Human Liver Spheroids as a Model to Study Aetiology and Treatment of Hepatic Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Spheroid Cultures
2.2. Functional Responses
2.3. Induction of a NASH-Like Phenotype
2.4. Drug Screening
2.5. Cell Viability
2.6. RNA Isolation and cDNA Synthesis
2.7. Gene Expression Analysis
2.8. Donor Genotyping
2.9. Immunohistochemistry
2.10. Statistical Analysis
2.11. Ethical Approval
3. Results
3.1. Human Liver Spheroids Stably Express Hepatocyte Markers
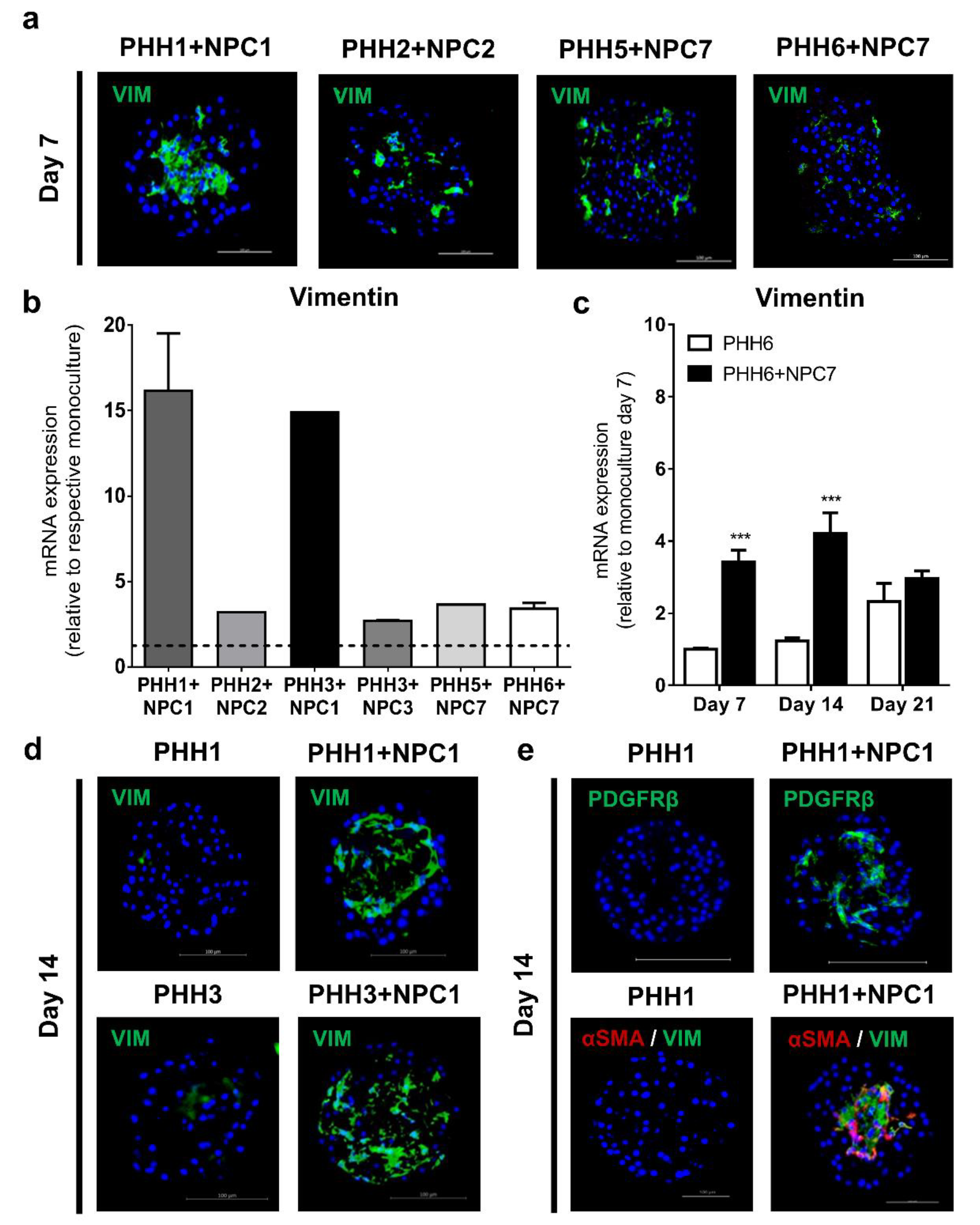
3.2. Liver Non-Parenchymal Cells Are Integrated in Human Liver Spheroids
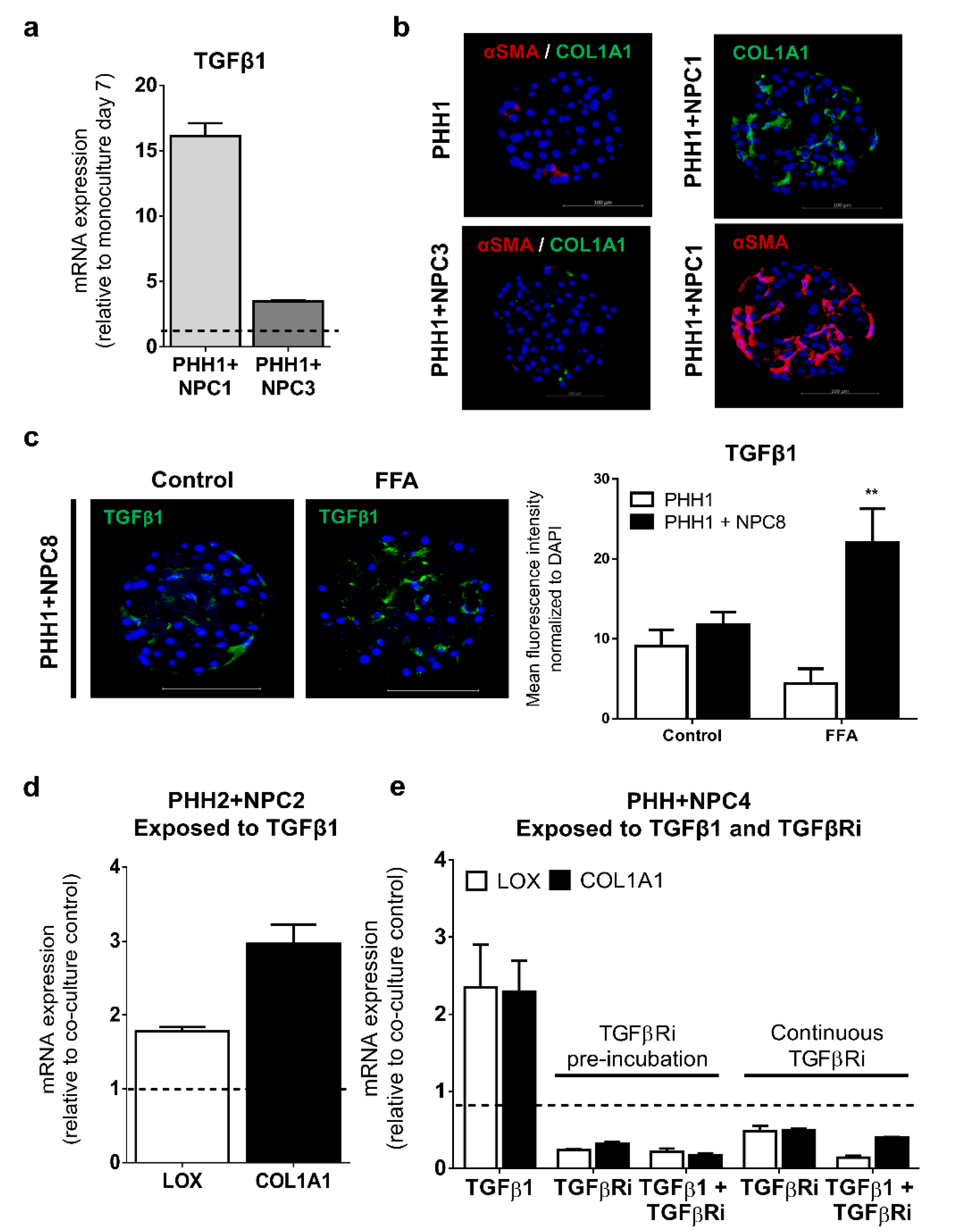
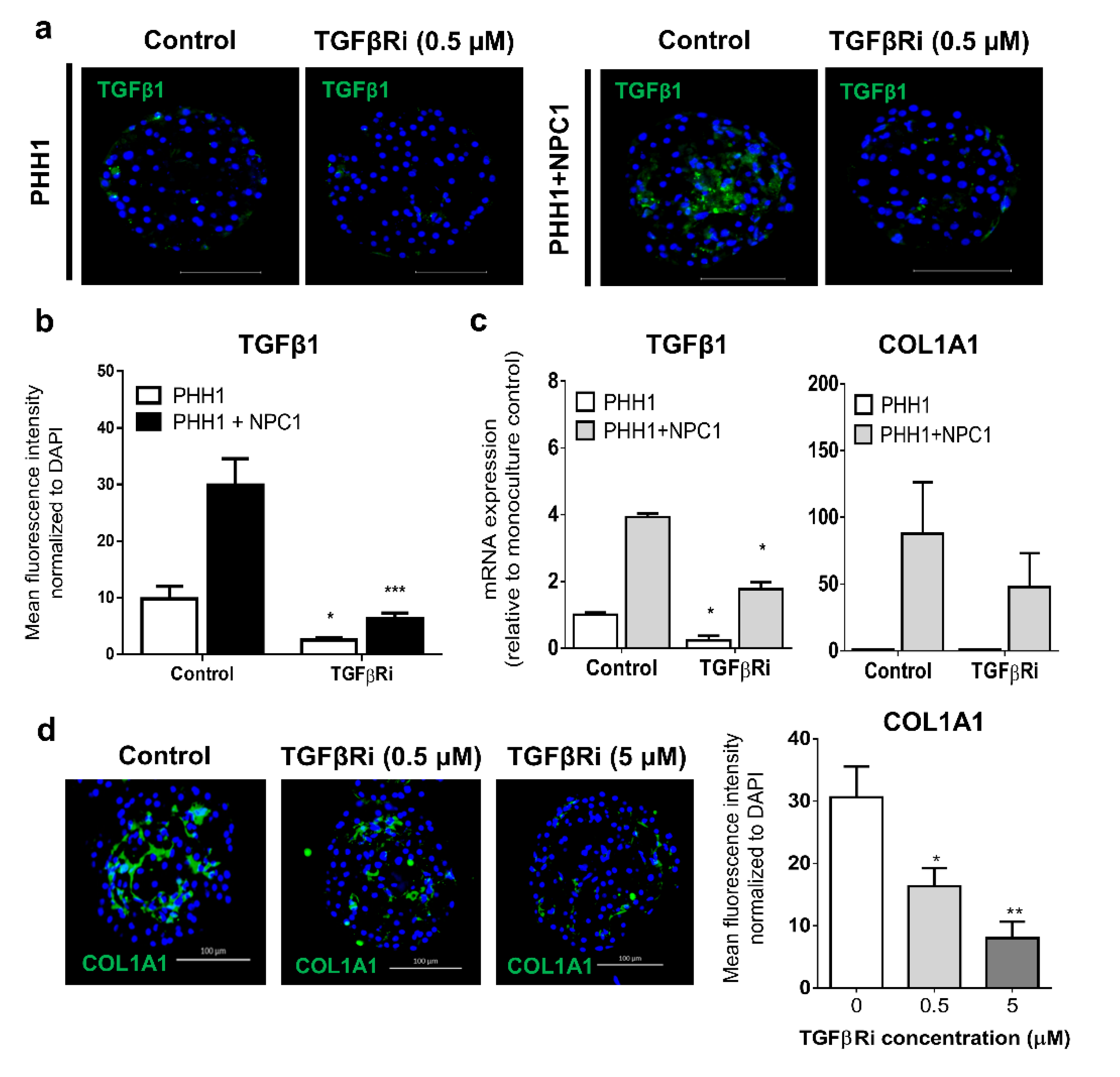
3.3. TGFβ Signaling Influences the Fibrogenic Response of Human Liver Spheroids
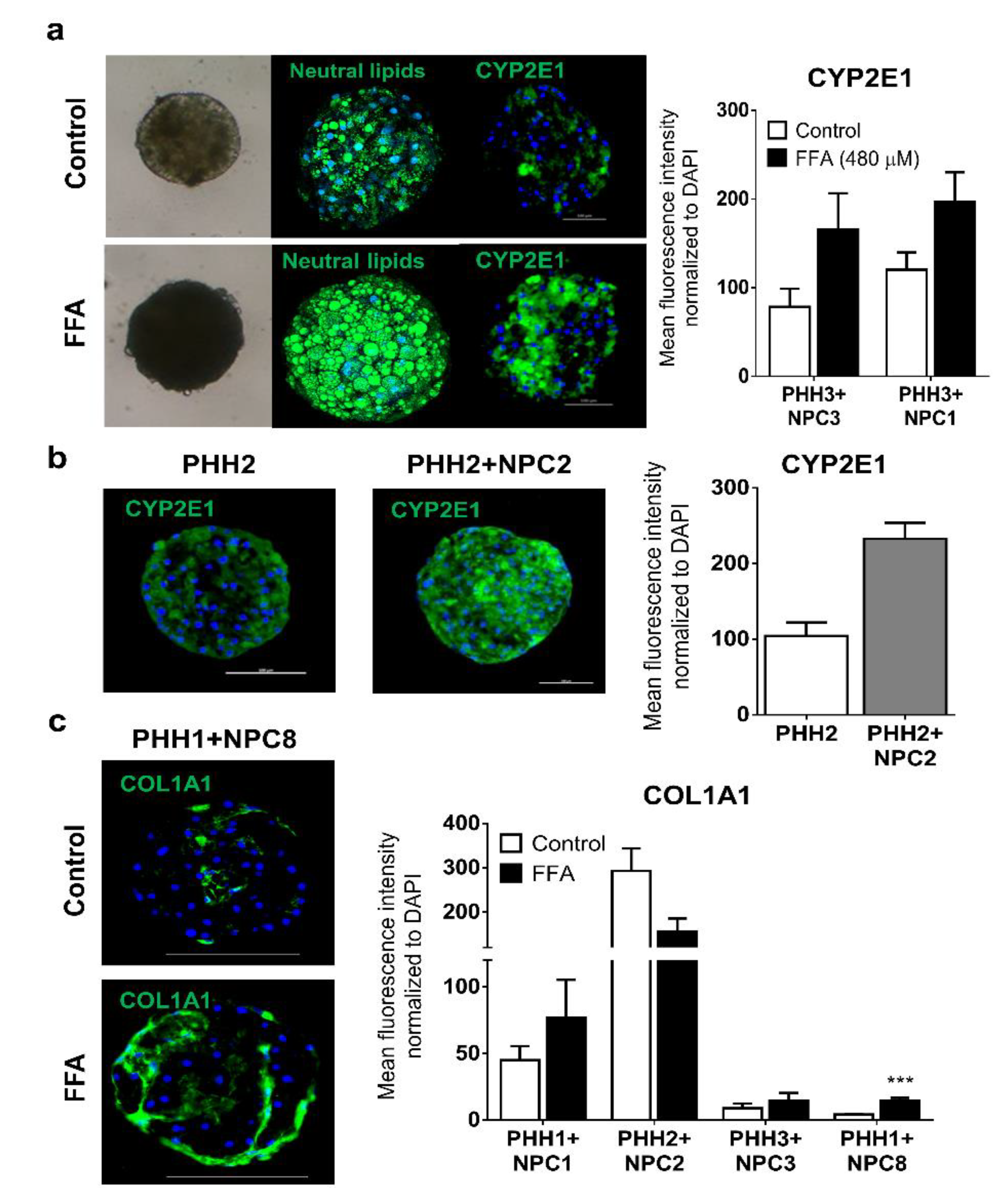
3.4. Human Liver Spheroids can Mimic Attributes seen in NASH Patients when Exposed to FFA
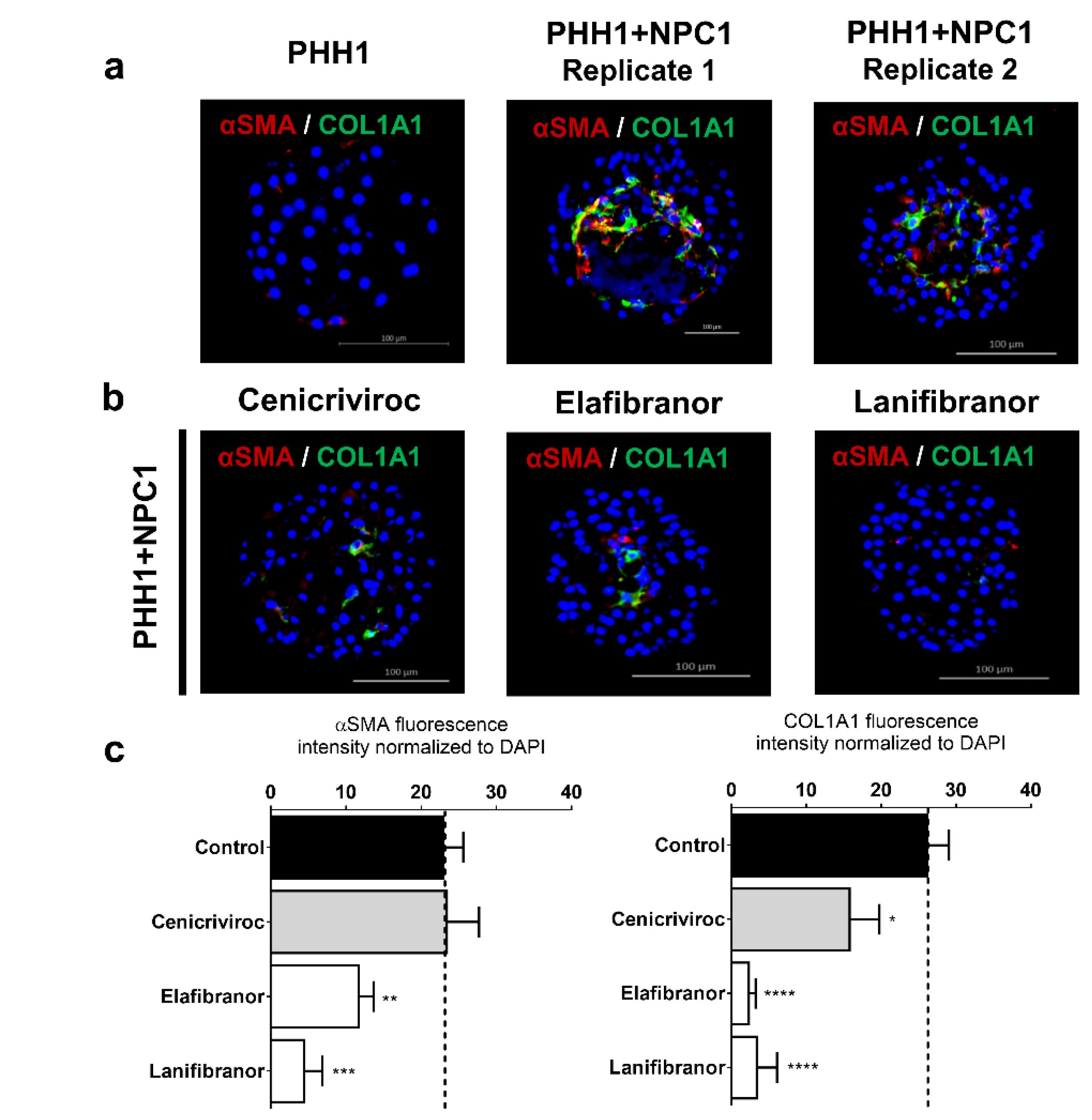
3.5. Human Liver Spheroids can be used as a Screening Platform for Anti-NASH Drugs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caldwell, S.; Crespo, D.M. The spectrum expanded: Cryptogenic cirrhosis and the natural history of non-Alcoholic fatty liver disease. J. Hepatol. 2004, 40, 578–584. [Google Scholar] [CrossRef]
- Kumar, K.S.; Malet, P.F. Nonalcoholic Steatohepatitis. Mayo Clin. Proc. 2000, 75, 733–739. [Google Scholar] [CrossRef]
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Matteoni, C.A.; Younossi, Z.M.; Gramlich, T.; Boparai, N.; Liu, Y.; McCullough, A. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology 1999, 116, 1413–1419. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Sookoian, S.; Pirola, C.J. Genetic predisposition in nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2017, 23, 1–12. [Google Scholar] [CrossRef]
- Walton, K.; Johnson, K.E.; Harrison, C.A. Targeting TGF-β Mediated SMAD Signaling for the Prevention of Fibrosis. Front. Pharmacol. 2017, 8, 461. [Google Scholar] [CrossRef] [PubMed]
- Connolly, J.J.; Ooka, K.; Lim, J. Future Pharmacotherapy for Non-alcoholic Steatohepatitis (NASH): Review of Phase 2 and 3 Trials. J. Clin. Transl. Hepatol. 2018, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Abdelmalek, M.F.; McColgan, B.J.; McHutchison, J.G.; Subramanian, G.M.; Myers, R.P.; Younossi, Z.; Ratziu, V.; Muir, A.J.; Afdhal, N.; et al. Simtuzumab Is Ineffective for Patients with Bridging Fibrosis or Compensated Cirrhosis Caused by Nonalcoholic Steatohepatitis. Gastroenterology 2018, 155, 1140–1153. [Google Scholar] [CrossRef]
- Lau, J.K.C.; Zhang, X.; Yu, J. Animal models of non-alcoholic fatty liver disease: Current perspectives and recent advances. J. Pathol. 2016, 241, 36–44. [Google Scholar] [CrossRef]
- Dooley, S.; Dijke, P.T. TGF-β in progression of liver disease. Cell Tissue Res. 2011, 347, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Mederacke, I.; Hsu, C.C.; Troeger, J.S.; Huebener, P.; Mu, X.; Dapito, D.H.; Pradère, J.-P.; Schwabe, R.F. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat. Commun. 2013, 4, 2823. [Google Scholar] [CrossRef] [PubMed]
- Prestigiacomo, V.; Weston, A.; Messner, S.; Lampart, F.; Suter-Dick, L. Pro-fibrotic compounds induce stellate cell activation, ECM-remodelling and Nrf2 activation in a human 3D-multicellular model of liver fibrosis. PLoS ONE 2017, 12, e0179995. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Miyajima, A. Liver regeneration and fibrosis after inflammation. Inflamm. Regen. 2016, 36, 19. [Google Scholar] [CrossRef]
- Lauschke, V.M.; Hendriks, D.; Bell, C.C.; Andersson, T.B.; Ingelman-Sundberg, M. Novel 3D Culture Systems for Studies of Human Liver Function and Assessments of the Hepatotoxicity of Drugs and Drug Candidates. Chem. Res. Toxicol. 2016, 29, 1936–1955. [Google Scholar] [CrossRef]
- Poloznikov, A. In vitro and in silico liver models: Current trends, challenges and opportunities. ALTEX 2018, 35, 397–412. [Google Scholar] [CrossRef]
- Tetsuka, K.; Ohbuchi, M.; Tabata, K. Recent Progress in Hepatocyte Culture Models and Their Application to the Assessment of Drug Metabolism, Transport, and Toxicity in Drug Discovery: The Value of Tissue Engineering for the Successful Development of a Microphysiological System. J. Pharm. Sci. 2017, 106, 2302–2311. [Google Scholar] [CrossRef]
- Bell, C.C.; Hendriks, D.; Moro, S.M.L.; Ellis, E.; Walsh, J.; Renblom, A.; Puigvert, L.F.; Dankers, A.C.A.; Jacobs, F.; Snoeys, J.; et al. Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Sci. Rep. 2016, 6, 25187. [Google Scholar] [CrossRef]
- Vorrink, S.U.; Ullah, S.; Schmidt, S.; Nandania, J.; Velagapudi, V.; Beck, O.; Ingelman-Sundberg, M.; Lauschke, V.M. Endogenous and xenobiotic metabolic stability of primary human hepatocytes in long-term 3D spheroid cultures revealed by a combination of targeted and untargeted metabolomics. FASEB J. 2017, 31, 2696–2708. [Google Scholar] [CrossRef]
- Hendriks, D.F.G.; Hurrell, T.; Riede, J.; Van Der Horst, M.; Tuovinen, S.; Ingelman-Sundberg, M. Mechanisms of chronic fialuridine hepatotoxicity as revealed in primary human hepatocyte spheroids. Toxicol. Sci. 2019, 171, 385–395. [Google Scholar] [CrossRef]
- Proctor, W.R.; Foster, A.J.; Vogt, J.; Summers, C.; Middleton, B.; Pilling, M.; Shienson, D.; Kijanska, M.; Ströbel, S.; Kelm, J.M.; et al. Utility of spherical human liver microtissues for prediction of clinical drug-induced liver injury. Arch. Toxicol. 2017, 91, 2849–2863. [Google Scholar] [CrossRef]
- Vorrink, S.U.; Zhou, Y.; Ingelman-Sundberg, M.; Lauschke, V.M. Prediction of Drug-Induced Hepatotoxicity Using Long-Term Stable Primary Hepatic 3D Spheroid Cultures in Chemically Defined Conditions. Toxicol. Sci. 2018, 163, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, D.; Puigvert, L.F.; Messner, S.; Mortiz, W.; Ingelman-Sundberg, M. Hepatic 3D spheroid models for the detection and study of compounds with cholestatic liability. Sci. Rep. 2016, 6, 35434. [Google Scholar] [CrossRef]
- Cordero-Herrera, I.; Kozyra, M.; Zhuge, Z.; Haworth, S.M.; Moretti, C.; Peleli, M.; Caldeira-Dias, M.; Jahandideh, A.; Huirong, H.; Cruz, J.D.C.; et al. AMP-activated protein kinase activation and NADPH oxidase inhibition by inorganic nitrate and nitrite prevent liver steatosis. Proc. Natl. Acad. Sci. USA 2018, 116, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Kozyra, M.; Johansson, I.; Nordling, Å.; Ullah, S.; Lauschke, V.M.; Ingelman-Sundberg, M. Human hepatic 3D spheroids as a model for steatosis and insulin resistance. Sci. Rep. 2018, 8, 14297. [Google Scholar] [CrossRef] [PubMed]
- Prill, S.; Caddeo, A.; Baselli, G.; Jamialahmadi, O.; Dongiovanni, P.; Rametta, R.; Kanebratt, K.P.; Pujia, A.; Pingitore, P.; Mancina, R.M.; et al. The TM6SF2 E167K genetic variant induces lipid biosynthesis and reduces apolipoprotein B secretion in human hepatic 3D spheroids. Sci. Rep. 2019, 9, 11585–11612. [Google Scholar] [CrossRef]
- Kane, B.J.; Zinner, M.J.; Yarmush, M.L.; Toner, M. Liver-Specific Functional Studies in a Microfluidic Array of Primary Mammalian Hepatocytes. Anal. Chem. 2006, 78, 4291–4298. [Google Scholar] [CrossRef]
- Khetani, S.R.; Bhatia, S.N. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 2007, 26, 120–126. [Google Scholar] [CrossRef]
- Kidambi, S.; Yarmush, R.S.; Novik, E.; Chao, P.; Yarmush, M.L.; Nahmias, Y. Oxygen-mediated enhancement of primary hepatocyte metabolism, functional polarization, gene expression, and drug clearance. Proc. Natl. Acad. Sci. USA 2009, 106, 15714–15719. [Google Scholar] [CrossRef]
- Nguyen, D.; Funk, J.; Robbins, J.B.; Crogan-Grundy, C.; Presnell, S.C.; Singer, T.; Roth, A.B. Bioprinted 3D Primary Liver Tissues Allow Assessment of Organ-Level Response to Clinical Drug Induced Toxicity In Vitro. PLoS ONE 2016, 11, e0158674. [Google Scholar] [CrossRef]
- Norona, L.M.; Nguyen, D.G.; Gerber, D.A.; Presnell, S.C.; Lecluyse, E.L. Editor’s Highlight: Modeling Compound-Induced Fibrogenesis In Vitro Using Three-Dimensional Bioprinted Human Liver Tissues. Toxicol. Sci. 2016, 154, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Bale, S.S.; Geerts, S.; Jindal, R.; Yarmush, M.L. Isolation and co-culture of rat parenchymal and non-parenchymal liver cells to evaluate cellular interactions and response. Sci. Rep. 2016, 6, 25329. [Google Scholar] [CrossRef] [PubMed]
- Baze, A.; Parmentier, C.; Hendriks, D.; Hurrell, T.; Heyd, B.; Bachellier, P.; Schuster, C.; Ingelman-Sundberg, M.; Richert, L.; Bachellier, P. Three-Dimensional Spheroid Primary Human Hepatocytes in Monoculture and Coculture with Nonparenchymal Cells. Tissue Eng. Part C: Methods 2018, 24, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Cole, B.K.; Feaver, R.E.; Wamhoff, B.R.; Dash, A. Non-alcoholic fatty liver disease (NAFLD) models in drug discovery. Exp. Opin. Drug Discov. 2017, 13, 193–205. [Google Scholar] [CrossRef]
- Marchisello, S.; Di Pino, A.; Scicali, R.; Urbano, F.; Piro, S.; Purrello, F.; Rabuazzo, A.M. Pathophysiological, Molecular and Therapeutic Issues of Nonalcoholic Fatty Liver Disease: An Overview. Int. J. Mol. Sci. 2019, 20, 1948. [Google Scholar] [CrossRef]
- Sumida, Y.; Yoneda, M. Current and future pharmacological therapies for NAFLD/NASH. J. Gastroenterol. 2018, 53, 362–376. [Google Scholar] [CrossRef]
- Eriksson, J.E.; Dechat, T.; Grin, B.; Helfand, B.; Mendez, M.; Pallari, H.-M.; Goldman, R.D. Introducing intermediate filaments: From discovery to disease. J. Clin. Investig. 2009, 119, 1763–1771. [Google Scholar] [CrossRef]
- Gottfried, E.; Kunz-Schughart, L.A.; Weber, A.; Rehli, M.; Peuker, A.; Müller, A.; Kastenberger, M.; Brockhoff, G.; Andreesen, R.; Kreutz, M. Expression of CD68 in Non-Myeloid Cell Types. Scand. J. Immunol. 2008, 67, 453–463. [Google Scholar] [CrossRef]
- Viñas, O.; Bataller, R.; Sancho-Bru, P.; Ginès, P.; Berenguer, C.; Enrich, C.; Nicolás, J.M.; Ercilla, G.; Gallart, T.; Vives, J. Human hepatic stellate cells show features of antigen-presenting cells and stimulate lymphocyte proliferation. Hepatology 2003, 38, 919–929. [Google Scholar] [CrossRef]
- Schon, H.-T.; Weiskirchen, R. Immunomodulatory effects of transforming growth factor-β in the liver. HepatoBiliary Surg. Nutr. 2014, 3, 386–406. [Google Scholar]
- Giraudi, P.J.; Becerra, V.J.B.; Marin, V.; Chavez-Tapia, N.C.; Tiribelli, C.; Rosso, N. The importance of the interaction between hepatocyte and hepatic stellate cells in fibrogenesis induced by fatty accumulation. Exp. Mol. Pathol. 2015, 98, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Linhart, K.; Bartsch, H.; Seitz, H.K. The role of reactive oxygen species (ROS) and cytochrome P-450 2E1 in the generation of carcinogenic etheno-DNA adducts. Redox Boil. 2014, 3, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Weltman, M.D.; Farrell, G.C.; Hall, P.; Ingelman-Sundberg, M.; Liddle, C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology 1998, 27, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Cederbaum, A.I. Cytochrome P450s and Alcoholic Liver Disease. Curr. Pharm. Des. 2018, 24, 1502–1517. [Google Scholar] [CrossRef] [PubMed]
- Arab, J.P.; Arrese, M.; Trauner, M. Recent Insights into the Pathogenesis of Nonalcoholic Fatty Liver Disease. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 321–350. [Google Scholar] [CrossRef] [PubMed]
- Pingitore, P.; Sasidharan, K.; Ekstrand, M.; Prill, S.; Lindén, D.; Romeo, S. Human Multilineage 3D Spheroids as a Model of Liver Steatosis and Fibrosis. Int. J. Mol. Sci. 2019, 20, 1629. [Google Scholar] [CrossRef] [PubMed]
- Van Grunsven, L.A. 3D in vitro models of liver fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 133–146. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Qin, H.; Teufel, U.; Engelmann, G.; Flechtenmacher, C.; Peccerella, T.; Millonig, G.; Hoffmann, G.; Mueller, S.; Seitz, H.-K. Detection of hepatic highly carcinogenic, exocyclic etheno-DNA-adducts in patients with alcoholic and and non-alcoholic fatty liver disease and in children with non-alcoholic steatoheopatitis. J. Hepatol. 2012, 56, S520. [Google Scholar] [CrossRef]
- Nieto, N.; Friedman, S.L.; Cederbaum, A.I. Cytochrome P450 2E1-derived Reactive Oxygen Species Mediate Paracrine Stimulation of Collagen I Protein Synthesis by Hepatic Stellate Cells. J. Boil. Chem. 2002, 277, 9853–9864. [Google Scholar] [CrossRef]
- Xu, J.; Ma, H.-Y.; Liang, S.; Sun, M.; Karin, G.; Koyama, Y.; Hu, R.; Quehenberger, O.; Davidson, N.O.; Dennis, E.A.; et al. The role of human cytochrome P450 2E1 in liver inflammation and fibrosis. Hepatol. Commun. 2017, 1, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Abdelmegeed, M.A.; Banerjee, A.; Yoo, S.H.; Jang, S.; Gonzalez, F.J.; Song, B.-J. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. J. Hepatol. 2012, 57, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.; Konkel, A.; Fischer, R.; Schunck, W.-H. Cytochrome P450–dependent metabolism of ω-6 and ω-3 long-chain polyunsaturated fatty acids. Pharmacol. Rep. 2010, 62, 536–547. [Google Scholar] [CrossRef]
- Boubia, B.; Poupardin, O.; Barth, M.; Binet, J.; Peralba, P.; Mounier, L.; Jacquier, E.; Gauthier, E.; Lepais, V.; Chatar, M.; et al. Design, Synthesis, and Evaluation of a Novel Series of Indole Sulfonamide Peroxisome Proliferator Activated Receptor (PPAR) α/γ/δ Triple Activators: Discovery of Lanifibranor, a New Antifibrotic Clinical Candidate. J. Med. Chem. 2018, 6, 2246–2265. [Google Scholar] [CrossRef] [PubMed]

| Donor | PHH | NPC | Origin | Cultured NPCs | Genotype | Age | Gender | Ethnicity |
|---|---|---|---|---|---|---|---|---|
| Donor 1 | X | X | KLC | Yes | Heterozygous PNPLA3-I148M | 47 | Female | Caucasian |
| Donor 2 | X | X | KLC | Yes | Homozygous PNPLA3-E434K | 61 | Male | Caucasian |
| Donor 3 | X | X | KLC | Yes | Heterozygous PNPLA3-I148M | 25 | Female | Caucasian |
| Donor 4 | X | X | KLC | Yes | No polymorphisms | 86 | Male | Caucasian |
| Donor 5 | X | BioIVT | n/a | No polymorphisms | 30 | Female | Caucasian | |
| Donor 6 | X | BioIVT | n/a | Heterozygous TM6SF2-E167K | 22 | Male | Caucasian | |
| Donor 7 | X | BioIVT | Unknown | Homozygous PNPLA3-I148M | Unknown | Female | Unknown | |
| Donor 8 | X | Lonza | Yes | No polymorphisms | 55 | Female | Caucasian |
| Donor | Baseline Fibrillary Matrix | 2xFFA and/or 3xFFA | Exogenous TGFβ | LPS | ALK5 Inhibitor (TGFβRi) |
|---|---|---|---|---|---|
| PHH1+NPC1 | High | Mild increase in COL1A1 mRNA and protein expression # | Minor increases in COL1A1 mRNA and protein expression # | Increased IL-6 # | TGFβ, LOX and COL1A1 mRNA expression reduced # Significantly reduced COL1A1 protein deposition # |
| PHH1+NPC3 | Low | No increase in COL1A1 or αSMA protein expression # | No increase in COL1A1 mRNA or protein expression * | ND | TGFβ, LOX, and COL1A1 mRNA expression reduced * |
| PHH1+NPC8 | Interme-diate | Increase in COL1A and αSMA protein expression * | ND | ND | ND |
| PHH2+NPC2 | High | Increased COL1A1 mRNA expressionsion # | Increased COL1A1 mRNA expression No protein increase * | Increased IL-6 * | ND |
| PHH3+NPC1 | High | No increase in COL1A1 or αSMA protein expression # | No increase in COL1A1 mRNA or protein expression * | ND | TGFβ, LOX, and COL1A1 mRNA expression reduced * |
| PHH3+NPC3 | Low | Minor increase in COL1A1 or αSMA protein expression # | No increase in COL1A1 mRNA or protein expression # | Increased IL-6 # | TGFβ, LOX, and COL1A1 mRNA expression reduced * |
| PHH4+NPC4 | Low | No increase in COL1A1 mRNA or protein expression # | No increase in COL1A1 mRNA or protein expression * | No response * | TGFβ induced increase in LOX and COL1A1 mRNA expression attenuated by TGFβRi # |
| PHH5+NPC7 | Low | Increased COL1A1 mRNA expressionNo protein increase * | Increased COL1A1 mRNA expressionNo protein increase * | Increased IL-6 # | ND |
| PHH6+NPC7 | Low | Increased COL1A1 mRNA expressionNo protein increase # | Increased COL1A1 mRNA expressionNo protein increase * | Increased IL-6 * | ND |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hurrell, T.; Kastrinou-Lampou, V.; Fardellas, A.; Hendriks, D.F.G.; Nordling, Å.; Johansson, I.; Baze, A.; Parmentier, C.; Richert, L.; Ingelman-Sundberg, M. Human Liver Spheroids as a Model to Study Aetiology and Treatment of Hepatic Fibrosis. Cells 2020, 9, 964. https://doi.org/10.3390/cells9040964
Hurrell T, Kastrinou-Lampou V, Fardellas A, Hendriks DFG, Nordling Å, Johansson I, Baze A, Parmentier C, Richert L, Ingelman-Sundberg M. Human Liver Spheroids as a Model to Study Aetiology and Treatment of Hepatic Fibrosis. Cells. 2020; 9(4):964. https://doi.org/10.3390/cells9040964
Chicago/Turabian StyleHurrell, Tracey, Vlasia Kastrinou-Lampou, Achilleas Fardellas, Delilah F. G. Hendriks, Åsa Nordling, Inger Johansson, Audrey Baze, Céline Parmentier, Lysiane Richert, and Magnus Ingelman-Sundberg. 2020. "Human Liver Spheroids as a Model to Study Aetiology and Treatment of Hepatic Fibrosis" Cells 9, no. 4: 964. https://doi.org/10.3390/cells9040964
APA StyleHurrell, T., Kastrinou-Lampou, V., Fardellas, A., Hendriks, D. F. G., Nordling, Å., Johansson, I., Baze, A., Parmentier, C., Richert, L., & Ingelman-Sundberg, M. (2020). Human Liver Spheroids as a Model to Study Aetiology and Treatment of Hepatic Fibrosis. Cells, 9(4), 964. https://doi.org/10.3390/cells9040964





