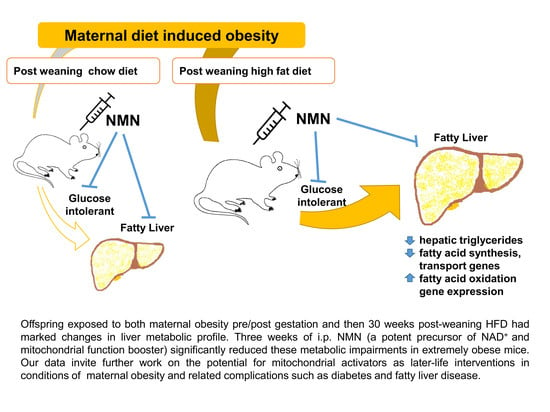
Administration of Nicotinamide Mononucleotide (NMN) Reduces Metabolic Impairment in Male Mouse Offspring from Obese Mothers
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Maternal Diet had a Significant Impact on Pup Body Weight
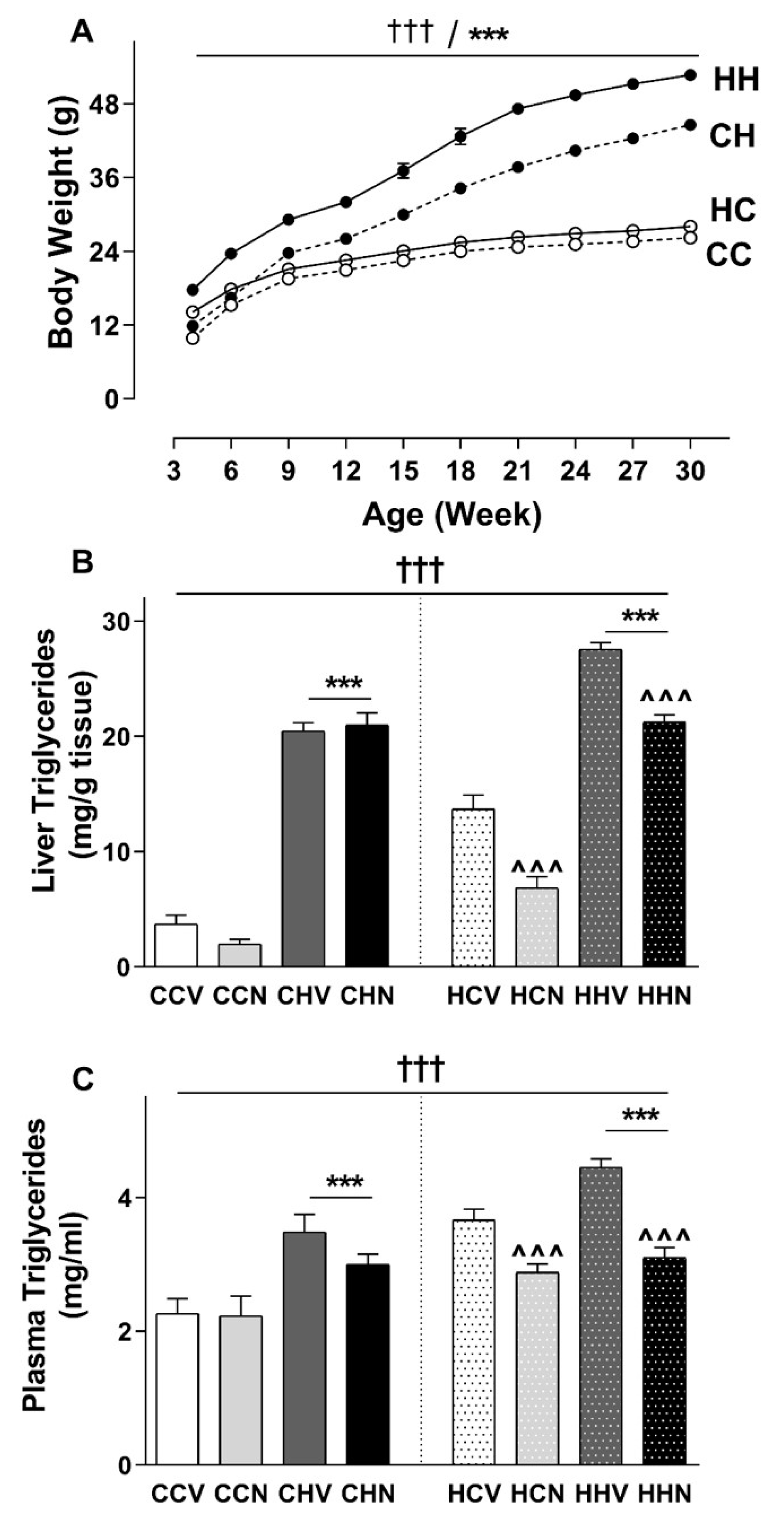
3.2. Consumption of HFD Increased Final Body Weight and Tissue Mass with a Recovery Phenotype by NMN
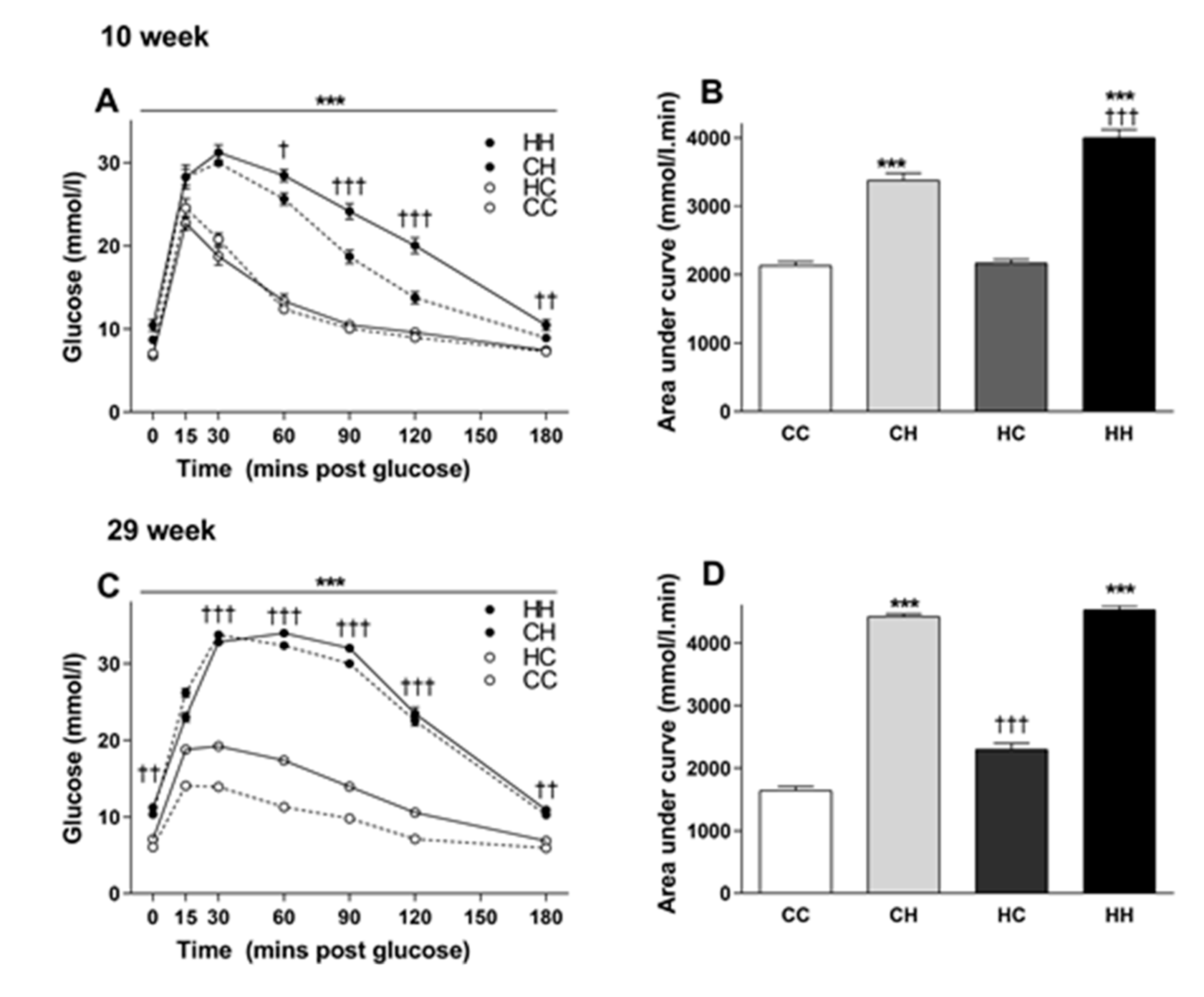
3.3. The Effects of Maternal and Post-weaning Diet on Glucose Tolerance at 10 Weeks of Age
3.4. The Effects of Maternal and Post-weaning Diet on Glucose Tolerance at 29 Weeks of Age
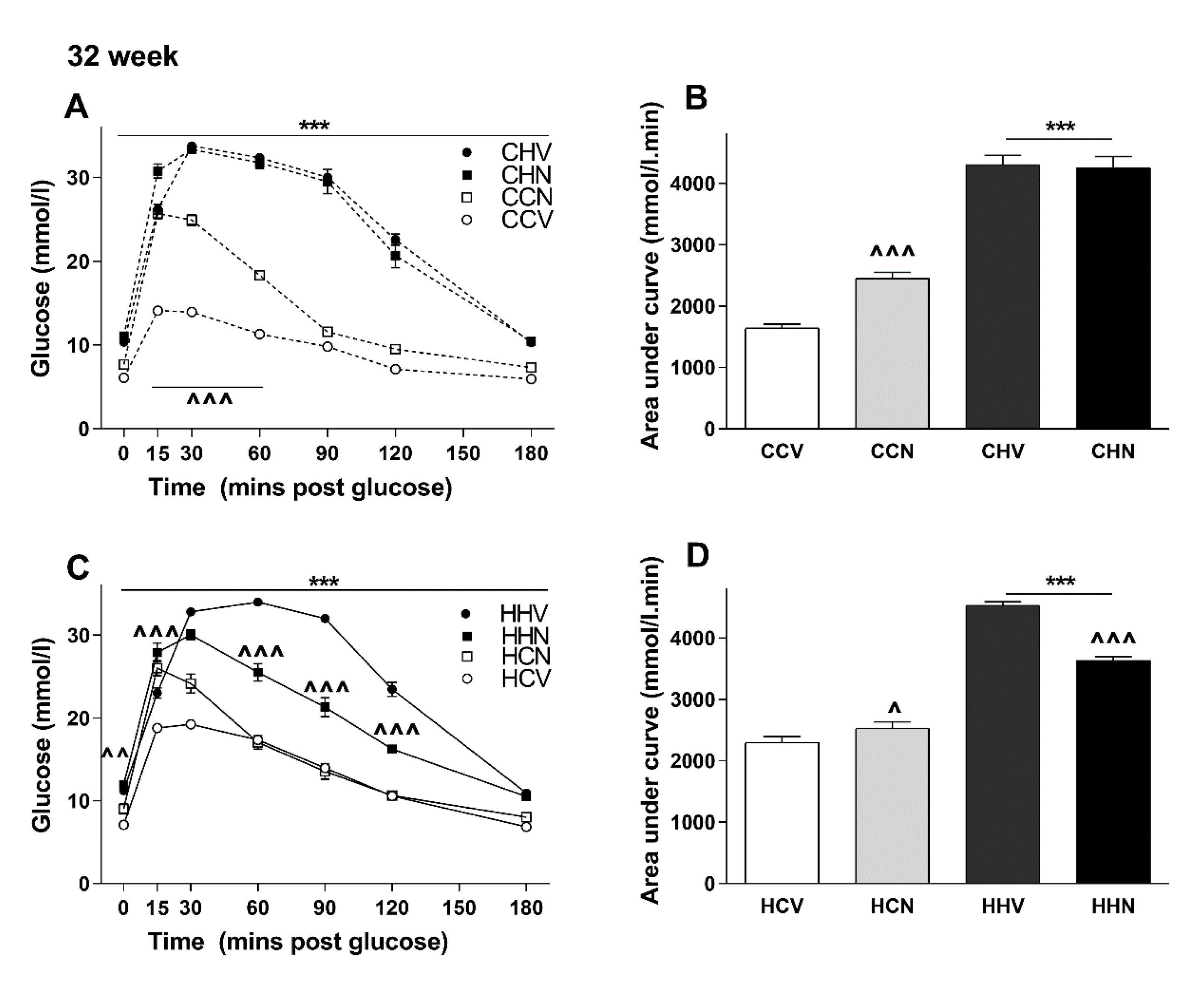
3.5. NMN Treatment Was Beneficial Only for the Most Metabolically Compromised Mice Group
3.6. Impacts of HFD and NMN Treatment on Insulin
3.7. Hepatic Triglyceride Was Increased Due to HFD Consumption and Recovered by NMN Treatment
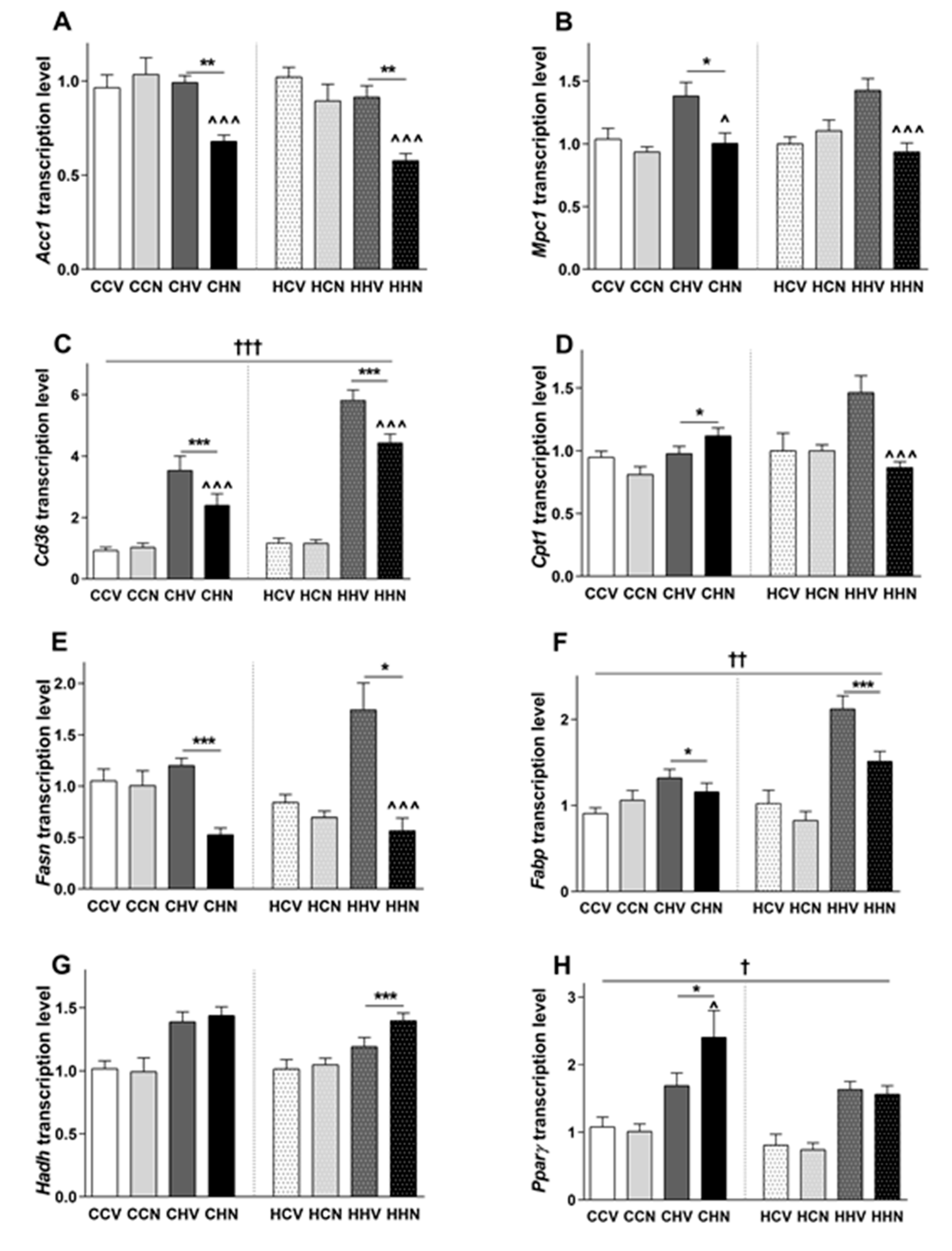
3.8. Changes in Gene Expression Responsible for Glucose and Fatty Acid Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Caballero, B. The Global Epidemic of Obesity: An Overview. Epidemiologic Rev. 2007, 29, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dietz, W.H. Are we making progress in the prevention and control of childhood obesity? It all depends on how you look at it. Obesity 2016, 24, 991–992. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xue, Y. Pediatric obesity: Causes, symptoms, prevention and treatment. Exp. Ther. Med. 2015, 11, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Suzuki, M. Parental obesity and overweight affect the body-fat accumulation in the offspring: The possible effect of a high-fat diet through epigenetic inheritance. Obes. Rev. 2006, 7, 201–208. [Google Scholar] [CrossRef]
- Vickers, M.H. Early Life Nutrition, Epigenetics and Programming of Later Life Disease. Nutrients 2014, 6, 2165–2178. [Google Scholar] [CrossRef]
- Morris, M.J. Early life influences on obesity risk: Maternal overnutrition and programming of obesity. Expert Rev. Endocrinol. Metab. 2009, 4, 625–637. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.; Eriksson, J.G.; Broekman, B.F.P. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2016, 5, 53–64. [Google Scholar] [CrossRef]
- Ayonrinde, O.T.; Oddy, W.H.; Adams, L.A.; Mori, T.; Beilin, L.; De Klerk, N.; Olynyk, J.K. Infant nutrition and maternal obesity influence the risk of non-alcoholic fatty liver disease in adolescents. J. Hepatol. 2017, 67, 568–576. [Google Scholar] [CrossRef]
- Boyle, K.; Patinkin, Z.; Shapiro, A.L.; Bader, C.; Vanderlinden, L.; Kechris, K.; Janssen, R.C.; Ford, R.J.; Smith, B.K.; Steinberg, G.R.; et al. Maternal obesity alters fatty acid oxidation, AMPK activity, and associated DNA methylation in mesenchymal stem cells from human infants. Mol. Metab. 2017, 6, 1503–1516. [Google Scholar] [CrossRef]
- Lowell, B.B.; Yoon, B.; Häkkinen, H.; Landman, U.; Wörz, A.S.; Antonietti, J.-M.; Abbet, S.; Judai, K.; Heiz, U. Mitochondrial Dysfunction and Type 2 Diabetes. Science 2005, 307, 384–387. [Google Scholar] [CrossRef]
- Jheng, H.-F.; Tsai, P.-J.; Guo, S.-M.; Kuo, L.-H.; Chang, C.-S.; Su, I.-J.; Chang, C.-R.; Tsai, Y.-S. Mitochondrial Fission Contributes to Mitochondrial Dysfunction and Insulin Resistance in Skeletal Muscle. Mol. Cell. Boil. 2011, 32, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Pirinen, E.; Sasso, G.L.; Auwerx, J. Mitochondrial sirtuins and metabolic homeostasis. Best Pr. Res. Clin. Endocrinol. Metab. 2012, 26, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sauve, A.A. NAD + metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2016, 1864, 1787–1800. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Mills, K.F.; Yoon, M.J.; Imai, S.-I. Nicotinamide Mononucleotide, a Key NAD+ Intermediate, Treats the Pathophysiology of Diet- and Age-Induced Diabetes in Mice. Cell Metab. 2011, 14, 528–536. [Google Scholar] [CrossRef]
- Gariani, K.; Menzies, K.J.; Ryu, D.; Wegner, C.J.; Wang, X.; Ropelle, E.R.; Moullan, N.; Zhang, H.; Perino, A.; Lemos, V.; et al. Eliciting the mitochondrial unfolded protein response via NAD repletion reverses fatty liver disease. Hepatology 2015. [Google Scholar] [CrossRef]
- Cantó, C.; Houtkooper, R.H.; Pirinen, E.; Youn, U.Y.; Oosterveer, M.H.; Cen, Y.; Fernandez-Marcos, P.J.; Yamamoto, H.; Andreux, P.A.; Cettour-Rose, P.; et al. The NAD+ Precursor Nicotinamide Riboside Enhances Oxidative Metabolism and Protects against High-Fat Diet-Induced Obesity. Cell Metab. 2012, 15, 838–847. [Google Scholar] [CrossRef]
- Mills, K.F.; Yoshida, S.; Stein, L.R.; Grozio, A.; Kubota, S.; Sasaki, Y.; Redpath, P.; Migaud, M.E.; Apte, R.S.; Uchida, K.; et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016, 24, 795–806. [Google Scholar] [CrossRef]
- Uddin, G.M.; Youngson, N.A.; Doyle, B.M.; Sinclair, D.A.; Morris, M.J. Nicotinamide mononucleotide (NMN) supplementation ameliorates the impact of maternal obesity in mice: Comparison with exercise. Sci. Rep. 2017, 7, 15063. [Google Scholar] [CrossRef]
- Oostvogels, A.J.; Hof, M.H.; Gademan, M.G.; Roseboom, T.J.; Stronks, K.; Vrijkotte, T.G. Does maternal pre-pregnancy overweight or obesity influence offspring’s growth patterns from birth up to 7 years? ABCD-Study Early Human Dev. 2017, 113, 62–70. [Google Scholar] [CrossRef]
- Rodriguez, L.; Otero, P.; Panadero, M.I.; Rodrigo, S.; Álvarez-Millán, J.J.; Bocos, C. Maternal Fructose Intake Induces Insulin Resistance and Oxidative Stress in Male, but Not Female, Offspring. J. Nutr. Metab. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Desai, M.; Jellyman, J.K.; Han, G.; Beall, M.; Lane, R.H.; Ross, M.G.; Han, G. Maternal obesity and high-fat diet program offspring metabolic syndrome. Am. J. Obstet. Gynecol. 2014, 211, 237-e1. [Google Scholar] [CrossRef]
- Lecoutre, S.; Deracinois, B.; Laborie, C.; Eberlé, D.; Guinez, C.; Panchenko, P.; Lesage, J.; Vieau, D.; Junien, C.; Gabory, A.; et al. Depot- and sex-specific effects of maternal obesity in offspring’s adipose tissue. J. Endocrinol. 2016, 230, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Uddin, G.M.; Youngson, N.A.; Sinclair, D.A.; Morris, M.J. Head to Head Comparison of Short-Term Treatment with the NAD+ Precursor Nicotinamide Mononucleotide (NMN) and 6 Weeks of Exercise in Obese Female Mice. Front. Pharmacol. 2016, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Long, N.M.; George, L.A.; Uthlaut, A.B.; Smith, D.T.; Nijland, M.; Nathanielsz, P.W.; Ford, S. Maternal obesity and increased nutrient intake before and during gestation in the ewe results in altered growth, adiposity, and glucose tolerance in adult offspring1. J. Anim. Sci. 2010, 88, 3546–3553. [Google Scholar] [CrossRef] [PubMed]
- Elahi, M.M.; Cagampang, F.R.; Mukhtar, D.; Anthony, F.W.; Ohri, S.K.; Hanson, M. Long-term maternal high-fat feeding from weaning through pregnancy and lactation predisposes offspring to hypertension, raised plasma lipids and fatty liver in mice. Br. J. Nutr. 2009, 102, 514. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Simar, D.; Lambert, K.; Mercier, J.; Morris, M.J. Maternal and Postnatal Overnutrition Differentially Impact Appetite Regulators and Fuel Metabolism. Endocrinology 2008, 149, 5348–5356. [Google Scholar] [CrossRef]
- Xiao, D.; Kou, H.; Zhang, L.; Guo, Y.; Wang, H. Prenatal Food Restriction with Postweaning High-fat Diet Alters Glucose Metabolic Function in Adult Rat Offspring. Arch. Med. Res. 2017, 48, 35–45. [Google Scholar] [CrossRef]
- Bahari, H.; Caruso, V.; Morris, M.J. Late-Onset Exercise in Female Rat Offspring Ameliorates the Detrimental Metabolic Impact of Maternal Obesity. Endocrinology 2013, 154, 3610–3621. [Google Scholar] [CrossRef]
- Yoshino, J.; Baur, J.; Imai, S.-I. NAD+ Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab. 2018, 27, 513–528. [Google Scholar] [CrossRef]
- Trammell, S.; Weidemann, B.J.; Chadda, A.; Yorek, M.S.; Holmes, A.; Coppey, L.J.; Obrosov, A.; Kardon, R.; Yorek, M.; Brenner, C. Nicotinamide Riboside Opposes Type 2 Diabetes and Neuropathy in Mice. Sci. Rep. 2016, 6, 26933. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, X.; Hua, X.; Liu, J.; Fan, M.; Li, G.; Song, J.; Xu, T.; Li, Z.-Y.; Guan, Y.; et al. Hepatic NAD+ deficiency as a therapeutic target for non-alcoholic fatty liver disease in ageing. Br. J. Pharmacol. 2016, 173, 2352–2368. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Z.-F.; Zheng, G.-H.; Wang, A.; Sun, C.-H.; Qin, S.-P.; Zhuang, J.; Lu, J.; Ma, D.; Zheng, Y.-L. The Inhibitory Effects of Purple Sweet Potato Color on Hepatic Inflammation Is Associated with Restoration of NAD+ Levels and Attenuation of NLRP3 Inflammasome Activation in High-Fat-Diet-Treated Mice. Molecules 2017, 22, 1315. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Byun, J.; Zhai, P.; Ikeda, Y.; Oka, S.; Sadoshima, J. Nicotinamide Mononucleotide, an Intermediate of NAD+ Synthesis, Protects the Heart from Ischemia and Reperfusion. PLoS ONE 2014, 9, e98972. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.F.; Chavez, J.D.; Garcia-Menendez, L.; Choi, Y.S.; Roe, N.D.; Chiao, Y.A.; Edgar, J.S.; Goo, Y.A.; Goodlett, D.R.; Bruce, J.E.; et al. Normalization of NAD+ Redox Balance as a Therapy for Heart Failure. Circulation 2016, 134, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, X.; Yang, Y.; Takata, T.; Sakurai, T. Nicotinamide mononucleotide protects against β-amyloid oligomer-induced cognitive impairment and neuronal death. Brain Res. 2016, 1643, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.P.; Price, N.L.; Ling, A.J.; Moslehi, J.J.; Montgomery, M.; Rajman, L.; White, J.P.; Teodoro, J.; Wrann, C.D.; Hubbard, B.; et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 2013, 155, 1624–1638. [Google Scholar] [CrossRef]
- Caton, P.W.; Kieswich, J.; Yaqoob, M.M.; Holness, M.J.; Sugden, M.C. Nicotinamide mononucleotide protects against pro-inflammatory cytokine-mediated impairment of mouse islet function. Diabetologia 2011, 54, 3083–3092. [Google Scholar] [CrossRef]
- Fu, L.; Bruckbauer, A.; Li, F.; Cao, Q.; Cui, X.; Wu, R.; Shi, H.; Zemel, M.; Xue, B. Interaction between metformin and leucine in reducing hyperlipidemia and hepatic lipid accumulation in diet-induced obese mice. Metabolism 2015, 64, 1426–1434. [Google Scholar] [CrossRef]
- Chan, Y.L.; Saad, S.; Simar, D.; Oliver, B.; McGrath, K.C.; Van Reyk, D.; Bertrand, P.; Gorrie, C.; Pollock, C.; Chen, H.; et al. Short term exendin-4 treatment reduces markers of metabolic disorders in female offspring of obese rat dams. Int. J. Dev. Neurosci. 2015, 46, 67–75. [Google Scholar] [CrossRef]
- Dall, M.; Penke, M.; Sulek, K.; Matz-Soja, M.; Holst, B.; Garten, A.; Kiess, W.; Treebak, J.T. Hepatic NAD+ levels and NAMPT abundance are unaffected during prolonged high-fat diet consumption in C57BL/6JBomTac mice. Mol. Cell. Endocrinol. 2018, 473, 245–256. [Google Scholar] [CrossRef]
- Costford, S.R.; Brouwers, B.; Hopf, M.E.; Sparks, L.M.; Dispagna, M.; Gomes, A.P.; Cornnell, H.H.; Petucci, C.; Phelan, P.; Xie, H.; et al. Skeletal muscle overexpression of nicotinamide phosphoribosyl transferase in mice coupled with voluntary exercise augments exercise endurance. Mol. Metab. 2018, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Stromsdorfer, K.L.; Yamaguchi, S.; Yoon, M.J.; Moseley, A.C.; Franczyk, M.P.; Kelly, S.C.; Qi, N.; Imai, S.-I.; Yoshino, J. NAMPT-Mediated NAD(+) Biosynthesis in Adipocytes Regulates Adipose Tissue Function and Multi-organ Insulin Sensitivity in Mice. Cell Rep. 2016, 16, 1851–1860. [Google Scholar] [CrossRef] [PubMed]
- Kruse, M.; Seki, Y.; Vuguin, P.; Du, X.Q.; Fiallo, A.; Glenn, A.S.; Singer, S.; Breuhahn, K.; Katz, E.B.; Charron, M.J. High-fat intake during pregnancy and lactation exacerbates high-fat diet-induced complications in male offspring in mice. Endocrinology 2013, 154, 3565–3576. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.E.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef]
- Xiao, D.; Huang, X.; Lawrence, J.; Yang, S.; Zhang, L. Fetal and Neonatal Nicotine Exposure Differentially Regulates Vascular Contractility in Adult Male and Female Offspring. J. Pharmacol. Exp. Ther. 2006, 320, 654–661. [Google Scholar] [CrossRef]
- Raipuria, M.; Bahari, H.; Morris, M.J. Effects of Maternal Diet and Exercise during Pregnancy on Glucose Metabolism in Skeletal Muscle and Fat of Weanling Rats. PLoS ONE 2015, 10, e0120980. [Google Scholar] [CrossRef]

| Offspring from Lean Mothers | Offspring from Obese Mothers | Maternal Diet | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CCV | CCN | CHV | CHN | HCV | HCN | HHV | HHN | ||
| Final Body Weight (g) | 25.6 ± 0.4 | 25.2 ± 0.3 | 47.2 ± 1.0 *** | 41.8 ± 0.9 ^^^ | 27.8 ± 0.5 | 26.8 ± 0.6 | 52.4 ± 1.1 *** | 51.0 ± 1.4 | ††† |
| Liver mass (mg) | 945 ± 55 | 952 ± 35 | 1668 ± 136 *** | 1425 ± 89 | 1059 ± 75 | 935 ± 36 | 2603 ± 170 *** | 2456 ± 198 | ††† |
| Quadriceps mass (mg) | 287 ± 13 | 311 ± 13 | 329 ± 11 * | 335 ± 12 | 322 ± 3 | 302 ± 9 | 333 ± 15 *** | 361 ± 14 | |
| RP mass (mg) | 84 ± 9 | 108 ± 11 | 639 ± 19 *** | 520 ± 15 ^^^ | 201 ± 19 | 195 ± 22 | 729 ± 44 *** | 633 ± 29 ^ | ††† |
| Liver (% BW) | 3.68 ± 0.20 | 3.79 ± 0.12 | 3.68 ± 0.22 | 3.38 ± 0.15 | 3.78 ± 0.22 | 3.49 ± 0.10 | 4.96 ± 0.28 *** | 4.52 ± 0.24 | ††† |
| Quadriceps (% BW) | 1.12 ± 0.05 | 1.22 ± 0.06 | 0.69 ± 0.02 *** | 0.81 ± 0.03 | 1.15 ± 0.03 | 1.11 ± 0.02 | 0.57 ± 0.07 *** | 0.71 ± 0.04 ^ | † |
| RP (% BW) | 0.33 ± 0.04 | 0.39 ± 0.03 | 1.34 ± 0.03 *** | 1.25 ± 0.06 | 0.71 ± 0.06 | 0.71 ± 0.07 | 1.41 ± 0.08 *** | 1.24 ± 0.05 | ††† |
| Basal glucose (mmol/L) Before NMN at 10th week | 7.05 ± 0.23 | NA | 8.72 ± 0.31 ** | NA | 6.75 ± 0.27 | NA | 10.42 ± 0.70 *** | NA | †† |
| Basal glucose (mmol/L) After NMN at 32nd week | 6.08 ± 0.17 | 7.68 ± 0.31 ^^ | 10.34 ± 0.22 *** | 11.05 ± 0.45 | 7.08 ± 0.12 | 9.01 ± 0.60 ^^ | 11.22 ± 0.28 *** | 11.94 ± 0.30 | |
| Plasma Insulin (ng/mL) | 0.15 ± 0.02 | 0.23 ± 0.05 | 2.10 ± 0.63 *** | 3.02 ± 0.48 | 0.45 ± 0.09 | 0.64 ± 0.08 | 1.57 ± 0.25 *** | 8.23 ± 1.30 ^^^ | ††† |
| Pgc1 gene expression | 0.94 ± 0.06 | 0.95 ± 0.13 | 0.72 ± 0.07 | 1.18 ± 0.23 ^ | 1.03 ± 0.14 | 1.01 ± 0.09 | 0.58 ± 0.04 *** | 0.63 ± 0.04 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uddin, G.M.; Youngson, N.A.; Chowdhury, S.S.; Hagan, C.; Sinclair, D.A.; Morris, M.J. Administration of Nicotinamide Mononucleotide (NMN) Reduces Metabolic Impairment in Male Mouse Offspring from Obese Mothers. Cells 2020, 9, 791. https://doi.org/10.3390/cells9040791
Uddin GM, Youngson NA, Chowdhury SS, Hagan C, Sinclair DA, Morris MJ. Administration of Nicotinamide Mononucleotide (NMN) Reduces Metabolic Impairment in Male Mouse Offspring from Obese Mothers. Cells. 2020; 9(4):791. https://doi.org/10.3390/cells9040791
Chicago/Turabian StyleUddin, Golam M., Neil A. Youngson, Sabiha S. Chowdhury, Christopher Hagan, David A. Sinclair, and Margaret J. Morris. 2020. "Administration of Nicotinamide Mononucleotide (NMN) Reduces Metabolic Impairment in Male Mouse Offspring from Obese Mothers" Cells 9, no. 4: 791. https://doi.org/10.3390/cells9040791
APA StyleUddin, G. M., Youngson, N. A., Chowdhury, S. S., Hagan, C., Sinclair, D. A., & Morris, M. J. (2020). Administration of Nicotinamide Mononucleotide (NMN) Reduces Metabolic Impairment in Male Mouse Offspring from Obese Mothers. Cells, 9(4), 791. https://doi.org/10.3390/cells9040791