Graphene Oxide Nanosheets for Localized Hyperthermia—Physicochemical Characterization, Biocompatibility, and Induction of Tumor Cell Death
Abstract
1. Introduction
2. Materials and Methods
2.1. Formation of Reduced Graphene Oxide (rGO)
2.2. Formation of Poly(Ethylene Glycol) Modified Reduced Graphene Oxide (rGO-PEG)
2.3. Sterility of Graphene Derivatives
2.4. Detection of Endotoxin Contamination
2.5. Human Material
2.6. Detection of Complement Activation
2.7. Measurement of Plasma Coagulation
2.8. Detection of Platelet Aggregation
2.9. Measurement of Plasma Membrane Stability
2.10. Detection of lactate dehydrogenase (LDH) release
2.11. Detection of Neutrophilic Degranulation
2.12. Detection of Neutrophil Extracellular Traps (Nets)
2.13. Quantification of Cell Migration
2.14. Phagocytosis
2.15. Gamma Irradiation (X-rays)
2.16. Graphene-Induced Hyperthermia (GIHT)
2.17. Statistics
3. Results
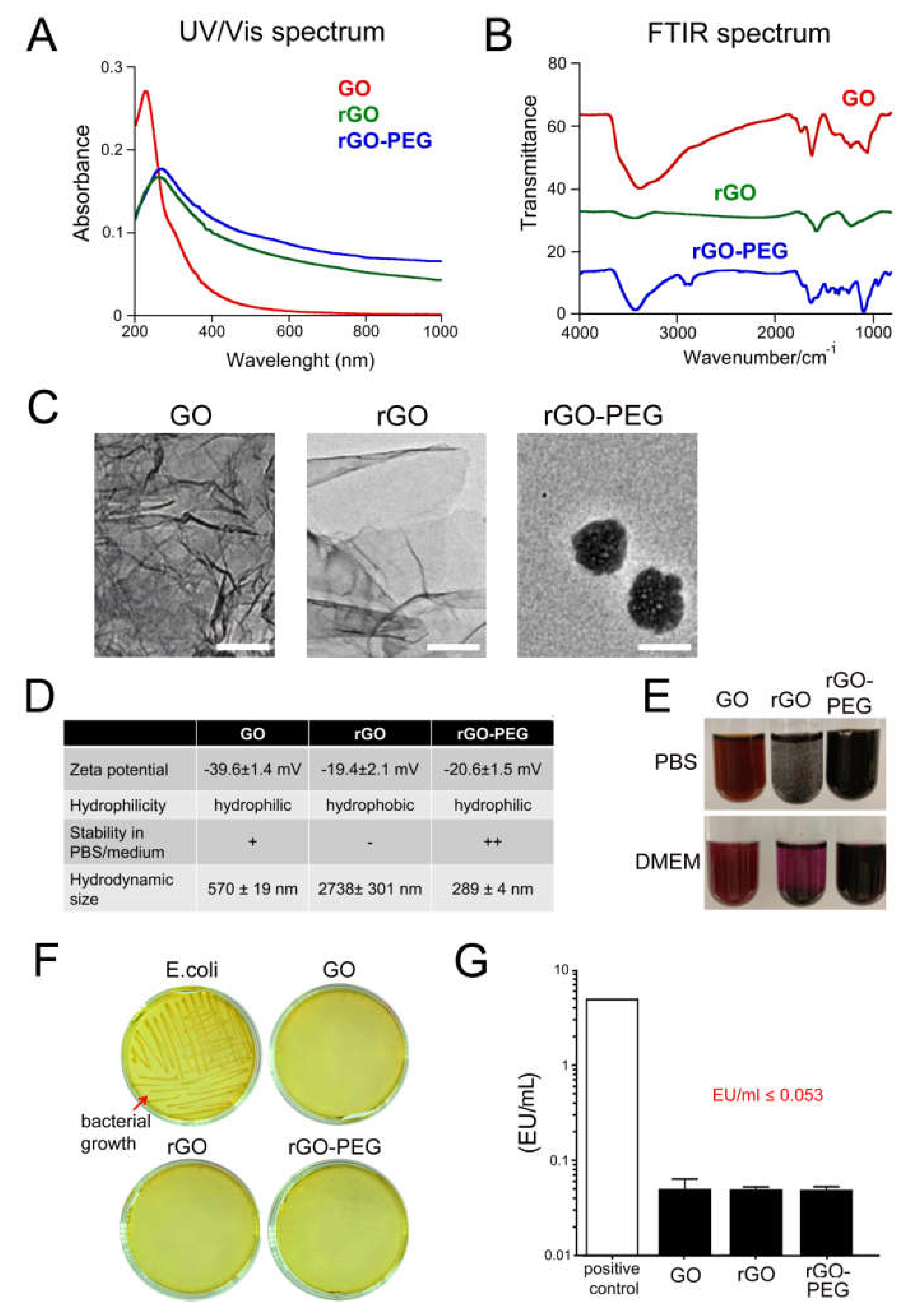
3.1. Physicochemical Characterization of Graphene Oxide Nanosheets and Its Derivatives
3.2. Sterility of Graphene Oxide Nanosheets and Its Derivatives
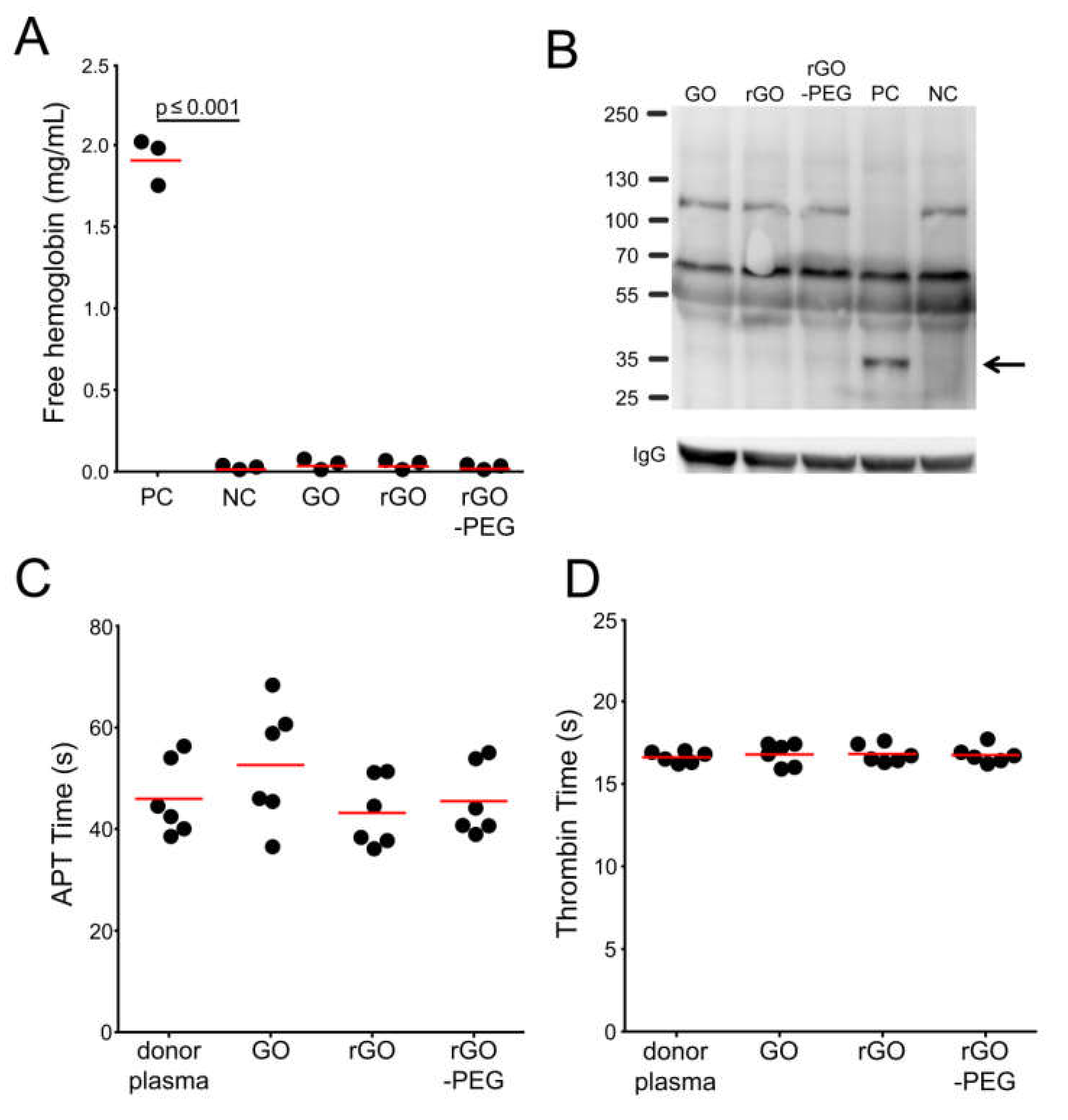
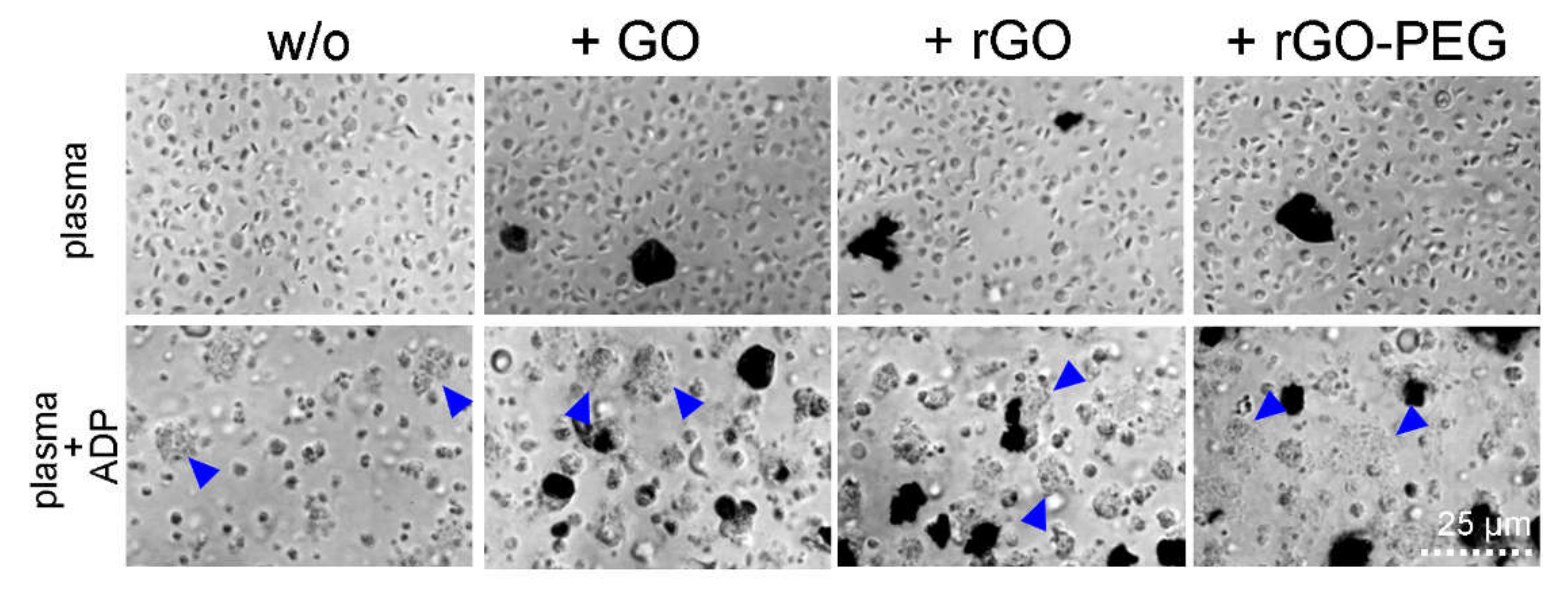
3.3. Hemocompatibility of Graphene Oxide Nanosheets and Its Derivatives
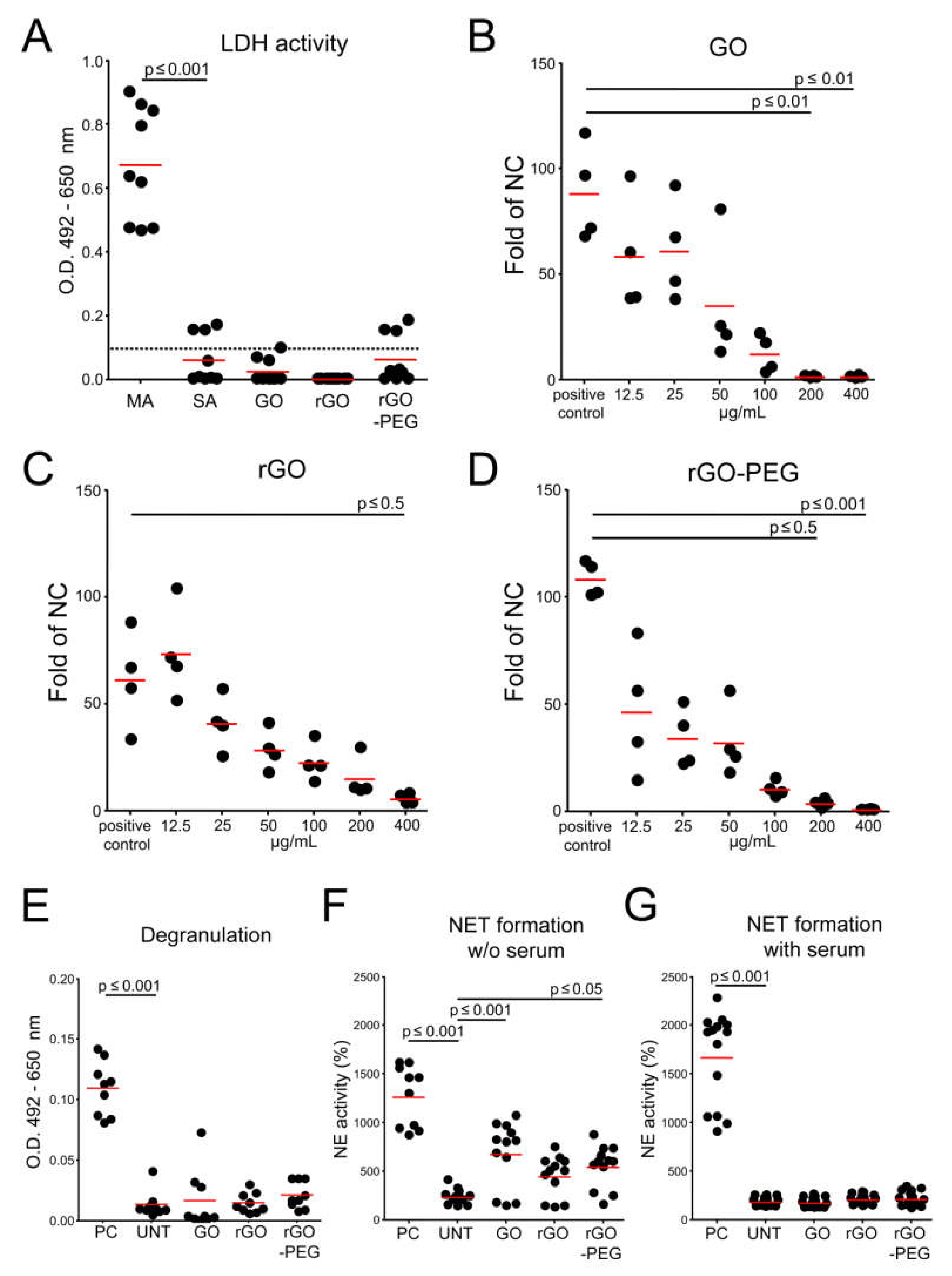
3.4. Interaction of Graphene Oxide Nanosheets and Its Derivatives with Leukocytes
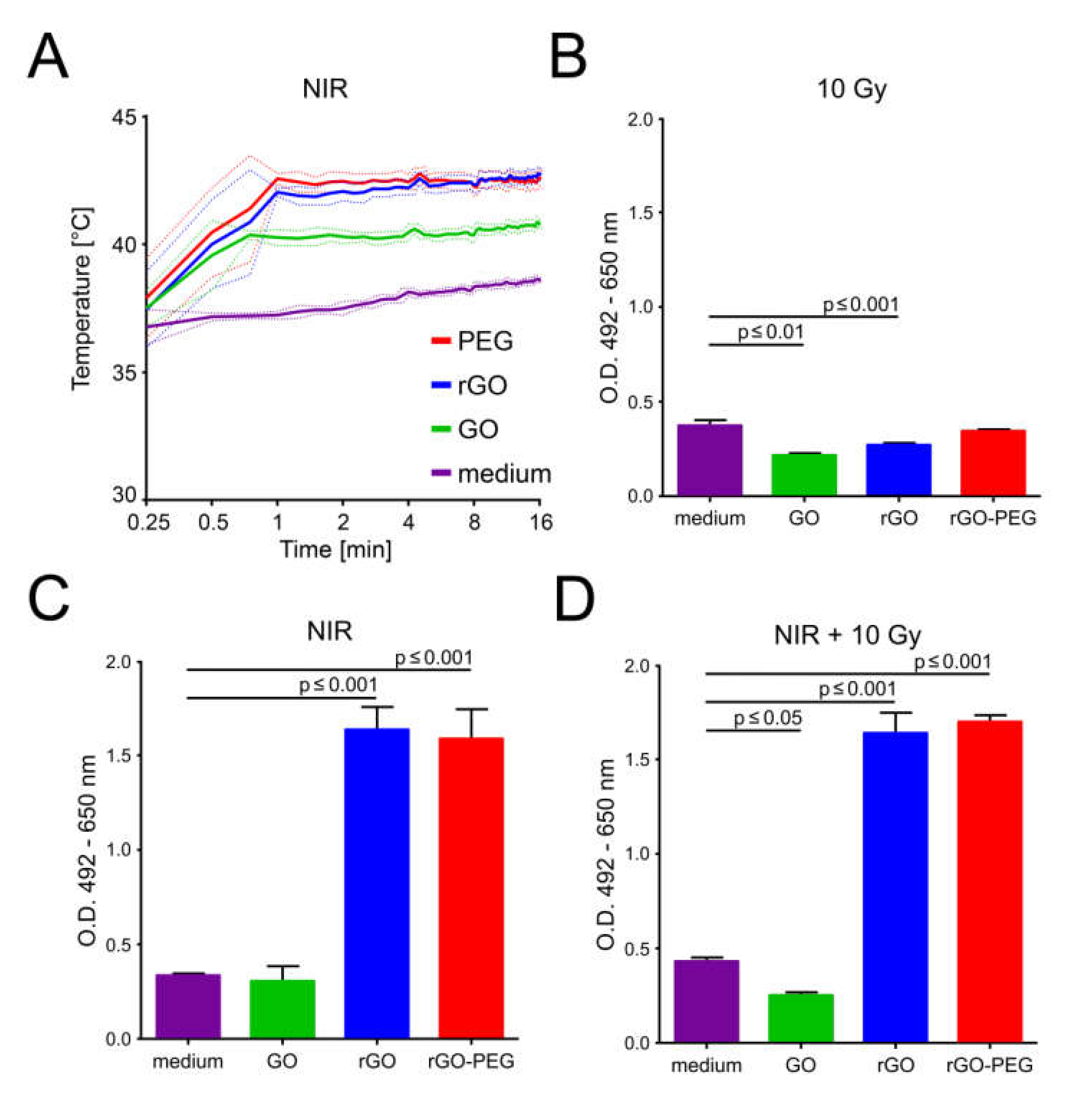
3.5. Graphene-Induced Hyperthermia (GITH) and Tumor Cell Death
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Turcheniuk, K.; Hage, C.-H.; Heliot, L.; Railian, S.; Zaitsev, V.; Spadavecchia, J.; Boukherroub, R.; Szunerits, S. Infrared Photothermal Therapy with Water Soluble Reduced Graphene Oxide: Shape, Size and Reduction Degree Effects. Nano LIFE 2015, 5, 1540002. [Google Scholar] [CrossRef]
- England, C.G.; Im, H.-J.; Feng, L.; Chen, F.; Graves, S.; Hernandez, R.; Orbay, H.; Xu, C.; Cho, S.Y.; Nickles, R.J.; et al. Re-assessing the enhanced permeability and retention effect in peripheral arterial disease using radiolabeled long circulating nanoparticles. Biomaterials 2016, 100, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Lucky, S.S.; Idris, N.M.; Li, Z.; Huang, K.; Soo, K.C.; Zhang, Y. Titania Coated Upconversion Nanoparticles for Near-Infrared Light Triggered Photodynamic Therapy. ACS Nano 2015, 9, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu, F.; Oz, Y.; Quéniat, G.; Abderrahmani, A.; Foulon, C.; Lecoeur, M.; Sanyal, R.; Sanyal, R.; Boukherroub, R.; Szunerits, S. Photothermally triggered on-demand insulin release from reduced graphene oxide modified hydrogels. J. Control. Release 2017, 246, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Altinbasak, I.; Jijie, R.; Barras, A.; Golba, B.; Sanyal, R.; Bouckaert, J.; Drider, D.; Bilyy, R.; Dumych, T.; Paryzhak, S.; et al. Reduced Graphene-Oxide-Embedded Polymeric Nanofiber Mats: An “On-Demand” Photothermally Triggered Antibiotic Release Platform. ACS Appl. Mater. Interfaces 2018, 10, 41098–41106. [Google Scholar] [CrossRef]
- Hildebrandt, B. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. 2002, 43, 33–56. [Google Scholar] [CrossRef]
- Banu, H.; Stanley, B.; Faheem, S.M.; Seenivasan, R.; Premkumar, K.; VasanthaKumar, G. Thermal Chemosensitization of Breast Cancer Cells to Cyclophosphamide Treatment Using Folate Receptor Targeted Gold Nanoparticles. Plasmon 2014, 9, 1341–1349. [Google Scholar] [CrossRef]
- Westra, A.; Dewey, W. Variation in Sensitivity to Heat Shock during the Cell-cycle of Chinese Hamster Cells in Vitro. Int. J. Radiat. Boil. Relat. Stud. Physics, Chem. Med. 1971, 19, 467–477. [Google Scholar] [CrossRef]
- Vidair, C.A.; Dewey, W.C. Division-associated and division-independent hyperthermic cell death: Comparison with other cytotoxic agents. Int. J. Hyperth. 1991, 7, 51–60. [Google Scholar] [CrossRef]
- McRae, A.D.; Esrick, M.A.; Mueller, S.C. Non-invasive, in-vivo electrical impedance of EMT-6 tumours during hyperthermia: Correlation with morphology and tumour-growth-delay. Int. J. Hyperth. 1997, 13, 1–20. [Google Scholar] [CrossRef]
- Dahl, O. Interaction of Heat and Drugs In Vitro and In Vivo. In Thermoradiotherapy and Thermochemotherapy: Biology, Physiology, Physics; Seegenschmiedt, M.H., Fessenden, P., Vernon, C.C., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 103–121. [Google Scholar]
- Turcheniuk, K.; Hage, C.H.; Heliot, L.; Railian, S.; Zaitsev, V.; Spadavecchia, J.; Boukherroub, R.; Szunerits, S. Infrared photothermal therapy with water soluble reduced graphene oxide: Shape, size and reduction degree effects. Nano Life 2015, 5, 1540002. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Hage, C.H.; Spadavecchia, J.; Serrano, A.Y.; Larroulet, I.; Pesquera, A.; Zurutuza, A.; Pisfil, M.G.; Héliot, L.; Boukaert, J.; et al. Plasmonic photothermal destruction of uropathogenic E. coli with reduced graphene oxide and core/shell nanocomposites of gold nanorods/reduced graphene oxide. J. Mater. Chem. B 2015, 3, 375–386. [Google Scholar] [CrossRef]
- Rother, R.P.; Bell, L.; Hillmen, P.; Gladwin, M.T. The Clinical Sequelae of Intravascular Hemolysis and Extracellular Plasma Hemoglobin. JAMA 2005, 293, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; D’Agnillo, F.; Vallelian, F.; Pereira, C.P.; Williams, M.C.; Jia, Y.; Schaer, D.; Buehler, P.W. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J. Clin. Investig. 2012, 122, 1444–1458. [Google Scholar] [CrossRef] [PubMed]
- Boretti, F.S.; Buehler, P.W.; D’Agnillo, F.; Kluge, K.; Glaus, T.; Butt, O.I.; Jia, Y.; Goede, J.; Pereira, C.P.; Maggiorini, M.; et al. Sequestration of extracellular hemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs. J. Clin. Investig. 2009, 119, 2271–2280. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, L.E.; Bilyy, R.; Biermann, M.; Kienhöfer, D.; Maueröder, C.; Hahn, J.; Brauner, J.M.; Weidner, D.; Chen, J.; Scharin-Mehlmann, M.; et al. Nanoparticles size-dependently initiate self-limiting NETosis-driven inflammation. Proc. Natl. Acad. Sci. USA 2016, 113, E5856–E5865. [Google Scholar]
- Lee, K.; Yu, Y. Lipid bilayer disruption induced by amphiphilic Janus nanoparticles: The non-monotonic effect of charged lipids. Soft Matter 2019, 15, 2373–2380. [Google Scholar] [CrossRef] [PubMed]
- Cho, H. Complement regulation: Physiology and disease relevance. Korean J. Pediatr. 2015, 58, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Al-Hanbali, O.; Rutt, K.J.; Sarker, D.K.; Hunter, A.; Moghimi, S.M. Concentration dependent structural ordering of poloxamine 908 on polystyrene nanoparticles and their modulatory role on complement consumption. J. Nanosci. Nanotechnol. 2006, 6, 3126–3133. [Google Scholar] [CrossRef]
- Bertholon, I.; Vauthier, C.; Labarre, D. Complement Activation by Core-Shell Poly(isobutylcyanoacrylate)-Polysaccharide Nanoparticles: Influences of Surface Morphology, Length, and Type of Polysaccharide. Pharm. Res. 2006, 23, 1313–1323. [Google Scholar] [CrossRef]
- Laloy, J.; Minet, V.; Alpan, L.; Mullier, F.; Beken, S.; Toussaint, O.; Lucas, S.; Dogné, J.-M. Impact of Silver Nanoparticles on Haemolysis, Platelet Function and Coagulation. Nanobiomedicine 2014, 1, 4. [Google Scholar] [CrossRef]
- Gonçalves, D.; Chiasson, S.; Girard, D. Activation of human neutrophils by titanium dioxide (TiO2) nanoparticles. Toxicol. Vitr. 2010, 24, 1002–1008. [Google Scholar] [CrossRef]
- Babin, K.; Antoine, F.; Goncalves, D.M.; Girard, D. TiO2, CeO2 and ZnO nanoparticles and modulation of the degranulation process in human neutrophils. Toxicol. Lett. 2013, 221, 57–63. [Google Scholar] [CrossRef]
- Podolska, M.J.; Mahajan, A.; Hahn, J.; Knopf, J.; Maueröder, C.; Petru, L.; Ullmann, M.; Schett, G.; Leppkes, M.; Herrmann, M.; et al. Treatment with DNases rescues hidden neutrophil elastase from aggregated NETs. J. Leukoc. Boil. 2019, 106, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Gorbet, M.B.; Sefton, M.V. Endotoxin: The uninvited guest. Biomaterials 2005, 26, 6811–6817. [Google Scholar] [CrossRef] [PubMed]
- Vallhov, H.; Qin, J.; Johansson, S.M.; Ahlborg, N.; Muhammed, M.A.; Scheynius, A.; Gabrielsson, S. The Importance of an Endotoxin-Free Environment during the Production of Nanoparticles Used in Medical Applications. Nano Lett. 2006, 6, 1682–1686. [Google Scholar] [CrossRef] [PubMed]
- Brooks, R.A.; Wimhurst, J.A.; Rushton, N. Endotoxin contamination of particles produces misleading inflammatory cytokine responses from macrophages in vitro. J. Bone Joint Surg. British Vol. 2002, 84, 295–299. [Google Scholar] [CrossRef]
- Chapekar, M.S.; Zaremba, T.G.; Kuester, R.K.; Hitchins, V.M. Synergistic induction of tumor necrosis factor alpha by bacterial lipopolysaccharide and lipoteichoic acid in combination with polytetrafluoroethylene particles in a murine macrophage cell line RAW 264.7. J. Biomed. Mater. Res. 1996, 31, 251–256. [Google Scholar] [CrossRef]
- Malyala, P.; Singh, M. Endotoxin Limits in Formulations for Preclinical Research. J. Pharm. Sci. 2008, 97, 2041–2044. [Google Scholar] [CrossRef]
- Fung, M.; Bowen, D.L. Silver Products for Medical Indications: Risk-Benefit Assessment. J. Toxicol. Clin. Toxicol. 1996, 34, 119–126. [Google Scholar] [CrossRef]
- Zhang, X.-D.; Wu, H.-Y.; Wu, D.; Wang, Y.-Y.; Chang, J.-H.; Zhai, Z.-B.; Meng, A.; Liu, P.-X.; Zhang, L.-A.; Fan, F.-Y. Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int. J. Nanomed. 2010, 5, 771–781. [Google Scholar] [CrossRef]
- Patton, J.S.; Byron, P.R. Inhaling medicines: Delivering drugs to the body through the lungs. Nat. Rev. Drug Discov. 2007, 6, 67–74. [Google Scholar] [CrossRef]
- Yang, W.; Peters, J.I.; Williams, R.O. Inhaled nanoparticles—A current review. Int. J. Pharm. 2008, 356, 239–247. [Google Scholar] [CrossRef]
- Trop, M.; Novak, M.; Rödl, S.; Hellbom, B.; Kroell, W.; Goessler, W. Silver-Coated Dressing Acticoat Caused Raised Liver Enzymes and Argyria-like Symptoms in Burn Patient. J. Trauma: Inj. Infect. Crit. Care 2006, 60, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Weigmann, H.-J.; Rickmeyer, C.; Barthelmes, H.; Schaefer, H.; Mueller, G.; Sterry, W. Penetration of Titanium Dioxide Microparticles in a Sunscreen Formulation into the Horny Layer and the Follicular Orifice. Ski. Pharmacol. Physiol. 1999, 12, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Schipper, M.L.; Nakayama-Ratchford, N.; Davis, C.R.; Kam, N.W.S.; Chu, P.; Liu, Z.; Sun, X.; Dai, H.; Gambhir, S.S. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat. Nanotechnol. 2008, 3, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.-S.; Kim, S.; Han, B.-S.; Son, W.-C.; Jeong, J. Comparison of gene expression profiles in mice liver following intravenous injection of 4 and 100nm-sized PEG-coated gold nanoparticles. Toxicol. Lett. 2009, 191, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Duch, M.C.; Budinger, G.R.S.; Liang, Y.T.; Soberanes, S.; Urich, D.; Chiarella, S.; Campochiaro, L.A.; González, Á.; Chandel, N.S.; Hersam, M.C.; et al. Minimizing Oxidation and Stable Nanoscale Dispersion Improves the Biocompatibility of Graphene in the Lung. Nano Lett. 2011, 11, 5201–5207. [Google Scholar] [CrossRef]
- Yang, K.; Gong, H.; Shi, X.; Wan, J.; Zhang, Y.; Liu, Z. In vivo biodistribution and toxicology of functionalized nano-graphene oxide in mice after oral and intraperitoneal administration. Biomaterials 2013, 34, 2787–2795. [Google Scholar] [CrossRef]
- Unterweger, H.; Janko, C.; Schwarz, M.; Dézsi, L.; Urbanics, R.; Matuszak, J.; Őrfi, E.; Fülöp, T.; Bäuerle, T.; Szebeni, J.; et al. Non-immunogenic dextran-coated superparamagnetic iron oxide nanoparticles: A biocompatible, size-tunable contrast agent for magnetic resonance imaging. Int. J. Nanomed. 2017, 12, 5223–5238. [Google Scholar] [CrossRef]
- Adiseshaiah, P.P.; Hall, J.B.; McNeil, S.E. Nanomaterial standards for efficacy and toxicity assessment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 2, 99–112. [Google Scholar] [CrossRef]
- Chen, F.; Cai, W. Tumor Vasculature Targeting: A Generally Applicable Approach for Functionalized Nanomaterials. Small 2014, 10, 1887–1893. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.-T.; Liu, Z. Graphene in Mice: Ultrahigh In Vivo Tumor Uptake and Efficient Photothermal Therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef]
- Yang, K.; Wan, J.; Zhang, S.; Tian, B.; Zhang, Y.; Liu, Z. The influence of surface chemistry and size of nanoscale graphene oxide on photothermal therapy of cancer using ultra-low laser power. Biomaterials 2012, 33, 2206–2214. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of Graphene Oxide. Nanoscale Res. Lett. 2010, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Neubert, E.; Senger-Sander, S.N.; Manzke, V.S.; Busse, J.; Polo, E.; Scheidmann, S.E.F.; Schön, M.P.; Kruss, S.; Erpenbeck, L. Serum and Serum Albumin Inhibit in vitro Formation of Neutrophil Extracellular Traps (NETs). Front. Immunol. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggård, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Monopoli, M.P.; Walczyk, D.; Campbell, A.; Elia, G.; Lynch, I.; Bombelli, F.B.; Dawson, K.A. Physical−Chemical Aspects of Protein Corona: Relevance toin Vitroandin VivoBiological Impacts of Nanoparticles. J. Am. Chem. Soc. 2011, 133, 2525–2534. [Google Scholar] [CrossRef]
- Saha, K.; Moyano, D.F.; Rotello, V.M. Protein coronas suppress the hemolytic activity of hydrophilic and hydrophobic nanoparticles. Mater. Horizons 2014, 1, 102–105. [Google Scholar] [CrossRef]
- Walkey, C.D.; Chan, W.C. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012, 41, 2780–2799. [Google Scholar] [CrossRef]
- Hellstrand, E.; Lynch, I.; Andersson, A.; Drakenberg, T.; Dahlbäck, B.; Dawson, K.A.; Linse, S.; Cedervall, T. Complete high-density lipoproteins in nanoparticle corona. FEBS J. 2009, 276, 3372–3381. [Google Scholar] [CrossRef]
- Fleischer, C.C.; Payne, C.K. Nanoparticle-Cell Interactions: Molecular Structure of the Protein Corona and Cellular Outcomes. Accounts Chem. Res. 2014, 47, 2651–2659. [Google Scholar] [CrossRef]
- Fleischer, C.C.; Payne, C.K. Secondary Structure of Corona Proteins Determines the Cell Surface Receptors Used by Nanoparticles. J. Phys. Chem. B 2014, 118, 14017–14026. [Google Scholar] [CrossRef]
- Kotchey, G.P.; Hasan, S.A.; Kapralov, O.; Ha, S.H.; Kim, K.; Shvedova, A.A.; Kagan, V.E.; Star, A. A Natural Vanishing Act: The Enzyme-Catalyzed Degradation of Carbon Nanomaterials. Accounts Chem. Res. 2012, 45, 1770–1781. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.; Mukherjee, S.P.; Gallud, A.; Burkert, S.C.; Bistarelli, S.; Bellucci, S.; Bottini, M.; Star, A.; Fadeel, B. Biological interactions of carbon-based nanomaterials: From coronation to degradation. Nanomed. Nanotechnol. Boil. Med. 2015, 12, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Russier, J.; Oudjedi, L.; Piponnier, M.; Bussy, C.; Prato, M.; Kostarelos, K.; Lounis, B.; Bianco, A.; Cognet, L. Direct visualization of carbon nanotube degradation in primary cells by photothermal imaging. Nanoscale 2017, 9, 4642–4645. [Google Scholar] [CrossRef] [PubMed]
- Kotchey, G.P.; Allen, B.L.; Vedala, H.; Yanamala, N.; Kapralov, O.; Tyurina, Y.Y.; Klein-Seetharaman, J.; Kagan, V.E.; Star, A. The Enzymatic Oxidation of Graphene Oxide. ACS Nano 2011, 5, 2098–2108. [Google Scholar] [CrossRef]
- Kurapati, R.; Mukherjee, S.P.; Martín, C.; Bepete, G.; Vázquez, E.; Penicaud, A.; Fadeel, B.; Bianco, A. Degradation of Single-Layer and Few-Layer Graphene by Neutrophil Myeloperoxidase. Angew. Chem. 2018, 130, 11896–11901. [Google Scholar] [CrossRef]
- Kurapati, R.; Bianco, A. Peroxidase mimicking DNAzymes degrade graphene oxide. Nanoscale 2018, 10, 19316–19321. [Google Scholar] [CrossRef]
- Daems, W.T.; Van Der Rhee, H.J. Peroxidase and Catalase in Monocytes, Macrophages, Epithelioid Cells and Giant Cells of the Rat. In Mononuclear Phagocytes; Springer Science and Business Media LLC: Basel, Swtzerland, 1980; pp. 43–60. [Google Scholar]
- Gref, R.; Luck, M.; Quellec, P.; Marchand, M.; Dellacherie, É.; Harnisch, S.; Blunk, T.; Müller, R. ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): Influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf. B: Biointerfaces 2000, 18, 301–313. [Google Scholar] [CrossRef]
- Orth, M.; Lauber, K.; Niyazi, M.; Friedl, A.A.; Li, M.; Maihöfer, C.; Schüttrumpf, L.; Ernst, A.; Niemöller, O.M.; Belka, C. Current concepts in clinical radiation oncology. Radiat. Environ. Biophys. 2013, 53, 1–29. [Google Scholar] [CrossRef]
- Gorayski, P.; Burmeister, B.; Foote, M. Radiotherapy for cutaneous melanoma: Current and future applications. Futur. Oncol. 2015, 11, 525–534. [Google Scholar] [CrossRef]
- Hader, M.; Frey, B.; Fietkau, R.; Hecht, M.; Gaipl, U.S. Immune biological rationales for the design of combined radio- and immunotherapies. Cancer Immunol. Immunother. 2020, 69, 293–306. [Google Scholar] [CrossRef]
- Schildkopf, P.; Frey, B.; Mantel, F.; Ott, O.; Weiss, E.-M.; Sieber, R.; Janko, C.; Sauer, D.M.R.; Fietkau, R.; Gaipl, U.S. Application of hyperthermia in addition to ionizing irradiation fosters necrotic cell death and HMGB1 release of colorectal tumor cells. Biochem. Biophys. Res. Commun. 2010, 391, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Wu, Y.; Duan, Y.; Luan, X.; Zhang, Q.; An, Q. Combined Photothermal and Surface-Enhanced Raman Spectroscopy Effect from Spiky Noble Metal Nanoparticles Wrapped within Graphene-Polymer Layers: Using Layer-by-layer Modified Reduced Graphene Oxide as Reactive Precursors. ACS Appl. Mater. Interfaces 2015, 7, 19353–19361. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.R.; Ravichandran, K. Clearance of apoptotic cells: Implications in health and disease. J. Cell Boil. 2010, 189, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Gaipl, U.S.; Muñoz, L.E.; Rödel, F.; Pausch, F.; Frey, B.; Brachvogel, B.; Von Der Mark, K.; Pöschl, E. Modulation of the immune system by dying cells and the phosphatidylserine-ligand annexin A5. Autoimmunity 2007, 40, 254–259. [Google Scholar] [CrossRef]
- Voll, R.E.; Herrmann, M.; Roth, E.A.; Stach, C.; Kalden, J.R.; Girkontaite, I. Immunosuppressive effects of apoptotic cells. Nature 1997, 390, 350–351. [Google Scholar] [CrossRef]
- Muñoz, L.E.; Lauber, K.; Schiller, M.; Manfredi, A.A.; Herrmann, M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat. Rev. Rheumatol. 2010, 6, 280–289. [Google Scholar] [CrossRef]
- Urbonaviciute, V.; Fürnrohr, B.G.; Meister, S.; Muñoz, L.E.; Heyder, P.; De Marchis, F.; Bianchi, M.E.; Kirschning, C.; Wagner, H.; Manfredi, A.A.; et al. Induction of inflammatory and immune responses by HMGB1–nucleosome complexes: Implications for the pathogenesis of SLE. J. Exp. Med. 2008, 205, 3007–3018. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podolska, M.J.; Barras, A.; Alexiou, C.; Frey, B.; Gaipl, U.; Boukherroub, R.; Szunerits, S.; Janko, C.; Muñoz, L.E. Graphene Oxide Nanosheets for Localized Hyperthermia—Physicochemical Characterization, Biocompatibility, and Induction of Tumor Cell Death. Cells 2020, 9, 776. https://doi.org/10.3390/cells9030776
Podolska MJ, Barras A, Alexiou C, Frey B, Gaipl U, Boukherroub R, Szunerits S, Janko C, Muñoz LE. Graphene Oxide Nanosheets for Localized Hyperthermia—Physicochemical Characterization, Biocompatibility, and Induction of Tumor Cell Death. Cells. 2020; 9(3):776. https://doi.org/10.3390/cells9030776
Chicago/Turabian StylePodolska, Malgorzata J., Alexandre Barras, Christoph Alexiou, Benjamin Frey, Udo Gaipl, Rabah Boukherroub, Sabine Szunerits, Christina Janko, and Luis E. Muñoz. 2020. "Graphene Oxide Nanosheets for Localized Hyperthermia—Physicochemical Characterization, Biocompatibility, and Induction of Tumor Cell Death" Cells 9, no. 3: 776. https://doi.org/10.3390/cells9030776
APA StylePodolska, M. J., Barras, A., Alexiou, C., Frey, B., Gaipl, U., Boukherroub, R., Szunerits, S., Janko, C., & Muñoz, L. E. (2020). Graphene Oxide Nanosheets for Localized Hyperthermia—Physicochemical Characterization, Biocompatibility, and Induction of Tumor Cell Death. Cells, 9(3), 776. https://doi.org/10.3390/cells9030776








