Chlorpromazine Induces Basolateral Aquaporin-2 Accumulation via F-Actin Depolymerization and Blockade of Endocytosis in Renal Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Western Blotting
2.3. Cell Immunofluorescence
2.4. Transferrin Endocytosis Assay
2.5. In Situ Kidney Tissue Slices
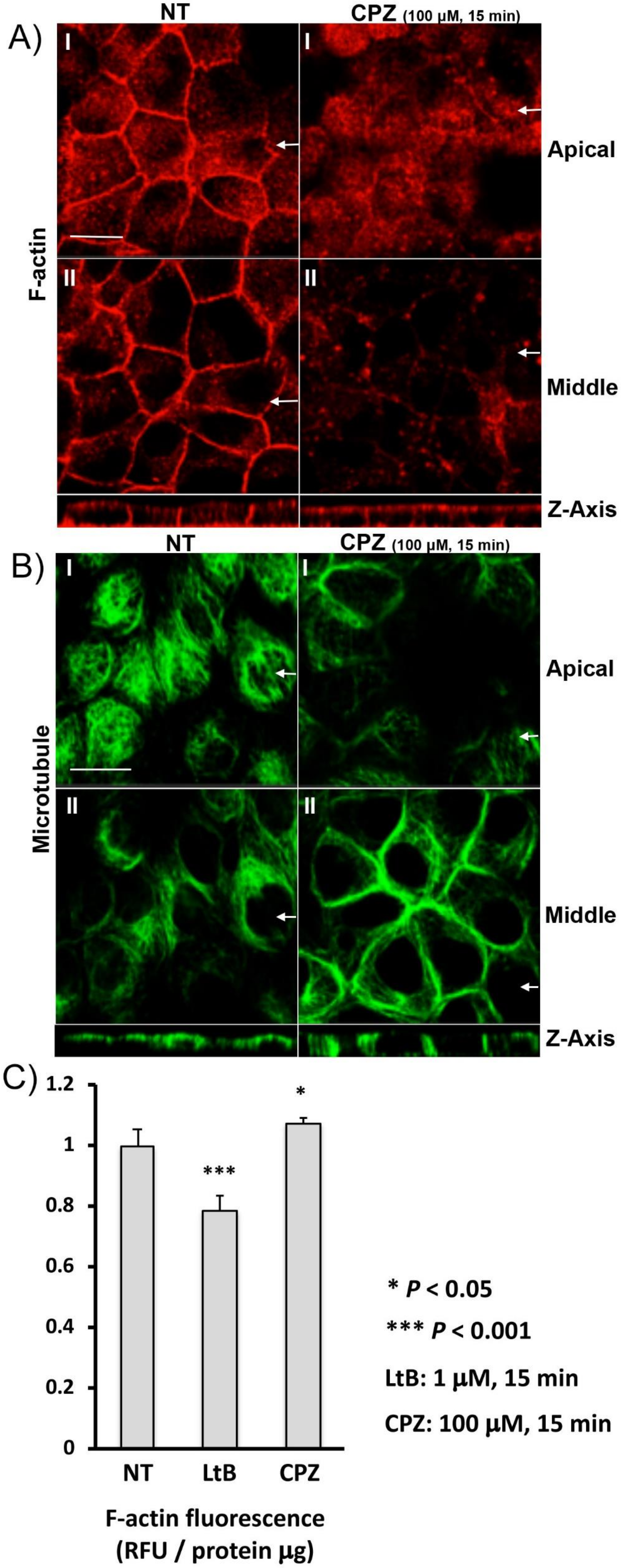
2.6. F-Actin Quantification
2.7. Statistical Analyses
3. Results
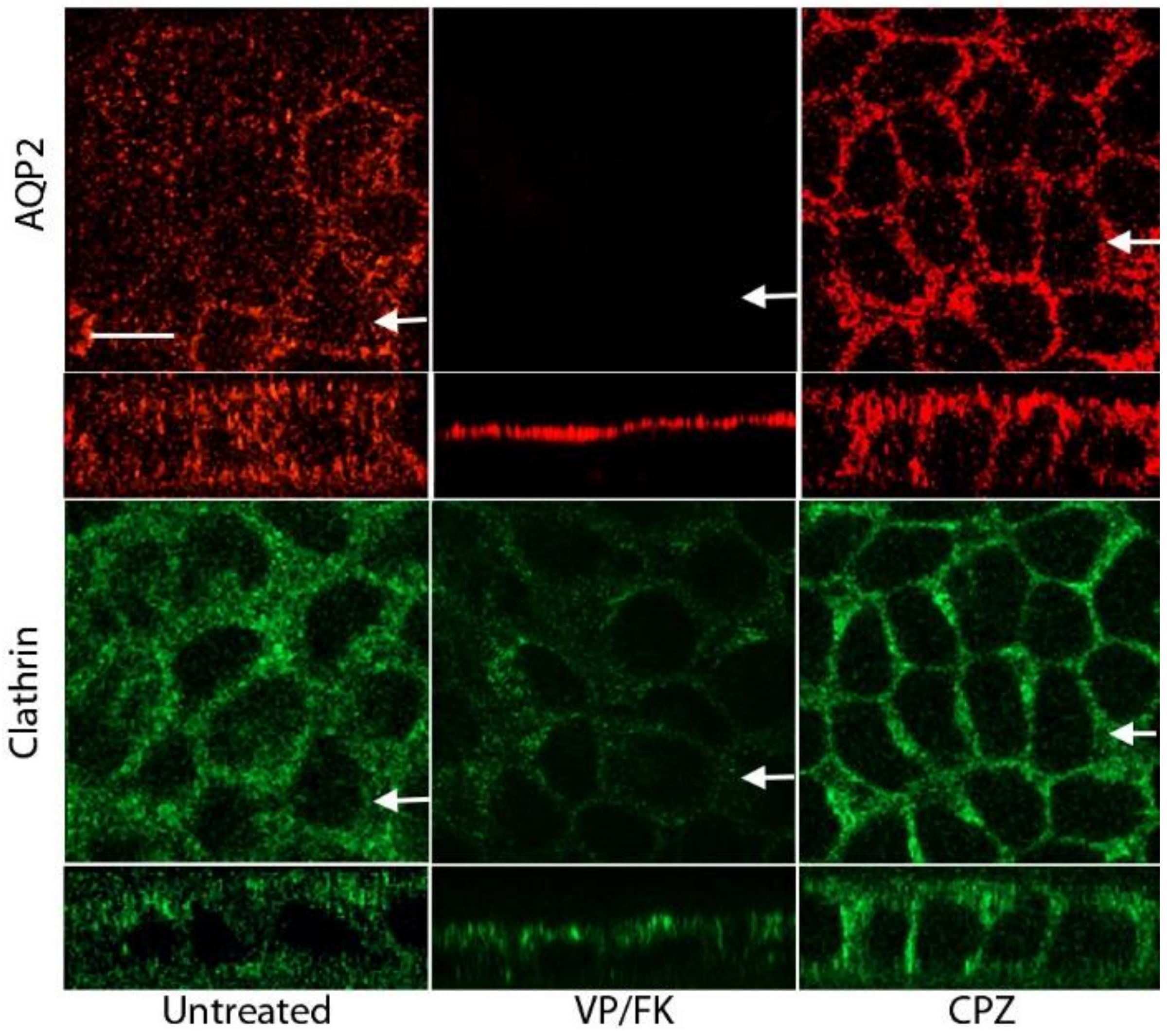
3.1. AQP2 Accumulates in the Basolateral Plasma Membrane After CPZ Treatment
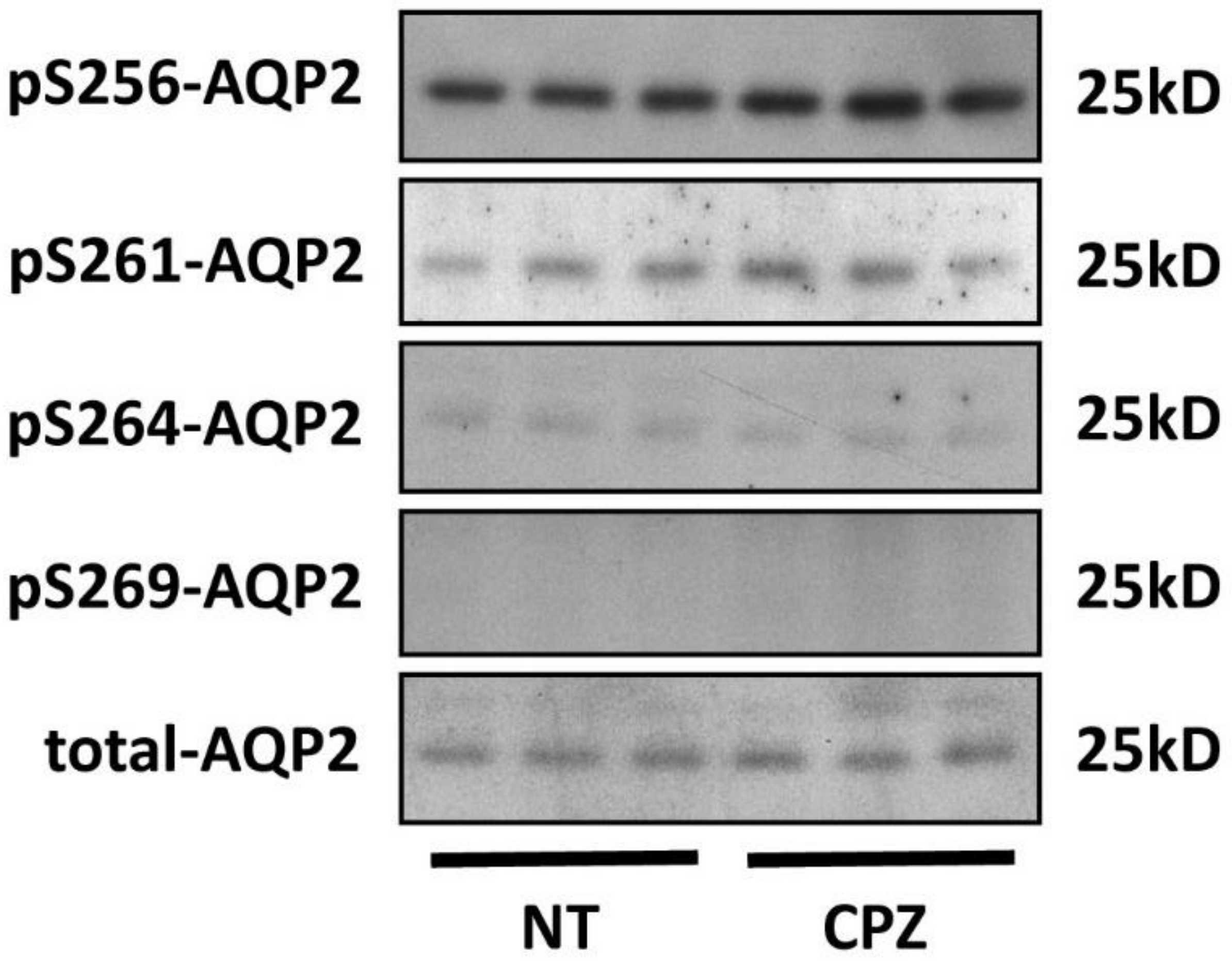
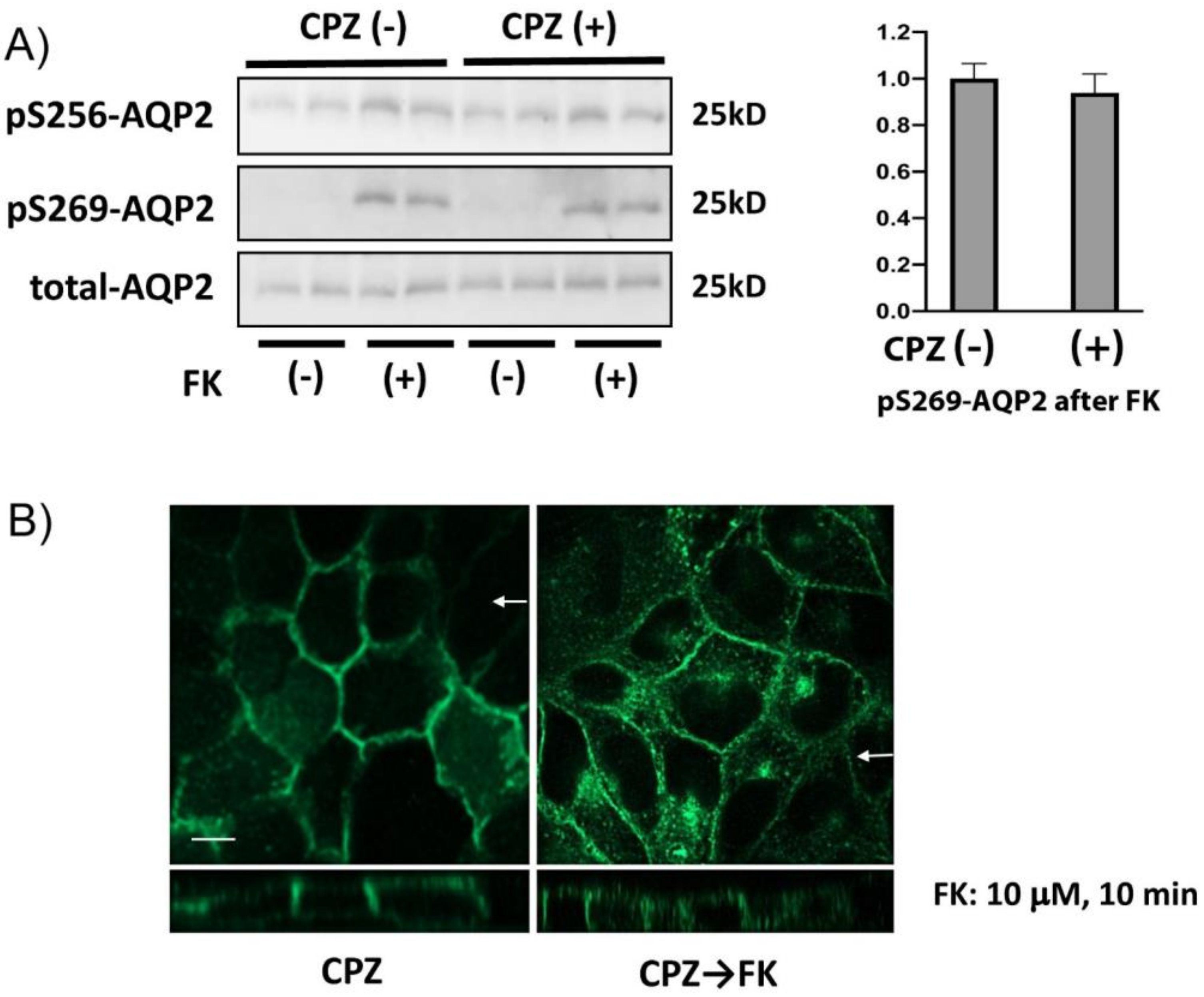
3.2. CPZ Does Not Modify AQP2 Phosphorylation
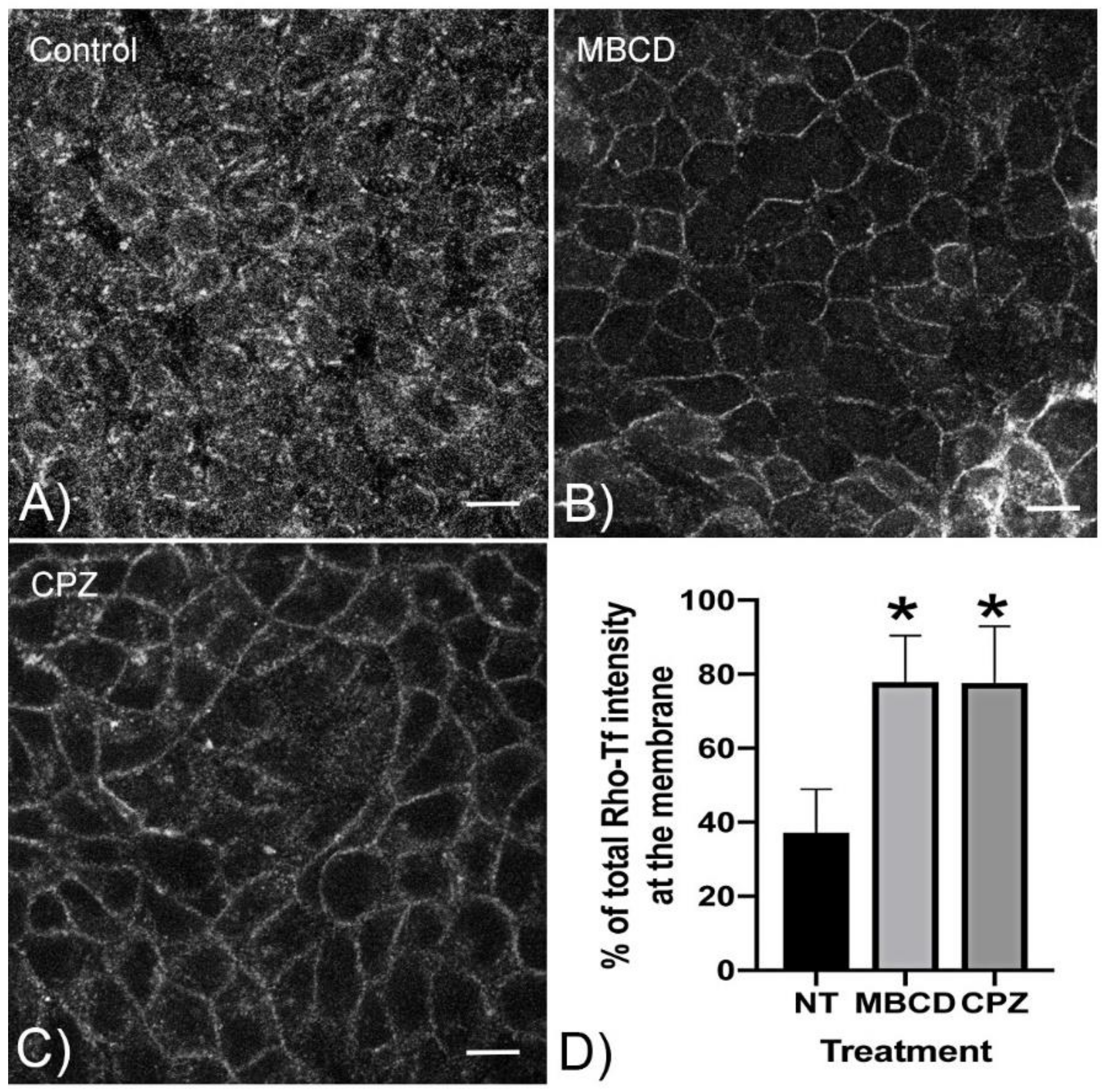
3.3. CPZ Inhibits Clathrin-Mediated Endocytosis of Transferrin in MDCK Cells
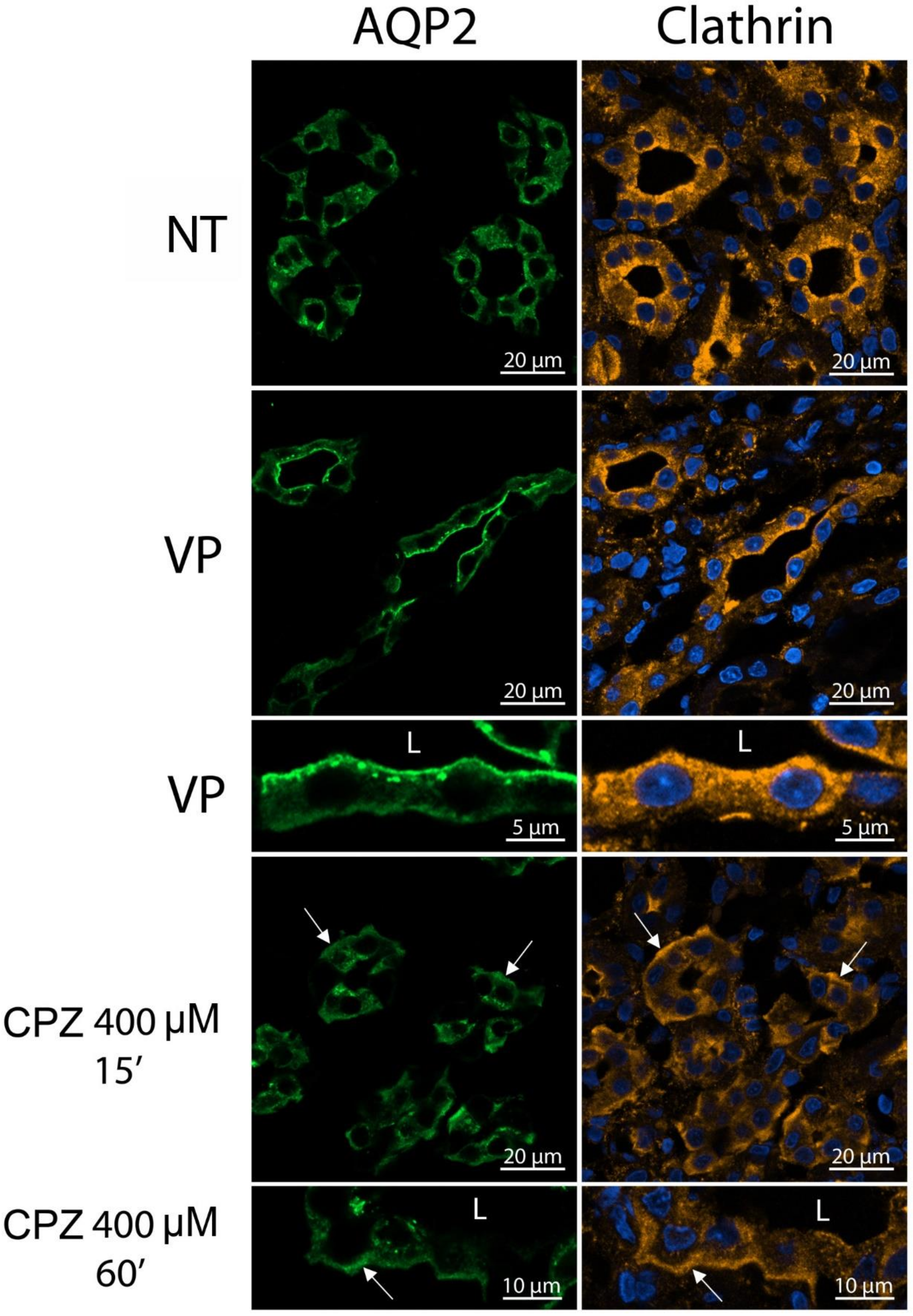
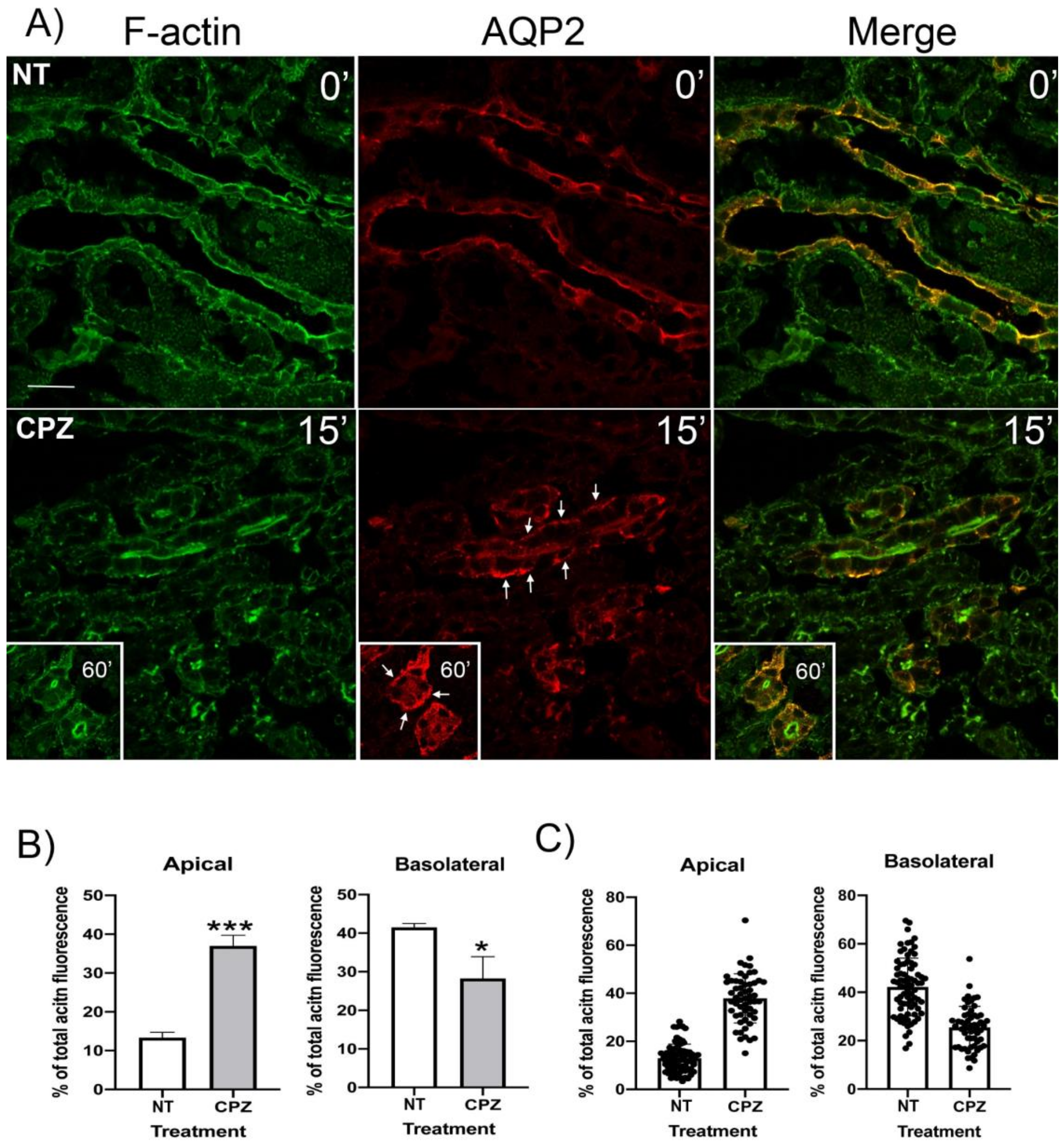
3.4. CPZ Causes Basolateral Accumulation of AQP2 and Clathrin in Kidney Slices
3.5. CPZ Decreases Basolateral F-Actin Staining in MDCK Cells
3.6. CPZ Decreases Basolateral and Increases Apical F-Actin in Kidney Slices
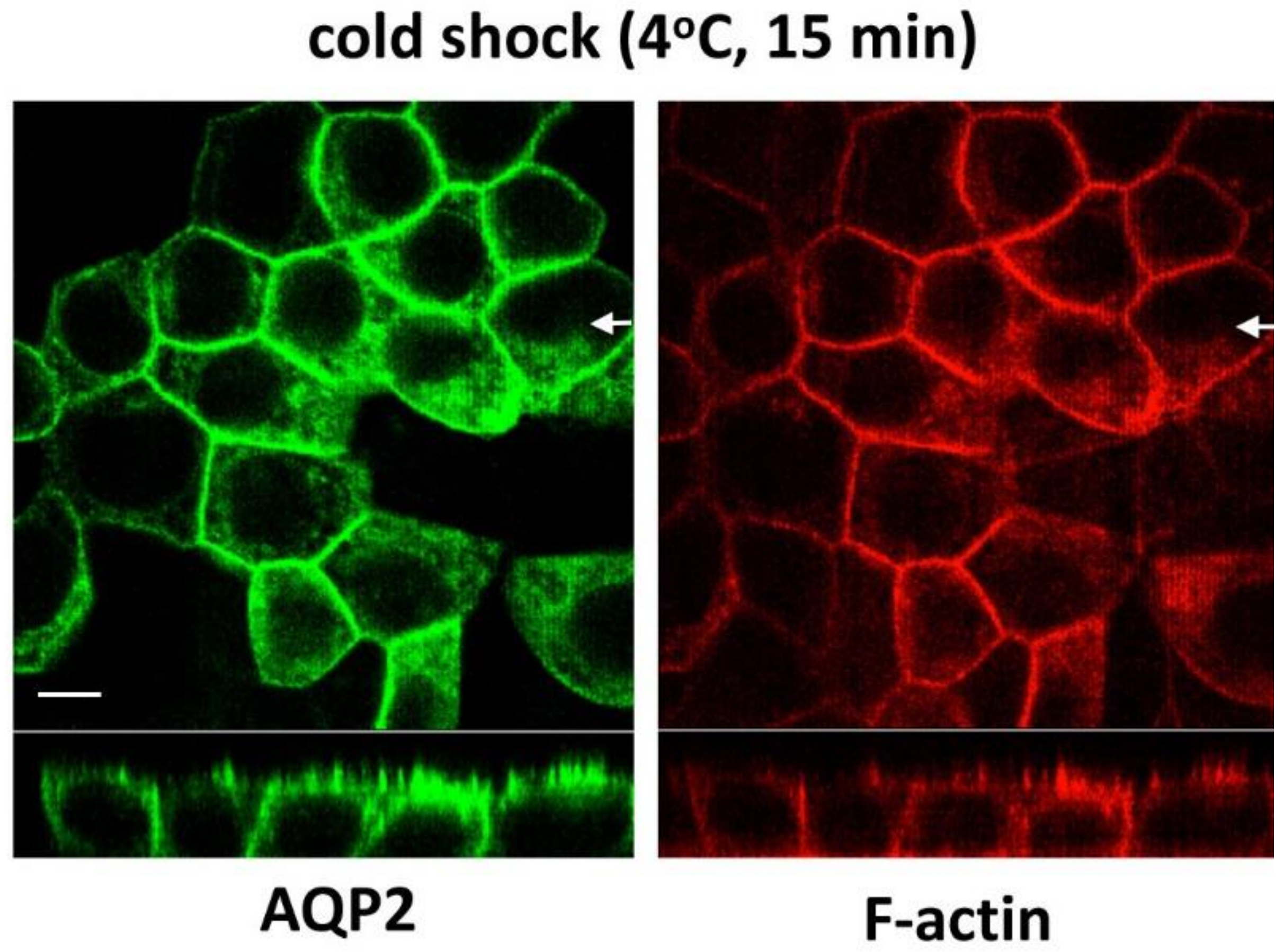
3.7. Cold Shock Increases Basolateral AQP2 Without Depolymerizing F-Actin
3.8. CPZ Inhibits Forskolin-Induced Apical Membrane Accumulation of AQP2
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Cheung, P.W.; Bouley, R.; Brown, D. Targeting the Trafficking of Kidney Water Channels for Therapeutic Benefit. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Fushimi, K.; Uchida, S.; Harat, Y.; Hirata, Y.; Marumo, F.; Sasaki, S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nat. 1993, 361, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Chou, C.L.; Marples, D.; Christensen, E.I.; Kishore, B.; Knepper, M.A. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc. Natl. Acad. Sci. USA 1995, 92, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Vukićević, T.; Schulz, M.; Faust, D.; Klussmann, E. The Trafficking of the Water Channel Aquaporin-2 in Renal Principal Cells—a Potential Target for Pharmacological Intervention in Cardiovascular Diseases. Front. Pharmacol. 2016, 7, 95. [Google Scholar] [CrossRef]
- Brown, D.; Hasler, U.; Nunes, P.; Bouley, R.; Lu, H.A. Phosphorylation events and the modulation of aquaporin 2 cell surface expression. Curr. Opin. Nephrol. Hypertens. 2008, 17, 491–498. [Google Scholar] [CrossRef]
- Hoffert, J.D.; Pisitkun, T.; Wang, G.; Shen, R.-F.; Knepper, M.A. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: Regulation of aquaporin-2 phosphorylation at two sites. Proc. Natl. Acad. Sci. 2006, 103, 7159–7164. [Google Scholar] [CrossRef]
- Hoffert, J.D.; Fenton, R.A.; Moeller, H.B.; Simons, B.; Tchapyjnikov, D.; McDill, B.W.; Yu, M.-J.; Pisitkun, T.; Chen, F.; Knepper, M.A. Vasopressin-stimulated increase in phosphorylation at Ser269 potentiates plasma membrane retention of aquaporin-2. J. Boil. Chem. 2008, 283, 24617–24627. [Google Scholar] [CrossRef]
- Moeller, H.B.; Knepper, M.A.; Fenton, R.A. Serine 269 phosphorylated aquaporin-2 is targeted to the apical membrane of collecting duct principal cells. Kidney Int. 2008, 75, 295–303. [Google Scholar] [CrossRef]
- Hoffert, J.D.; Nielsen, J.; Yu, M.-J.; Pisitkun, T.; Schleicher, S.M.; Nielsen, S.; Knepper, M.A. Dynamics of aquaporin-2 serine-261 phosphorylation in response to short-term vasopressin treatment in collecting duct. Am. J. Physiol. Physiol. 2007, 292, F691–F700. [Google Scholar] [CrossRef]
- Arthur, J.; Huang, J.; Nomura, N.; Jin, W.W.; Li, W.; Cheng, X.; Brown, D.; Lu, H.A.J. Characterization of the putative phosphorylation sites of the AQP2 C terminus and their role in AQP2 trafficking in LLC-PK1 cells. Am. J. Physiol. Physiol. 2015, 309, F673–F679. [Google Scholar] [CrossRef]
- Lu, H.; Sun, T.-X.; Bouley, R.; Blackburn, K.; McLaughlin, M.; Brown, D. Inhibition of endocytosis causes phosphorylation (S256)-independent plasma membrane accumulation of AQP2. Am. J. Physiol. Physiol. 2004, 286, F233–F243. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.M.; McKee, M.; Brown, D. Methyl-β-cyclodextrin induces vasopressin-independent apical accumulation of aquaporin-2 in the isolated, perfused rat kidney. Am. J. Physiol. Physiol. 2006, 291, F246–F253. [Google Scholar] [CrossRef][Green Version]
- Bouley, R.; Hawthorn, G.; Russo, L.M.; Lin, H.Y.; Ausiello, D.A.; Brown, D. Aquaporin 2 (AQP2) and vasopressin type 2 receptor (V2R) endocytosis in kidney epithelial cells: AQP2 is located in ‘endocytosis-resistant’ membrane domains after vasopressin treatment. Boil. Cell 2006, 98, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Y.; Bouley, R.; Chen, Y.; Matsuzaki, T.; Nunes, P.; Hasler, U.; Brown, D.; Lu, H.A.J. Simvastatin enhances aquaporin-2 surface expression and urinary concentration in vasopressin-deficient Brattleboro rats through modulation of Rho GTPase. Am. J. Physiol. Physiol. 2011, 301, F309–F318. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Hasler, P.; Hasler, U.; McKee, M.; Lu, H.A.J.; Bouley, R.; Brown, D. A fluorimetry-based ssYFP secretion assay to monitor vasopressin-induced exocytosis in LLC-PK1 cells expressing aquaporin-2. Am. J. Physiol. Physiol. 2008, 295, C1476–C1487. [Google Scholar] [CrossRef]
- Knepper, M.A.; Nielsen, S. Kinetic model of water and urea permeability regulation by vasopressin in collecting duct. Am. J. Physiol. Physiol. 1993, 265, F214–F224. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.A.J.; Matsuzaki, T.; Eswara, J.; McKee, M.; Brown, D.; Sun, T.-X.; Yi, X.-H.; Bouley, R. Heat Shock Protein 70 Interacts with Aquaporin-2 and Regulates Its Trafficking*. J. Boil. Chem. 2007, 282, 28721–28732. [Google Scholar] [CrossRef]
- Moeller, H.B.; Fenton, R.A. Cell biology of vasopressin-regulated aquaporin-2 trafficking. Pflügers Archiv - Eur. J. Physiol. 2012, 464, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Moeller, H.B.; Praetorius, J.; Rutzler, M.; Fenton, R.A. Phosphorylation of aquaporin-2 regulates its endocytosis and protein–protein interactions. Proc. Natl. Acad. Sci. USA 2009, 107, 424–429. [Google Scholar] [CrossRef]
- Noda, Y.; Horikawa, S.; Kanda, E.; Yamashita, M.; Meng, H.; Eto, K.; Li, Y.; Kuwahara, M.; Hirai, K.; Pack, C.; et al. Reciprocal interaction with G-actin and tropomyosin is essential for aquaporin-2 trafficking. J. Cell Boil. 2008, 182, 587–601. [Google Scholar] [CrossRef]
- Yui, N.; Lu, H.J.; Bouley, R.; Brown, D. AQP2 is necessary for vasopressin- and forskolin-mediated filamentous actin depolymerization in renal epithelial cells. Boil. Open 2011, 1, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Brown, D. The ins and outs of aquaporin-2 trafficking. Am. J. Physiol. Physiol. 2003, 284, F893–F901. [Google Scholar] [CrossRef]
- Coleman, R.A.; Wu, D.C.; Liu, J.; Wade, J.B. Expression of aquaporins in the renal connecting tubule. Am. J. Physiol. Physiol. 2000, 279, F874–F883. [Google Scholar] [CrossRef] [PubMed]
- De Seigneux, S.; Nielsen, J.; Olesen, E.; Dimke, H.; Kwon, T.-H.; Frøkiær, J.; Nielsen, S. Long-term aldosterone treatment induces decreased apical but increased basolateral expression of AQP2 in CCD of rat kidney. Am. J. Physiol. Physiol. 2007, 293, F87–F99. [Google Scholar] [CrossRef] [PubMed]
- Jeon, U.S.; Joo, K.W.; Na, K.Y.; Kim, Y.S.; Lee, J.S.; Kim, J.; Kim, G.-H.; Nielsen, S.; Knepper, M.A.; Han, J.S. Oxytocin induces apical and basolateral redistribution of aquaporin-2 in rat kidney. Nephron 2003, 93, e36–e45. [Google Scholar] [CrossRef] [PubMed]
- Van Balkom, B.W.M.; Van Raak, M.; Breton, S.; Pastor-Soler, N.; Van Der Sluijs, P.; Brown, D.; Bouley, R.; Deen, P.M.T. Hypertonicity Is Involved in Redirecting the Aquaporin-2 Water Channel into the Basolateral, Instead of the Apical, Plasma Membrane of Renal Epithelial Cells. J. Boil. Chem. 2002, 278, 1101–1107. [Google Scholar] [CrossRef]
- Grindstaff, K.K.; Yeaman, C.; Anandasabapathy, N.; Hsu, S.C.; Rodriguez-Boulan, E.; Scheller, R.H.; Nelson, W.J. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell 1998, 93, 731–740. [Google Scholar] [CrossRef]
- Barile, M.; Pisitkun, T.; Yu, M.-J.; Chou, C.-L.; Verbalis, M.J.; Shen, R.-F.; Knepper, M.A. Large-Scale Protein Identification in Intracellular Aquaporin-2 Vesicles from Renal Inner Medullary Collecting Duct. Mol. Cell. Proteom. 2005, 4, 1095–1106. [Google Scholar] [CrossRef]
- Chen, Y.; Rice, W.; Gu, Z.; Li, J.; Huang, J.; Brenner, M.B.; Van Hoek, A.; Xiong, J.; Gundersen, G.G.; Norman, J.C.; et al. Aquaporin 2 Promotes Cell Migration and Epithelial Morphogenesis. J. Am. Soc. Nephrol. 2012, 23, 1506–1517. [Google Scholar] [CrossRef]
- Yui, N.; Lu, H.A.J.; Chen, Y.; Nomura, N.; Bouley, R.; Brown, D. Basolateral targeting and microtubule-dependent transcytosis of the aquaporin-2 water channel. Am. J. Physiol. Physiol. 2012, 304, C38–C48. [Google Scholar] [CrossRef]
- Loo, C.S.; Chen, C.-W.; Wang, P.-J.; Chen, P.-Y.; Lin, S.-Y.; Khoo, K.-H.; Fenton, R.A.; Knepper, M.A.; Yu, M.-J. Quantitative apical membrane proteomics reveals vasopressin-induced actin dynamics in collecting duct cells. Proc. Natl. Acad. Sci. USA 2013, 110, 17119–17124. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, C.T. Caring about the other 47% of the water channels. Focus on "Basolateral targeting and microtubule-dependent transcytosis of the aquaporin-2 water channel". Am. J. Physiol. Physiol. 2012, 304, C33–C35. [Google Scholar] [CrossRef] [PubMed]
- Mykoniatis, A.; Shen, L.; Fedor-Chaiken, M.; Tang, J.; Tang, X.; Worrell, R.T.; Delpire, E.; Turner, J.R.; Matlin, K.S.; Bouyer, P.G.; et al. Phorbol 12-myristate 13-acetate-induced endocytosis of the Na-K-2Cl cotransporter in MDCK cells is associated with a clathrin-dependent pathway. Am. J. Physiol. Physiol. 2009, 298, C85–C97. [Google Scholar] [CrossRef] [PubMed]
- Yui, N.; Okutsu, R.; Sohara, E.; Rai, T.; Ohta, A.; Noda, Y.; Sasaki, S.; Uchida, S. FAPP2 is required for aquaporin-2 apical sorting at trans-Golgi network in polarized MDCK cells. Am. J. Physiol. Physiol. 2009, 297, C1389–C1396. [Google Scholar] [CrossRef]
- Deen, P.M.; Rijss, J.P.; Mulders, S.M.; Errington, R.J.; Van Baal, J.; Van Os, C.H. Aquaporin-2 transfection of Madin-Darby canine kidney cells reconstitutes vasopressin-regulated transcellular osmotic water transport. J. Am. Soc. Nephrol. 1997, 8, 1493–1501. [Google Scholar]
- Cheung, P.W.; Nomura, N.; Nair, A.V.; Pathomthongtaweechai, N.; Ueberdiek, L.; Lu, H.A.J.; Brown, D.; Bouley, R. EGF Receptor Inhibition by Erlotinib Increases Aquaporin 2–Mediated Renal Water Reabsorption. J. Am. Soc. Nephrol. 2016, 27, 3105–3116. [Google Scholar] [CrossRef] [PubMed]
- Bouley, R.; Breton, S.; Sun, T.-X.; McLaughlin, M.; Nsumu, N.N.; Lin, H.Y.; Ausiello, D.A.; Brown, D. Nitric oxide and atrial natriuretic factor stimulate cGMP-dependent membrane insertion of aquaporin 2 in renal epithelial cells. J. Clin. Investig. 2000, 106, 1115–1126. [Google Scholar] [CrossRef]
- Bouley, R.; Lu, H.A.J.; Nunes-Hasler, P.; Da Silva, N.; McLaughlin, M.; Chen, Y.; Brown, D. Calcitonin has a vasopressin-like effect on aquaporin-2 trafficking and urinary concentration. J. Am. Soc. Nephrol. 2010, 22, 59–72. [Google Scholar] [CrossRef]
- Sasaki, S.; Yui, N.; Noda, Y. Actin directly interacts with different membrane channel proteins and influences channel activities: AQP2 as a model. Biochim. Biophys. Acta (BBA) - Biomembr. 2014, 1838, 514–520. [Google Scholar] [CrossRef]
- Tajika, Y.; Matsuzaki, T.; Suzuki, T.; Aoki, T.; Hagiwara, H.; Kuwahara, M.; Sasaki, S.; Takata, K. Aquaporin-2 Is Retrieved to the Apical Storage Compartment via Early Endosomes and Phosphatidylinositol 3-Kinase-Dependent Pathway. Endocrinology 2004, 145, 4375–4383. [Google Scholar] [CrossRef]
- Simon, H.; Gao, Y.; Franki, N.; Hays, R.M. Vasopressin depolymerizes apical F-actin in rat inner medullary collecting duct. Am. J. Physiol. Physiol. 1993, 265, C757–C762. [Google Scholar] [CrossRef] [PubMed]
- Harrold, M.W.; A Chang, Y.; A Wallace, R.; Farooqui, T.; Wallace, L.J.; Uretsky, N.; Miller, D.D. Charged analogues of chlorpromazine as dopamine antagonists. J. Med. Chem. 1987, 30, 1631–1635. [Google Scholar] [CrossRef]
- Prozialeck, W.C.; Wallace, T.L.; Weiss, B. Differential inhibition of calmodulin-sensitive phosphodiesterase and Ca++-adenosine triphosphatase by chlorpromazine-linked calmodulin. J. Pharmacol. Exp. Ther. 1987, 243, 171–179. [Google Scholar] [PubMed]
- Hernáez, B.; Alonso, C. Dynamin- and Clathrin-Dependent Endocytosis in African Swine Fever Virus Entry. J. Virol. 2009, 84, 2100–2109. [Google Scholar] [CrossRef] [PubMed]
- Sieczkarski, S.B.; Whittaker, G.R. Dissecting virus entry via endocytosis. J. Gen. Virol. 2002, 83, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Rothberg, K.G.; Anderson, R.G. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Boil. 1993, 123, 1107–1117. [Google Scholar] [CrossRef]
- Milzani, A.D.G.; Dalle-Donne, I. Effects of Chlorpromazine on Actin Polymerization: Slackening of Filament Elongation and Filament Annealing. Arch. Biochem. Biophys. 1999, 369, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Hays, R.M.; Condeelis, J.; Gao, Y.; Simon, H.; Ding, G.; Franki, N. The effect of vasopressin on the cytoskeleton of the epithelial cell. Pediatr. Nephrol. 1993, 7, 672–679. [Google Scholar] [CrossRef]
- Tamma, G.; Carmosino, M.; Svelto, M.; Valenti, G. Bradykinin Signaling Counteracts cAMP-Elicited Aquaporin 2 Translocation in Renal Cells. J. Am. Soc. Nephrol. 2005, 16, 2881–2889. [Google Scholar] [CrossRef]
- Tamma, G.; Klussmann, E.; Maric, K.; Aktories, K.; Svelto, M.; Rosenthal, W.; Valenti, G. Rho inhibits cAMP-induced translocation of aquaporin-2 into the apical membrane of renal cells. Am. J. Physiol. Physiol. 2001, 281, F1092–F1101. [Google Scholar] [CrossRef]
- Tamma, G. cAMP-induced AQP2 translocation is associated with RhoA inhibition through RhoA phosphorylation and interaction with RhoGDI. J. Cell Sci. 2003, 116, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Tamma, G.; Wiesner, B.; Furkert, J.; Hahm, D.; Oksche, A.; Schaefer, M.; Valenti, G.; Rosenthal, W.; Klussmann, E. The prostaglandin E2 analogue sulprostone antagonizes vasopressin-induced antidiuresis through activation of Rho. J. Cell Sci. 2003, 116, 3285–3294. [Google Scholar] [CrossRef] [PubMed]
- Valenti, G.; Procino, G.; Carmosino, M.; Frigeri, A.; Mannucci, R.; Nicoletti, I.; Svelto, M. The phosphatase inhibitor okadaic acid induces AQP2 translocation independently from AQP2 phosphorylation in renal collecting duct cells. J. Cell Sci. 2000, 113, 113. [Google Scholar]
- Ghosh, D.; Nieves-Cintron, M.; Tajada, S.; Brust-Mascher, I.; Horne, M.C.; Hell, J.W.; Dixon, R.E.; Santana, L.F.; Navedo, M.F. Dynamic L-type CaV1.2 channel trafficking facilitates CaV1.2 clustering and cooperative gating. Biochim. Biophys. Acta (BBA) - Bioenerg. 2018, 1865, 1341–1355. [Google Scholar] [CrossRef]
- Huang, J.; Imamura, T.; Babendure, J.L.; Lu, J.-C.; Olefsky, J. Disruption of Microtubules Ablates the Specificity of Insulin Signaling to GLUT4 Translocation in 3T3-L1 Adipocytes. J. Boil. Chem. 2005, 280, 42300–42306. [Google Scholar] [CrossRef]
- Karpushev, A.V.; Ilatovskaya, D.V.; Pavlov, T.S.; Negulyaev, Y.A.; Staruschenko, A. Intact Cytoskeleton Is Required for Small G Protein Dependent Activation of the Epithelial Na+ Channel. PLoS ONE 2010, 5, e8827. [Google Scholar] [CrossRef]
- Schappi, J.M.; Krbanjevic, A.; Rasenick, M.M. Tubulin, actin and heterotrimeric G proteins: Coordination of signaling and structure. Biochim. Biophys. Acta (BBA) - Bioenerg. 2013, 1838, 674–681. [Google Scholar] [CrossRef]
- Taylor, J.B.; Hogue, L.A.; Lipuma, J.J.; Walter, M.J.; Brody, S.L.; Cannon, C.L. Entry of Burkholderia organisms into respiratory epithelium: CFTR, microfilament and microtubule dependence. J. Cyst. Fibros. 2009, 9, 36–43. [Google Scholar] [CrossRef]
- Klingner, C.; Cherian, A.V.; Fels, J.; Diesinger, P.M.; Aufschnaiter, R.; Maghelli, N.; Keil, T.; Beck, G.; Tolić, I.M.; Bathe, M.; et al. Isotropic actomyosin dynamics promote organization of the apical cell cortex in epithelial cells. J. Cell Boil. 2014, 207, 107–121. [Google Scholar] [CrossRef]
- Tajika, Y.; Matsuzaki, T.; Suzuki, T.; Ablimit, A.; Aoki, T.; Hagiwara, H.; Kuwahara, M.; Sasaki, S.; Takata, K. Differential regulation of AQP2 trafficking in endosomes by microtubules and actin filaments. Histochem. Cell Boil. 2005, 124, 1–12. [Google Scholar] [CrossRef]
- Devergne, O.; Tsung, K.; Barcelo, G.; Schüpbach, T. Polarized deposition of basement membrane proteins depends on Phosphatidylinositol synthase and the levels of Phosphatidylinositol 4,5-bisphosphate. Proc. Natl. Acad. Sci. USA 2014, 111, 7689–7694. [Google Scholar] [CrossRef] [PubMed]
- Fiévet, B.; Louvard, D.; Arpin, M. ERM proteins in epithelial cell organization and functions. Biochim. Biophys. Acta (BBA) - Bioenerg. 2007, 1773, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Gassama-Diagne, A.; Yu, W.; Ter Beest, M.; Martin-Belmonte, F.; Kierbel, A.; Engel, J.; Mostov, K.E. Phosphatidylinositol-3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nature 2006, 8, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Martin-Belmonte, F.; Gassama, A.; Datta, A.; Yu, W.; Rescher, U.; Gerke, V.; Mostov, K. PTEN-Mediated Apical Segregation of Phosphoinositides Controls Epithelial Morphogenesis through Cdc42. Cell 2007, 128, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Rice, W.L.; Li, W.; Mamuya, F.; McKee, M.; Paunescu, T.; Lu, H.A.J. Polarized Trafficking of AQP2 Revealed in Three Dimensional Epithelial Culture. PLoS ONE 2015, 10, e0131719. [Google Scholar] [CrossRef]
- Yui, N.; Ando, F.; Sasaki, S.; Uchida, S. Ser-261 phospho-regulation is involved in pS256 and pS269-mediated aquaporin-2 apical translocation. Biochem. Biophys. Res. Commun. 2017, 490, 1039–1044. [Google Scholar] [CrossRef]
- Sun, T.-X.; Van Hoek, A.; Huang, Y.; Bouley, R.; McLaughlin, M.; Brown, D. Aquaporin-2 localization in clathrin-coated pits: Inhibition of endocytosis by dominant-negative dynamin. Am. J. Physiol. Physiol. 2002, 282, F998–F1011. [Google Scholar] [CrossRef]
- Procino, G.; Barbieri, C.; Carmosino, M.; Tamma, G.; Milano, S.; De Benedictis, L.; Mola, M.G.; Fernandez, Y.L.; Valenti, G.; Svelto, M. Fluvastatin modulates renal water reabsorption in vivo through increased AQP2 availability at the apical plasma membrane of collecting duct cells. Pflügers Archiv - Eur. J. Physiol. 2011, 462, 753–766. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouley, R.; Yui, N.; Terlouw, A.; Cheung, P.W.; Brown, D. Chlorpromazine Induces Basolateral Aquaporin-2 Accumulation via F-Actin Depolymerization and Blockade of Endocytosis in Renal Epithelial Cells. Cells 2020, 9, 1057. https://doi.org/10.3390/cells9041057
Bouley R, Yui N, Terlouw A, Cheung PW, Brown D. Chlorpromazine Induces Basolateral Aquaporin-2 Accumulation via F-Actin Depolymerization and Blockade of Endocytosis in Renal Epithelial Cells. Cells. 2020; 9(4):1057. https://doi.org/10.3390/cells9041057
Chicago/Turabian StyleBouley, Richard, Naofumi Yui, Abby Terlouw, Pui W. Cheung, and Dennis Brown. 2020. "Chlorpromazine Induces Basolateral Aquaporin-2 Accumulation via F-Actin Depolymerization and Blockade of Endocytosis in Renal Epithelial Cells" Cells 9, no. 4: 1057. https://doi.org/10.3390/cells9041057
APA StyleBouley, R., Yui, N., Terlouw, A., Cheung, P. W., & Brown, D. (2020). Chlorpromazine Induces Basolateral Aquaporin-2 Accumulation via F-Actin Depolymerization and Blockade of Endocytosis in Renal Epithelial Cells. Cells, 9(4), 1057. https://doi.org/10.3390/cells9041057




