An Overview on ERAP Roles in Infectious Diseases
Abstract
1. Introduction
1.1. Biological Functions of ERAP1/2
1.2. ERAPs Polymorphisms (SNPs)
1.2.1. ERAP1 Genetic Variants
1.2.2. ERAP2 Genetic Variants
2. ERAPs and Infectious Diseases
2.1. ERAPs and Hepatitis C Virus (HCV)
2.2. ERAPs and Influenza Virus (Flu)
2.3. ERAPs and Human Cytomegalovirus (HCMV)
2.4. ERAPs and Human Papilloma Virus (HPV)
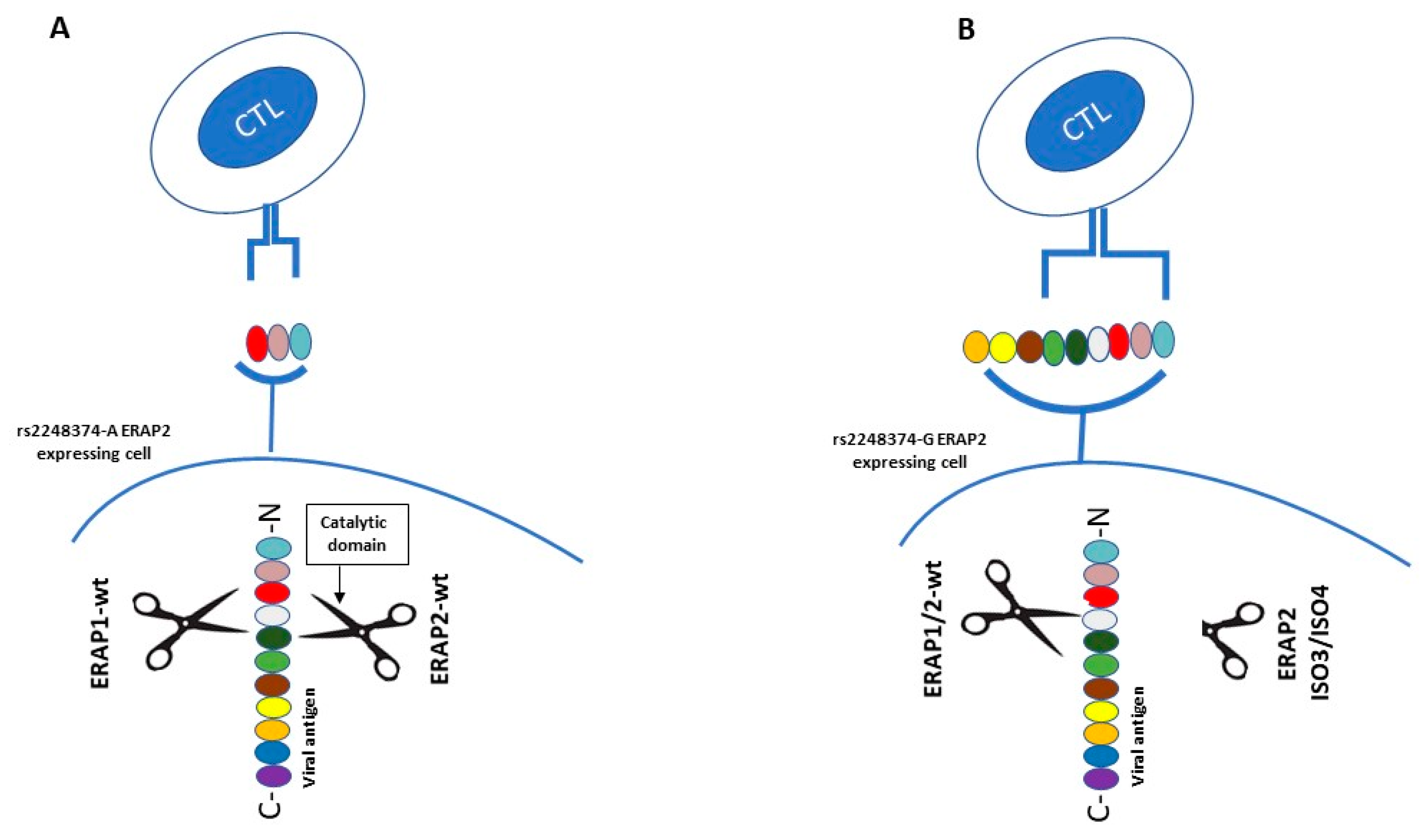
2.5. ERAPs and Human Immunodeficiency Virus (HIV)
2.6. ERAPs and other Microrganisms
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Woon, A.P.; Purcell, A.W. The use of proteomics to understand antiviral immunity. Semin. Cell Dev. Biol. 2018, 84, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Neefjes, J.; Jongsma, M.L.M.; Paul, P.; Bakke, O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat. Rev. Immunol. 2011, 11, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Vyas, J.M.; Van der Veen, A.G.; Ploegh, H.L. The known unknowns of antigen processing and presentation. Nat. Rev. Immunol. 2008, 8, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Hattori, A.; Tsujimoto, M. Endoplasmic reticulum aminopeptidases: Biochemistry, physiology and pathology. J. Biochem. 2013, 154, 219–228. [Google Scholar] [CrossRef]
- Chen, H.; Li, L.; Weimershaus, M.; Evnouchidou, I.; van Endert, P.; Bouvier, M. ERAP1-ERAP2 dimers trim MHC I-Bound precursor peptides; implications for understanding peptide editing. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-C.; Momburg, F.; Bhutani, N.; Goldberg, A.L. The ER aminopeptidase, ERAP1, trims precursors to lengths of MHC class I peptides by a “molecular ruler” mechanism. Proc. Natl. Acad. Sci. USA 2005, 102, 17107–17112. [Google Scholar] [CrossRef]
- Saveanu, L.; Carroll, O.; Lindo, V.; Del Val, M.; Lopez, D.; Lepelletier, Y.; Greer, F.; Schomburg, L.; Fruci, D.; Niedermann, G.; et al. Concerted peptide trimming by human ERAP1 and ERAP2 aminopeptidase complexes in the endoplasmic reticulum. Nat. Immunol. 2005, 6, 689–697. [Google Scholar] [CrossRef]
- Evnouchidou, I.; Weimershaus, M.; Saveanu, L.; van Endert, P. ERAP1–ERAP2 Dimerization Increases Peptide-Trimming Efficiency. J. Immunol. 2014. [Google Scholar] [CrossRef]
- Kochan, G.; Krojer, T.; Harvey, D.; Fischer, R.; Chen, L.; Vollmar, M.; von Delft, F.; Kavanagh, K.L.; Brown, M.A.; Bowness, P.; et al. Crystal structures of the endoplasmic reticulum aminopeptidase-1 (ERAP1) reveal the molecular basis for N-Terminal peptide trimming. Proc. Natl. Acad. Sci. USA 2011, 108, 7745–7750. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Chang, S.-C.; Evnouchidou, I.; York, I.A.; Zikos, C.; Rock, K.L.; Goldberg, A.L.; Stratikos, E.; Stern, L.J. Structural Basis For Antigenic Peptide Precursor Processing by the Endoplasmic Reticulum Aminopeptidase ERAP1. Nat. Struct. Mol. Biol. 2011, 18, 604–613. [Google Scholar] [CrossRef]
- Serwold, T.; Gaw, S.; Shastri, N. ER aminopeptidases generate a unique pool of peptides for MHC class I molecules. Nat. Immunol. 2001, 2, 644–651. [Google Scholar] [CrossRef] [PubMed]
- López de Castro, J.A. How ERAP1 and ERAP2 Shape the Peptidomes of Disease-Associated MHC-I Proteins. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Rouhani, F.N.; Hawari, F.; Levine, S.J. Shedding of the Type II IL-1 Decoy Receptor Requires a Multifunctional Aminopeptidase, Aminopeptidase Regulator of TNF Receptor Type 1 Shedding. J. Immunol. 2003, 171, 6814–6819. [Google Scholar] [CrossRef]
- Cui, X.; Rouhani, F.N.; Hawari, F.; Levine, S.J. An Aminopeptidase, ARTS-1, Is Required for Interleukin-6 Receptor Shedding. J. Biol. Chem. 2003, 278, 28677–28685. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Ogawa, K.; Nakamura, T.J.; Hattori, A.; Tsujimoto, M. Substrate-Dependent nitric oxide synthesis by secreted endoplasmic reticulum aminopeptidase 1 in macrophages. J. Biochem. 2015, 157, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Aldhamen, Y.A.; Seregin, S.S.; Rastall, D.P.W.; Aylsworth, C.F.; Pepelyayeva, Y.; Busuito, C.J.; Godbehere-Roosa, S.; Kim, S.; Amalfitano, A. Endoplasmic Reticulum Aminopeptidase-1 Functions Regulate Key Aspects of the Innate Immune Response. PLoS ONE 2013, 8, e69539. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y. Role of Aminopeptidase in Angiogenesis. Biol. Pharm. Bull. 2004, 27, 772–776. [Google Scholar] [CrossRef]
- Tanioka, T.; Hattori, A.; Masuda, S.; Nomura, Y.; Nakayama, H.; Mizutani, S.; Tsujimoto, M. Human Leukocyte-Derived Arginine Aminopeptidase THE THIRD MEMBER OF THE OXYTOCINASE SUBFAMILY OF AMINOPEPTIDASES. J. Biol. Chem. 2003, 278, 32275–32283. [Google Scholar] [CrossRef]
- Goto, Y.; Ogawa, Y.; Tsumoto, H.; Miura, Y.; Nakamura, T.J.; Ogawa, K.; Akimoto, Y.; Kawakami, H.; Endo, T.; Yanoshita, R.; et al. Contribution of the exosome-Associated form of secreted endoplasmic reticulum aminopeptidase 1 to exosome-Mediated macrophage activation. Biochim. et Biophys. Acta (BBA)-Mol. Cell Res. 2018, 1865, 874–888. [Google Scholar] [CrossRef]
- Saulle, I.; Ibba, S.V.; Torretta, E.; Vittori, C.; Fenizia, C.; Piancone, F.; Minisci, D.; Lori, E.M.; Trabattoni, D.; Gelfi, C.; et al. Endoplasmic Reticulum Associated Aminopeptidase 2 (ERAP2) Is Released in the Secretome of Activated MDMs and Reduces in vitro HIV-1 Infection. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- De Castro, J.A.L.; Stratikos, E. Intracellular antigen processing by ERAP2: Molecular mechanism and roles in health and disease. Hum. Immunol. 2019, 80, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Cifaldi, L.; Romania, P.; Lorenzi, S.; Locatelli, F.; Fruci, D. Role of Endoplasmic Reticulum Aminopeptidases in Health and Disease: From Infection to Cancer. Int. J. Mol. Sci. 2012, 13, 8338–8352. [Google Scholar] [CrossRef] [PubMed]
- Stamogiannos, A.; Koumantou, D.; Papakyriakou, A.; Stratikos, E. Effects of polymorphic variation on the mechanism of Endoplasmic Reticulum Aminopeptidase 1. Mol. Immunol. 2015, 67, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Reeves, E.; Edwards, C.J.; Elliott, T.; James, E. Naturally occurring ERAP1 haplotypes encode functionally distinct alleles with fine substrate specificity. J. Immunol. 2013, 191, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Evnouchidou, I.; Kamal, R.P.; Seregin, S.S.; Goto, Y.; Tsujimoto, M.; Hattori, A.; Voulgari, P.V.; Drosos, A.A.; Amalfitano, A.; York, I.A.; et al. Cutting Edge: Coding Single Nucleotide Polymorphisms of Endoplasmic Reticulum Aminopeptidase 1 Can Affect Antigenic Peptide Generation In Vitro by Influencing Basic Enzymatic Properties of the Enzyme. J. Immunol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Hattori, A.; Ishii, Y.; Tsujimoto, M. Reduced activity of the hypertension-associated Lys528Arg mutant of human adipocyte-derived leucine aminopeptidase (A-LAP)/ER-aminopeptidase-1. FEBS Letters 2006, 580, 1833–1838. [Google Scholar] [CrossRef]
- Martín-Esteban, A.; Gómez-Molina, P.; Sanz-Bravo, A.; de Castro, J.A.L. Combined Effects of Ankylosing Spondylitis-Associated ERAP1 Polymorphisms Outside the Catalytic and Peptide-Binding Sites on the Processing of Natural HLA-B27 Ligands. J. Biol. Chem. 2014, 289, 3978–3990. [Google Scholar]
- Ombrello, M.; Kastner, D.; Remmers, E. Endoplasmic reticulum-Associated amino-Peptidase 1 and rheumatic disease: Genetics. Curr. Opin. Rheumatol. 2015, 27, 349–356. [Google Scholar] [CrossRef]
- Reeves, E.; Colebatch-Bourn, A.; Elliott, T.; Edwards, C.J.; James, E. Functionally distinct ERAP1 allotype combinations distinguish individuals with Ankylosing Spondylitis. Proc. Natl. Acad. Sci. USA 2014, 111, 17594–17599. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, N.; Zhou, Z.; Shi, L. Influence of ERAP1 and ERAP2 gene polymorphisms on disease susceptibility in different populations. Hum. Immunol. 2019, 80, 325–334. [Google Scholar] [CrossRef]
- Evnouchidou, I.; Birtley, J.; Seregin, S.; Papakyriakou, A.; Zervoudi, E.; Samiotaki, M.; Panayotou, G.; Giastas, P.; Petrakis, O.; Georgiadis, D.; et al. A common SNP in ER aminopeptidase 2 induces a specificity switch that leads to altered antigen processing. J. Immunol. 2012, 189, 2383–2392. [Google Scholar] [CrossRef] [PubMed]
- Andrés, A.M.; Dennis, M.Y.; Kretzschmar, W.W.; Cannons, J.L.; Lee-Lin, S.-Q.; Hurle, B.; Schwartzberg, P.L.; Williamson, S.H.; Bustamante, C.D.; Nielsen, R.; et al. Balancing Selection Maintains a Form of ERAP2 that Undergoes Nonsense-Mediated Decay and Affects Antigen Presentation. PLoS Genet. 2010, 6. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.J.; Chen, J.; Villani, A.-C.; Gate, R.E.; Subramaniam, M.; Bhangale, T.; Lee, M.N.; Raj, T.; Raychowdhury, R.; Li, W.; et al. Genetic analysis of isoform usage in the human anti-Viral response reveals influenza-Specific regulation of ERAP2 transcripts under balancing selection. Genome Res. 2018, 28, 1812–1825. [Google Scholar] [CrossRef] [PubMed]
- Vanhille, D.L.; Hill, L.D.; Hilliard, D.D.; Lee, E.D.; Teves, M.E.; Srinivas, S.; Kusanovic, J.P.; Gomez, R.; Stratikos, E.; Elovitz, M.A.; et al. A novel ERAP2 haplotype structure in a Chilean population: Implications for ERAP2 protein expression and preeclampsia risk. Mol. Genet. Genom. Med. 2013, 1, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, J.J.W.; Van Setten, J.; Ripke, S.; Van ‘T Slot, R.; Mulder, F.; Missotten, T.; Baarsma, G.S.; Francioli, L.C.; Pulit, S.L.; De Kovel, C.G.F.; et al. A genome-Wide association study identifies a functional ERAP2 haplotype associated with birdshot chorioretinopathy. Hum. Mol. Genet. 2014, 23, 6081–6087. [Google Scholar] [CrossRef] [PubMed]
- Paladini, F.; Fiorillo, M.T.; Vitulano, C.; Tedeschi, V.; Piga, M.; Cauli, A.; Mathieu, A.; Sorrentino, R. An allelic variant in the intergenic region between ERAP1 and ERAP2 correlates with an inverse expression of the two genes. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Forni, D.; Cagliani, R.; Tresoldi, C.; Pozzoli, U.; Gioia, L.D.; Filippi, G.; Riva, S.; Menozzi, G.; Colleoni, M.; Biasin, M.; et al. An Evolutionary Analysis of Antigen Processing and Presentation across Different Timescales Reveals Pervasive Selection. PLoS Genet. 2014, 10, e1004189. [Google Scholar] [CrossRef][Green Version]
- Mozzi, A.; Pontremoli, C.; Sironi, M. Genetic susceptibility to infectious diseases: Current status and future perspectives from genome-Wide approaches. Infect. Genet. Evol. 2018, 66, 286–307. [Google Scholar] [CrossRef]
- Hansen, T.H.; Bouvier, M. MHC class I antigen presentation: Learning from viral evasion strategies. Nat. Rev. Immunol. 2009, 9, 503–513. [Google Scholar] [CrossRef]
- Niepmann, M. Hepatitis C Virus RNA Translation. In Hepatitis C Virus: From Molecular Virology to Antiviral Therapy; Bartenschlager, R., Ed.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2013; pp. 143–166. ISBN 978-3-642-27340-7. [Google Scholar]
- Lavanchy, D. Evolving epidemiology of hepatitis C virus. Clin. Microbiol. Infect. 2011, 17, 107–115. [Google Scholar] [CrossRef]
- Zarębska-Michaluk, D. Viral hepatitis C treatment shortening–What is the limit? Clin. Exp. Hepatol. 2019, 5, 265–270. [Google Scholar] [CrossRef]
- Paul, D.; Madan, V.; Bartenschlager, R. Hepatitis C Virus RNA Replication and Assembly: Living on the Fat of the Land. Cell Host Microbe 2014, 16, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, V. Hepatitis C Virus RNA Replication. In Hepatitis C Virus: From Molecular Virology to Antiviral Therapy; Bartenschlager, R., Ed.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2013; pp. 167–198. ISBN 978-3-642-27340-7. [Google Scholar]
- York, I.A.; Chang, S.-C.; Saric, T.; Keys, J.A.; Favreau, J.M.; Goldberg, A.L.; Rock, K.L. The ER aminopeptidase ERAP1 enhances or limits antigen presentation by trimming epitopes to 8–9 residues. Nat. Immunol. 2002, 3, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cao, D.; Shen, Y.; Li, Y.; Li, Y.; Shi, L.; Yu, J.; Li, C.; Zhang, X.; Sun, M.; et al. The ERAP gene is associated with HCV chronic infection in a Chinese Han population. Hum. Immunol. 2017, 78, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Kemming, J.; Reeves, E.; Nitschke, K.; Widmeier, V.; Emmerich, F.; Hermle, T.; Gostick, E.; Walker, A.; Timm, J.; Price, D.A.; et al. ERAP1 allotypes shape the epitope repertoire of virus-Specific CD8+ T cell responses in acute hepatitis C virus infection. J. Hepatol. 2019, 70, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Francis, M.E.; King, M.L.; Kelvin, A.A. Back to the Future for Influenza Preimmunity—Looking Back at Influenza Virus History to Infer the Outcome of Future Infections. Viruses 2019, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Akram, A.; Inman, R.D. Immunodominance: A pivotal principle in host response to viral infections. Clin. Immunol. 2012, 143, 99–115. [Google Scholar] [CrossRef]
- Evans, D.M.; Spencer, C.C.A.; Pointon, J.J.; Su, Z.; Harvey, D.; Kochan, G.; Oppermann, U.; Dilthey, A.; Pirinen, M.; Stone, M.A.; et al. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat. Genet. 2011, 43, 761–767. [Google Scholar] [CrossRef]
- Saveanu, L.; Carroll, O.; Hassainya, Y.; Endert, P.V. Complexity, contradictions, and conundrums: Studying post-Proteasomal proteolysis in HLA class I antigen presentation. Immunol. Rev. 2005, 207, 42–59. [Google Scholar] [CrossRef]
- HLA-B27, but Not HLA-B7, Immunodominance to Influenza Is ERAP Dependent|The Journal of Immunology. Available online: https://www-jimmunol-org.pros.lib.unimi.it:2050/content/192/12/5520 (accessed on 11 March 2020).
- Cannon, M.J.; Schmid, D.S.; Hyde, T.B. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med Virol. 2010, 20, 202–213. [Google Scholar] [CrossRef]
- Reeves, M.; Sinclair, J. Aspects of Human Cytomegalovirus Latency and Reactivation. In Human Cytomegalovirus; Shenk, T.E., Stinski, M.F., Eds.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 297–313. ISBN 978-3-540-77349-8. [Google Scholar]
- Britt, W.J. Congenital Human Cytomegalovirus Infection and the Enigma of Maternal Immunity. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Kenneson, A.; Cannon, M.J. Review and meta-Analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med Virol. 2007, 17, 253–276. [Google Scholar] [CrossRef] [PubMed]
- Lanzieri, T.M.; Dollard, S.C.; Bialek, S.R.; Grosse, S.D. Systematic review of the birth prevalence of congenital cytomegalovirus infection in developing countries. Int. J. Infect. Dis. 2014, 22, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, A.P.; van Son, W.J.; Jiwa, N.M.; van der Bij, W.; Schirm, J.; van der Giessen, M.; The, T.H. Recent advances in the diagnosis of active cytomegalovirus infection after organ transplantation. Transplant. Proc. 1990, 22, 226–228. [Google Scholar]
- Modlin, J.F.; Arvin, A.M.; Fast, P.; Myers, M.; Plotkin, S.; Rabinovich, R. Vaccine Development to Prevent Cytomegalovirus Disease: Report from the National Vaccine Advisory Committee. Clin. Infect. Dis. 2004, 39, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Gerna, G.; Kabanova, A.; Lilleri, D. Human Cytomegalovirus Cell Tropism and Host Cell Receptors. Vaccines 2019, 7, 70. [Google Scholar] [CrossRef]
- Gibson, W. Structure and Formation of the Cytomegalovirus Virion. In Human Cytomegalovirus; Shenk, T.E., Stinski, M.F., Eds.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 187–204. ISBN 978-3-540-77349-8. [Google Scholar]
- Yurochko, A.D. Human Cytomegalovirus Modulation of Signal Transduction. In Human Cytomegalovirus; Shenk, T.E., Stinski, M.F., Eds.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 205–220. ISBN 978-3-540-77349-8. [Google Scholar]
- Yu, Y.; Clippinger, A.J.; Alwine, J.C. Viral affects on metabolism: Changes in glucose and glutamine utilization during human cytomegalovirus infection. Trends Microbiol. 2011, 19, 360–367. [Google Scholar] [CrossRef]
- Milbradt, J.; Auerochs, S.; Marschall, M. Cytomegaloviral proteins pUL50 and pUL53 are associated with the nuclear lamina and interact with cellular protein kinase C. J. Gen. Virol. 2007, 88, 2642–2650. [Google Scholar] [CrossRef]
- Hamirally, S.; Kamil, J.P.; Ndassa-Colday, Y.M.; Lin, A.J.; Jahng, W.J.; Baek, M.-C.; Noton, S.; Silva, L.A.; Simpson-Holley, M.; Knipe, D.M.; et al. Viral Mimicry of Cdc2/Cyclin-Dependent Kinase 1 Mediates Disruption of Nuclear Lamina during Human Cytomegalovirus Nuclear Egress. PLoS Pathog. 2009, 5. [Google Scholar] [CrossRef]
- Buchkovich, N.J.; Maguire, T.G.; Alwine, J.C. Role of the Endoplasmic Reticulum Chaperone BiP, SUN Domain Proteins, and Dynein in Altering Nuclear Morphology during Human Cytomegalovirus Infection. J. Virol. 2010, 84, 7005–7017. [Google Scholar] [CrossRef]
- Milbradt, J.; Kraut, A.; Hutterer, C.; Sonntag, E.; Schmeiser, C.; Ferro, M.; Wagner, S.; Lenac, T.; Claus, C.; Pinkert, S.; et al. Proteomic Analysis of the Multimeric Nuclear Egress Complex of Human Cytomegalovirus. Mol. Cell Proteom. 2014, 13, 2132–2146. [Google Scholar] [CrossRef] [PubMed]
- Alwine, J.C. The Human Cytomegalovirus Assembly Compartment: A Masterpiece of Viral Manipulation of Cellular Processes That Facilitates Assembly and Egress. PLoS Pathog. 2012, 8, e1002878. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Pellett, P.E. Spatial Relationships between Markers for Secretory and Endosomal Machinery in Human Cytomegalovirus-Infected Cells versus Those in Uninfected Cells. J. Virol. 2011, 85, 5864–5879. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Vasanji, A.; Pellett, P.E. Three-Dimensional Structure of the Human Cytomegalovirus Cytoplasmic Virion Assembly Complex Includes a Reoriented Secretory Apparatus. J. Virol. 2007, 81, 11861–11869. [Google Scholar] [CrossRef] [PubMed]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of mRNA Translation and Stability by microRNAs. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Shin, J.; Kim, Y.; Evnouchidou, I.; Kim, D.; Kim, Y.-K.; Kim, Y.-E.; Ahn, J.-H.; Riddell, S.R.; et al. Human cytomegalovirus microRNA miR-US4-1 inhibits CD8+ T cell response by targeting the aminopeptidase ERAP1. Nat. Immunol. 2011, 12, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Romania, P.; Cifaldi, L.; Pignoloni, B.; Starc, N.; D′Alicandro, V.; Melaiu, O.; Li Pira, G.; Giorda, E.; Carrozzo, R.; Bergvall, M.; et al. Identification of a Genetic Variation in ERAP1 Aminopeptidase that Prevents Human Cytomegalovirus miR-UL112-5p-Mediated Immunoevasion. Cell Rep. 2017, 20, 846–853. [Google Scholar] [CrossRef]
- Dunne, E.F.; Unger, E.R.; Sternberg, M.; McQuillan, G.; Swan, D.C.; Patel, S.S.; Markowitz, L.E. Prevalence of HPV infection among females in the United States. JAMA 2007, 297, 813–819. [Google Scholar] [CrossRef]
- Chabeda, A.; Yanez, R.J.R.; Lamprecht, R.; Meyers, A.E.; Rybicki, E.P.; Hitzeroth, I.I. Therapeutic vaccines for high-risk HPV-Associated diseases. Papillomavirus Res. 2017, 5, 46–58. [Google Scholar] [CrossRef]
- Moody, C.A.; Laimins, L.A. Human papillomavirus oncoproteins: Pathways to transformation. Nat. Rev. Cancer 2010, 10, 550–560. [Google Scholar] [CrossRef]
- Münger, K.; Baldwin, A.; Edwards, K.M.; Hayakawa, H.; Nguyen, C.L.; Owens, M.; Grace, M.; Huh, K. Mechanisms of human papillomavirus-induced oncogenesis. J. Virol. 2004, 78, 11451–11460. [Google Scholar] [CrossRef] [PubMed]
- Hummel, M.; Hudson, J.B.; Laimins, L.A. Differentiation-Induced and constitutive transcription of human papillomavirus type 31b in cell lines containing viral episomes. J. Virol. 1992, 66, 6070–6080. [Google Scholar] [CrossRef] [PubMed]
- Ozbun, M.A.; Meyers, C. Characterization of late gene transcripts expressed during vegetative replication of human papillomavirus type 31b. J. Virol. 1997, 71, 5161–5172. [Google Scholar] [CrossRef] [PubMed]
- Bedell, M.A.; Hudson, J.B.; Golub, T.R.; Turyk, M.E.; Hosken, M.; Wilbanks, G.D.; Laimins, L.A. Amplification of human papillomavirus genomes in vitro is dependent on epithelial differentiation. J. Virol. 1991, 65, 2254–2260. [Google Scholar] [CrossRef] [PubMed]
- Heemels, M.-T.; Ploegh, H. Generation, Translocation, and Presentation of Mhc Class I-Restricted Peptides. Annu. Rev. Biochem. 1995, 64, 463–491. [Google Scholar] [CrossRef]
- Mehta, A.M.; Jordanova, E.S.; Corver, W.E.; van Wezel, T.; Uh, H.-W.; Kenter, G.G.; Fleuren, G.J. Single nucleotide polymorphisms in antigen processing machinery component ERAP1 significantly associate with clinical outcome in cervical carcinoma. Genes Chromosomes Cancer 2009, 48, 410–418. [Google Scholar] [CrossRef]
- Mehta, A.M.; Jordanova, E.S.; Kenter, G.G.; Ferrone, S.; Fleuren, G.-J. Association of antigen processing machinery and HLA class I defects with clinicopathological outcome in cervical carcinoma. Cancer Immunol. Immunother. 2008, 57, 197–206. [Google Scholar] [CrossRef]
- Hasim, A.; Abudula, M.; Aimiduo, R.; Ma, J.-Q.; Jiao, Z.; Akula, G.; Wang, T.; Abudula, A. Post-Transcriptional and Epigenetic Regulation of Antigen Processing Machinery (APM) Components and HLA-I in Cervical Cancers from Uighur Women. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Mehta, A.M.; Osse, M.; Kolkman-Uljee, S.; Fleuren, G.J.; Jordanova, E.S. Molecular Backgrounds of ERAP1 Downregulation in Cervical Carcinoma. Anal. Cell. Pathol. 2015, 2015, 1–5. [Google Scholar] [CrossRef]
- Mehta, A.M.; Spaans, V.M.; Mahendra, N.B.; Osse, E.M.; Vet, J.N.I.; Purwoto, G.; Surya, I.G.D.; Cornian, S.; Peters, A.A.; Fleuren, G.J.; et al. Differences in genetic variation in antigen-Processing machinery components and association with cervical carcinoma risk in two Indonesian populations. Immunogenetics 2015, 67, 267–275. [Google Scholar] [CrossRef]
- Mehta, A.M.; Jordanova, E.S.; van Wezel, T.; Uh, H.-W.; Corver, W.E.; Kwappenberg, K.M.C.; Verduijn, W.; Kenter, G.G.; van der Burg, S.H.; Fleuren, G.J. Genetic variation of antigen processing machinery components and association with cervical carcinoma. Genes Chromosomes Cancer 2007, 46, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Chow, R.A.; Caeiro, J.L.; Chen, S.-J.; Garcia-Bertrand, R.L.; Herrera, R.J. Genetic characterization of four Austronesian-speaking populations. J. Hum. Genet. 2005, 50, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Shepard, E.M.; Chow, R.A.; Suafo’a, E.; Addison, D.; Pérez-Miranda, A.M.; Garcia-Bertrand, R.L.; Herrera, R.J. Autosomal STR variation in five Austronesian populations. Hum. Biol. 2005, 77, 825–851. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vet, J.N.I.; de Boer, M.A.; van den Akker, B.E.W.M.; Siregar, B.; Lisnawati; Budiningsih, S.; Tyasmorowati, D.; Moestikaningsih; Cornain, S.; Peters, A.A.W.; et al. Prevalence of human papillomavirus in Indonesia: A population-based study in three regions. Br. J. Cancer 2008, 99, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, A.; Winter, J.; Reuschenbach, M.; Blatnik, R.; Klevenz, A.; Bertrand, M.; Hoppe, S.; von Knebel Doeberitz, M.; Grabowska, A.K.; Riemer, A.B. ERAP1 overexpression in HPV-induced malignancies: A possible novel immune evasion mechanism. OncoImmunology 2017, 6, e1336594. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-Based map of the human proteome. Science 2015, 347. [Google Scholar] [CrossRef]
- Keller, M.; Ebstein, F.; Bürger, E.; Textoris-Taube, K.; Gorny, X.; Urban, S.; Zhao, F.; Dannenberg, T.; Sucker, A.; Keller, C.; et al. The proteasome immunosubunits, PA28 and ER-Aminopeptidase 1 protect melanoma cells from efficient MART-126-35-specific T-cell recognition. Eur. J. Immunol. 2015, 45, 3257–3268. [Google Scholar] [CrossRef]
- James, E.; Bailey, I.; Sugiyarto, G.; Elliott, T. Induction of Protective Antitumor Immunity through Attenuation of ERAAP Function. J. Immunol. 2013. [Google Scholar] [CrossRef]
- Zervoudi, E.; Saridakis, E.; Birtley, J.R.; Seregin, S.S.; Reeves, E.; Kokkala, P.; Aldhamen, Y.A.; Amalfitano, A.; Mavridis, I.M.; James, E.; et al. Rationally designed inhibitor targeting antigen-Trimming aminopeptidases enhances antigen presentation and cytotoxic T-Cell responses. Proc. Natl. Acad. Sci. USA 2013, 110, 19890–19895. [Google Scholar] [CrossRef]
- Reeves, E.; Wood, O.; Ottensmeier, C.H.; King, E.V.; Thomas, G.J.; Elliott, T.; James, E. HPV epitope processing differences correlate with ERAP1 allotype and extent of CD8+ T cell tumor infiltration in OPSCC. Cancer Immunol. Res. 2019, 7, 1202–1213. [Google Scholar] [CrossRef]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, Density, and Location of Immune Cells Within Human Colorectal Tumors Predict Clinical Outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef] [PubMed]
- Leffers, N.; Gooden, M.J.M.; de Jong, R.A.; Hoogeboom, B.-N.; ten Hoor, K.A.; Hollema, H.; Boezen, H.M.; van der Zee, A.G.J.; Daemen, T.; Nijman, H.W. Prognostic significance of tumor-Infiltrating T-Lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol. Immunother. 2008, 58, 449. [Google Scholar] [CrossRef] [PubMed]
- Noble, F.; Mellows, T.; McCormick Matthews, L.H.; Bateman, A.C.; Harris, S.; Underwood, T.J.; Byrne, J.P.; Bailey, I.S.; Sharland, D.M.; Kelly, J.J.; et al. Tumour infiltrating lymphocytes correlate with improved survival in patients with oesophageal adenocarcinoma. Cancer Immunol. Immunother. 2016, 65, 651–662. [Google Scholar] [CrossRef]
- Piersma, S.J.; Jordanova, E.S.; van Poelgeest, M.I.E.; Kwappenberg, K.M.C.; van der Hulst, J.M.; Drijfhout, J.W.; Melief, C.J.M.; Kenter, G.G.; Fleuren, G.J.; Offringa, R.; et al. High Number of Intraepithelial CD8+ Tumor-Infiltrating Lymphocytes Is Associated with the Absence of Lymph Node Metastases in Patients with Large Early-Stage Cervical Cancer. Cancer Res. 2007, 67, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Welters, M.J.P.; Ma, W.; Santegoets, S.J.A.M.; Goedemans, R.; Ehsan, I.; Jordanova, E.S.; van Ham, V.J.; van Unen, V.; Koning, F.; van Egmond, S.I.; et al. Intratumoral HPV16-Specific T Cells Constitute a Type I–Oriented Tumor Microenvironment to Improve Survival in HPV16-Driven Oropharyngeal Cancer. Clin. Cancer Res. 2018, 24, 634–647. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, E.; Le Teuff, G.; Marguet, S.; Lantuejoul, S.; Dunant, A.; Graziano, S.; Pirker, R.; Douillard, J.-Y.; Le Chevalier, T.; Filipits, M.; et al. Prognostic Effect of Tumor Lymphocytic Infiltration in Resectable Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 1223–1230. [Google Scholar] [CrossRef]
- Albers, A.; Abe, K.; Hunt, J.; Wang, J.; Lopez-Albaitero, A.; Schaefer, C.; Gooding, W.; Whiteside, T.L.; Ferrone, S.; DeLeo, A.; et al. Antitumor Activity of Human Papillomavirus Type 16 E7–Specific T Cells against Virally Infected Squamous Cell Carcinoma of the Head and Neck. Cancer Res. 2005, 65, 11146–11155. [Google Scholar] [CrossRef]
- van Steenwijk, P.J.D.V.; Heusinkveld, M.; Ramwadhdoebe, T.H.; Löwik, M.J.; van der Hulst, J.M.; Goedemans, R.; Piersma, S.J.; Kenter, G.G.; van der Burg, S.H. An Unexpectedly Large Polyclonal Repertoire of HPV-Specific T Cells Is Poised for Action in Patients with Cervical Cancer. Cancer Res. 2010, 70, 2707–2717. [Google Scholar] [CrossRef]
- Ward, M.J.; Thirdborough, S.M.; Mellows, T.; Riley, C.; Harris, S.; Suchak, K.; Webb, A.; Hampton, C.; Patel, N.N.; Randall, C.J.; et al. Tumour-Infiltrating lymphocytes predict for outcome in HPV-Positive oropharyngeal cancer. Br. J. Cancer 2014, 110, 489–500. [Google Scholar] [CrossRef]
- HIV/AIDS. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed on 2 February 2020).
- Waymack, J.R.; Sundareshan, V. Acquired Immune Deficiency Syndrome (AIDS). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Tenzer, S.; Wee, E.; Burgevin, A.; Stewart-Jones, G.; Friis, L.; Lamberth, K.; Chang, C.; Harndahl, M.; Weimershaus, M.; Gerstoft, J.; et al. Antigen processing influences HIV-Specific cytotoxic T lymphocyte immunodominance. Nat. Immunol. 2009, 10, 636–646. [Google Scholar] [CrossRef]
- Draenert, R.; Le Gall, S.; Pfafferott, K.J.; Leslie, A.J.; Chetty, P.; Brander, C.; Holmes, E.C.; Chang, S.-C.; Feeney, M.E.; Addo, M.M.; et al. Immune Selection for Altered Antigen Processing Leads to Cytotoxic T Lymphocyte Escape in Chronic HIV-1 Infection. J. Exp. Med. 2004, 199, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Cagliani, R.; Riva, S.; Biasin, M.; Fumagalli, M.; Pozzoli, U.; Lo Caputo, S.; Mazzotta, F.; Piacentini, L.; Bresolin, N.; Clerici, M.; et al. Genetic diversity at endoplasmic reticulum aminopeptidases is maintained by balancing selection and is associated with natural resistance to HIV-1 infection. Hum. Mol. Genet. 2010, 19, 4705–4714. [Google Scholar] [CrossRef] [PubMed]
- Biasin, M.; Sironi, M.; Saulle, I.; de Luca, M.; la Rosa, F.; Cagliani, R.; Forni, D.; Agliardi, C.; lo Caputo, S.; Mazzotta, F.; et al. Endoplasmic reticulum aminopeptidase 2 haplotypes play a role in modulating susceptibility to HIV infection. AIDS 2013, 27, 1697–1706. [Google Scholar] [CrossRef]
- Dinter, J.; Gourdain, P.; Lai, N.Y.; Duong, E.; Bracho-Sanchez, E.; Rucevic, M.; Liebesny, P.H.; Xu, Y.; Shimada, M.; Ghebremichael, M.; et al. Different Antigen-Processing Activities in Dendritic Cells, Macrophages, and Monocytes Lead to Uneven Production of HIV Epitopes and Affect CTL Recognition. J. Immunol. 2014, 193, 4322–4334. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fischer, R.; Peng, Y.; Reeves, E.; McHugh, K.; Ternette, N.; Hanke, T.; Dong, T.; Elliott, T.; Shastri, N.; et al. Critical Role of Endoplasmic Reticulum Aminopeptidase 1 in Determining the Length and Sequence of Peptides Bound and Presented by HLA–B27. Arthritis Rheumatol. 2014, 66, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.F.; Bourgeois, S.; Chaponda, M.; Takeshita, L.Y.; Morris, A.P.; Castro, E.M.C.; Alfirevic, A.; Jones, A.R.; Rigden, D.J.; Haldenby, S.; et al. Genome-Wide association study of nevirapine hypersensitivity in a sub-Saharan African HIV-Infected population. J. Antimicrob. Chemother. 2017, 72, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Bergthaler, A.; Graw, F.; Flatz, L.; Bonilla, W.V.; Siegrist, C.-A.; Lambert, P.-H.; Regoes, R.R.; Pinschewer, D.D. Protective Efficacy of Individual CD8+ T Cell Specificities in Chronic Viral Infection. J. Immunol. 2015. [Google Scholar] [CrossRef]
- York, I.A.; Brehm, M.A.; Zendzian, S.; Towne, C.F.; Rock, K.L. Endoplasmic reticulum aminopeptidase 1 (ERAP1) trims MHC class I-presented peptides in vivo and plays an important role in immunodominance. Proc. Natl. Acad. Sci. USA 2006, 103, 9202–9207. [Google Scholar] [CrossRef]
- Firat, E.; Saveanu, L.; Aichele, P.; Staeheli, P.; Huai, J.; Gaedicke, S.; Nil, A.; Besin, G.; Kanzler, B.; van Endert, P.; et al. The Role of Endoplasmic Reticulum-Associated Aminopeptidase 1 in Immunity to Infection and in Cross-Presentation. J. Immunol. 2007, 178, 2241–2248. [Google Scholar] [CrossRef]
- Yan, J.; Parekh, V.V.; Mendez-Fernandez, Y.; Olivares-Villagómez, D.; Dragovic, S.; Hill, T.; Roopenian, D.C.; Joyce, S.; Van Kaer, L. In vivo role of ER-Associated peptidase activity in tailoring peptides for presentation by MHC class Ia and class Ib molecules. J. Exp. Med. 2006, 203, 647–659. [Google Scholar] [CrossRef]
- Tsai, F.-J.; Lee, Y.-C.; Chang, J.-S.; Huang, L.-M.; Huang, F.-Y.; Chiu, N.-C.; Chen, M.-R.; Chi, H.; Lee, Y.-J.; Chang, L.-C.; et al. Identification of Novel Susceptibility Loci for Kawasaki Disease in a Han Chinese Population by a Genome-Wide Association Study. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, N.; Gonzalez, F.; Schaeffer, M.; Joncker, N.T.; Cheng, T.; Shastri, A.J.; Robey, E.A.; Shastri, N. Immunodominant, protective response to the parasite Toxoplasma gondii requires antigen processing in the endoplasmic reticulum. Nat. Immunol. 2008, 9, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.G.; Mui, E.; Cong, H.; Witola, W.; Montpetit, A.; Muench, S.P.; Sidney, J.; Alexander, J.; Sette, A.; Grigg, M.; et al. Identification of T. gondii epitopes, adjuvants, & host genetic factors that influence protection of mice & humans. Vaccine 2010, 28, 3977–3989. [Google Scholar] [PubMed]
- Lorente, E.; Barriga, A.; Johnstone, C.; Mir, C.; Jiménez, M.; López, D. Concerted In Vitro Trimming of Viral HLA-B27-Restricted Ligands by Human ERAP1 and ERAP2 Aminopeptidases. PLoS ONE 2013, 8, e79596. [Google Scholar] [CrossRef] [PubMed]
- Seregin, S.S.; Rastall, D.P.W.; Evnouchidou, I.; Aylsworth, C.F.; Quiroga, D.; Kamal, R.P.; Godbehere-Roosa, S.; Blum, C.F.; York, I.A.; Stratikos, E.; et al. Endoplasmic reticulum aminopeptidase-1 alleles associated with increased risk of Ankylosing Spondylitis reduce HLA-B27 mediated presentation of multiple antigens. Autoimmunity 2013, 46, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Pepelyayeva, Y.; Rastall, D.P.W.; Aldhamen, Y.A.; O′Connell, P.; Raehtz, S.; Alyaqoub, F.S.; Blake, M.K.; Raedy, A.M.; Angarita, A.M.; Abbas, A.M.; et al. ERAP1 deficient mice have reduced Type 1 regulatory T cells and develop skeletal and intestinal features of Ankylosing Spondylitis. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
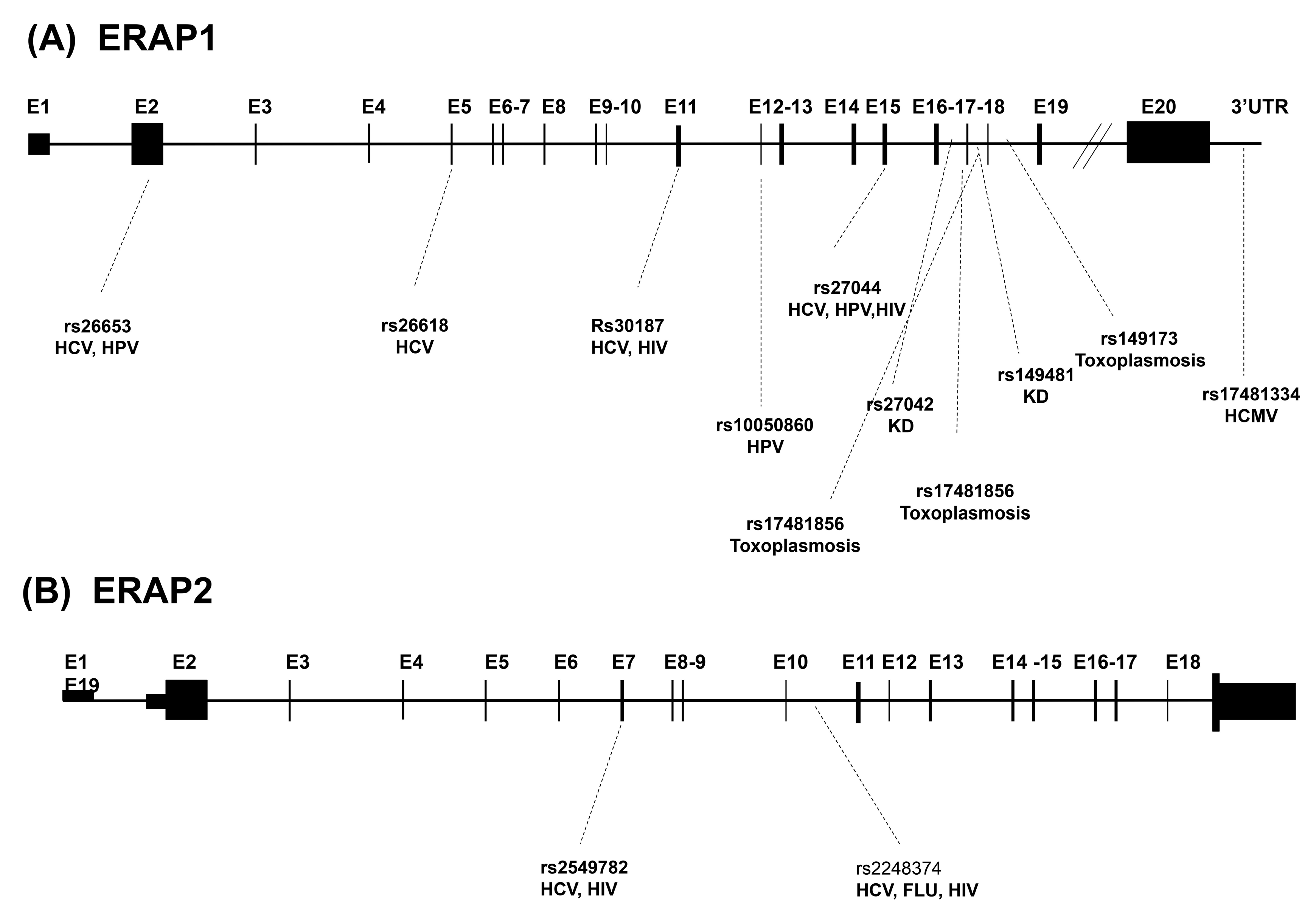
| (A) | |||
| ERAP1 SNPs | Region | Variation | Infectious disease(s) |
| rs30187 | Exon 11 | K528R | HCV, HIV |
| rs27044 | Exon 15 | Q730E | HCV, HIV, HPV |
| rs10050860 | Exon 12 | D575N | HPV |
| rs26618 | Exon 5 | M276I | HCV |
| rs26653 | Exon 2 | P127R | HCV, HPV |
| rs17481856 | Exon 17 | L848L | Toxoplasmosis |
| rs17481334 | 3’ UTR | None | HCMV |
| rs149481 | Intron 17 | None | KD |
| rs27042 | Intron 16 | None | KD |
| rs149173 | Intron 18 | None | Toxoplasmosis |
| rs17481856 | Intron 17 | None | Toxoplasmosis |
| (B) | |||
| ERAP2 SNPs | Region | Variation | Infectious disease(s) |
| rs2549782 | Exon 7 | K392N | HCV, HIV |
| rs2248374 | Intron 10 | HCV, Influenza, HIV | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saulle, I.; Vicentini, C.; Clerici, M.; Biasin, M. An Overview on ERAP Roles in Infectious Diseases. Cells 2020, 9, 720. https://doi.org/10.3390/cells9030720
Saulle I, Vicentini C, Clerici M, Biasin M. An Overview on ERAP Roles in Infectious Diseases. Cells. 2020; 9(3):720. https://doi.org/10.3390/cells9030720
Chicago/Turabian StyleSaulle, Irma, Chiara Vicentini, Mario Clerici, and Mara Biasin. 2020. "An Overview on ERAP Roles in Infectious Diseases" Cells 9, no. 3: 720. https://doi.org/10.3390/cells9030720
APA StyleSaulle, I., Vicentini, C., Clerici, M., & Biasin, M. (2020). An Overview on ERAP Roles in Infectious Diseases. Cells, 9(3), 720. https://doi.org/10.3390/cells9030720







