in Regenerative Medicine
Even if stem cell therapy displays great potential in the field of regenerative medicine and MSCs have been already successfully used in several clinical applications (see clinicaltrial.gov database) [58], their use also presents some risks, such as cell rejection, undesired immune response [59], possible contamination with viruses [60], low recovery rate [61,62,63], problematic transport and storage of cells before their use [64,65,66], and adverse effects associated with their harvest in the case of bone marrow mesenchymal stem cells. Other important risks of MSCs are tumorigenicity, proinflammation, and fibrosis. Moreover, for cell therapy applications, their ex vivo expansion is necessary, although a reduction in their effectiveness was observed during in vitro cultivation, due to aging, loss of stemness and undesired differentiation. Finally, it is well documented that proliferation and differentiation capability of MSCs are also negatively influenced by donor age.
The discovery of iPSCs by Takahashi and collaborators [15] allows researchers to eliminate some of the disadvantage of MSCs. However, genetic instability along with plasticity and self-renewal, common to both MSCs and iPSCs, could lead to tumor formation in the host tissue [67,68,69,70]. Notwithstanding, regardless of the promise of stem cell research to treat untreatable disease, such as Parkinson’s disease, spinal cord injuries, macular degeneration, amyotrophic lateral sclerosis, their use as a routine therapy is still a long way off. Indeed, to exert their regenerative potential, stem cells need to home the injured site, engraft it, and due to their self-renewal and differentiation capability replace damaged cells. Unfortunately, numerous preclinical studies have shown a low grafting rate. Indeed, systemic stem cell delivery by intravenous injection determines the capture of the majority of cells into the capillary beds, especially in the lungs, but also in spleen, liver, and kidney. Because of the systemic clearance [58,71], only a small amount of stem cells reaches the target site [68,72,73,74,75,76,77]. These limitations could be overcome by directly injecting stem cells into the damaged site, e.g., direct myocardial infusion after infarction. Despite this, problems still remain for the successfully stem cell engraftment and in situ differentiation: for example a low cell survival rate was found in the injured site [78,79,80]. Furthermore, genetic instability of stem cells, especially iPSCs, along with plasticity and self-renewal could lead to tumor formation in the host tissue, should be still evaluated [67,68].
Experimental data obtained by animal models of myocardial infarction and brain stroke showed that ADSC treatment improved both cardiac and neurological function. However, there are no unambiguous evidences demonstrating that the amelioration of symptoms was due to ADSC transdifferentiation into functioning cardiomyocytes or neurons [78,81,82,83,84]. MSCs therapeutic effects might be due to the secretion of bioactive factors, collectively named secretome, as well as extracellular vesicles (EVs) [73,85,86,87,88,89,90,91,92,93,94,95,96]. The secretome consists of bioactive factors released by stem cells both actively and passively; it includes soluble protein factors (i.e., growth factors and cytokines) and EVs, which transfer to recipient cell proteins, lipids, and nucleic acid [97]. EV paracrine effects were firstly described by Gnecchi et al. [98]. MSC secretome composition is dependent on origin of the stem cells and culture conditions. Moreover, it is also influenced by preconditioning treatments of the stem cells during cell culture [95,99,100]. For example, Magne et al. demonstrated that IL-1β priming of gingival MSCs led to secretome changes, potentiating therapeutic effects in both in vivo and in vitro models of skin trauma. Their results showed an increase in keratinocyte migration and an enhanced dermal-epidermal junction protein deposition. It is possible to hypothesize that the effects reported by the use of IL-1β primed MSCs are due to the release of EVs containing growth factors, cytokines, and extracellular proteins [101].
In selected animal models of diseases, MSC supernatant EVs showed a similar therapeutic effect of MSCs alone, confirming their role in tissue repair [89,102].
5. Extracellular Vesicles (EVs)
It is now well demonstrated that cell-to-cell communication is fundamental for multicellular organisms both during development and also in adult tissues. This communication can be mediated either by direct cell-to-cell contact, realized by cell adhesion molecules (CAMs), or by transfer of secreted molecules, kept inside EVs [103]. For many years since their discovery [104,105] EVs have been considered as cellular debris and their study was overlooked until the 21st century due to insufficient research techniques. Today, it is now accepted that almost all cell types, included stem cells, release EVs through an active and specific pathway, and they have been found in almost all the body fluid (e.g., serum, plasma, milk, cerebrospinal fluid, saliva and urine) [106,107,108].
EVs consist of a heterogeneous population of vesicles surrounded by a lipid bilayer containing transmembrane proteins, DNA, RNA, and other metabolites. The lipid envelope protects bioactive molecules from extracellular enzymatic degradation. EVs were classified into exosomes, microvesicles and apoptotic bodies according to their biogenesis, surface markers, function and size (Figure 1, Table 1) [109,110]. Exosomes are derived from the multivesicular endosomal system and are secreted when the multivesicular bodies are fused with the plasma membrane. Their size is below of 50 nm in diameter and they are enriched in endosome-derived components (e.g., Alix, Tsg101) [109,111]. On the contrary, microvesicles (MVs) arise directly from the outward budding of the plasma membrane, followed by a fission event similar to the abscission step observed in cytokinesis [112]. These particles range in size between 50 nm and 1 μm [109]. MVs formation requires increased cytosolic calcium ions, molecular rearrangements within the plasma membrane in terms of lipid and protein composition (e.g., phosphatydylserine exposure), and degradation of membrane cytoskeleton [113,114,115]. Conversely, apoptotic bodies are larger than exosomes and MVs [116], and are produced by the blebbing of aging or dying cells [117,118]. However, since this last type of EVs is less studied, they will not be discussed further.
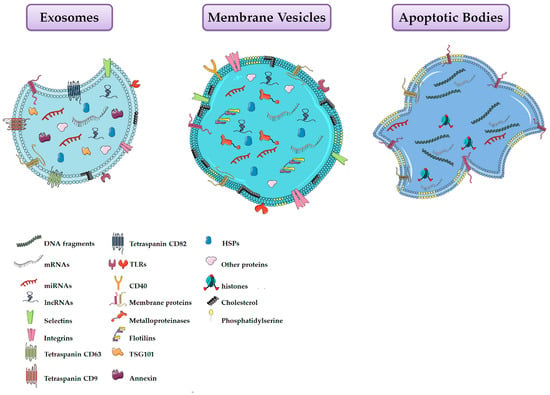
Figure 1.
Schematic representation of the three main types of extracellular vesicles released by cells.

Table 1.
Summary of extracellular vesicles properties.
Recently, as some confusion existed in the literature regarding the terms used by the researchers to describe vesicles present in the extracellular space (e.g., exosomes, shedding vesicles, melanosomes, prostasomes, ectosomes, microvesicles, argosomes, oncosomes), the International Society for Extracellular Vesicles (ISEV) have recommended researchers to use the term “extracellular vesicles (EVs)” for all types of vesicles, as the generic term for particles naturally released from the cell that are delimited by a lipid bilayer and cannot replicate, i.e., do not contain a functional nucleus [119]. Following this recommendation, we use the term EVs throughout the entire paper.
Today EVs are considered key elements of cell-cell communication, containing not only bioactive molecules, but also whole organelles (i.e., mitochondria and ribosomes) [120,121].
6. Extracellular Vesicles Composition
The composition of EVs depend mainly on their cell origin and on the stimuli they receive, under both physiological and pathological conditions. Indeed, it is well recognized that targeting of bioactive molecules within EVs is a selective process, being specific molecules included or excluded [122,123,124,125]. After their release into the extracellular environment many EVs rapidly break down, liberating their cargo in the surrounding microenvironment. Otherwise, EVs can deliver their cargo to target cells via different pathways: direct interaction, resulting in EV fusion with the target cell membrane or in their endocytosis, and interaction mediated by ligand-receptor binding [126,127,128] (Figure 2).
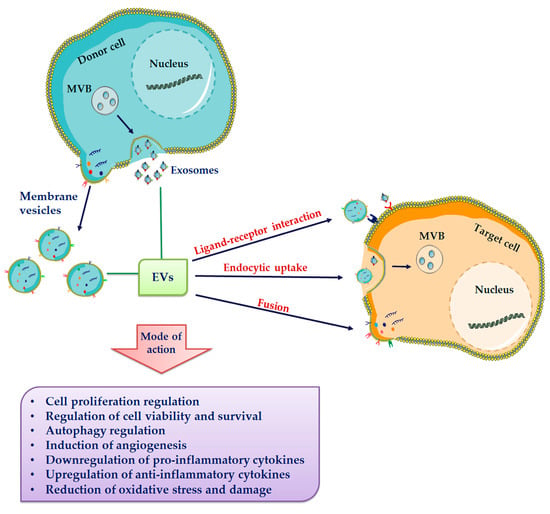
Figure 2.
Extracellular vesicle (EVs) release and uptake by target cells. EVs, including exosomes and membrane vesicles, are released in the extracellular milieu. All subtypes of EVs share a general composition with an outer lipid bilayer and various proteins, lipids, and nucleic acids. EVs have been suggested to be internalized into target cells by various uptake mechanisms including membrane fusion, different endocytic pathways and receptor-mediated endocytosis.
Multi-omics studies allowed to characterize EVs content, essential for their biological functions. Proteomic analysis of EVs have demonstrated that proteins released through vesicles are involved in intercellular communication and play a pivotal role in cell signaling, differentiation, cell adhesion, angiogenesis, and apoptosis. They include growth factors, cytokines, chemokines, extracellular matrix proteins, metalloproteinases, etc. [129]. In particular, it has been demonstrated that cells use EVs for unconventional protein export. In fact, some cytosolic proteins (e.g., FGF-2, IL-1β) which lack exocytosis signals necessary for the classical endoplasmic reticulum-Golgi secretory pathway are released within EVs [130,131].
One of the first proteomic characterization of MSCs was performed in 2012 by Lai et al., and Kim et al. [132,133]. These authors by two different methods identified 857 and 730 proteins, respectively. Among the common subset of 315 proteins, both cytoplasmic and membrane proteins were identified. Specific markers of MSCs, such as CD63, CD9, CD109, CD151, CD81, CD248, and CD276, as well as surface receptors (e.g., PDGF-RB, EGF-R), signaling molecules (RHO, CDC42, MAPK1, Wnt5B) responsible for self-renewal and cell differentiation, have been also identified [133]. In addition, proteins implicated in EV biogenesis, intracellular trafficking and fusion were characterized (RAB1A, RAB2A, RAB5A/B/C, RAB7A, RAB8). Finally, Kim et al. identified proteins implicated in cell adhesion, migration, and morphogenesis [133].
Nowadays, there are three public databases containing data from proteins, mRNAs, miRNAs, and lipids enclosed in prokaryotic, non-mammalian eukaryotic, and mammalian EVs: EVpedia, Exocarta, and Vesiclepedia [134,135,136].
One of the best characterized functions of MSC-derived EVs is to improve angiogenesis, whose efficiency varies according to their tissue of origin. The enriched angiogenic proteins identified in EVs include VEGF, vWF, TGF-β1, IGF-1, and IL-8 [137,138,139]. Literature data have demonstrated that conditions such as hypoxia, serum deprivation, exposure to IFN-γ, TNF-α, are able to influence EV angiogenic factors concentration. For example, serum deprivation and hypoxia increase the release of VEGF, FGF-2, HGF, IGF, and TGF-β within EVs derived from ADSCs, bone marrow MSCs and placenta MSCs [95,99].
Recent attention has also focused on the capacity of EVs to induce epigenetic changes in target cells [140,141,142,143]. These changes could be due to the transfer through EVs of genetic information between donor and target cells, making them similar to the producing cells. Indeed, it is now well demonstrated that EVs in addition to growth factor and other proteins also contain nucleic acid, especially RNA (i.e., mRNA, miRNA, tRNA, lncRNA).
Particularly important is the transfer of miRNA, which are small noncoding RNA that are 19 to 23 nucleotides long. miRNAs are powerful regulators of gene expression due to translational inhibition or to degradation of target mRNAs [144,145]. Detailed analysis of miRNA content in MSC-EVs demonstrated that there are profound differences between vesicles and cellular content, suggesting an active process of sorting and packaging of miRNA into EVs, as already demonstrated for protein sorting [146]. Several of the identified miRNAs are involved in angiogenesis modulation. They target and modulate the expression of regulatory angiogenic genes encoding for MMPs and growth factors such as VEGF, PDGF, FGF, and EGF [147,148].
Furthermore, EVs are also enriched in tRNAs [149], which contribute to maintaining stem cell potency, stimulating cell survival and inhibiting cell differentiation [150]. Several evidences have also demonstrated the presence of long noncoding RNAs (lncRNAs) within EVs [151]. These RNAs are able to induce epigenetic modification by binding to specific genomic loci and recruiting epigenetic regulators. In this way, EVs may induce epigenetic modifications in target cells.
EVs also contain bioactive lipids, as well as lipid metabolic enzymes. Lipids have been implicated in multiple aspects of EV biogenesis and function.
As for proteins and RNAs, EV lipid composition is distinct from that of cells of origin. In particular, they contain cholesterol, sphingomyelin, glycosphingolipid, ganglioside GM3, and phosphatidylserine [152]. Cholesterols, sphingomyelins and phosphatidylserine are the major components of lipid rafts, with all three lipids showing increased abundance in EVs when compared to their secreting cells. The presence of lipid raft-associated proteins, including flotillin-1, suggested that lipid rafts could influence selective protein sorting into EVs. They also transport arachidonic acid, phosphatidic acid, prostaglandins, leukotriens [153], and are enriched in spingosine-1-phosphate, a signaling lipid that with the other lipids of the EVs induce several biological responses, including cell proliferation and migration [154]. Lipid composition is also fundamental to determine EV-target cell interaction [155] and can be influenced by MSC culture conditions.
7. Extracellular Vesicles-Based Regenerative Medicine
As described above, it appears evident that positive effects of MSC transplantation is most likely due to their paracrine activity, rather than to their homing and differentiation in the injured tissues. According to these observations it is conceivable that EVs may replace the stem cells used in regenerative medicine, playing their biological functions.
Recently, more and more in vitro and in vivo studies have demonstrated that stem cell conditioned media alone, and in particular EVs, can replicate the reparative effects observed by using stem cells (Figure 3) [156,157,158].
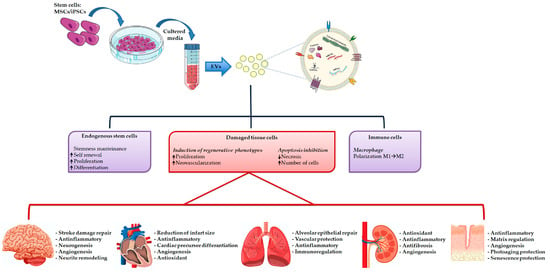
Figure 3.
Extracellular vesicles isolation and their therapeutically potential in tissue repair.
Most of the literature concerning EVs released by MSCs is isolated from different tissues. That is why we barely mention the topic and for more in depth information we refer to some recent reviews [159,160].
Many studies showed that the protective effects exerted by MSC-EVs in the respiratory injury model are dependent on the regulation through miRNAs of inflammation and proliferation [161,162,163]. It is interesting to know that in a mouse model of hemorrhagic shock and laparotomy-induced lung injury, Potter et al. demonstrated that both MSCs and EVs ameliorate the lung condition, with several differences in their molecular effect [164].
EVs have been proved to be a good candidate to substitute MSCs in mild acute and acute kidney injury, in particular by inhibiting epithelial cell apoptosis, promoting wound regeneration, and by stimulating angiogenesis [165,166,167,168,169]. MSC-EVs are also efficient in chronic kidney diseases [170,171,172,173]. It was also demonstrated that, in acute renal injury, EVs both in vivo and in vitro are responsible for inflammation reduction through the expression of the receptor CCR2, which reduces migration and activation of macrophages [174]. Autophagy induction is another mechanism used by MSC EVs to ameliorate tubular function [175,176].
The protective effects of MSC-EVs in cardiovascular diseases have been already observed in 2010 [177]. It is interesting to note that in some cases, the effects of the EVs were significantly better than those of MSCs [178]. Generally, EVs can promote myocardial regeneration and restore cardiac function by directly activating cardiac precursor cells and inducing their differentiation, through the promotion of cardiomyocytes survival and proliferation. EVs are also able to inhibit cardiomyocytes apoptosis, to stimulate angiogenesis, and finally to regulate the inflammatory response in the damaged area [179,180,181,182]. One of the possible mechanism is related to the increase in cardiomyocytes of Bcl-2 expression, responsible for reduction of cardiac cell apoptosis, promotion of proliferation and angiogenesis [183]. Apoptosis reduction could also be due to autophagy activation via the PI3K/Akt/mTOR pathway [184]. Similarly increased angiogenesis is induced by the presence within EVs of the extracellular matrix metalloproteinase inducer EMMPRIN, VEGF, MMP9 and miRNA-210 [185,186]. Other miRNAs involved in myocardium protection are miRNA-21a-5p, miRNA-21, miRNA-125-5p, and miRNA93-5p [187,188,189,190]. Besides, overexpression of miRNA-30d inhibited autophagy and promoted polarization of macrophages into the anti-inflammatory M2 type [182]. The expression of inflammatory factors was also down-regulated by the miRNA-126 [191].
Particularly interesting and promising are the results obtained by using MSC-EVs in neurological and neurodegenerative diseases. In fact, differently from MSCs, EVs because of their lipid structure can cross the blood-brain barrier [192,193]. In addition, they have an extremely low immunogenicity that allow them to exert an immunoregulatory role avoiding clearance to which MSCs are subjected. Doeppner et al. showed that MSC-EVs in stroke treatment had the same tissue regeneration capability as MSCs [193]. In an another study, EVs had even better recovery effects in stroke damage repair, most likely due to their higher blood-brain barrier permeability than MSCs [194]. EVs have been also successfully used in brain trauma. Indeed, Chuang et al. discovered that MSC-EVs could promote nerve and endothelial cell regeneration, increase VEGF production involved in angiogenesis [195], and also reduce the local inflammatory response [196]. A reduction of the inflammatory response was observed by Kim et al. too [197,198]. A diminished inflammation was even observed in spinal cord injury, most likely via neuronal apoptosis inhibition, angiogenesis stimulation, A1 astrocytes inactivation and up-regulation of anti-inflammatory IL-10 and TGF-β as well as down-regulation of the pro-inflammatory TNF-α and IL-1β [184,199,200,201,202]. An essential function after spinal cord injury and brain ischemia was also attributed to the miRNA-113b [197,203].
8. Induced Pluripotent Stem Cell Derived EVs in Regenerative Medicine
Despite all the advantages so far considered for the MSCs, and although MSCs are used to treat several kinds of diseases completely different from each other, they remain multipotent stem cells, and their ability to differentiate in some cell types (e.g., neural precursor cells) is reduced. Furthermore, the number of MSCs that can be obtained by a single donor is limited. On the other end, the use of pluripotent embryonic stem cells carries potential ethical problems with respect to their clinical use. For this reason, the development of iPSCs opened a new scenario in regenerative medicine. Despite their potentiality, clinical application of iPSCs is limited because of their tumorigenic potential, low engraftment, and cell retention.
As described above, more and more in vitro and preclinical studies have demonstrated that stem cells exert their regenerative potential via paracrine mechanisms, which include EV release. Due to their lower immunogenicity, easier handling, and having no risk of tumor formation, researchers recently focused on their potential as possible alternatives to stem cells [159,204,205,206], studying experimental model diseases treated with MSC-EVs. To date, a total of eight clinical trials on the application of EVs for disease treatment were identified [160]. Accordingly, many researchers investigated the role of EVs released by iPSCs in tissue regeneration. Several proteomic analyses have identified cytokines, chaperones, signaling molecules, plasma membrane proteins, isolated from human pluripotent stem cells [207,208], and it is reasonable to hypothesize that iPSC-EVs contain many kinds of these proteins. Therefore, many researchers shifted their attention to the EVs released by transplanted cells. To date, many studies have highlighted the importance of EVs and their miRNA in cell-to-cell communication within the cardiovascular system, also because the myocardial infarction is a leading cause of death worldwide [209,210]. Wang et al. demonstrated that iPSC-EVs can be taken up by cardiomyocytes in vitro, producing cytoprotection against H2O2 induced oxidative stress. Additionally, in vivo EV transplantation protected cardiac cells by inhibiting their apoptosis via caspase 3/7 inhibition [211]. These data raise the possibility that iPSC-EVs could be protective for cardiomyocytes. EV cytoprotective effect is also related to their miRNA content, in particular miRNA-21 and miRNA-210. Xuan et al. generated cardiac progenitor cells (CPCs) starting from iPSCs by treating them with the small molecule ISX-9, with antioxidant and regenerative properties, and used EVs derived from these cells to repair post-infarcted heart [212]. They found that the cardioprotective EV effect was in part due to their miRNA content. In particular, CPCISX-9-derived EVs were highly enriched with miRNA-520/-373 family members. miRNA-373 was originally identified as a human embryonic stem cell specific miRNA and is involved in the regulation of cell proliferation, apoptosis, senescence, migration and invasion following hypoxia [213]. miRNA-373 inhibited TGF-β, hypoxia-induced fibrotic genes upregulation (i.e., GDF-11 and ROCK-2), and myofibroblast differentiation in fibroblasts. Additionally, in vivo data confirmed the therapeutic role of CPCISX-9-derived EVs. EVs were responsible for cardiomyocyte proliferation and angiogenesis, too. These studies confirmed that EVs can be used for the treatment of ischemic heart by overcoming the limitation of cell based therapy [213].
Oh et al. demonstrated that exosomes derived from human iPSCs exert a protective role on skin fibroblasts against photoaging and natural senescence [214]. They found that iPSC-EVs stimulated both proliferation and migration of dermal fibroblasts, increased the expression level of collagen type-I in photoaged and senescent fibroblasts, reduced the expression level of MMP-1/3 during senescence [214].
As described above, several data have demonstrated the beneficial effects of MSC-EVs in both acute and chronic kidney disease. Lee et al. have already demonstrated that also iPSCs can protect the kidney through the induction of an anti-apoptotic, anti-inflammatory, and antioxidant response [215]. Collino et al. evaluated the protective and regenerative potential of iPSC-EVs in an acute kidney injury model to explore the possibility of their use in kidney regenerative medicine [216]. In their study, the authors demonstrated the main mechanism associated with mitochondria protection. Indeed, after EV administration, the mitochondrial mass of renal cells was maintained as well as the ΔΨm, fundamental ATP generation, and cell survival. Even if this protective mechanism is similar to that exerted by ADSC-EVs, IPSCs-EVs showed a higher efficiency in renal cell protection. Furthermore, iPSC-EVs have been involved in immune response modulation, with a decrease in infiltrating macrophages, and in inflammation modulation, through macrophage M2 polarization. A modulation of oxidative stress was also observed [216].
Furthermore, iPSC secretome were used to treat pulmonary fibrosis. In particular, Gazdhar et al. have demonstrated that the secretome induces alveolar epithelial repair in vitro and also reduces lung fibrosis in vivo [217].
9. EVs as a Cargo Delivery System
Several data have demonstrated that EVs can be engineered for nucleic acid delivery to target cells, using either cell preconditioning or exogenous transfection, especially resulting in an enrichment of natural miRNAs.
Next generation sequencing revealed that EVs originating from hypoxic human neural stem cells have an increased levels of 53 miRNAs and a reduction in 26 miRNAs [218]. Similarly, microarray analysis of EV released by cardiac progenitor cells under hypoxic stress condition showed an upregulation of 11 miRNAs compared to EVs secreted from normoxic cells [219]. Moreover, Xiao et al. have demonstrated that EVs enriched in miRNA-21, released by cardiac progenitor cells stress-treated via H2O2 incubation, protected cardiomyocytes from damage in ischemic conditions [220].
However, preconditioning offers only a limited control in the specific types of enriched miRNA, and standardization also remains a problem, as different conditions can lead to different types and levels of cargo enrichment.
Conversely, transfection or transduction, an indirect method of cargo loading, can offer more much control than preconditioning. Han et al. have transfected umbilical cord MSCs with miRNA-675 mimic, leading to the secretion of EVs that showed an increased expression of this miRNA [38]. When they injected the miR-675-enriched EVs delivered in silk fibroin hydrogels in a murine model for aging-induced vascular dysfunction, they observed a promotion of blood perfusion in ischemic hindlimbs and reduction of proinflammatory molecules expression [221]. Furthermore, lentiviral transduction have used to engineer MSCs to overexpress, for example, miRNA-let7c, and secrete EVs enriched in this miRNA [186]. The enriched EVs were showed to mediate the improved transfer of miR-let7c to the kidneys, attenuating renal fibrosis.
In addition to miRNA, siRNAs are another class of small RNAs which have been exogenously loaded within EVs. siRNA loading is similar to that of miRNA loading, with electroporation being the most common method of active loading [222]. In a model of acute lung injury, EVs isolated from iPSCs derived by reprogramming renal epithelial cells were subjected to electroporation to introduce siRNA against ICAM-1, which is involved in the inflammatory response [223].
10. EVs Functionalized Scaffolds in Regenerative Medicine
In recent years, regenerative medicine has gone a step further thanks to the use of engineered constructs (i.e., scaffolds) [224]. Several biomedical researches have focused on the use of scaffolds functionalized with EVs, trying to improve regenerative capacity. In particular, EVs used to engineer different types of scaffolds are those derived from MSCs due to their proangiogenic properties.
EVs can be immobilized on a variety of polymer-based scaffolds. For example, Xie and co-workers coated decalcified bone matrix (DBM) with bone marrow-derived MSC EVs using fibronectin as the immobilizing agent. The authors showed that the implanted EV-functionalized scaffolds promoted bone regeneration in a murine model. Significant new bone matrix was observed, with higher levels of CD31-positive vessels within the matrix compared to unmodified scaffolds, confirming the ability of the EVs to promote bone formation and vascularization [225].
Similar results were obtained with EVs derived from other stem cell sources combined with other type of scaffolds. Li and collaborators have used a PLGA/pDA (poly lactic-co-glycolic acid) scaffold engineered with hADSC-EVs in the murine model of critical-sized calvarial bone defects. They observed a significantly higher bone tissue and mature collagen formation in hADSC-EV modified scaffolds compared to the unmodified ones [226].
To stimulate wound healing, EV-modified sponges and patches were used. Shi et al. successfully loaded gingival MSCs, onto a chitosan/silk hydrogel sponge, to cover surgically-induced skin in diabetic rat [227]. Quantification of wound size showed that the EV-modified sponges had a significantly greater wound closing ability because of their aptitude in angiogenesis and collagen deposition [227].
EV-modified hydrogel patches begun to emerge as a therapeutic strategy for cardiac recovery and regeneration. EVs secreted from iPSC-derived cardiomyocytes were encapsulated in hydrogel patches. Their application to rat myocardium promoted arrhythmic recovery, decreased cardiomyocyte apoptosis after infarction, and reduced infarct size and cell hypertrophy [228].
11. Vantages and Disadvantages in the Use of Stem Cell-EV Based Therapies
To date, more and more data have demonstrated that EVs can substitute stem cells in regenerative medicine and tissue regeneration. EVs display several advantages over cells; (1) they do not solicit host immune response due to their low immunogenicity, so there is no need to match the donor and the recipient. (2) they can be constantly released by immortalized stem cells, overcoming the low amount of cells isolated, especially adult ones, compared to the large number needed for therapy; (3) they avoid tumor formation because of their inability to self-replicate; (4) due to their lipidic structure EVs are easily stored for a long time at −80 °C without losing their bioactivity, in addition to being more resistant to freezing and thawing than stem cells; (5) EVs can cross some barriers, such as blood brain barrier [229], and, because of their dimensions, move into wound areas and capillary; (6) there is no risk of infection transmission; and, (7) EVs containing specific cargo (i.e., nucleic acids, proteins) can easily obtained. Indeed, it is possible to manipulate EV releasing stem cells to improve their beneficial effect. EV content can be modified for example by hypoxia and stress preconditioning [219], serum deprivation, or by genetic modification and epigenetic reprogramming of EV-producing stem cells. In addition, genetically engineered EVs containing small therapeutic molecules can be used as a delivery system [222]. Exposure to TNF-α or IFN-γ also modifies EV content.
Even if stem cell isolated EVs are much safer than cell therapy for bench-to-bedside translation, there are also problems with their use; (1) contrasting data regard the duration of EV effects, as it is not clear regarding their half-life. On the contrary, engrafted donor stem cells can continuously release EVs by having long term beneficial effect; (2) in some cases (e.g., iPSC-EVs) EVs stimulate fibroblast proliferation increasing scar tissue formation; (3) possible inefficient endocytosis by target cells has to be evaluated; (4) EV properties can vary between cell batches and passage number, making standardized therapies difficult to manufacture; (5) the current standard methodologies for EVs isolating involves long culture times to obtain hundreds of millions of cells and intensive ultracentrifugation steps, all of which are expensive and require a great deal of time.
Before using EVs for a cell free therapy, some aspects must be considered. It is necessary to define univocal methods to select the most appropriate source for their release. In fact, different cell sources release EVs with different compositions, which affect the effects on different diseases. Only a few studies have identified the whole proteome contained in MSC-EVs, and the comparison clearly indicates several differences in their composition [132,133]. It is also essential to develop a unified protocol of cell culture conditions, quantification, and for collecting EVs. In particular, regarding EV isolation, the techniques most commonly used are ultracentrifugation and polymeric precipitation, but they permit the coprecipitation of EVs and culture medium components; in addition, the recovery is very low. To be used in clinical trials, EVs must have specific requirement, such as purity and sterility above all, but also their composition and potency need to be determined [230]. Biodistribution of EVs need to be investigated.
Additionally, due to the still limited number of clinical trials using EVs for tissue regeneration the safety and the optimal dose should be tested and GMP procedures have to be set up.
12. Conclusions and Future Perspective in EV-Based Therapies in Regenerative Medicine
The data summarized in this review suggest that EVs could be used in regenerative medicine in a cell free therapy, as they mimic stem cell effect.
Their potential in tissue regeneration is mainly due to their ability to regulate the host immune response, promote the migration and the proliferation of damaged cells, tissue neovascularization. Preliminary EVs positive effect were already observed in skin, heart, kidney, lung reconstruction, and also in neurodegenerative diseases.
Even if EVs-based tissue engineering represents a promising clinical strategy in the field of regenerative medicine, and many studies on EVs have improved our knowledge on their origin, there are still many challenges to overcome. The researchers proposed distinct methods for EV isolation, although different culture conditions, such as FBS, supplements, cell seeding density, age of the donor cells, and oxygen concentrations, may affect EV production and composition. Therefore, before their use in clinical trial, a standard protocol needs to be formulated for EV recovery. Furthermore, to improve the therapeutic effects of EVs it is necessary to improve the methods to edit stem cells to introduce within them specific molecules (i.e., miRNAs and lncRNAs).
Author Contributions
F.G. designed the study; writing—original draft preparation M.M.B., P.C., and F.G.; M.M.B. constructed the figures. Manuscript was proofread by all the authors. All authors contributed to the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
FFABR 2017 from Italian Ministry of University and Research (Miur) to FG.
Acknowledgments
We are grateful to Gabriella Sconzo for helpful comments on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guild, W.R.; Harrison, J.H.; Merrill, J.P.; Murray, J. Successful homotransplantation of the kidney in an identical twin. Trans. Am. Clin. Climatol. Assoc. 1955, 67, 167–173. [Google Scholar] [PubMed]
- Gatti, R.A.; Meuwissen, H.J.; Allen, H.D.; Hong, R.; Good, R.A. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet 1968, 2, 1366–1369. [Google Scholar] [CrossRef]
- Weissman, I.L. Translating stem and progenitor cell biology to the clinic: Barriers and opportunities. Science 2000, 287, 1442–1446. [Google Scholar] [CrossRef] [PubMed]
- Menasche, P.; Vanneaux, V.; Hagege, A.; Bel, A.; Cholley, B.; Cacciapuoti, I.; Parouchev, A.; Benhamouda, N.; Tachdjian, G.; Tosca, L.; et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: First clinical case report. Eur. Heart J. 2015, 36, 2011–2017. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef]
- Rossant, J. Stem cells from the Mammalian blastocyst. Stem Cells 2001, 19, 477–482. [Google Scholar] [CrossRef]
- Jaenisch, R.; Young, R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell 2008, 132, 567–582. [Google Scholar] [CrossRef]
- De Miguel, M.P.; Fuentes-Julian, S.; Alcaina, Y. Pluripotent stem cells: Origin, maintenance and induction. Stem Cell Rev. Rep. 2010, 6, 633–649. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Zuba-Surma, E.; Kucia, M.; Poniewierska, A.; Suszynska, M.; Ratajczak, J. Pluripotent and multipotent stem cells in adult tissues. Adv. Med. Sci. 2012, 57, 1–17. [Google Scholar] [CrossRef]
- Bentzinger, C.F.; Wang, Y.X.; von Maltzahn, J.; Rudnicki, M.A. The emerging biology of muscle stem cells: Implications for cell-based therapies. BioEssays News Rev. Mol. Cell. Dev. Biol. 2013, 35, 231–241. [Google Scholar] [CrossRef]
- Beck, B.; Blanpain, C. Mechanisms regulating epidermal stem cells. EMBO J. 2012, 31, 2067–2075. [Google Scholar] [CrossRef] [PubMed]
- Ilic, D.; Polak, J.M. Stem cells in regenerative medicine: Introduction. Br. Med. Bull. 2011, 98, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Bongso, A.; Richards, M. History and perspective of stem cell research. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 827–842. [Google Scholar] [CrossRef] [PubMed]
- Rossant, J. Stem cells and early lineage development. Cell 2008, 132, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- de Lazaro, I.; Yilmazer, A.; Kostarelos, K. Induced pluripotent stem (iPS) cells: A new source for cell-based therapeutics? J. Control. Release Off. J. Control. Release Soc. 2014, 185, 37–44. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, X.; Li, X. Exosomes derived from mesenchymal stem cells. Int. J. Mol. Sci. 2014, 15, 4142–4157. [Google Scholar] [CrossRef]
- Omole, A.E.; Fakoya, A.O.J. Ten years of progress and promise of induced pluripotent stem cells: Historical origins, characteristics, mechanisms, limitations, and potential applications. PeerJ 2018, 6, e4370. [Google Scholar] [CrossRef]
- Malik, N.; Rao, M.S. A review of the methods for human iPSC derivation. Methods Mol. Biol. 2013, 997, 23–33. [Google Scholar]
- Mohammadian, M.; Shamsasenjan, K.; Lotfi Nezhad, P.; Talebi, M.; Jahedi, M.; Nickkhah, H.; Minayi, N.; Movassagh Pour, A. Mesenchymal stem cells: New aspect in cell-based regenerative therapy. Adv. Pharm. Bull. 2013, 3, 433–437. [Google Scholar]
- Gallico, G.G., 3rd; O’Connor, N.E.; Compton, C.C.; Kehinde, O.; Green, H. Permanent coverage of large burn wounds with autologous cultured human epithelium. N. Engl. J. Med. 1984, 311, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Thivolet, J.; Faure, M.; Demidem, A.; Mauduit, G. Long-term survival and immunological tolerance of human epidermal allografts produced in culture. Transplantation 1986, 42, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Rheinwald, J.G.; Green, H. Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells. Cell 1975, 6, 331–343. [Google Scholar] [CrossRef]
- Ronfard, V.; Rives, J.M.; Neveux, Y.; Carsin, H.; Barrandon, Y. Long-term regeneration of human epidermis on third degree burns transplanted with autologous cultured epithelium grown on a fibrin matrix. Transplantation 2000, 70, 1588–1598. [Google Scholar] [CrossRef]
- Hirsch, T.; Rothoeft, T.; Teig, N.; Bauer, J.W.; Pellegrini, G.; De Rosa, L.; Scaglione, D.; Reichelt, J.; Klausegger, A.; Kneisz, D.; et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature 2017, 551, 327–332. [Google Scholar] [CrossRef]
- Kroon, E.; Martinson, L.A.; Kadoya, K.; Bang, A.G.; Kelly, O.G.; Eliazer, S.; Young, H.; Richardson, M.; Smart, N.G.; Cunningham, J.; et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 2008, 26, 443–452. [Google Scholar] [CrossRef]
- Trivedi, H.L.; Vanikar, A.V.; Thakker, U.; Firoze, A.; Dave, S.D.; Patel, C.N.; Patel, J.V.; Bhargava, A.B.; Shankar, V. Human adipose tissue-derived mesenchymal stem cells combined with hematopoietic stem cell transplantation synthesize insulin. Transplant. Proc. 2008, 40, 1135–1139. [Google Scholar] [CrossRef]
- Menasche, P.; Alfieri, O.; Janssens, S.; McKenna, W.; Reichenspurner, H.; Trinquart, L.; Vilquin, J.T.; Marolleau, J.P.; Seymour, B.; Larghero, J.; et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: First randomized placebo-controlled study of myoblast transplantation. Circulation 2008, 117, 1189–1200. [Google Scholar] [CrossRef]
- Hagege, A.A.; Marolleau, J.P.; Vilquin, J.T.; Alheritiere, A.; Peyrard, S.; Duboc, D.; Abergel, E.; Messas, E.; Mousseaux, E.; Schwartz, K.; et al. Skeletal myoblast transplantation in ischemic heart failure: Long-term follow-up of the first phase I cohort of patients. Circulation 2006, 114, I108–I113. [Google Scholar] [CrossRef]
- Burt, R.K.; Loh, Y.; Cohen, B.; Stefoski, D.; Balabanov, R.; Katsamakis, G.; Oyama, Y.; Russell, E.J.; Stern, J.; Muraro, P.; et al. Autologous non-myeloablative haemopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: A phase I/II study. Lancet Neurol. 2009, 8, 244–253. [Google Scholar] [CrossRef]
- Bjorklund, L.M.; Sanchez-Pernaute, R.; Chung, S.; Andersson, T.; Chen, I.Y.; McNaught, K.S.; Brownell, A.L.; Jenkins, B.G.; Wahlestedt, C.; Kim, K.S.; et al. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc. Natl. Acad. Sci. USA 2002, 99, 2344–2349. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, O.; Kokaia, Z. Stem cells for the treatment of neurological disorders. Nature 2006, 441, 1094–1096. [Google Scholar] [CrossRef] [PubMed]
- Nasef, A.; Ashammakhi, N.; Fouillard, L. Immunomodulatory effect of mesenchymal stromal cells: Possible mechanisms. Regen. Med. 2008, 3, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.R.; Hare, J.M. Mesenchymal stem cells: Biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ. Res. 2011, 109, 923–940. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef]
- Yin, Y.; Li, X.; He, X.T.; Wu, R.X.; Sun, H.H.; Chen, F.M. Leveraging Stem Cell Homing for Therapeutic Regeneration. J. Dent. Res. 2017, 96, 601–609. [Google Scholar] [CrossRef]
- Caplan, A.I.; Correa, D. The MSC: An injury drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Kluter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Hass, R.; Kasper, C.; Bohm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. CCS 2011, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Curtis, L.A.; Janowska-Wieczorek, A.; McGann, L.E.; Elliott, J.A. Mesenchymal stromal cells derived from various tissues: Biological, clinical and cryopreservation aspects. Cryobiology 2015, 71, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, M.; Lin, L.; Chen, P.; Ma, K.T.; Zhou, C.Y.; Ao, Y.F. Runx2 overexpression enhances osteoblastic differentiation and mineralization in adipose--derived stem cells in vitro and in vivo. Calcif. Tissue Int. 2006, 79, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Xu, J.; Wu, X.; Xie, Z.; Luo, F.; Zhang, Z.; Zeng, L. Umbilical cord Wharton’s Jelly: A new potential cell source of mesenchymal stromal cells for bone tissue engineering. Tissue Eng. A 2009, 15, 2325–2334. [Google Scholar] [CrossRef]
- Huang, G.T.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells-current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Amine, M.; Malka, G. Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). Int. J. Mol. Sci. 2019, 20, 2523. [Google Scholar] [CrossRef]
- Mizuno, H. Adipose-derived stem and stromal cells for cell-based therapy: Current status of preclinical studies and clinical trials. Curr. Opin. Mol. Ther. 2010, 12, 442–449. [Google Scholar]
- Ruetze, M.; Richter, W. Adipose-derived stromal cells for osteoarticular repair: Trophic function versus stem cell activity. Expert Rev. Mol. Med. 2014, 16, e9. [Google Scholar] [CrossRef] [PubMed]
- Im, W.; Ban, J.; Lim, J.; Lee, M.; Lee, S.T.; Chu, K.; Kim, M. Extracts of adipose derived stem cells slows progression in the R6/2 model of Huntington’s disease. PLoS ONE 2013, 8, e59438. [Google Scholar] [CrossRef] [PubMed]
- Lataillade, J.J.; Doucet, C.; Bey, E.; Carsin, H.; Huet, C.; Clairand, I.; Bottollier-Depois, J.F.; Chapel, A.; Ernou, I.; Gourven, M.; et al. New approach to radiation burn treatment by dosimetry-guided surgery combined with autologous mesenchymal stem cell therapy. Regen. Med. 2007, 2, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Bey, E.; Prat, M.; Duhamel, P.; Benderitter, M.; Brachet, M.; Trompier, F.; Battaglini, P.; Ernou, I.; Boutin, L.; Gourven, M.; et al. Emerging therapy for improving wound repair of severe radiation burns using local bone marrow-derived stem cell administrations. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2010, 18, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Linard, C.; Brachet, M.; L’Homme, B.; Strup-Perrot, C.; Busson, E.; Bonneau, M.; Lataillade, J.J.; Bey, E.; Benderitter, M. Long-term effectiveness of local BM-MSCs for skeletal muscle regeneration: A proof of concept obtained on a pig model of severe radiation burn. Stem Cell Res. Ther. 2018, 9, 299. [Google Scholar] [CrossRef]
- Linard, C.; Busson, E.; Holler, V.; Strup-Perrot, C.; Lacave-Lapalun, J.V.; Lhomme, B.; Prat, M.; Devauchelle, P.; Sabourin, J.C.; Simon, J.M.; et al. Repeated autologous bone marrow-derived mesenchymal stem cell injections improve radiation-induced proctitis in pigs. Stem Cells Transl. Med. 2013, 2, 916–927. [Google Scholar] [CrossRef]
- Fortunel, N.O.; Chadli, L.; Coutier, J.; Lemaitre, G.; Auvre, F.; Domingues, S.; Bouissou-Cadio, E.; Vaigot, P.; Cavallero, S.; Deleuze, J.F.; et al. KLF4 inhibition promotes the expansion of keratinocyte precursors from adult human skin and of embryonic-stem-cell-derived keratinocytes. Nat. Biomed. Eng. 2019, 3, 985–997. [Google Scholar] [CrossRef]
- Ankrum, J.; Karp, J.M. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol. Med. 2010, 16, 203–209. [Google Scholar] [CrossRef]
- Ning, H.; Yang, F.; Jiang, M.; Hu, L.; Feng, K.; Zhang, J.; Yu, Z.; Li, B.; Xu, C.; Li, Y.; et al. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: Outcome of a pilot clinical study. Leukemia 2008, 22, 593–599. [Google Scholar] [CrossRef]
- Sundin, M.; Orvell, C.; Rasmusson, I.; Sundberg, B.; Ringden, O.; Le Blanc, K. Mesenchymal stem cells are susceptible to human herpesviruses, but viral DNA cannot be detected in the healthy seropositive individual. Bone Marrow Transplant. 2006, 37, 1051–1059. [Google Scholar] [CrossRef]
- Pilehvar-Soltanahmadi, Y.; Nouri, M.; Martino, M.M.; Fattahi, A.; Alizadeh, E.; Darabi, M.; Rahmati-Yamchi, M.; Zarghami, N. Cytoprotection, proliferation and epidermal differentiation of adipose tissue-derived stem cells on emu oil based electrospun nanofibrous mat. Exp. Cell Res. 2017, 357, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Nejati-Koshki, K.; Mortazavi, Y.; Pilehvar-Soltanahmadi, Y.; Sheoran, S.; Zarghami, N. An update on application of nanotechnology and stem cells in spinal cord injury regeneration. Biomed. Pharmacother. 2017, 90, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Saei Arezoumand, K.; Alizadeh, E.; Pilehvar-Soltanahmadi, Y.; Esmaeillou, M.; Zarghami, N. An overview on different strategies for the stemness maintenance of MSCs. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1255–1271. [Google Scholar] [CrossRef] [PubMed]
- Herberts, C.A.; Kwa, M.S.; Hermsen, H.P. Risk factors in the development of stem cell therapy. J. Transl. Med. 2011, 9, 29. [Google Scholar] [CrossRef]
- Lukomska, B.; Stanaszek, L.; Zuba-Surma, E.; Legosz, P.; Sarzynska, S.; Drela, K. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells Int. 2019, 2019, 9628536. [Google Scholar] [CrossRef]
- Celikkan, F.T.; Mungan, C.; Sucu, M.; Ulus, A.T.; Cinar, O.; Ili, E.G.; Can, A. Optimizing the transport and storage conditions of current Good Manufacturing Practice -grade human umbilical cord mesenchymal stromal cells for transplantation (HUC-HEART Trial). Cytotherapy 2019, 21, 64–75. [Google Scholar] [CrossRef]
- Filip, S.; Mokry, J.; Horacek, J.; English, D. Stem cells and the phenomena of plasticity and diversity: A limiting property of carcinogenesis. Stem Cells Dev. 2008, 17, 1031–1038. [Google Scholar] [CrossRef]
- Heslop, J.A.; Hammond, T.G.; Santeramo, I.; Tort Piella, A.; Hopp, I.; Zhou, J.; Baty, R.; Graziano, E.I.; Proto Marco, B.; Caron, A.; et al. Concise review: Workshop review: Understanding and assessing the risks of stem cell-based therapies. Stem Cells Transl. Med. 2015, 4, 389–400. [Google Scholar] [CrossRef]
- Rubio, D.; Garcia-Castro, J.; Martin, M.C.; de la Fuente, R.; Cigudosa, J.C.; Lloyd, A.C.; Bernad, A. Spontaneous human adult stem cell transformation. Cancer Res. 2005, 65, 3035–3039. [Google Scholar] [CrossRef]
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; Weinberg, R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007, 449, 557–563. [Google Scholar] [CrossRef]
- Galleu, A.; Riffo-Vasquez, Y.; Trento, C.; Lomas, C.; Dolcetti, L.; Cheung, T.S.; von Bonin, M.; Barbieri, L.; Halai, K.; Ward, S.; et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med. 2017, 9, eaam7828. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; O’Hara, M.D.; Laptev, A.V.; Halford, K.W.; Pollard, M.D.; Class, R.; Simon, D.; Livezey, K.; Prockop, D.J. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc. Natl. Acad. Sci. USA 1998, 95, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Pulin, A.A.; Seo, M.J.; Kota, D.J.; Ylostalo, J.; Larson, B.L.; Semprun-Prieto, L.; Delafontaine, P.; Prockop, D.J. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 2009, 5, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Dennis, J.E.; Muzic, R.F.; Lundberg, M.; Caplan, A.I. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs 2001, 169, 12–20. [Google Scholar] [CrossRef]
- Schrepfer, S.; Deuse, T.; Reichenspurner, H.; Fischbein, M.P.; Robbins, R.C.; Pelletier, M.P. Stem cell transplantation: The lung barrier. Transplant. Proc. 2007, 39, 573–576. [Google Scholar] [CrossRef]
- Hou, D.; Youssef, E.A.; Brinton, T.J.; Zhang, P.; Rogers, P.; Price, E.T.; Yeung, A.C.; Johnstone, B.H.; Yock, P.G.; March, K.L. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: Implications for current clinical trials. Circulation 2005, 112, I150–I156. [Google Scholar]
- Kidd, S.; Spaeth, E.; Dembinski, J.L.; Dietrich, M.; Watson, K.; Klopp, A.; Battula, V.L.; Weil, M.; Andreeff, M.; Marini, F.C. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells 2009, 27, 2614–2623. [Google Scholar] [CrossRef]
- Murry, C.E.; Soonpaa, M.H.; Reinecke, H.; Nakajima, H.; Nakajima, H.O.; Rubart, M.; Pasumarthi, K.B.; Virag, J.I.; Bartelmez, S.H.; Poppa, V.; et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature 2004, 428, 664–668. [Google Scholar] [CrossRef]
- Toma, C.; Wagner, W.R.; Bowry, S.; Schwartz, A.; Villanueva, F. Fate of culture-expanded mesenchymal stem cells in the microvasculature: In vivo observations of cell kinetics. Circ. Res. 2009, 104, 398–402. [Google Scholar] [CrossRef]
- Leiker, M.; Suzuki, G.; Iyer, V.S.; Canty, J.M., Jr.; Lee, T. Assessment of a nuclear affinity labeling method for tracking implanted mesenchymal stem cells. Cell Transplant. 2008, 17, 911–922. [Google Scholar] [CrossRef]
- Wang, L.; Deng, J.; Tian, W.; Xiang, B.; Yang, T.; Li, G.; Wang, J.; Gruwel, M.; Kashour, T.; Rendell, J.; et al. Adipose-derived stem cells are an effective cell candidate for treatment of heart failure: An MR imaging study of rat hearts. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1020–H1031. [Google Scholar] [CrossRef] [PubMed]
- Bayes-Genis, A.; Soler-Botija, C.; Farre, J.; Sepulveda, P.; Raya, A.; Roura, S.; Prat-Vidal, C.; Galvez-Monton, C.; Montero, J.A.; Buscher, D.; et al. Human progenitor cells derived from cardiac adipose tissue ameliorate myocardial infarction in rodents. J. Mol. Cell. Cardiol. 2010, 49, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Mazo, M.; Planat-Benard, V.; Abizanda, G.; Pelacho, B.; Leobon, B.; Gavira, J.J.; Penuelas, I.; Cemborain, A.; Penicaud, L.; Laharrague, P.; et al. Transplantation of adipose derived stromal cells is associated with functional improvement in a rat model of chronic myocardial infarction. Eur. J. Heart Fail. 2008, 10, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Greenberg, D.A. Tales of transdifferentiation. Exp. Neurol. 2003, 183, 255–257. [Google Scholar] [CrossRef]
- Togel, F.; Hu, Z.; Weiss, K.; Isaac, J.; Lange, C.; Westenfelder, C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am. J. Physiol. Ren. Physiol. 2005, 289, F31–F42. [Google Scholar] [CrossRef]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef]
- Gnecchi, M.; He, H.; Noiseux, N.; Liang, O.D.; Zhang, L.; Morello, F.; Mu, H.; Melo, L.G.; Pratt, R.E.; Ingwall, J.S.; et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 661–669. [Google Scholar]
- Teixeira, F.G.; Carvalho, M.M.; Sousa, N.; Salgado, A.J. Mesenchymal stem cells secretome: A new paradigm for central nervous system regeneration? Cell. Mol. Life Sci. CMLS 2013, 70, 3871–3882. [Google Scholar] [CrossRef]
- Madrigal, M.; Rao, K.S.; Riordan, N.H. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J. Transl. Med. 2014, 12, 260. [Google Scholar] [CrossRef]
- Chen, L.; Tredget, E.E.; Wu, P.Y.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef]
- Hung, S.C.; Pochampally, R.R.; Chen, S.C.; Hsu, S.C.; Prockop, D.J. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells 2007, 25, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Togel, F.; Weiss, K.; Yang, Y.; Hu, Z.; Zhang, P.; Westenfelder, C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am. J. Physiol. Ren. Physiol. 2007, 292, F1626–F1635. [Google Scholar] [CrossRef] [PubMed]
- van Poll, D.; Parekkadan, B.; Cho, C.H.; Berthiaume, F.; Nahmias, Y.; Tilles, A.W.; Yarmush, M.L. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology 2008, 47, 1634–1643. [Google Scholar] [CrossRef]
- Uemura, R.; Xu, M.; Ahmad, N.; Ashraf, M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ. Res. 2006, 98, 1414–1421. [Google Scholar] [CrossRef]
- Cunningham, C.J.; Redondo-Castro, E.; Allan, S.M. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2018, 38, 1276–1292. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, H.; Shen, H.; Wang, B.; Lei, G.; Tuan, R.S. Mesenchymal stem cell-derived extracellular matrix enhances chondrogenic phenotype of and cartilage formation by encapsulated chondrocytes in vitro and in vivo. Acta Biomater. 2018, 69, 71–82. [Google Scholar] [CrossRef]
- Beer, L.; Mildner, M.; Ankersmit, H.J. Cell secretome based drug substances in regenerative medicine: When regulatory affairs meet basic science. Ann. Transl. Med. 2017, 5, 170. [Google Scholar] [CrossRef]
- Gnecchi, M.; Danieli, P.; Malpasso, G.; Ciuffreda, M.C. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Methods Mol. Biol. 2016, 1416, 123–146. [Google Scholar]
- Liu, L.; Gao, J.; Yuan, Y.; Chang, Q.; Liao, Y.; Lu, F. Hypoxia preconditioned human adipose derived mesenchymal stem cells enhance angiogenic potential via secretion of increased VEGF and bFGF. Cell Biol. Int. 2013, 37, 551–560. [Google Scholar] [CrossRef]
- Tasso, R.; Gaetani, M.; Molino, E.; Cattaneo, A.; Monticone, M.; Bachi, A.; Cancedda, R. The role of bFGF on the ability of MSC to activate endogenous regenerative mechanisms in an ectopic bone formation model. Biomaterials 2012, 33, 2086–2096. [Google Scholar] [CrossRef]
- Magne, B.; Dedier, M.; Nivet, M.; Coulomb, B.; Banzet, S.; Lataillade, J.J.; Trouillas, M. IL-1beta-Primed Mesenchymal Stromal Cells Improve Epidermal Substitute Engraftment and Wound Healing via Matrix Metalloproteinases and Transforming Growth Factor-beta1. J. Investig. Dermatol. 2020, 140, 688–698 e621. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Van Balkom, B.W.M.; Bruno, S.; Choo, A.; Dominici, M.; Gimona, M.; Hill, A.F.; De Kleijn, D.; Koh, M.; Lai, R.C.; et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell. Vesicles 2019, 8, 1609206. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Behnke, O. Electron microscopical observations on the surface coating of human blood platelets. J. Ultrastruct. Res. 1968, 24, 51–69. [Google Scholar] [CrossRef]
- Chargaff, E.; West, R. The biological significance of the thromboplastic protein of blood. J. Biol. Chem. 1946, 166, 189–197. [Google Scholar] [PubMed]
- Keller, S.; Ridinger, J.; Rupp, A.K.; Janssen, J.W.; Altevogt, P. Body fluid derived exosomes as a novel template for clinical diagnostics. J. Transl. Med. 2011, 9, 86. [Google Scholar] [CrossRef]
- Caby, M.P.; Lankar, D.; Vincendeau-Scherrer, C.; Raposo, G.; Bonnerot, C. Exosomal-like vesicles are present in human blood plasma. Int. Immunol. 2005, 17, 879–887. [Google Scholar] [CrossRef]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Turturici, G.; Tinnirello, R.; Sconzo, G.; Geraci, F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: Advantages and disadvantages. Am. J. Physiol. Cell Physiol. 2014, 306, C621–C633. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Thery, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.D.; Homesley, H.D.; Doellgast, G.J. Binding of specific peroxidase-labeled antibody to placental-type phosphatase on tumor-derived membrane fragments. Cancer Res. 1980, 40, 4064–4069. [Google Scholar] [PubMed]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular vesicles in cancer: Exosomes, microvesicles and the emerging role of large oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, J.; Wysoczynski, M.; Hayek, F.; Janowska-Wieczorek, A.; Ratajczak, M.Z. Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia 2006, 20, 1487–1495. [Google Scholar] [CrossRef]
- Moskovich, O.; Fishelson, Z. Live cell imaging of outward and inward vesiculation induced by the complement c5b-9 complex. J. Biol. Chem. 2007, 282, 29977–29986. [Google Scholar] [CrossRef] [PubMed]
- Gyorgy, B.; Szabo, T.G.; Pasztoi, M.; Pal, Z.; Misjak, P.; Aradi, B.; Laszlo, V.; Pallinger, E.; Pap, E.; Kittel, A.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell. Mol. Life Sci. CMLS 2011, 68, 2667–2688. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.B.; Brennan, M.A.; Lotvall, J.; Breakefield, X.O.; El Andaloussi, S. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019, 11, eaav8521. [Google Scholar] [CrossRef]
- Dias, I.E.; Pinto, P.O.; Barros, L.C.; Viegas, C.A.; Dias, I.R.; Carvalho, P.P. Mesenchymal stem cells therapy in companion animals: Useful for immune-mediated diseases? BMC Vet. Res. 2019, 15, 358. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Hauser, P.; Wang, S.; Didenko, V.V. Apoptotic Bodies: Selective Detection in Extracellular Vesicles. Methods Mol. Biol. 2017, 1554, 193–200. [Google Scholar]
- Gregory, C.D.; Dransfield, I. Apoptotic Tumor Cell-Derived Extracellular Vesicles as Important Regulators of the Onco-Regenerative Niche. Front. Immunol. 2018, 9, 1111. [Google Scholar] [CrossRef]
- Hinger, S.A.; Cha, D.J.; Franklin, J.L.; Higginbotham, J.N.; Dou, Y.; Ping, J.; Shu, L.; Prasad, N.; Levy, S.; Zhang, B.; et al. Diverse Long RNAs Are Differentially Sorted into Extracellular Vesicles Secreted by Colorectal Cancer Cells. Cell Rep. 2018, 25, 715–725 e714. [Google Scholar] [CrossRef]
- Sharma, H.; Chinnappan, M.; Agarwal, S.; Dalvi, P.; Gunewardena, S.; O’Brien-Ladner, A.; Dhillon, N.K. Macrophage-derived extracellular vesicles mediate smooth muscle hyperplasia: Role of altered miRNA cargo in response to HIV infection and substance abuse. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 5174–5185. [Google Scholar] [CrossRef]
- Cocucci, E.; Racchetti, G.; Podini, P.; Meldolesi, J. Enlargeosome traffic: Exocytosis triggered by various signals is followed by endocytosis, membrane shedding or both. Traffic 2007, 8, 742–757. [Google Scholar] [CrossRef]
- Li, C.C.; Eaton, S.A.; Young, P.E.; Lee, M.; Shuttleworth, R.; Humphreys, D.T.; Grau, G.E.; Combes, V.; Bebawy, M.; Gong, J.; et al. Glioma microvesicles carry selectively packaged coding and non-coding RNAs which alter gene expression in recipient cells. RNA Biol. 2013, 10, 1333–1344. [Google Scholar] [CrossRef]
- Gasser, O.; Schifferli, J.A. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood 2004, 104, 2543–2548. [Google Scholar] [CrossRef] [PubMed]
- Janowska-Wieczorek, A.; Majka, M.; Kijowski, J.; Baj-Krzyworzeka, M.; Reca, R.; Turner, A.R.; Ratajczak, J.; Emerson, S.G.; Kowalska, M.A.; Ratajczak, M.Z. Platelet-derived microparticles bind to hematopoietic stem/progenitor cells and enhance their engraftment. Blood 2001, 98, 3143–3149. [Google Scholar] [CrossRef] [PubMed]
- Morel, O.; Toti, F.; Hugel, B.; Freyssinet, J.M. Cellular microparticles: A disseminated storage pool of bioactive vascular effectors. Curr. Opin. Hematol. 2004, 11, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Kupcova Skalnikova, H. Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie 2013, 95, 2196–2211. [Google Scholar] [CrossRef]
- Candela, M.E.; Geraci, F.; Turturici, G.; Taverna, S.; Albanese, I.; Sconzo, G. Membrane vesicles containing matrix metalloproteinase-9 and fibroblast growth factor-2 are released into the extracellular space from mouse mesoangioblast stem cells. J. Cell. Physiol. 2010, 224, 144–151. [Google Scholar] [CrossRef]
- MacKenzie, A.; Wilson, H.L.; Kiss-Toth, E.; Dower, S.K.; North, R.A.; Surprenant, A. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity 2001, 15, 825–835. [Google Scholar] [CrossRef]
- Lai, R.C.; Tan, S.S.; Teh, B.J.; Sze, S.K.; Arslan, F.; de Kleijn, D.P.; Choo, A.; Lim, S.K. Proteolytic Potential of the MSC Exosome Proteome: Implications for an Exosome-Mediated Delivery of Therapeutic Proteasome. Int. J. Proteom. 2012, 2012, 971907. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Choi, D.Y.; Yun, S.J.; Choi, S.M.; Kang, J.W.; Jung, J.W.; Hwang, D.; Kim, K.P.; Kim, D.W. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J. Proteome Res. 2012, 11, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Lee, J.; Kim, S.R.; Choi, D.S.; Yoon, Y.J.; Kim, J.H.; Go, G.; Nhung, D.; Hong, K.; Jang, S.C.; et al. EVpedia: A community web portal for extracellular vesicles research. Bioinformatics 2015, 31, 933–939. [Google Scholar] [CrossRef]
- Simpson, R.J.; Kalra, H.; Mathivanan, S. ExoCarta as a resource for exosomal research. J. Extracell. Vesicles 2012, 1. [Google Scholar] [CrossRef]
- Mathivanan, S.; Fahner, C.J.; Reid, G.E.; Simpson, R.J. ExoCarta 2012: Database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012, 40, D1241–D1244. [Google Scholar] [CrossRef]
- Hsiao, S.T.; Asgari, A.; Lokmic, Z.; Sinclair, R.; Dusting, G.J.; Lim, S.Y.; Dilley, R.J. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev. 2012, 21, 2189–2203. [Google Scholar] [CrossRef]
- Eirin, A.; Zhu, X.Y.; Puranik, A.S.; Woollard, J.R.; Tang, H.; Dasari, S.; Lerman, A.; van Wijnen, A.J.; Lerman, L.O. Comparative proteomic analysis of extracellular vesicles isolated from porcine adipose tissue-derived mesenchymal stem/stromal cells. Sci. Rep. 2016, 6, 36120. [Google Scholar] [CrossRef]
- Eirin, A.; Zhu, X.Y.; Puranik, A.S.; Tang, H.; McGurren, K.A.; van Wijnen, A.J.; Lerman, A.; Lerman, L.O. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017, 92, 114–124. [Google Scholar] [CrossRef]
- Aliotta, J.M.; Pereira, M.; Johnson, K.W.; de Paz, N.; Dooner, M.S.; Puente, N.; Ayala, C.; Brilliant, K.; Berz, D.; Lee, D.; et al. Microvesicle entry into marrow cells mediates tissue-specific changes in mRNA by direct delivery of mRNA and induction of transcription. Exp. Hematol. 2010, 38, 233–245. [Google Scholar] [CrossRef]
- Camussi, G.; Deregibus, M.C.; Bruno, S.; Grange, C.; Fonsato, V.; Tetta, C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am. J. Cancer Res. 2011, 1, 98–110. [Google Scholar] [PubMed]
- Camussi, G.; Deregibus, M.C.; Tetta, C. Paracrine/endocrine mechanism of stem cells on kidney repair: Role of microvesicle-mediated transfer of genetic information. Curr. Opin. Nephrol. Hypertens. 2010, 19, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef]
- Ma, T.; Chen, Y.; Chen, Y.; Meng, Q.; Sun, J.; Shao, L.; Yu, Y.; Huang, H.; Hu, Y.; Yang, Z.; et al. MicroRNA-132, Delivered by Mesenchymal Stem Cell-Derived Exosomes, Promote Angiogenesis in Myocardial Infarction. Stem Cells Int. 2018, 2018, 3290372. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and exosomal microRNA: Trafficking, sorting, and function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef]
- Salinas-Vera, Y.M.; Marchat, L.A.; Gallardo-Rincon, D.; Ruiz-Garcia, E.; Astudillo-De La Vega, H.; Echavarria-Zepeda, R.; Lopez-Camarillo, C. AngiomiRs: MicroRNAs driving angiogenesis in cancer (Review). Int. J. Mol. Med. 2019, 43, 657–670. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Wang, S.; Han, Q.; Zhao, R.C. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J. Cell Sci. 2016, 129, 2182–2189. [Google Scholar] [CrossRef]
- Baglio, S.R.; Rooijers, K.; Koppers-Lalic, D.; Verweij, F.J.; Perez Lanzon, M.; Zini, N.; Naaijkens, B.; Perut, F.; Niessen, H.W.; Baldini, N.; et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res. Ther. 2015, 6, 127. [Google Scholar] [CrossRef]
- De Luca, L.; Trino, S.; Laurenzana, I.; Simeon, V.; Calice, G.; Raimondo, S.; Podesta, M.; Santodirocco, M.; Di Mauro, L.; La Rocca, F.; et al. MiRNAs and piRNAs from bone marrow mesenchymal stem cell extracellular vesicles induce cell survival and inhibit cell differentiation of cord blood hematopoietic stem cells: A new insight in transplantation. Oncotarget 2016, 7, 6676–6692. [Google Scholar] [CrossRef]
- Fatima, F.; Nawaz, M. Vesiculated Long Non-Coding RNAs: Offshore Packages Deciphering Trans-Regulation between Cells, Cancer Progression and Resistance to Therapies. Non-Coding RNA 2017, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.S.; Kim, D.K.; Kim, Y.K.; Gho, Y.S. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics 2013, 13, 1554–1571. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Sun, C.; Sun, Y.; Li, H.; Yang, L.; Wu, D.; Gao, Q.; Jiang, X. Lipid, Protein, and MicroRNA Composition Within Mesenchymal Stem Cell-Derived Exosomes. Cell. Reprogramming 2018, 20, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Yang, K.; Liang, Z.; Wan, Y.; Cheng, Y.; Ma, D.; Zhang, H.; Hou, W.; Fu, P. Sphingosine-1-phosphate mediates the therapeutic effects of bone marrow mesenchymal stem cell-derived microvesicles on articular cartilage defect. Transl. Res. J. Lab. Clin. Med. 2018, 193, 42–53. [Google Scholar] [CrossRef]
- Miyanishi, M.; Tada, K.; Koike, M.; Uchiyama, Y.; Kitamura, T.; Nagata, S. Identification of Tim4 as a phosphatidylserine receptor. Nature 2007, 450, 435–439. [Google Scholar] [CrossRef]
- Yang, D.; Wang, W.; Li, L.; Peng, Y.; Chen, P.; Huang, H.; Guo, Y.; Xia, X.; Wang, Y.; Wang, H.; et al. The relative contribution of paracine effect versus direct differentiation on adipose-derived stem cell transplantation mediated cardiac repair. PLoS ONE 2013, 8, e59020. [Google Scholar] [CrossRef]
- Salgado, A.J.; Reis, R.L.; Sousa, N.J.; Gimble, J.M. Adipose tissue derived stem cells secretome: Soluble factors and their roles in regenerative medicine. Curr. Stem Cell Res. Ther. 2010, 5, 103–110. [Google Scholar] [CrossRef]
- Drago, D.; Cossetti, C.; Iraci, N.; Gaude, E.; Musco, G.; Bachi, A.; Pluchino, S. The stem cell secretome and its role in brain repair. Biochimie 2013, 95, 2271–2285. [Google Scholar] [CrossRef]
- Yin, L.; Liu, X.; Shi, Y.; Ocansey, D.K.W.; Hu, Y.; Li, X.; Zhang, C.; Xu, W.; Qian, H. Therapeutic Advances of Stem Cell-Derived Extracellular Vesicles in Regenerative Medicine. Cells 2020, 9, 707. [Google Scholar] [CrossRef]
- Zhang, B.; Tian, X.; Hao, J.; Xu, G.; Zhang, W. Mesenchymal Stem Cell-Derived Extracellular Vesicles in Tissue Regeneration. Cell Transplant. 2020, 29, 963689720908500. [Google Scholar] [CrossRef]
- Lee, C.; Mitsialis, S.A.; Aslam, M.; Vitali, S.H.; Vergadi, E.; Konstantinou, G.; Sdrimas, K.; Fernandez-Gonzalez, A.; Kourembanas, S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 2012, 126, 2601–2611. [Google Scholar] [CrossRef]
- Aliotta, J.M.; Pereira, M.; Wen, S.; Dooner, M.S.; Del Tatto, M.; Papa, E.; Goldberg, L.R.; Baird, G.L.; Ventetuolo, C.E.; Quesenberry, P.J.; et al. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovasc. Res. 2016, 110, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.D.; Shi, L.; Monsel, A.; Li, X.Y.; Zhu, H.L.; Zhu, Y.G.; Qu, J.M. Mesenchymal Stem Cell Microvesicles Attenuate Acute Lung Injury in Mice Partly Mediated by Ang-1 mRNA. Stem Cells 2017, 35, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Potter, D.R.; Miyazawa, B.Y.; Gibb, S.L.; Deng, X.; Togaratti, P.P.; Croze, R.H.; Srivastava, A.K.; Trivedi, A.; Matthay, M.; Holcomb, J.B.; et al. Mesenchymal stem cell-derived extracellular vesicles attenuate pulmonary vascular permeability and lung injury induced by hemorrhagic shock and trauma. J. Trauma Acute Care Surg. 2018, 84, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Gatti, S.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Cantaluppi, V.; Tetta, C.; Camussi, G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2011, 26, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Grange, C.; Collino, F.; Deregibus, M.C.; Cantaluppi, V.; Biancone, L.; Tetta, C.; Camussi, G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS ONE 2012, 7, e33115. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Tapparo, M.; Collino, F.; Chiabotto, G.; Deregibus, M.C.; Soares Lindoso, R.; Neri, F.; Kholia, S.; Giunti, S.; Wen, S.; et al. Renal Regenerative Potential of Different Extracellular Vesicle Populations Derived from Bone Marrow Mesenchymal Stromal Cells. Tissue Eng. A 2017, 23, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Gu, D.; Xing, X.; Cheng, Z.; Gong, D.; Zhang, G.; Zhu, Y. Human mesenchymal stromal cell-derived extracellular vesicles alleviate renal ischemic reperfusion injury and enhance angiogenesis in rats. Am. J. Transl. Res. 2016, 8, 4289–4299. [Google Scholar]
- Zou, X.; Zhang, G.; Cheng, Z.; Yin, D.; Du, T.; Ju, G.; Miao, S.; Liu, G.; Lu, M.; Zhu, Y. Microvesicles derived from human Wharton’s Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res. Ther. 2014, 5, 40. [Google Scholar] [CrossRef]
- He, J.; Wang, Y.; Lu, X.; Zhu, B.; Pei, X.; Wu, J.; Zhao, W. Micro-vesicles derived from bone marrow stem cells protect the kidney both in vivo and in vitro by microRNA-dependent repairing. Nephrology 2015, 20, 591–600. [Google Scholar] [CrossRef]
- He, J.; Wang, Y.; Sun, S.; Yu, M.; Wang, C.; Pei, X.; Zhu, B.; Wu, J.; Zhao, W. Bone marrow stem cells-derived microvesicles protect against renal injury in the mouse remnant kidney model. Nephrology 2012, 17, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Lee, H.G.; Kim, B.S.; Ahn, S.H.; Jung, A.; Lee, M.; Lee, J.E.; Kim, H.J.; Ha, S.K.; Park, H.C. Mesenchymal stem cell-derived microparticles ameliorate peritubular capillary rarefaction via inhibition of endothelial-mesenchymal transition and decrease tubulointerstitial fibrosis in unilateral ureteral obstruction. Stem Cell Res. Ther. 2015, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Eirin, A.; Zhu, X.Y.; Puranik, A.S.; Woollard, J.R.; Tang, H.; Dasari, S.; Lerman, A.; van Wijnen, A.J.; Lerman, L.O. Integrated transcriptomic and proteomic analysis of the molecular cargo of extracellular vesicles derived from porcine adipose tissue-derived mesenchymal stem cells. PLoS ONE 2017, 12, e0174303. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Liu, J.; Zhang, F.; Wang, Y.; Qin, Y.; Zhou, Z.; Qiu, J.; Fan, Y. CCR2 Positive Exosome Released by Mesenchymal Stem Cells Suppresses Macrophage Functions and Alleviates Ischemia/Reperfusion-Induced Renal Injury. Stem Cells Int. 2016, 2016, 1240301. [Google Scholar] [CrossRef]
- Jia, H.; Liu, W.; Zhang, B.; Wang, J.; Wu, P.; Tandra, N.; Liang, Z.; Ji, C.; Yin, L.; Hu, X.; et al. HucMSC exosomes-delivered 14-3-3zeta enhanced autophagy via modulation of ATG16L in preventing cisplatin-induced acute kidney injury. Am. J. Transl. Res. 2018, 10, 101–113. [Google Scholar]
- Wang, B.; Jia, H.; Zhang, B.; Wang, J.; Ji, C.; Zhu, X.; Yan, Y.; Yin, L.; Yu, J.; Qian, H.; et al. Pre-incubation with hucMSC-exosomes prevents cisplatin-induced nephrotoxicity by activating autophagy. Stem Cell Res. Ther. 2017, 8, 75. [Google Scholar] [CrossRef]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef]
- Shao, L.; Zhang, Y.; Lan, B.; Wang, J.; Zhang, Z.; Zhang, L.; Xiao, P.; Meng, Q.; Geng, Y.J.; Yu, X.Y.; et al. MiRNA-Sequence Indicates That Mesenchymal Stem Cells and Exosomes Have Similar Mechanism to Enhance Cardiac Repair. BioMed Res. Int. 2017, 2017, 4150705. [Google Scholar] [CrossRef]
- Pan, W.; Zhu, Y.; Meng, X.; Zhang, C.; Yang, Y.; Bei, Y. Immunomodulation by Exosomes in Myocardial Infarction. J. Cardiovasc. Transl. Res. 2019, 12, 28–36. [Google Scholar] [CrossRef]
- Teng, X.; Chen, L.; Chen, W.; Yang, J.; Yang, Z.; Shen, Z. Mesenchymal Stem Cell-Derived Exosomes Improve the Microenvironment of Infarcted Myocardium Contributing to Angiogenesis and Anti-Inflammation. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015, 37, 2415–2424. [Google Scholar] [CrossRef]
- Arslan, F.; Lai, R.C.; Smeets, M.B.; Akeroyd, L.; Choo, A.; Aguor, E.N.; Timmers, L.; van Rijen, H.V.; Doevendans, P.A.; Pasterkamp, G.; et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013, 10, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wang, H.; Jin, M.; Yang, X.; Ji, H.; Jiang, Y.; Zhang, H.; Wu, F.; Wu, G.; Lai, X.; et al. Exosomes from MiR-30d-5p-ADSCs Reverse Acute Ischemic Stroke-Induced, Autophagy-Mediated Brain Injury by Promoting M2 Microglial/Macrophage Polarization. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 47, 864–878. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, X.; Cao, W.; Ma, J.; Sun, L.; Qian, H.; Zhu, W.; Xu, W. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Relieve Acute Myocardial Ischemic Injury. Stem Cells Int. 2015, 2015, 761643. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, X.; Gong, X.; Wang, G. Human umbilical cord mesenchymal stem cells derived exosomes exert antiapoptosis effect via activating PI3K/Akt/mTOR pathway on H9C2 cells. J. Cell. Biochem. 2019, 120, 14455–14464. [Google Scholar] [CrossRef]
- Vrijsen, K.R.; Maring, J.A.; Chamuleau, S.A.; Verhage, V.; Mol, E.A.; Deddens, J.C.; Metz, C.H.; Lodder, K.; van Eeuwijk, E.C.; van Dommelen, S.M.; et al. Exosomes from Cardiomyocyte Progenitor Cells and Mesenchymal Stem Cells Stimulate Angiogenesis Via EMMPRIN. Adv. Healthc. Mater. 2016, 5, 2555–2565. [Google Scholar] [CrossRef]
- Wang, N.; Chen, C.; Yang, D.; Liao, Q.; Luo, H.; Wang, X.; Zhou, F.; Yang, X.; Yang, J.; Zeng, C.; et al. Mesenchymal stem cells-derived extracellular vesicles, via miR-210, improve infarcted cardiac function by promotion of angiogenesis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2085–2092. [Google Scholar] [CrossRef]
- Luther, K.M.; Haar, L.; McGuinness, M.; Wang, Y.; Lynch Iv, T.L.; Phan, A.; Song, Y.; Shen, Z.; Gardner, G.; Kuffel, G.; et al. Exosomal miR-21a-5p mediates cardioprotection by mesenchymal stem cells. J. Mol. Cell. Cardiol. 2018, 119, 125–137. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, Z.; Webster, K.A.; Chen, J.; Hu, H.; Zhou, Y.; Zhao, J.; Wang, L.; Wang, Y.; Zhong, Z.; et al. Enhanced Cardioprotection by Human Endometrium Mesenchymal Stem Cells Driven by Exosomal MicroRNA-21. Stem Cells Transl. Med. 2017, 6, 209–222. [Google Scholar] [CrossRef]
- Xiao, C.; Wang, K.; Xu, Y.; Hu, H.; Zhang, N.; Wang, Y.; Zhong, Z.; Zhao, J.; Li, Q.; Zhu, D.; et al. Transplanted Mesenchymal Stem Cells Reduce Autophagic Flux in Infarcted Hearts via the Exosomal Transfer of miR-125b. Circ. Res. 2018, 123, 564–578. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, M.; Deng, S.; Lu, J.; Huang, H.; Zhang, Y.; Gong, P.; Shen, X.; Ruan, H.; Jin, M.; et al. miR-93-5p-Containing Exosomes Treatment Attenuates Acute Myocardial Infarction-Induced Myocardial Damage. Mol. Ther. Nucleic Acids 2018, 11, 103–115. [Google Scholar] [CrossRef]
- Luo, Q.; Guo, D.; Liu, G.; Chen, G.; Hang, M.; Jin, M. Exosomes from MiR-126-Overexpressing Adscs Are Therapeutic in Relieving Acute Myocardial Ischaemic Injury. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 44, 2105–2116. [Google Scholar]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Doeppner, T.R.; Herz, J.; Gorgens, A.; Schlechter, J.; Ludwig, A.K.; Radtke, S.; de Miroschedji, K.; Horn, P.A.; Giebel, B.; Hermann, D.M. Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Postischemic Immunosuppression. Stem Cells Transl. Med. 2015, 4, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Moon, G.J.; Sung, J.H.; Kim, D.H.; Kim, E.H.; Cho, Y.H.; Son, J.P.; Cha, J.M.; Bang, O.Y. Application of Mesenchymal Stem Cell-Derived Extracellular Vesicles for Stroke: Biodistribution and MicroRNA Study. Transl. Stroke Res. 2019, 10, 509–521. [Google Scholar] [CrossRef]
- Chuang, T.J.; Lin, K.C.; Chio, C.C.; Wang, C.C.; Chang, C.P.; Kuo, J.R. Effects of secretome obtained from normoxia-preconditioned human mesenchymal stem cells in traumatic brain injury rats. J. Trauma Acute Care Surg. 2012, 73, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chopp, M.; Meng, Y.; Katakowski, M.; Xin, H.; Mahmood, A.; Xiong, Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J. Neurosurg. 2015, 122, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Nishida, H.; An, S.Y.; Shetty, A.K.; Bartosh, T.J.; Prockop, D.J. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc. Natl. Acad. Sci. USA 2016, 113, 170–175. [Google Scholar] [CrossRef]
- Patel, N.A.; Moss, L.D.; Lee, J.Y.; Tajiri, N.; Acosta, S.; Hudson, C.; Parag, S.; Cooper, D.R.; Borlongan, C.V.; Bickford, P.C. Long noncoding RNA MALAT1 in exosomes drives regenerative function and modulates inflammation-linked networks following traumatic brain injury. J. Neuroinflammation 2018, 15, 204. [Google Scholar] [CrossRef]
- Nerem, R.M. Cell-based therapies: From basic biology to replacement, repair, and regeneration. Biomaterials 2007, 28, 5074–5077. [Google Scholar] [CrossRef]
- Shiue, S.J.; Rau, R.H.; Shiue, H.S.; Hung, Y.W.; Li, Z.X.; Yang, K.D.; Cheng, J.K. Mesenchymal stem cell exosomes as a cell-free therapy for nerve injury-induced pain in rats. Pain 2019, 160, 210–223. [Google Scholar] [CrossRef]
- Sun, G.; Li, G.; Li, D.; Huang, W.; Zhang, R.; Zhang, H.; Duan, Y.; Wang, B. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 89, 194–204. [Google Scholar] [CrossRef]
- Wang, L.; Pei, S.; Han, L.; Guo, B.; Li, Y.; Duan, R.; Yao, Y.; Xue, B.; Chen, X.; Jia, Y. Mesenchymal Stem Cell-Derived Exosomes Reduce A1 Astrocytes via Downregulation of Phosphorylated NFkappaB P65 Subunit in Spinal Cord Injury. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 50, 1535–1559. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.M.; Gibbs, K.M.; Davila, J.; Campbell, N.; Sung, S.; Todorova, T.I.; Otsuka, S.; Sabaawy, H.E.; Hart, R.P.; Schachner, M. MicroRNA miR-133b is essential for functional recovery after spinal cord injury in adult zebrafish. Eur. J. Neurosci. 2011, 33, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Lener, T.; Gimona, M.; Aigner, L.; Borger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef] [PubMed]
- Lelek, J.; Zuba-Surma, E.K. Perspectives for Future Use of Extracellular Vesicles from Umbilical Cord- and Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells in Regenerative Therapies-Synthetic Review. Int. J. Mol. Sci. 2020, 21, 799. [Google Scholar] [CrossRef]
- Abbaszadeh, H.; Ghorbani, F.; Derakhshani, M.; Movassaghpour, A.; Yousefi, M. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles: A novel therapeutic paradigm. J. Cell. Physiol. 2020, 235, 706–717. [Google Scholar] [CrossRef]
- Hughes, C.S.; Nuhn, A.A.; Postovit, L.M.; Lajoie, G.A. Proteomics of human embryonic stem cells. Proteomics 2011, 11, 675–690. [Google Scholar] [CrossRef]
- Baharvand, H.; Hajheidari, M.; Ashtiani, S.K.; Salekdeh, G.H. Proteomic signature of human embryonic stem cells. Proteomics 2006, 6, 3544–3549. [Google Scholar] [CrossRef]
- Ong, S.G.; Lee, W.H.; Huang, M.; Dey, D.; Kodo, K.; Sanchez-Freire, V.; Gold, J.D.; Wu, J.C. Cross talk of combined gene and cell therapy in ischemic heart disease: Role of exosomal microRNA transfer. Circulation 2014, 130, S60–S69. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, W.; Wani, M.; Yu, X.; Ashraf, M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS ONE 2014, 9, e88685. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Li, Y.; Chen, L.; Wang, X.; Guo, W.; Zhang, X.; Qin, G.; He, S.H.; Zimmerman, A.; et al. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int. J. Cardiol. 2015, 192, 61–69. [Google Scholar] [CrossRef]
- Xuan, W.; Wang, L.; Xu, M.; Weintraub, N.L.; Ashraf, M. miRNAs in Extracellular Vesicles from iPS-Derived Cardiac Progenitor Cells Effectively Reduce Fibrosis and Promote Angiogenesis in Infarcted Heart. Stem Cells Int. 2019, 2019, 3726392. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Cao, C.; Xu, X.; Wang, J. Diverse functions of miR-373 in cancer. J. Transl. Med. 2015, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Lee, J.; Kim, Y.J.; Rhee, W.J.; Park, J.H. Exosomes Derived from Human Induced Pluripotent Stem Cells Ameliorate the Aging of Skin Fibroblasts. Int. J. Mol. Sci. 2018, 19, 1715. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Chien, Y.; Chiou, G.Y.; Lin, C.H.; Chiou, C.H.; Tarng, D.C. Induced pluripotent stem cells without c-Myc attenuate acute kidney injury via downregulating the signaling of oxidative stress and inflammation in ischemia-reperfusion rats. Cell Transplant. 2012, 21, 2569–2585. [Google Scholar] [CrossRef] [PubMed]
- Collino, F.; Lopes, J.A.; Tapparo, M.; Tortelote, G.G.; Kasai-Brunswick, T.H.; Lopes, G.M.C.; Almeida, D.B.; Skovronova, R.; Wendt, C.H.C.; Miranda, K.R.; et al. Extracellular Vesicles Derived from Induced Pluripotent Stem Cells Promote Renoprotection in Acute Kidney Injury Model. Cells 2020, 9, 453. [Google Scholar] [CrossRef]
- Gazdhar, A.; Grad, I.; Tamo, L.; Gugger, M.; Feki, A.; Geiser, T. The secretome of induced pluripotent stem cells reduces lung fibrosis in part by hepatocyte growth factor. Stem Cell Res. Ther. 2014, 5, 123. [Google Scholar] [CrossRef][Green Version]
- Zhang, G.; Chen, L.; Guo, X.; Wang, H.; Chen, W.; Wu, G.; Gu, B.; Miao, W.; Kong, J.; Jin, X.; et al. Comparative Analysis of microRNA Expression Profiles of Exosomes Derived from Normal and Hypoxic Preconditioning Human Neural Stem Cells by Next Generation Sequencing. J. Biomed. Nanotechnol. 2018, 14, 1075–1089. [Google Scholar] [CrossRef]
- Gray, W.D.; French, K.M.; Ghosh-Choudhary, S.; Maxwell, J.T.; Brown, M.E.; Platt, M.O.; Searles, C.D.; Davis, M.E. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ. Res. 2015, 116, 255–263. [Google Scholar] [CrossRef]
- Xiao, J.; Pan, Y.; Li, X.H.; Yang, X.Y.; Feng, Y.L.; Tan, H.H.; Jiang, L.; Feng, J.; Yu, X.Y. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Dis. 2016, 7, e2277. [Google Scholar] [CrossRef]
- Han, C.; Zhou, J.; Liu, B.; Liang, C.; Pan, X.; Zhang, Y.; Zhang, Y.; Wang, Y.; Shao, L.; Zhu, B.; et al. Delivery of miR-675 by stem cell-derived exosomes encapsulated in silk fibroin hydrogel prevents aging-induced vascular dysfunction in mouse hindlimb. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.; Ma, J.; Wang, C.; Yu, J.; Qiao, Y.; Hei, F. Exosomes from iPSCs Delivering siRNA Attenuate Intracellular Adhesion Molecule-1 Expression and Neutrophils Adhesion in Pulmonary Microvascular Endothelial Cells. Inflammation 2017, 40, 486–496. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Xie, H.; Wang, Z.; Zhang, L.; Lei, Q.; Zhao, A.; Wang, H.; Li, Q.; Cao, Y.; Jie Zhang, W.; Chen, Z. Extracellular Vesicle-functionalized Decalcified Bone Matrix Scaffolds with Enhanced Pro-angiogenic and Pro-bone Regeneration Activities. Sci. Rep. 2017, 7, 45622. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Zhang, P.; Tang, Y.; Zhou, M.; Jiang, W.; Zhang, X.; Wu, G.; Zhou, Y. Tissue-Engineered Bone Immobilized with Human Adipose Stem Cells-Derived Exosomes Promotes Bone Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 5240–5254. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Qian, Z.; Liu, D.; Sun, J.; Wang, X.; Liu, H.; Xu, J.; Guo, X. GMSC-Derived Exosomes Combined with a Chitosan/Silk Hydrogel Sponge Accelerates Wound Healing in a Diabetic Rat Skin Defect Model. Front. Physiol. 2017, 8, 904. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lee, B.W.; Nakanishi, K.; Villasante, A.; Williamson, R.; Metz, J.; Kim, J.; Kanai, M.; Bi, L.; Brown, K.; et al. Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat. Biomed. Eng. 2018, 2, 293–303. [Google Scholar] [CrossRef]
- Xin, H.; Katakowski, M.; Wang, F.; Qian, J.Y.; Liu, X.S.; Ali, M.M.; Buller, B.; Zhang, Z.G.; Chopp, M. MicroRNA cluster miR-17-92 Cluster in Exosomes Enhance Neuroplasticity and Functional Recovery After Stroke in Rats. Stroke 2017, 48, 747–753. [Google Scholar] [CrossRef]
- Gimona, M.; Pachler, K.; Laner-Plamberger, S.; Schallmoser, K.; Rohde, E. Manufacturing of Human Extracellular Vesicle-Based Therapeutics for Clinical Use. Int. J. Mol. Sci. 2017, 18, 1190. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).