Epigenetic Regulation of Neuregulin-1 Tunes White Adipose Stem Cell Differentiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Stem Cell Culture
2.2. Generation of Clonal ASC Line
2.3. Decitabine (DAC) Treatment and Adipose Differentiation
2.4. NRG1 Recombinant Protein Experiment in 6-Well Dishes
2.5. Adipocyte Differentiation Quantitation with Oil Red O in 24-Well Dishes
2.6. NRG1 Isoform Expression Analysis
2.7. Microarray Analysis and Volcano Plot
2.8. Bisulfite Conversion and PCR
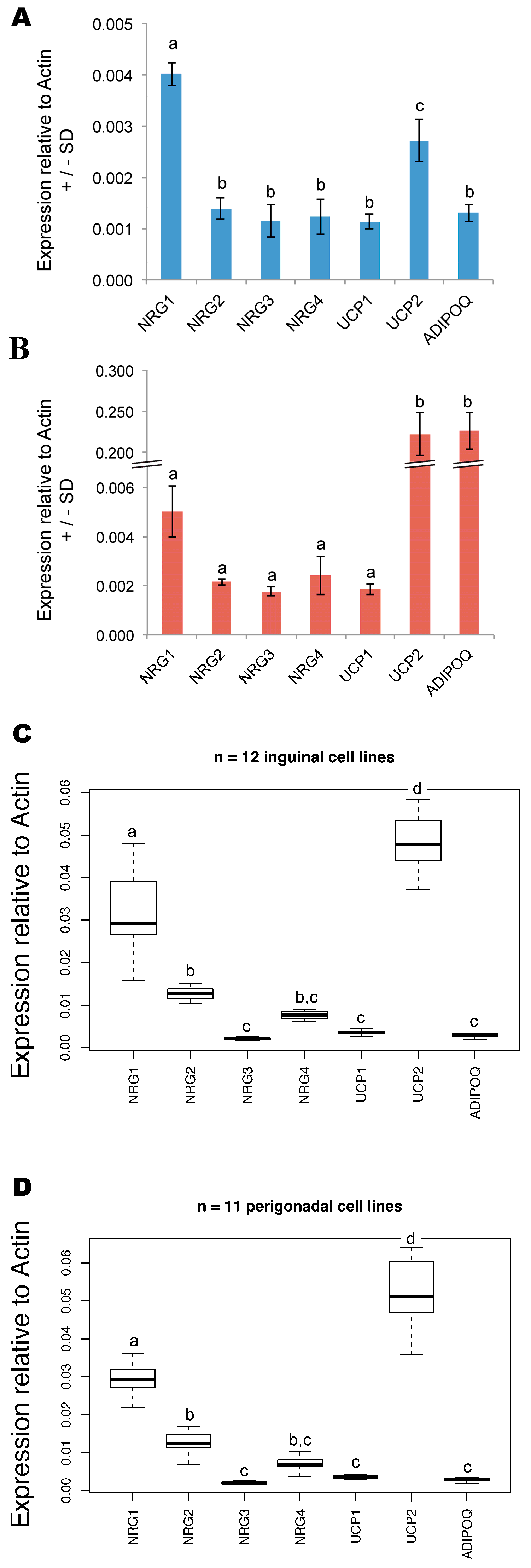
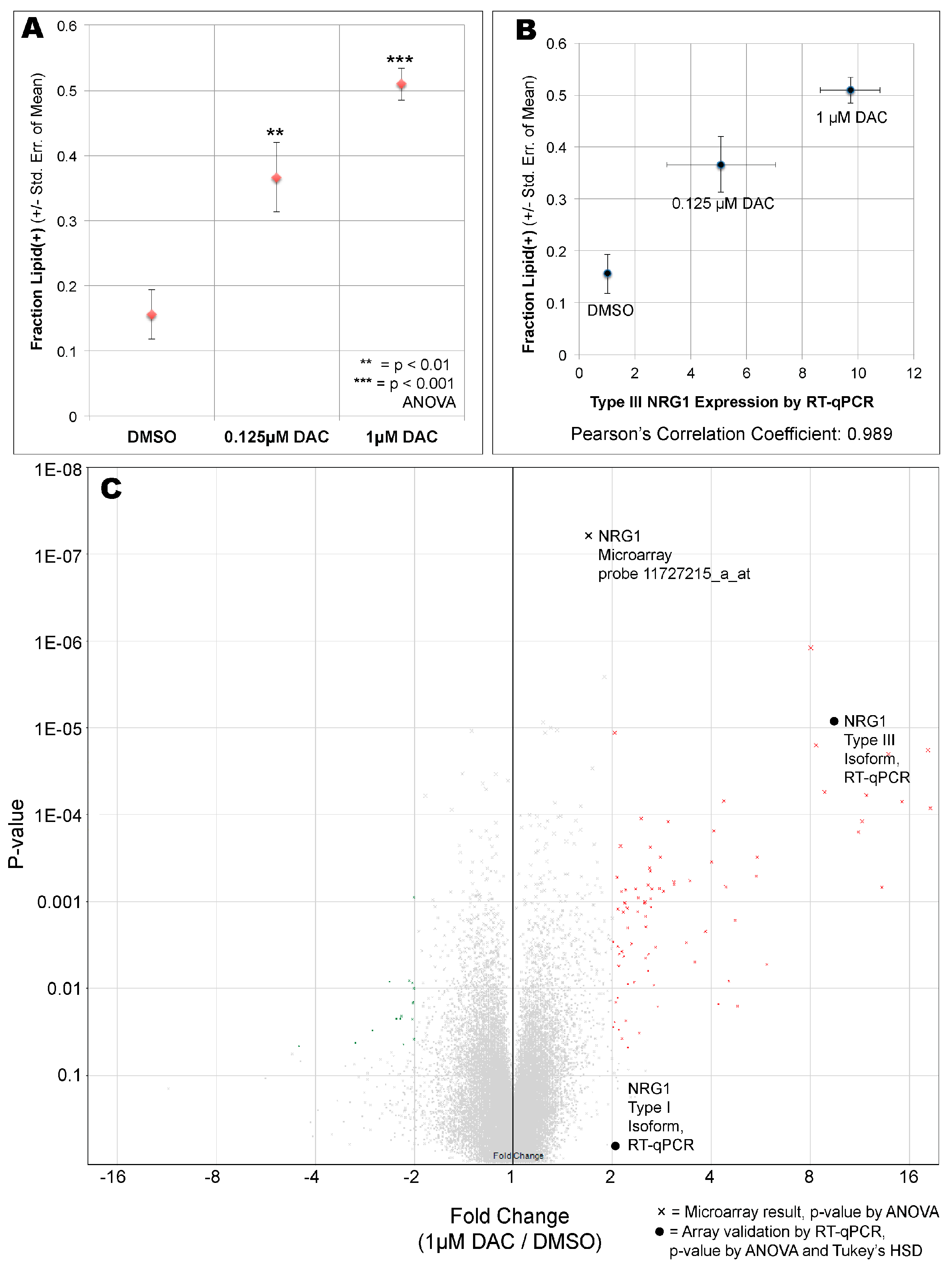
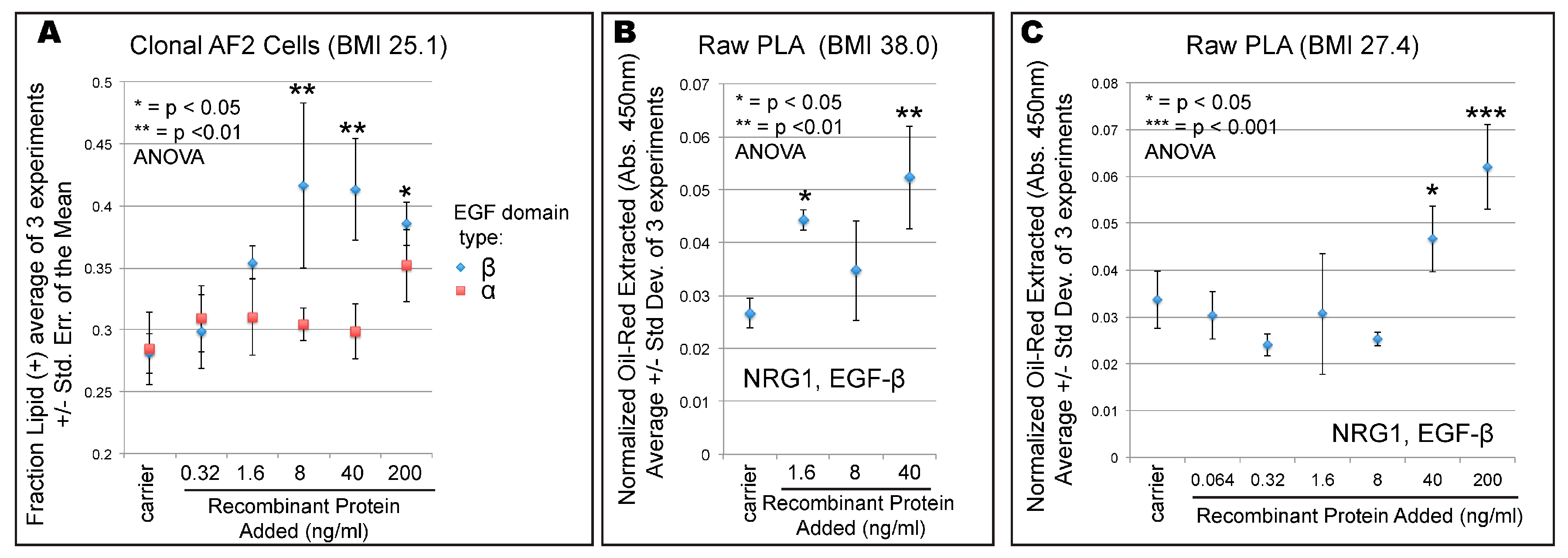
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS Data Brief 2017, 1–8. Available online: https://www.ncbi.nlm.nih.gov/pubmed/29155689.
- Upadhyay, J.; Farr, O.; Perakakis, N.; Ghaly, W.; Mantzoros, C. Obesity as a Disease. Med. Clin. North Am. 2018, 102, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, B.; Hedjazifar, S.; Gogg, S.; Hammarstedt, A.; Smith, U. Insulin resistance and impaired adipogenesis. Trends Endocrinol. Metab. 2015, 26, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Boyko, E.J.; Leonetti, D.L.; McNeely, M.J.; Newell-Morris, L.; Kahn, S.E.; Fujimoto, W.Y. Visceral adiposity and the risk of impaired glucose tolerance: A prospective study among Japanese Americans. Diabetes Care 2003, 26, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Rosito, G.A.; Massaro, J.M.; Hoffmann, U.; Ruberg, F.L.; Mahabadi, A.A.; Vasan, R.S.; O’Donnell, C.J.; Fox, C.S. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: The Framingham Heart Study. Circulation 2008, 117, 605–613. [Google Scholar] [CrossRef]
- Bluher, M. The distinction of metabolically “healthy” from “unhealthy” obese individuals. Curr. Opin. Lipidol. 2010, 21, 38–43. [Google Scholar] [CrossRef]
- Goncalves, C.G.; Glade, M.J.; Meguid, M.M. Metabolically healthy obese individuals: Key protective factors. Nutrition 2016, 32, 14–20. [Google Scholar] [CrossRef]
- Oh, Y.H.; Moon, J.H.; Kim, H.J.; Kong, M.H. Visceral-to-subcutaneous fat ratio as a predictor of the multiple metabolic risk factors for subjects with normal waist circumference in Korea. Diabetes Metab. Syndr. Obes. 2017, 10, 505–511. [Google Scholar] [CrossRef]
- Shafqat, M.N.; Haider, M. Subcutaneous to visceral fat ratio: A possible risk factor for metabolic syndrome and cardiovascular diseases. Diabetes Metab. Syndr. Obes. 2018, 11, 129–130. [Google Scholar] [CrossRef]
- Hwang, Y.-C.; Hayashi, T.; Fujimoto, W.Y.; Kahn, S.E.; Leonetti, D.L.; McNeely, M.J.; Boyko, E.J. Visceral abdominal fat accumulation predicts the conversion of metabolically healthy obese subjects to an unhealthy phenotype. Int. J. Obes. 2015, 39, 1365–1370. [Google Scholar] [CrossRef]
- Virtue, S.; Vidal-Puig, A. It’s not how fat you are, it’s what you do with it that counts. PLoS Biol. 2008, 6, e237. [Google Scholar] [CrossRef] [PubMed]
- Virtue, S.; Vidal-Puig, A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome—an allostatic perspective. Biochim. Biophys. Acta 2010, 1801, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, B.; Smith, U. Regulation of white adipogenesis and its relation to ectopic fat accumulation and cardiovascular risk. Atherosclerosis 2015, 241, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Ross, R.; Després, J.-P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Lessard, J.; Laforest, S.; Pelletier, M.; Leboeuf, M.; Blackburn, L.; Tchernof, A. Low abdominal subcutaneous preadipocyte adipogenesis is associated with visceral obesity, visceral adipocyte hypertrophy, and a dysmetabolic state. Adipocyte 2014, 3, 197–205. [Google Scholar] [CrossRef]
- Gustafson, B.; Nerstedt, A.; Smith, U. Reduced subcutaneous adipogenesis in human hypertrophic obesity is linked to senescent precursor cells. Nat. Commun. 2019, 10, 2757. [Google Scholar] [CrossRef]
- Gustafson, B.; Gogg, S.; Hedjazifar, S.; Jenndahl, L.; Hammarstedt, A.; Smith, U. Inflammation and impaired adipogenesis in hypertrophic obesity in man. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E999–E1003. [Google Scholar] [CrossRef]
- Isakson, P.; Hammarstedt, A.; Gustafson, B.; Smith, U. Impaired preadipocyte differentiation in human abdominal obesity: Role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes 2009, 58, 1550–1557. [Google Scholar] [CrossRef]
- Gustafson, B.; Hammarstedt, A.; Andersson, C.X.; Smith, U. Inflamed adipose tissue: A culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2276–2283. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Falls, D.L. Neuregulins: Functions, forms, and signaling strategies. Exp. Cell Res. 2003, 284, 14–30. [Google Scholar] [CrossRef]
- Mei, L.; Xiong, W.-C. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat. Rev. Neurosci. 2008, 9, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.; Birchmeier, C. Multiple essential functions of neuregulin in development. Nature 1995, 378, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.; Bucay, N.; Kane, D.J.; Martin, L.E.; Tarpley, J.E.; Theill, L.E. Neuregulins with an Ig-like domain are essential for mouse myocardial and neuronal development. Proc. Natl. Acad. Sci. USA 1996, 93, 4833–4838. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cao, R.; Zhang, X.; Huang, L.; Sun, L.; Zhao, J.; Ma, J.; Han, C. Recent Progress in Rare Oncogenic Drivers and Targeted Therapy for Non-Small Cell Lung Cancer. Onco. Targets Ther. 2019, 12, 10343–10360. [Google Scholar] [CrossRef]
- Trombetta, D.; Rossi, A.; Fabrizio, F.P.; Sparaneo, A.; Graziano, P.; Fazio, V.M.; Muscarella, L.A. NRG1-ErbB Lost in Translation: A New Paradigm for Lung Cancer? Curr. Med. Chem. 2017, 24, 4213–4228. [Google Scholar] [CrossRef]
- Schmid, R.S.; McGrath, B.; Berechid, B.E.; Boyles, B.; Marchionni, M.; Sestan, N.; Anton, E.S. Neuregulin 1-erbB2 signaling is required for the establishment of radial glia and their transformation into astrocytes in cerebral cortex. Proc. Natl. Acad. Sci. USA 2003, 100, 4251–4256. [Google Scholar] [CrossRef]
- Sato, T.; Sato, F.; Kamezaki, A.; Sakaguchi, K.; Tanigome, R.; Kawakami, K.; Sehara-Fujisawa, A. Neuregulin 1 Type II-ErbB Signaling Promotes Cell Divisions Generating Neurons from Neural Progenitor Cells in the Developing Zebrafish Brain. PLoS ONE 2015, 10, e0127360. [Google Scholar] [CrossRef]
- Pirotte, D.; Wislet-Gendebien, S.; Cloes, J.M.; Rogister, B. Neuregulin-1 modulates the differentiation of neural stem cells in vitro trough an interaction with the Swi/Snf complex. Mol. Cell. Neurosci. 2010, 43, 72–80. [Google Scholar] [CrossRef]
- Bersell, K.; Arab, S.; Haring, B.; Kuhn, B. Neuregulin1/ErbB4 Signaling Induces Cardiomyocyte Proliferation and Repair of Heart Injury. Cell 2009, 138, 257–270. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, G.; Wu, Y.; Guan, Y.; Cui, L.; Lei, X.; Zhang, J.; Mou, L.; Sun, B.; Dai, Q. Neuregulin-1 enhances differentiation of cardiomyocytes from embryonic stem cells. Med. Biol. Eng. Comput. 2009, 47, 41–48. [Google Scholar] [CrossRef]
- Díaz-Herráez, P.; Garbayo, E.; Simón-Yarza, T.; Formiga, F.R.; Prosper, F.; Blanco-Prieto, M.J. Adipose-derived stem cells combined with neuregulin-1 delivery systems for heart tissue engineering. Eur. J. Pharm. Biopharm. 2013, 85, 143–150. [Google Scholar] [CrossRef]
- Liang, X.; Ding, Y.; Zhang, Y.; Chai, Y.-H.; He, J.; Chiu, S.-M.; Gao, F.; Tse, H.-F.; Lian, Q. Activation of NRG1-ERBB4 signaling potentiates mesenchymal stem cell-mediated myocardial repairs following myocardial infarction. Cell Death Dis. 2015, 6, e1765. [Google Scholar] [CrossRef] [PubMed]
- Bruun, K.; Schermer, E.; Sivendra, A.; Valaik, E.; Wise, R.B.; Said, R.; Bracht, J.R. Therapeutic applications of adipose-derived stem cells in cardiovascular disease. Am. J. Stem Cells 2018, 7, 94–103. [Google Scholar] [PubMed]
- Chua, Y.L.; Ito, Y.; Pole, J.C.M.; Newman, S.; Chin, S.-F.; Stein, R.C.; Ellis, I.O.; Caldas, C.; O’Hare, M.J.; Murrell, A.; et al. The NRG1 gene is frequently silenced by methylation in breast cancers and is a strong candidate for the 8p tumour suppressor gene. Oncogene 2009, 28, 4041–4052. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-P.; Yu, C.-Y.; Chen, H.-F.; Chen, P.-H.; Chuang, C.-Y.; Lin, S.-J.; Huang, S.-T.; Chan, W.-H.; Ueng, T.-H.; Ho, H.-N.; et al. Factors from human embryonic stem cell-derived fibroblast-like cells promote topology-dependent hepatic differentiation in primate embryonic and induced pluripotent stem cells. J. Biol. Chem. 2010, 285, 33510–33519. [Google Scholar] [CrossRef]
- Christian, M. Transcriptional fingerprinting of “browning” white fat identifies NRG4 as a novel adipokine. Adipocyte 2015, 4, 50–54. [Google Scholar] [CrossRef]
- Paffhausen, E.S.; Ylowais, Y.; Chao, C.W.; Callihan, E.C.; Creswell, K.; Bracht, J.R. Discovery of a stem-like multipotent cell fate. Am. J. Stem Cells 2018, 7, 25–37. [Google Scholar]
- Fleury, C.; Neverova, M.; Collins, S.; Raimbault, S.; Champigny, O.; Levi-Meyrueis, C.; Bouillaud, F.; Seldin, M.F.; Surwit, R.S.; Ricquier, D.; et al. Uncoupling protein-2: A novel gene linked to obesity and hyperinsulinemia. Nat. Genet. 1997, 15, 269–272. [Google Scholar] [CrossRef]
- Pecqueur, C.; Alves-Guerra, M.C.; Gelly, C.; Levi-Meyrueis, C.; Couplan, E.; Collins, S.; Ricquier, D.; Bouillaud, F.; Miroux, B. Uncoupling protein 2, in vivo distribution, induction upon oxidative stress, and evidence for translational regulation. J. Biol. Chem. 2001, 276, 8705–8712. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, E.B.M.; Sparks, L.M.; Divoux, A.; van Gisbergen, M.W.; Broeders, E.P.M.; Jörgensen, J.A.; Schaart, G.; Bouvy, N.D.; van Marken Lichtenbelt, W.D.; Schrauwen, P. Genetic Markers of Brown Adipose Tissue Identity and In Vitro Brown Adipose Tissue Activity in Humans. Obesity 2018, 26, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Luong, Q.; Sharma, R.; Dreyfuss, J.M.; Ussar, S.; Kahn, C.R. Developmental and functional heterogeneity of white adipocytes within a single fat depot. EMBO J. 2019, 38, e99291. [Google Scholar] [CrossRef] [PubMed]
- Kwan, R.; Looi, K.; Omary, M.B. Absence of keratins 8 and 18 in rodent epithelial cell lines associates with keratin gene mutation and DNA methylation: Cell line selective effects on cell invasion. Exp. Cell Res. 2015, 335, 12–22. [Google Scholar] [CrossRef][Green Version]
- Ferrario, C.; Lavagni, P.; Gariboldi, M.; Miranda, C.; Losa, M.; Cleris, L.; Formelli, F.; Pilotti, S.; Pierotti, M.A.; Greco, A. Metallothionein 1G acts as an oncosupressor in papillary thyroid carcinoma. Lab. Invest. 2008, 88, 474–481. [Google Scholar] [CrossRef]
- Giri, A.K.; Aittokallio, T. DNMT Inhibitors Increase Methylation in the Cancer Genome. Front. Pharmacol. 2019, 10, 385. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Zhang, J.; Cheng, L.; Wu, X.; Dong, W.; Yang, X.; Li, T.; Liu, X.; Xu, Y.; Li, X.; et al. A Phase II, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J. Am. Coll. Cardiol. 2010, 55, 1907–1914. [Google Scholar] [CrossRef]
- Jabbour, A.; Hayward, C.S.; Keogh, A.M.; Kotlyar, E.; McCrohon, J.A.; England, J.F.; Amor, R.; Liu, X.; Li, X.Y.; Zhou, M.D.; et al. Parenteral administration of recombinant human neuregulin-1 to patients with stable chronic heart failure produces favourable acute and chronic haemodynamic responses. Eur. J. Heart Fail. 2011, 13, 83–92. [Google Scholar] [CrossRef]
- Ennequin, G.; Boisseau, N.; Caillaud, K.; Chavanelle, V.; Etienne, M.; Li, X.; Montaurier, C.; Sirvent, P. Neuregulin 1 affects leptin levels, food intake and weight gain in normal-weight, but not obese, db/db mice. Diabetes Metab. 2015, 41, 168–172. [Google Scholar] [CrossRef]
- Vandekerckhove, L.; Vermeulen, Z.; Liu, Z.Z.; Boimvaser, S.; Patzak, A.; Segers, V.F.M.; De Keulenaer, G.W. Neuregulin-1 attenuates development of nephropathy in a type 1 diabetes mouse model with high cardiovascular risk. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E495–E504. [Google Scholar] [CrossRef]
- Ennequin, G.; Boisseau, N.; Caillaud, K.; Chavanelle, V.; Etienne, M.; Li, X.; Sirvent, P. Neuregulin 1 Improves Glucose Tolerance in db/db Mice. PLoS ONE 2015, 10, e0130568. [Google Scholar] [CrossRef] [PubMed]
- Caillaud, K.; Boisseau, N.; Ennequin, G.; Chavanelle, V.; Etienne, M.; Li, X.; Denis, P.; Dardevet, D.; Lacampagne, A.; Sirvent, P. Neuregulin 1 improves glucose tolerance in adult and old rats. Diabetes Metab. 2016, 42, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Snodgrass-Belt, P.; Gilbert, J.L.; Davis, F.C. Central administration of transforming growth factor-alpha and neuregulin-1 suppress active behaviors and cause weight loss in hamsters. Brain Res. 2005, 1038, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Heal, D.J.; Smith, S.L.; Jones, R.B. Central regulation of food intake and energy expenditure. Neuropharmacology 2012, 63, 1–2. [Google Scholar] [CrossRef]
- Geisberg, C.A.; Wang, G.; Safa, R.N.; Smith, H.M.; Anderson, B.; Peng, X.Y.; Veerkamp, B.; Zhao, D.X.; Blakemore, D.; Yu, C.; et al. Circulating neuregulin-1beta levels vary according to the angiographic severity of coronary artery disease and ischemia. Coron. Artery Dis. 2011, 22, 577–582. [Google Scholar] [CrossRef]
- Moondra, V.; Sarma, S.; Buxton, T.; Safa, R.; Cote, G.; Storer, T.; Lebrasseur, N.K.; Sawyer, D.B. Serum Neuregulin-1beta as a Biomarker of Cardiovascular Fitness. Open Biomark. J. 2009, 2, 1–5. [Google Scholar] [CrossRef]
- Galvez-Contreras, A.Y.; Quiñones-Hinojosa, A.; Gonzalez-Perez, O. The role of EGFR and ErbB family related proteins in the oligodendrocyte specification in germinal niches of the adult mammalian brain. Front. Cell. Neurosci. 2013, 7, 258. [Google Scholar] [CrossRef]
- Pareja, F.; Pines, G.; Yarden, Y. Receptor Tyrosine Kinases: Family and Subfamilies. In The EGFR/ERBB Receptor Family; Wheeler, D., Yarden, Y., Eds.; Springer: Basel, Switzerland, 2015; pp. 107–164. [Google Scholar]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef]
- Sandholm, N.; Salem, R.M.; McKnight, A.J.; Brennan, E.P.; Forsblom, C.; Isakova, T.; McKay, G.J.; Williams, W.W.; Sadlier, D.M.; Mäkinen, V.-P.; et al. New susceptibility loci associated with kidney disease in type 1 diabetes. PLoS Genet. 2012, 8, e1002921. [Google Scholar] [CrossRef]
- Böger, C.A.; Sedor, J.R. GWAS of diabetic nephropathy: Is the GENIE out of the bottle? PLoS Genet. 2012, 8, e1002989. [Google Scholar] [CrossRef]
- Dekker, J.; Mirny, L. The 3D Genome as Moderator of Chromosomal Communication. Cell 2016, 164, 1110–1121. [Google Scholar] [CrossRef] [PubMed]
- Todaro, G.J.; Green, H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell Biol. 1963, 17, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Wu, R.; Yang, X.; Kou, S.; MacDougald, O.A.; Yu, L.; Shi, H.; Xue, B. Inhibiting DNA methylation switches adipogenesis to osteoblastogenesis by activating Wnt10a. Sci. Rep. 2016, 6, 25283. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wu, R.; Shan, W.; Yu, L.; Xue, B.; Shi, H. DNA Methylation Biphasically Regulates 3T3-L1 Preadipocyte Differentiation. Mol. Endocrinol. 2016, 30, 677–687. [Google Scholar] [CrossRef]
- Mazzu, Y.Z.; Hu, Y.; Soni, R.K.; Mojica, K.M.; Qin, L.-X.; Agius, P.; Waxman, Z.M.; Mihailovic, A.; Socci, N.D.; Hendrickson, R.C.; et al. miR-193b-Regulated Signaling Networks Serve as Tumor Suppressors in Liposarcoma and Promote Adipogenesis in Adipose-Derived Stem Cells. Cancer Res. 2017, 77, 5728–5740. [Google Scholar] [CrossRef]
- Zych, J.; Stimamiglio, M.A.; Senegaglia, A.C.; Brofman, P.R.S.; Dallagiovanna, B.; Goldenberg, S.; Correa, A. The epigenetic modifiers 5-aza-2′-deoxycytidine and trichostatin A influence adipocyte differentiation in human mesenchymal stem cells. Braz. J. Med. Biol. Res. 2013, 46, 405–416. [Google Scholar] [CrossRef]
- Bracht, J.R.; Vieira-Potter, V.; de Souza Santos, R.; Oz, O.; Palmer, B.; Clegg, D. The Role of Estrogens in the Adipose Tissue Milieu. Ann. N. Y. Acad. Sci. 2019, 1641, 127–143. [Google Scholar] [CrossRef]
- van den Dungen, M.W.; Murk, A.J.; Kok, D.E.; Steegenga, W.T. Comprehensive DNA Methylation and Gene Expression Profiling in Differentiating Human Adipocytes. J. Cell. Biochem. 2016, 117, 2707–2718. [Google Scholar] [CrossRef]
- Zaim, M.; Karaman, S.; Cetin, G.; Isik, S. Donor age and long-term culture affect differentiation and proliferation of human bone marrow mesenchymal stem cells. Ann. Hematol. 2012, 91, 1175–1186. [Google Scholar] [CrossRef]
- Zhang, P.; Kuang, H.; He, Y.; Idiga, S.O.; Li, S.; Chen, Z.; Yang, Z.; Cai, X.; Zhang, K.; Potthoff, M.J.; et al. NRG1-Fc improves metabolic health via dual hepatic and central action. JCI Insight 2018, 3, 98522. [Google Scholar] [CrossRef]
- Ennequin, G.; Capel, F.; Caillaud, K.; Chavanelle, V.; Etienne, M.; Teixeira, A.; Li, X.; Boisseau, N.; Sirvent, P. Neuregulin 1 improves complex 2-mediated mitochondrial respiration in skeletal muscle of healthy and diabetic mice. Sci. Rep. 2017, 7, 1742. [Google Scholar] [CrossRef] [PubMed]

| Cell Line | BMI | Depot | Sex | Age | Passage at Experiment | Source | Figure |
|---|---|---|---|---|---|---|---|
| ASC080414A-derived clonal line = AF2 | 25.1 | Abdomen | F | 39 | p12 | Zen-Bio, Inc., Research Triangle Park, NC, USA | Figure 1A, Figure 2B, Figure 3 and Figure 4A |
| ASC072709 | 38.0 | Hip | F | 39 | p9 | Zen-Bio, Inc., Research Triangle Park, NC, USA | Figure 4B |
| Line 1107 | 27.4 | Abdomen | F | 40 | p8 | DeCicco-Skinner lab (AU) | Figure 4C |
| ASC012502 | 25.3 | Abdomen | M | 40 | p4 | Zen-Bio, Inc., Research Triangle Park, NC, USA | Figure 2C |
| Transcript Cluster ID | Fold Change (1 uM DAC vs. DMSO) | ANOVA p-Value (1 uM DAC vs. DMSO) | Gene Symbol | Description |
|---|---|---|---|---|
| 11752634_x_at | 18.58 | 0.000084 | KRT8 | keratin 8, type II |
| 11758298_x_at | 18.25 | 0.000018 | KRT8 | keratin 8, type II |
| 11756989_x_at | 15.22 | 0.000071 | KRT8 | keratin 8, type II |
| 11758184_x_at | 13.85 | 0.00002 | KRT8 | keratin 8, type II |
| 11717386_s_at | 13.22 | 0.000681 | MT1G | metallothionein 1G |
| 11758188_x_at | 11.85 | 0.00006 | KRT8 | keratin 8, type II |
| 11758301_x_at | 11.51 | 0.000118 | KRT8 | keratin 8, type II |
| 11758183_x_at | 11.23 | 0.000159 | KRT8 | keratin 8, type II |
| RT-qPCR_NRG1_Type III | 9.72 | 0.0000098 | NRG1 | Neuregulin-1 |
| 11727248_a_at | 8.85 | 0.000055 | MYH3 | myosin, heavy chain 3 |
| 11733121_s_at | 8.33 | 0.000016 | DAZL | deleted in azoospermia-like |
| 11715280_s_at | 8.03 | 0.000001 | KRT17 | keratin 17, type I |
| 11756072_s_at | 5.92 | 0.005264 | SAA1 | serum amyloid A1 |
| 11727092_x_at | 5.53 | 0.000309 | IL18 | interleukin 18 |
| 11730408_a_at | 5.48 | 0.00051 | C19orf33 | chromosome 19 open reading frame 33 |
| 11753131_x_at | 4.81 | 0.015946 | TM4SF1 | transmembrane 4 L six family member 1 |
| 11717387_x_at | 4.73 | 0.00164 | MT1G | metallothionein 1G |
| 11753130_at | 4.52 | 0.008248 | TM4SF1 | transmembrane 4 L six family member 1 |
| 11755287_x_at | 4.43 | 0.000674 | KRT8 | keratin 8, type II |
| 11756334_x_at | 4.37 | 0.00007 | ANXA3 | annexin A3 |
| 11753129_a_at | 4.21 | 0.015138 | TM4SF1 | transmembrane 4 L six family member 1 |
| 11724283_a_at | 4.08 | 0.000154 | ANXA3 | annexin A3 |
| 11718347_a_at | 4.01 | 0.000348 | S100P | S100 calcium binding protein P |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordero, A.D.; Callihan, E.C.; Said, R.; Alowais, Y.; Paffhausen, E.S.; Bracht, J.R. Epigenetic Regulation of Neuregulin-1 Tunes White Adipose Stem Cell Differentiation. Cells 2020, 9, 1148. https://doi.org/10.3390/cells9051148
Cordero AD, Callihan EC, Said R, Alowais Y, Paffhausen ES, Bracht JR. Epigenetic Regulation of Neuregulin-1 Tunes White Adipose Stem Cell Differentiation. Cells. 2020; 9(5):1148. https://doi.org/10.3390/cells9051148
Chicago/Turabian StyleCordero, Alyssa D., Evan C. Callihan, Rana Said, Yasir Alowais, Emily S. Paffhausen, and John R. Bracht. 2020. "Epigenetic Regulation of Neuregulin-1 Tunes White Adipose Stem Cell Differentiation" Cells 9, no. 5: 1148. https://doi.org/10.3390/cells9051148
APA StyleCordero, A. D., Callihan, E. C., Said, R., Alowais, Y., Paffhausen, E. S., & Bracht, J. R. (2020). Epigenetic Regulation of Neuregulin-1 Tunes White Adipose Stem Cell Differentiation. Cells, 9(5), 1148. https://doi.org/10.3390/cells9051148




