Hybrid Label-Free Molecular Microscopies for Simultaneous Visualization of Changes in Cell Wall Polysaccharides of Peach at Single- and Multiple-Cell Levels during Postharvest Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Measurement of Firmness and Cell Wall Materials
2.3. Label-Free Hyperspectral Molecular Imaging
2.4. Statistical Analysis
3. Results
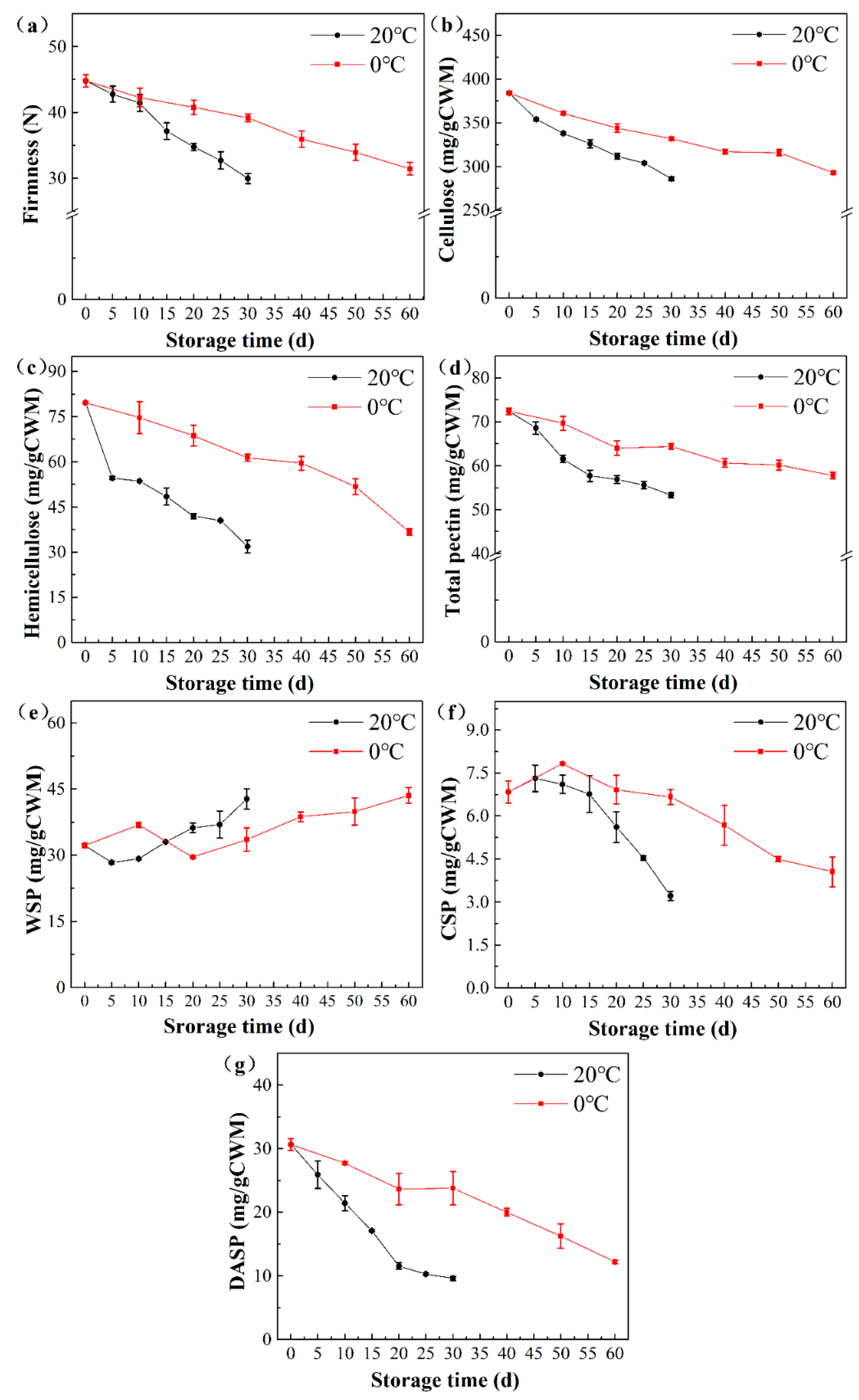
3.1. Firmness and Cell Wall Polysaccharide Content
3.2. CRM Hyperspectral Imaging
3.2.1. Assignment of Characteristic Peaks in RS
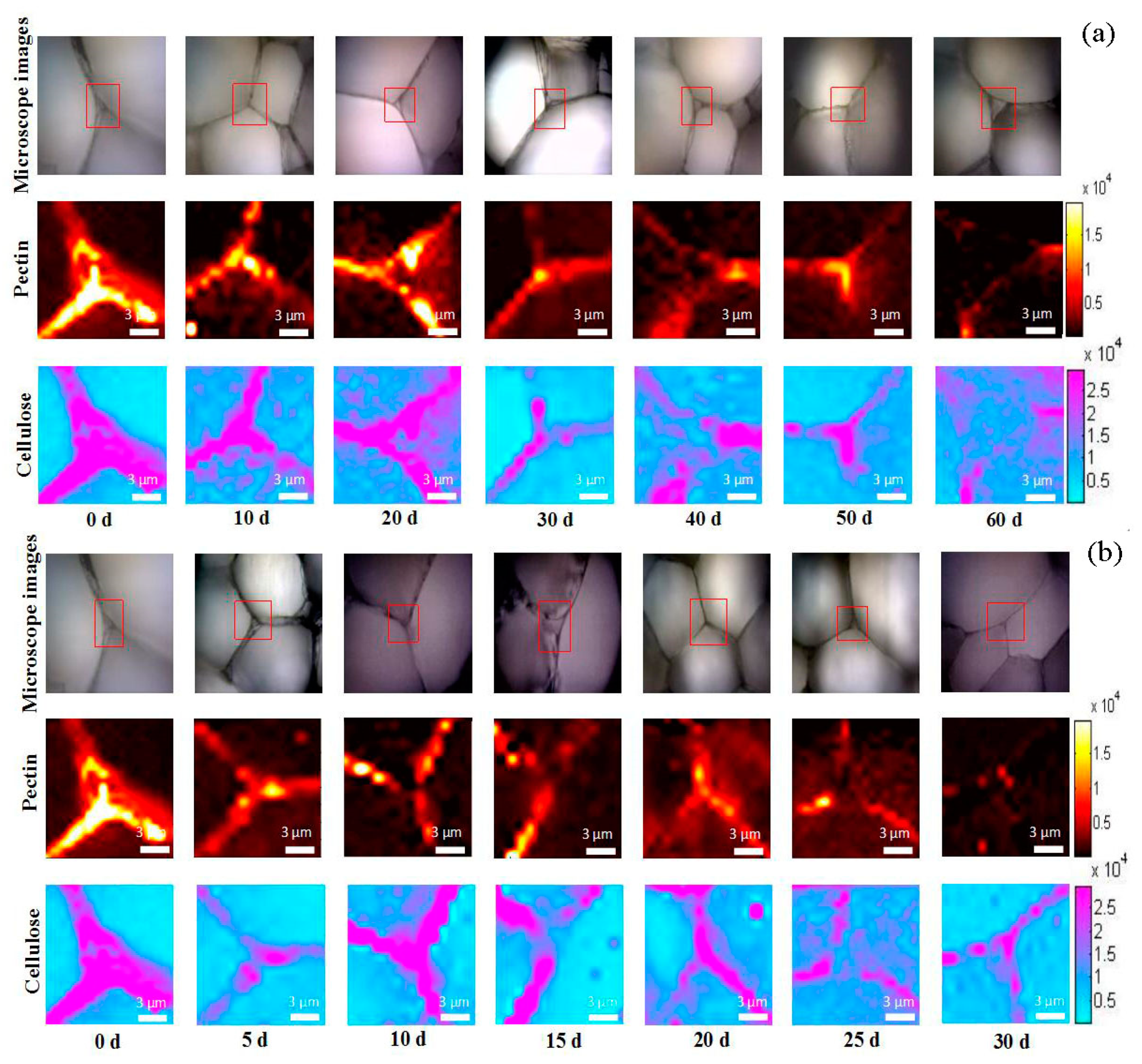
3.2.2. CRM Hyperspectral Images of Cell Wall Polysaccharides
3.3. FTIRM Hyperspectral Imaging
3.3.1. Analysis of IR of Peach Cell Wall
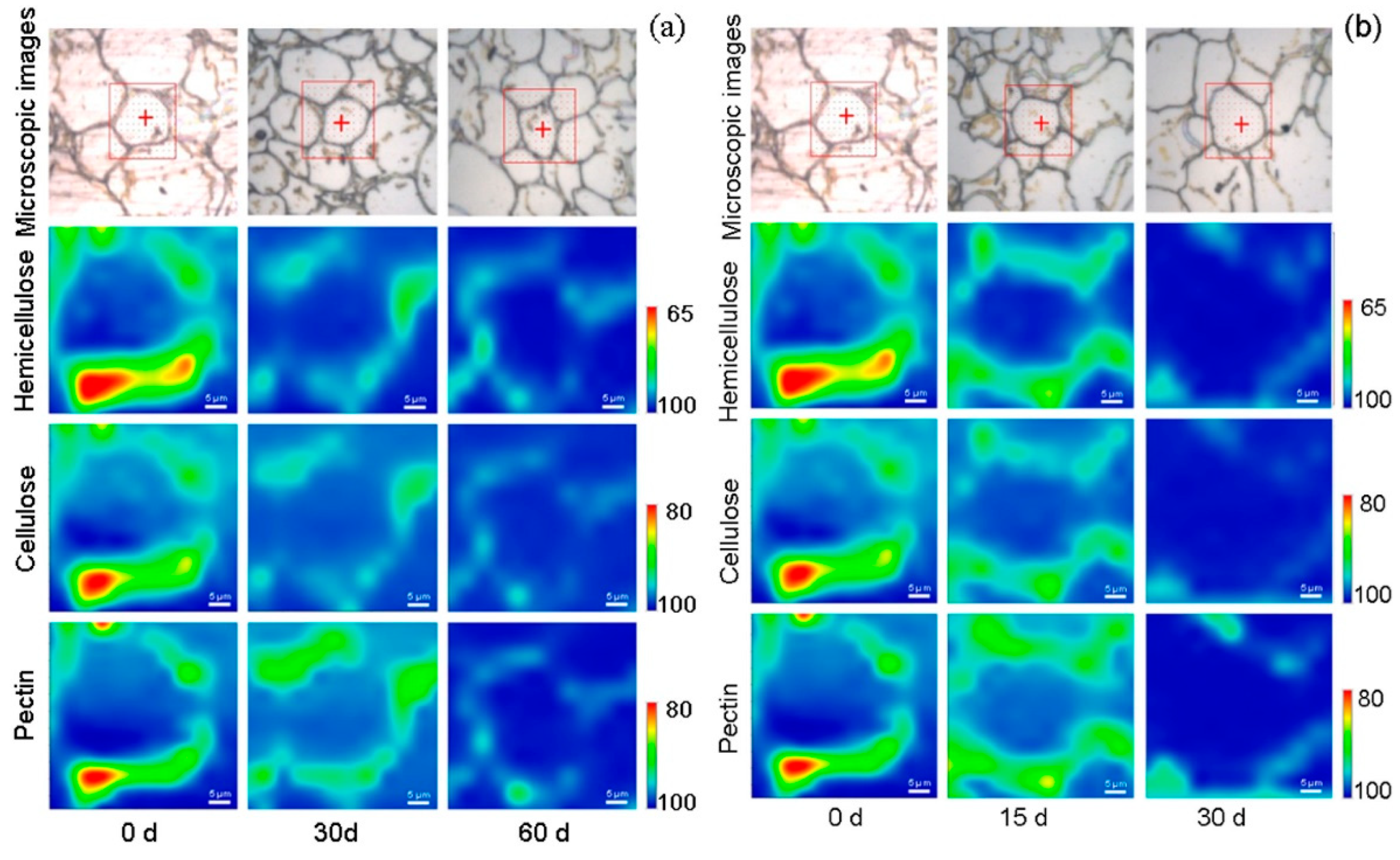
3.3.2. FTIRM Hyperspectral Images of Cell Wall Polysaccharides
3.4. SRS Hyperspectral Imaging
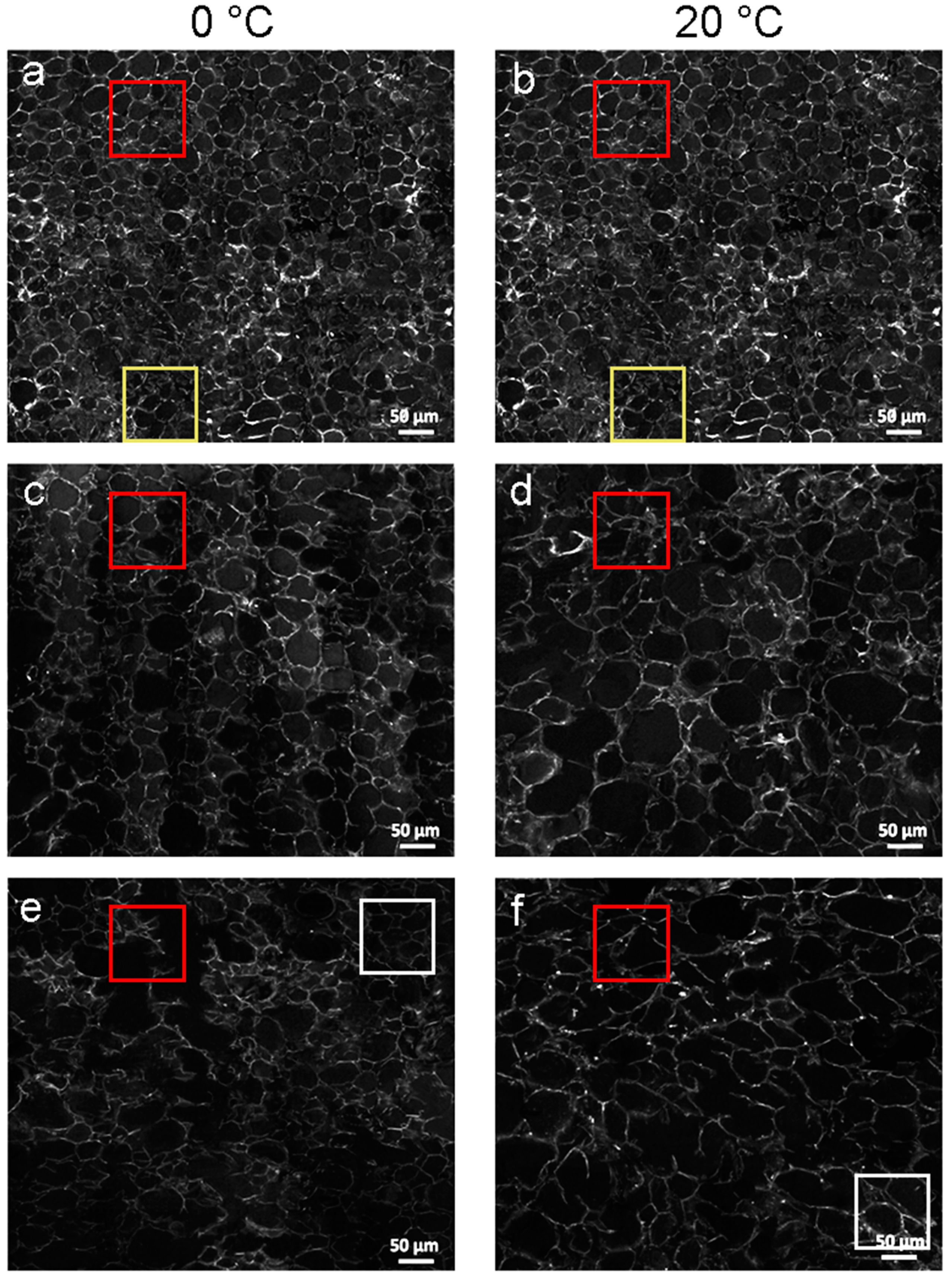
3.4.1. Analysis of SRS Images
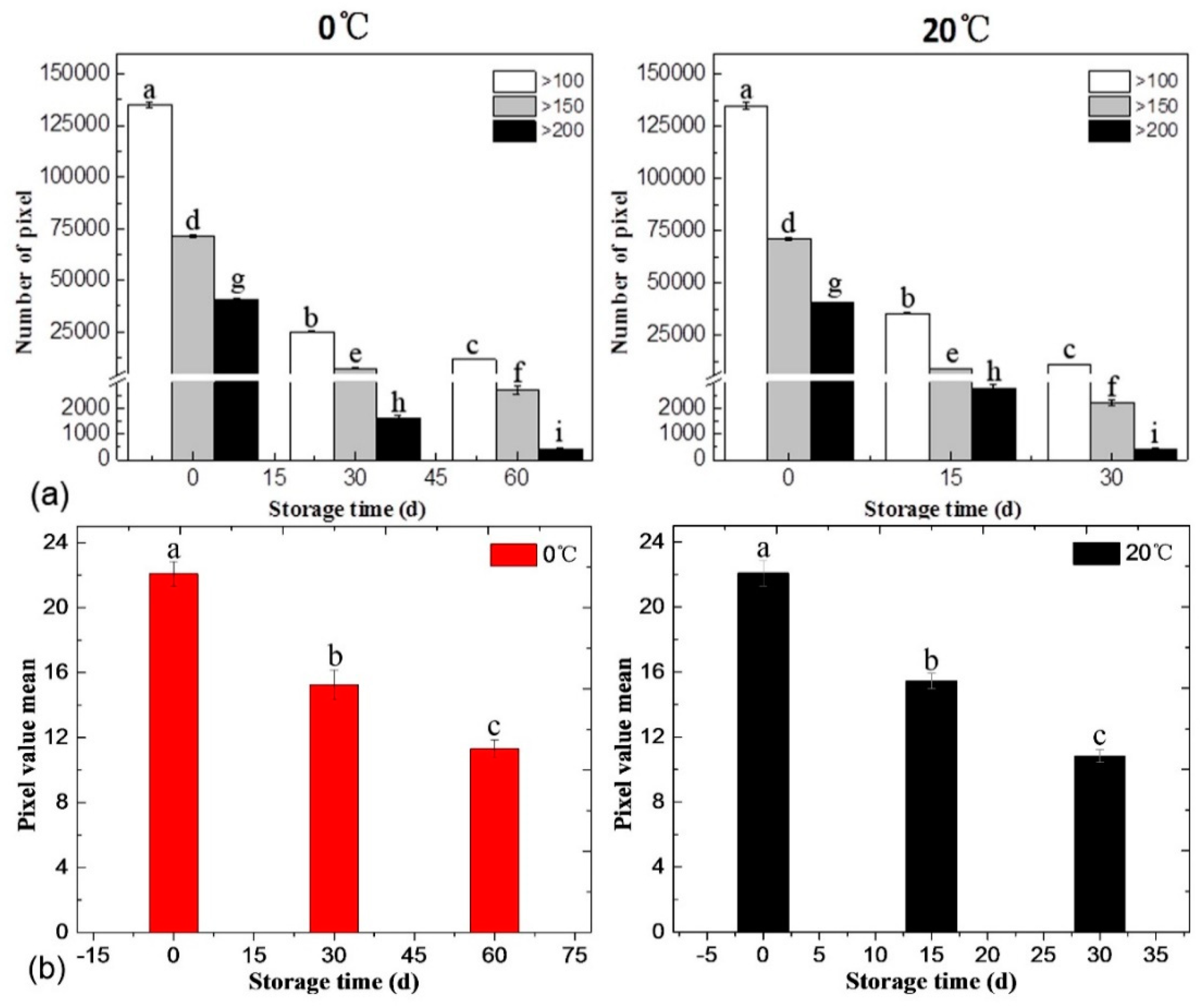
3.4.2. Statistical Analysis of SRS Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- De Baerdemaeker, J.; Jancsók, P.T.; Verlinden, B.E. Firmness and softening of fruits and vegetables. In Physical Methods in Agriculture; Blahovec, J., Kutílek, M., Eds.; Springer: Berlin, Germany, 2002; pp. 343–357. [Google Scholar]
- Ng, J.K.; Schröder, R.; Sutherland, P.W.; Hallett, I.C.; Hall, M.I.; Prakash, R.; Smith, B.G.; Melton, L.D.; Johnston, J.W. Cell wall structures leading to cultivar differences in softening rates develop early during apple (Malus x domestica) fruit growth. BMC Plant Biol. 2013, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- De Roeck, A.; Sila, D.N.; Duvetter, T.; Van Loey, A.; Hendrickx, M. Effect of high pressure/high temperature processing on cell wall pectic substances in relation to firmness of carrot tissue. Food Chem. 2008, 107, 1225–1235. [Google Scholar] [CrossRef]
- Manrique, G.D.; Lajolo, F.M. Cell wall polysaccharide modifications during postharvest ripening of papaya fruit (Carica papaya). Postharvest Biol. Technol. 2004, 33, 11–26. [Google Scholar] [CrossRef]
- Altartouri, B.; Geitmann, A. Understanding plant cell morphogenesis requires real-time monitoring of cell wall polymers. Curr. Opin. Plant Biol. 2015, 23, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lavis, L.D.; Betzig, E. Imaging live-cell dynamics and structure at the single-molecule level. Mol. Cell 2015, 58, 644–659. [Google Scholar] [CrossRef] [PubMed]
- Buda, G.J.; Isaacson, T.; Matas, A.J.; Paolillo, D.J.; Rose, J.K. Three-dimensional imaging of plant cuticle architecture using confocal scanning laser microscopy. Plant J. 2009, 60, 378–385. [Google Scholar] [CrossRef]
- Broderick, C.E.; Cooke, P.H.; Patil, B.; Murano, P.; Amiotcarlin, M.J. Fruit composition, tissues, and localization of antioxidants and capsaicinoids in Capsicum peppers by fluorescence microscopy. Acta Hortic. 2009, 841, 85–90. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Chylińska, M.; Pieczywek, P.M.; Rösch, P.; Schmitt, M.; Popp, J.; Zdunek, A. Raman imaging of changes in the polysaccharides distribution in the cell wall during apple fruit development and senescence. Planta 2016, 243, 935–945. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Benítez, J.J.; Domínguez, E.; Bayer, I.S.; Cingolani, R.; Athanassiou, A.; Heredia, A. Infrared and Raman spectroscopic features of plant cuticles: A review. Front Plant Sci. 2014, 5, 305. [Google Scholar] [CrossRef]
- Mohamad, W.F.W.; Buckow, R.; Augustin, M.; McNaughton, D. In situ quantification of β-carotene partitioning in oil-in-water emulsions by confocal Raman microscopy. Food Chem. 2017, 233, 197–203. [Google Scholar] [CrossRef]
- Zhu, N.; Wu, D.; Chen, K. Label-free visualization of fruit lignification: Raman molecular imaging of loquat lignified cells. Plant Methods 2018, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.-T.; Pu, H.; Sun, D.-W. Insights into the changes in chemical compositions of the cell wall of pear fruit infected by Alternaria alternata with confocal Raman microspectroscopy. Postharvest Biol. Tec. 2017, 132, 119–129. [Google Scholar] [CrossRef]
- Mazurek, S.; Mucciolo, A.; Humbel, B.M.; Nawrath, C. Transmission F ourier transform infrared microspectroscopy allows simultaneous assessment of cutin and cell-wall polysaccharides of A rabidopsis petals. Plant J. 2013, 74, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Yang, Z.; Han, L.; Jiang, X.; Ji, G. Study on in situ analysis of cellulose, hemicelluloses and lignin distribution linked to tissue structure of crop stalk internodal transverse section based on FTIR microspectroscopic imaging. Cellulose 2015, 22, 139–149. [Google Scholar] [CrossRef]
- González-Cabrera, M.; Domínguez-Vidal, A.; Ayora-Cañada, M. Hyperspectral FTIR imaging of olive fruit for understanding ripening processes. Postharvest Biol. Tec. 2018, 145, 74–82. [Google Scholar] [CrossRef]
- Huang, B.; Yan, S.; Xiao, L.; Ji, R.; Yang, L.; Miao, A.J.; Wang, P. Label-free imaging of nanoparticle uptake competition in single cells by hyperspectral stimulated raman scattering. Small 2018, 14, 1703246. [Google Scholar] [CrossRef] [PubMed]
- Tipping, W.; Lee, M.; Serrels, A.; Brunton, V.; Hulme, A. Stimulated Raman scattering microscopy: An emerging tool for drug discovery. Chem. Soc. Rev. 2016, 45, 2075–2089. [Google Scholar] [CrossRef]
- Renard, C.M. Variability in cell wall preparations: Quantification and comparison of common methods. Carbohyd. Polym. 2005, 60, 515–522. [Google Scholar] [CrossRef]
- Figueroa, C.R.; Opazo, M.C.; Vera, P.; Arriagada, O.; Díaz, M.; Moya-León, M.A. Effect of postharvest treatment of calcium and auxin on cell wall composition and expression of cell wall-modifying genes in the Chilean strawberry (Fragaria chiloensis) fruit. Food Chem. 2012, 132, 2014–2022. [Google Scholar] [CrossRef]
- Vicente, A.R.; Ortugno, C.; Rosli, H.; Powell, A.L.; Greve, L.C.; Labavitch, J.M. Temporal sequence of cell wall disassembly events in developing fruits. 2. Analysis of blueberry (Vaccinium species). J. Agr. Food Chem. 2007, 55, 4125–4130. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, N.; Wang, J.; Zhang, H.; Li, D.; Shi, J.; Li, R.; Weeda, S.; Zhao, B.; Ren, S. Melatonin promotes ripening and improves quality of tomato fruit during postharvest life. J. Exp. Bot. 2015, 66, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Madeira, J.V., Jr.; Contesini, F.J.; Calzado, F.; Rubio, M.V.; Zubieta, M.P.; Lopes, D.B.; de Melo, R.R. Agro-industrial residues and microbial enzymes: An overview on the eco-friendly bioconversion into high value-added products. In Biotechnology of Microbial Enzymes; Brahmachari, G., Ed.; Elsevier: Amsterdam, the Netherlands, 2017; pp. 475–511. [Google Scholar]
- Reid, J.G. Carbohydrate metabolism: Structural carbohydrates. In Plant Biochemistry; Dey, P.M., Harborne, J.B., Eds.; Elsevier: Amsterdam, the Netherlands, 1997; pp. 205–236. [Google Scholar]
- Brownleader, M.; Jackson, P.; Mobasheri, A.; Pantelides, A.; Sumar, S.; Trevan, M.; Dey, P. Molecular aspects of cell wall modifications during fruit ripening. Crit. Rev. Food Sci. 1999, 39, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Toivonen, P.M.; Brummell, D.A. Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetables. Postharvest Biol. Tec. 2008, 48, 1–14. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S. Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: A review. Int. J. Biol. Macromol. 2013, 61, 1–6. [Google Scholar] [CrossRef]
- Farhat, W.; Venditti, R.A.; Hubbe, M.; Taha, M.; Becquart, F.; Ayoub, A. A review of water-resistant hemicellulose-based materials: Processing and applications. ChemSusChem 2017, 10, 305–323. [Google Scholar] [CrossRef]
- Padayachee, A.; Day, L.; Howell, K.; Gidley, M. Complexity and health functionality of plant cell wall fibers from fruits and vegetables. Crit. Rev. Food Sci. 2017, 57, 59–81. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, S.; Liang, Y.-Z. Baseline correction using adaptive iteratively reweighted penalized least squares. Analyst 2010, 135, 1138–1146. [Google Scholar] [CrossRef]
- Synytsya, A.; Čopíková, J.; Matějka, P.; Machovič, V. Fourier transform Raman and infrared spectroscopy of pectins. Carbohyd. Polym. 2003, 54, 97–106. [Google Scholar] [CrossRef]
- Gierlinger, N.; Schwanninger, M. The potential of Raman microscopy and Raman imaging in plant research. J. Spectroscopy 2007, 21, 69–89. [Google Scholar] [CrossRef]
- Chylińska, M.; Szymańska-Chargot, M.; Zdunek, A. Imaging of polysaccharides in the tomato cell wall with Raman microspectroscopy. Plant Methods 2014, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Kacurakova, M.; Capek, P.; Sasinkova, V.; Wellner, N.; Ebringerova, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohyd. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Chatjigakis, A.; Pappas, C.; Proxenia, N.; Kalantzi, O.; Rodis, P.; Polissiou, M. FT-IR spectroscopic determination of the degree of esterification of cell wall pectins from stored peaches and correlation to textural changes. Carbohyd. Polym. 1998, 37, 395–408. [Google Scholar] [CrossRef]
- Kratchanova, M.; Pavlova, E.; Panchev, I. The effect of microwave heating of fresh orange peels on the fruit tissue and quality of extracted pectin. Carbohyd. Polym. 2004, 56, 181–185. [Google Scholar] [CrossRef]
- Egüés, I.; Sanchez, C.; Mondragon, I.; Labidi, J. Effect of alkaline and autohydrolysis processes on the purity of obtained hemicelluloses from corn stalks. Bioresource Technol. 2012, 103, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Barron, C.; Parker, M.; Mills, E.; Rouau, X.; Wilson, R. FTIR imaging of wheat endosperm cell walls in situ reveals compositional and architectural heterogeneity related to grain hardness. Planta 2005, 220, 667–677. [Google Scholar] [CrossRef]
- Gierlinger, N.; Schwanninger, M. Chemical imaging of poplar wood cell walls by confocal Raman microscopy. Plant Physiol. 2006, 140, 1246–1254. [Google Scholar] [CrossRef]
- Wiley, J.H.; Atalla, R.H. Band assignments in the Raman spectra of celluloses. Carbohyd. Res. 1987. [Google Scholar] [CrossRef]
- Wang, X.; Ding, Y.; Wang, Y.; Pan, L.; Niu, L.; Lu, Z.; Cui, G.; Zeng, W.; Wang, Z. Genes involved in ethylene signal transduction in peach (Prunus persica) and their expression profiles during fruit maturation. Sci Hortic-Amsterdam 2017, 224, 306–316. [Google Scholar] [CrossRef]
- Pei, M.; Gu, C.; Zhang, S. Genome-wide identification and expression analysis of genes associated with peach (Prunus persica) fruit ripening. Sci Hortic-Amsterdam 2019, 246, 317–327. [Google Scholar] [CrossRef]
- Pan, H.; Sheng, Y.; Gao, Z.; Chen, H.; Qi, Y.; Yi, X.; Qin, G.; Zhang, J. Transcriptome analysis of peach (Prunus persica L. Batsch) during the late stage of fruit ripening. Genet. Mol. Res. 2016, 15, gmr15049335. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, M.; Ji, M.; Wang, X.; Xiao, W.; Li, L.; Gao, D.; Chen, X.; Li, D. Transcriptome analysis of ripe peach (Prunus persica) fruit under low-dose UVB radiation. Sci Hortic-Amsterdam 2020, 259, 108757. [Google Scholar] [CrossRef]
- Francis, A.; Berry, K.; Chen, Y.; Figueroa, B.; Fu, D. Label-free pathology by spectrally sliced femtosecond stimulated Raman scattering (SRS) microscopy. PLoS ONE 2017, 12, e0178750. [Google Scholar] [CrossRef]
- Camp, C.H., Jr.; Lee, Y.J.; Heddleston, J.M.; Hartshorn, C.M.; Walker, A.R.H.; Rich, J.N.; Lathia, J.D.; Cicerone, M.T. High-speed coherent Raman fingerprint imaging of biological tissues. Nat. Photonics 2014, 8, 627. [Google Scholar] [CrossRef] [PubMed]
- Manganaris, G.A.; Vasilakakis, M.; Diamantidis, G.; Mignani, I. Diverse metabolism of cell wall components of melting and non-melting peach genotypes during ripening after harvest or cold storage. J. Sci. Food Agric. 2006, 86, 243–250. [Google Scholar] [CrossRef]
- Cao, S.; Bian, K.; Shi, L.; Chung, H.-H.; Chen, W.; Yang, Z. Role of melatonin in cell wall disassembly and chilling tolerance in cold-stored peach fruit. J. Agr. Food Chem. 2018, 66, 5663–5670. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Yang, S.; Yuan, Y. Lignin Involvement in Programmed Changes in Peach-Fruit Texture Indicated by Metabolite and Transcriptome Analyses. J. Agr. Food Chem. 2018, 66, 12627–12640. [Google Scholar] [CrossRef] [PubMed]
- Ciacciulli, A.; Chiozzotto, R.; Attanasio, G.; Cirilli, M.; Bassi, D. Identification of a melting type variant among peach (P. persica L. Batsch) fruit textures by a digital penetrometer. J. Texture Stud. 2018, 49, 370–377. [Google Scholar] [CrossRef]
- Chen, Y.; Hung, Y.-C.; Chen, M.; Lin, H. Effects of acidic electrolyzed oxidizing water on retarding cell wall degradation and delaying softening of blueberries during postharvest storage. LWT 2017, 84, 650–657. [Google Scholar] [CrossRef]
- Zhou, R.; Li, Y.; Yan, L.; Xie, J. Effect of edible coatings on enzymes, cell-membrane integrity, and cell wall constituents in relation to brittleness and firmness of Huanghua pears (Pyrus pyrifolia Nakai, cv. Huanghua) during storage. Food Chem. 2011, 124, 569–575. [Google Scholar] [CrossRef]
- Wang, L.; Jin, P.; Wang, J.; Jiang, L.; Shan, T.; Zheng, Y. Effect of β-aminobutyric acid on cell wall modification and senescence in sweet cherry during storage at 20 °C. Food Chem. 2015, 175, 471–477. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Nie, Y.; Zhu, N.; Yang, Y.; Zhu, C.; Ji, M.; Wu, D.; Chen, K. Hybrid Label-Free Molecular Microscopies for Simultaneous Visualization of Changes in Cell Wall Polysaccharides of Peach at Single- and Multiple-Cell Levels during Postharvest Storage. Cells 2020, 9, 761. https://doi.org/10.3390/cells9030761
Huang W, Nie Y, Zhu N, Yang Y, Zhu C, Ji M, Wu D, Chen K. Hybrid Label-Free Molecular Microscopies for Simultaneous Visualization of Changes in Cell Wall Polysaccharides of Peach at Single- and Multiple-Cell Levels during Postharvest Storage. Cells. 2020; 9(3):761. https://doi.org/10.3390/cells9030761
Chicago/Turabian StyleHuang, Weinan, Yating Nie, Nan Zhu, Yifan Yang, Changqing Zhu, Minbiao Ji, Di Wu, and Kunsong Chen. 2020. "Hybrid Label-Free Molecular Microscopies for Simultaneous Visualization of Changes in Cell Wall Polysaccharides of Peach at Single- and Multiple-Cell Levels during Postharvest Storage" Cells 9, no. 3: 761. https://doi.org/10.3390/cells9030761
APA StyleHuang, W., Nie, Y., Zhu, N., Yang, Y., Zhu, C., Ji, M., Wu, D., & Chen, K. (2020). Hybrid Label-Free Molecular Microscopies for Simultaneous Visualization of Changes in Cell Wall Polysaccharides of Peach at Single- and Multiple-Cell Levels during Postharvest Storage. Cells, 9(3), 761. https://doi.org/10.3390/cells9030761






