Stress Erythropoiesis is a Key Inflammatory Response
Abstract
1. Bone Marrow Erythropoiesis Maintains Homeostasis at Steady State
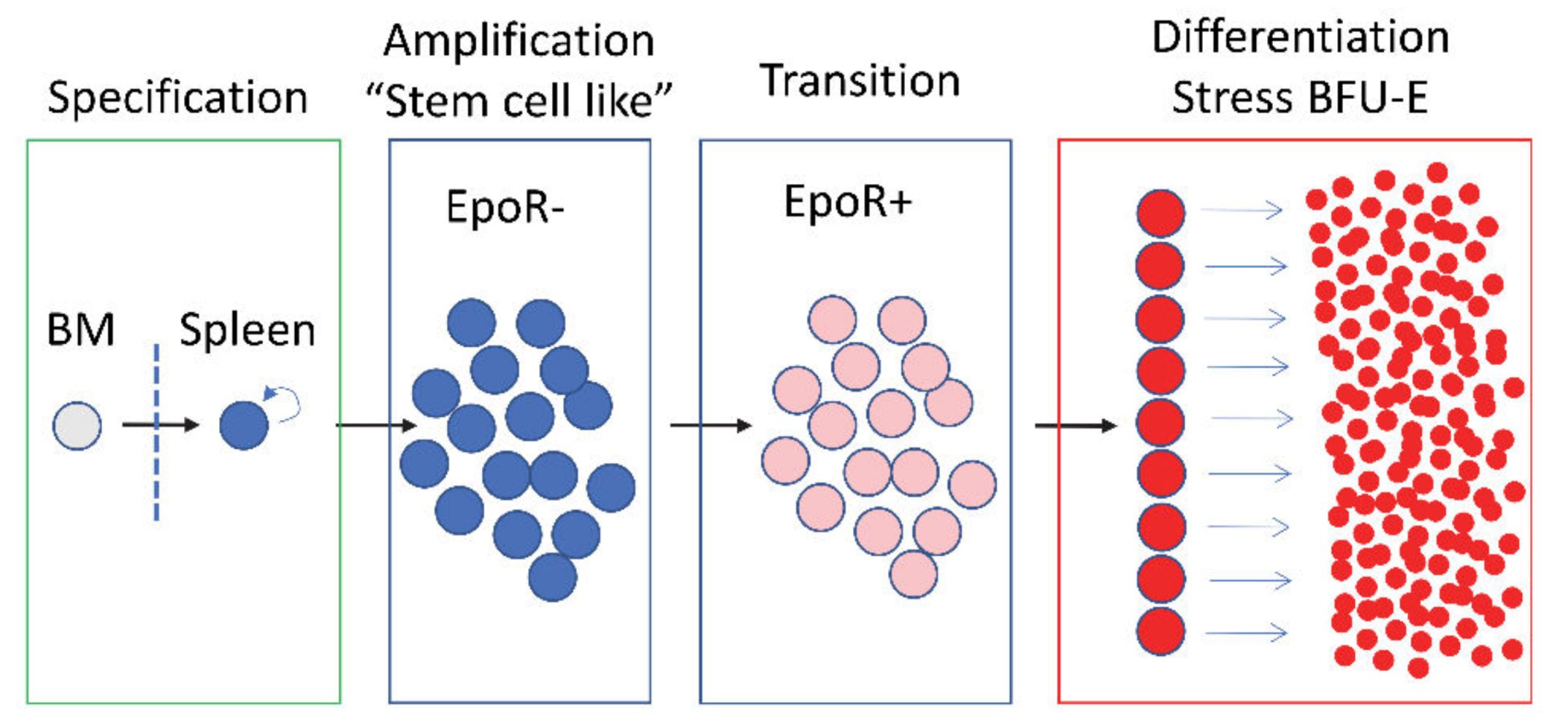
2. Stress Erythropoiesis Maintains Erythroid Homeostasis When Steady State Erythropoiesis is Impaired
3. Inflammation Inhibits Steady State Erythropoiesis
4. Inflammation Induces Stress Erythropoiesis
5. New Model: Stress Erythropoiesis is a Key Component of the Inflammatory Response
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Palis, J. Primitive and Definitive Erythropoiesis in Mammals. Front. Physiol. 2014, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Seu, K.G.; Papoin, J.; Fessler, R.; Hom, J.; Huang, G.; Mohandas, N.; Blanc, L.; Kalfa, T.A. Unraveling Macrophage Heterogeneity in Erythroblastic islands. Front. Immunol. 2017, 8, 1140. [Google Scholar] [CrossRef] [PubMed]
- Klei, T.R.; Meinderts, S.M.; Van Den Berg, T.K.; Van Bruggen, R. From the Cradle to the Grave: The Role of Macrophages in Erythropoiesis and Erythrophagocytosis. Front. Immunol. 2017, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.J.; Mcculloch, E.A.; Till, J.E. Erythropoietic Progenitors Capable of Colony formation in Culture: State of Differentiation. J. Cell. Physiol. 1973, 81, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Liu, J.; Heck, S.; Chasis, J.A.; An, X.; Mohandas, N. Resolving the Distinct Stages in Erythroid Differentiation Based on Dynamic Changes in Membrane Protein Expression during Erythropoiesis. Proc. Natl. Acad. Sci. USA 2009, 106, 17413–17418. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Lee, H.Y.; Da Rocha, E.L.; Zhang, C.; Lu, Y.F.; Li, D.; Feng, Y.; Ezike, J.; Elmes, R.R.; Barrasa, M.I.; et al. Tgf-Beta inhibitors Stimulate Red Blood Cell Production by Enhancing Self-Renewal of Bfu-E Erythroid Progenitors. Blood 2016, 128, 2637–2641. [Google Scholar] [CrossRef]
- Li, H.; Natarajan, A.; Ezike, J.; Barrasa, M.I.; Le, Y.; Feder, Z.A.; Yang, H.; Ma, C.; Markoulaki, S.; Lodish, H.F. Rate of Progression Through A Continuum of Transit-Amplifying Progenitor Cell States Regulates Blood Cell Production. Dev. Cell 2019, 49, 118–129.e7. [Google Scholar] [CrossRef]
- Tusi, B.K.; Wolock, S.L.; Weinreb, C.; Hwang, Y.; Hidalgo, D.; Zilionis, R.; Waisman, A.; Huh, J.R.; Klein, A.M.; Socolovsky, M. Population Snapshots Predict Early Haematopoietic and Erythroid Hierarchies. Nature 2018, 555, 54–60. [Google Scholar] [CrossRef]
- Welch, J.J.; Watts, J.A.; Vakoc, C.R.; Yao, Y.; Wang, H.; Hardison, R.C.; Blobel, G.A.; Chodosh, L.A.; Weiss, M.J. Global Regulation of Erythroid Gene Expression by Transcription Factor Gata-1. Blood 2004, 104, 3136–3147. [Google Scholar] [CrossRef]
- Zhang, J.; Socolovsky, M.; Gross, A.W.; Lodish, H.F. Role of Ras Signaling in Erythroid Differentiation of Mouse Fetal Liver Cells: Functional Analysis by A Flow Cytometry-Based Novel Culture System. Blood 2003, 102, 3938–3946. [Google Scholar] [CrossRef]
- Busch, K.; Klapproth, K.; Barile, M.; Flossdorf, M.; Holland-Letz, T.; Schlenner, S.M.; Reth, M.; Hofer, T.; Rodewald, H.R. Fundamental Properties of Unperturbed Haematopoiesis from Stem Cells in Vivo. Nature 2015, 518, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ramos, A.; Chapman, B.; Johnnidis, J.B.; Le, L.; Ho, Y.J.; Klein, A.; Hofmann, O.; Camargo, F.D. Clonal Dynamics of Native Haematopoiesis. Nature 2014, 514, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Karamitros, D.; Stoilova, B.; Aboukhalil, Z.; Hamey, F.; Reinisch, A.; Samitsch, M.; Quek, L.; Otto, G.; Repapi, E.; Doondeea, J.; et al. Single-Cell Analysis Reveals the Continuum of Human Lympho-Myeloid Progenitor Cells. Nat. Immunol. 2018, 19, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Fraticelli, A.E.; Wolock, S.L.; Weinreb, C.S.; Panero, R.; Patel, S.H.; Jankovic, M.; Sun, J.; Calogero, R.A.; Klein, A.M.; Camargo, F.D. Clonal Analysis of Lineage Fate in Native Haematopoiesis. Nature 2018, 553, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Pronk, C.J.; Rossi, D.J.; Mansson, R.; Attema, J.L.; Norddahl, G.L.; Chan, C.K.; Sigvardsson, M.; Weissman, I.L.; Bryder, D. Elucidation of the Phenotypic, Functional, and Molecular Topography of A Myeloerythroid Progenitor Cell Hierarchy. Cell Stem Cell 2007, 1, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Velten, L.; Haas, S.F.; Raffel, S.; Blaszkiewicz, S.; Islam, S.; Hennig, B.P.; Hirche, C.; Lutz, C.; Buss, E.C.; Nowak, D.; et al. Human Haematopoietic Stem Cell Lineage Commitment is A Continuous Process. Nat. Cell Biol. 2017, 19, 271–281. [Google Scholar] [CrossRef]
- Grover, A.; Mancini, E.; Moore, S.; Mead, A.J.; Atkinson, D.; Rasmussen, K.D.; O’carroll, D.; Jacobsen, S.E.; Nerlov, C. Erythropoietin Guides Multipotent Hematopoietic Progenitor Cells Toward An Erythroid Fate. J. Exp. Med. 2014, 211, 181–188. [Google Scholar] [CrossRef]
- Singh, R.P.; Grinenko, T.; Ramasz, B.; Franke, K.; Lesche, M.; Dahl, A.; Gassmann, M.; Chavakis, T.; Henry, I.; Wielockx, B. Hematopoietic Stem Cells But Not Multipotent Progenitors Drive Erythropoiesis during Chronic Erythroid Stress in Epo Transgenic Mice. Stem Cell Rep. 2018, 10, 1908–1919. [Google Scholar] [CrossRef]
- Oduro, K.A., Jr.; Liu, F.; Tan, Q.; Kim, C.K.; Lubman, O.; Fremont, D.; Mills, J.C.; Choi, K. Myeloid Skewing in Murine Autoimmune Arthritis Occurs in Hematopoietic Stem and Primitive Progenitor Cells. Blood 2012, 120, 2203–2213. [Google Scholar] [CrossRef]
- Boldin, M.P.; Taganov, K.D.; Rao, D.S.; Yang, L.; Zhao, J.L.; Kalwani, M.; Garcia-Flores, Y.; Luong, M.; Devrekanli, A.; Xu, J.; et al. Mir-146a is A Significant Brake On Autoimmunity, Myeloproliferation, and Cancer in Mice. J. Exp. Med. 2011, 208, 1189–1201. [Google Scholar] [CrossRef]
- Zhao, J.L.; Rao, D.S.; Boldin, M.P.; Taganov, K.D.; O’connell, R.M.; Baltimore, D. Nf-Kappab Dysregulation in Microrna-146a-Deficient Mice Drives the Development of Myeloid Malignancies. Proc. Natl. Acad. Sci. USA 2011, 108, 9184–9189. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.L.; Rao, D.S.; O’connell, R.M.; Garcia-Flores, Y.; Baltimore, D. Microrna-146a Acts As A Guardian of the Quality and Longevity of Hematopoietic Stem Cells in Mice. eLife 2013, 2, E00537. [Google Scholar] [CrossRef] [PubMed]
- Pietras, E.M. inflammation: A Key Regulator of Hematopoietic Stem Cell Fate in Health and Disease. Blood 2017, 130, 1693–1698. [Google Scholar] [CrossRef] [PubMed]
- Pietras, E.M.; Mirantes-Barbeito, C.; Fong, S.; Loeffler, D.; Kovtonyuk, L.V.; Zhang, S.; Lakshminarasimhan, R.; Chin, C.P.; Techner, J.M.; Will, B.; et al. Chronic interleukin-1 Exposure Drives Haematopoietic Stem Cells Towards Precocious Myeloid Differentiation At the Expense of Self-Renewal. Nat. Cell Biol. 2016, 18, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Ogawa, M. Erthropoietic Precursors in Mice With Phenylhydrazine-induced Anemia. Am. J. Hematol. 1976, 1, 453–458. [Google Scholar] [CrossRef]
- Hara, H.; Ogawa, M. Erythropoietic Precursors in Mice under Erythropoietic Stimulation and Suppression. Exp. Hematol. 1977, 5, 141–148. [Google Scholar]
- Lenox, L.E.; Perry, J.M.; Paulson, R.F. Bmp4 and Madh5 Regulate the Erythroid Response to Acute Anemia. Blood 2005, 105, 2741–2748. [Google Scholar] [CrossRef]
- Liao, C.; Prabhu, K.S.; Paulson, R.F. Monocyte-Derived Macrophages Expand the Murine Stress Erythropoietic Niche during the Recovery from Anemia. Blood 2018, 132, 2580–2593. [Google Scholar] [CrossRef]
- Paulson, R.F.; Shi, L.; Wu, D.C. Stress Erythropoiesis: New Signals and New Stress Progenitor Cells. Curr. Opin. Hematol. 2011, 18, 139–145. [Google Scholar] [CrossRef]
- Hara, H. Kinetics of Pluripotent Hemopoietic Precursors in Vitro after Erythropoietic Stimulation or Suppression. Exp. Hematol. 1980, 8, 345–350. [Google Scholar]
- Hara, H.; Ogawa, M. Erythropoietic Precursors in Murine Blood. Exp. Hematol. 1977, 5, 161–165. [Google Scholar] [PubMed]
- Cole, R.J.; Regan, T. Haemopoietic Progenitor Cells in Prenatal Congenitally Anaemic ‘Flexed-Tailed’ (F/F) Mice. Br. J. Haematol. 1976, 33, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.J.; Regan, T.; Tarbutt, R.G. Haemoglobin Synthesis in Reticulocytes of Prenatal F-F Anaemic Mice. Br. J. Haematol. 1972, 23, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.J.; Mcculloch, E.A.; Till, J.E. The Cellular Basis for the Defect in Haemopoiesis in Flexed-Tailed Mice. Iii. Restriction of the Defect to Erythropoietic Progenitors Capable of Transient Colony formation in Vivo. Br. J. Haematol. 1975, 30, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Mixter, R.; Hunt, H.R. Anemia in the Flexed Tailed Mouse, Mus Musculus. Genetics 1933, 18, 367–387. [Google Scholar]
- Coleman, D.L.; Russell, E.S.; Levin, E.Y. Enzymatic Studies of the Hemopoietic Defect in Flexed Mice. Genetics 1969, 61, 631–642. [Google Scholar]
- Hegde, S.; Lenox, L.E.; Lariviere, A.; Porayette, P.; Perry, J.M.; Yon, M.; Paulson, R.F. An intronic Sequence Mutated in Flexed-Tail Mice Regulates Splicing of Smad5. Mamm. Genome 2007, 18, 852–860. [Google Scholar] [CrossRef]
- Perry, J.M.; Harandi, O.F.; Paulson, R.F. Bmp4, Scf, and Hypoxia Cooperatively Regulate the Expansion of Murine Stress Erythroid Progenitors. Blood 2007, 109, 4494–4502. [Google Scholar] [CrossRef]
- Perry, J.M.; Harandi, O.F.; Porayette, P.; Hegde, S.; Kannan, A.K.; Paulson, R.F. Maintenance of the Bmp4-Dependent Stress Erythropoiesis Pathway in the Murine Spleen Requires Hedgehog Signaling. Blood 2009, 113, 911–918. [Google Scholar] [CrossRef]
- Xiang, J.; Wu, D.C.; Chen, Y.; Paulson, R.F. In Vitro Culture of Stress Erythroid Progenitors Identifies Distinct Progenitor Populations and Analogous Human Progenitors. Blood 2015, 125, 1803–1812. [Google Scholar] [CrossRef]
- Bennett, L.F.; Liao, C.; Quickel, M.D.; Yeoh, B.S.; Vijay-Kumar, M.; Hankey-Giblin, P.; Prabhu, K.S.; Paulson, R.F. Inflammation induces Stress Erythropoiesis Through Heme-Dependent Activation of Spi-C. Sci. Signal. 2019, 12. [Google Scholar] [CrossRef]
- Harandi, O.F.; Hedge, S.; Wu, D.C.; Mckeone, D.; Paulson, R.F. Murine Erythroid Short-Term Radioprotection Requires A Bmp4-Dependent, Self-Renewing Population of Stress Erythroid Progenitors. J. Clin. Investig. 2010, 120, 4507–4519. [Google Scholar] [CrossRef]
- Liao, C.; Hardison, R.C.; Kennett, M.J.; Carlson, B.A.; Paulson, R.F.; Prabhu, K.S. Selenoproteins Regulate Stress Erythroid Progenitors and Spleen Microenvironment during Stress Erythropoiesis. Blood 2018, 131, 2568–2580. [Google Scholar] [CrossRef]
- Hao, S.; Xiang, J.; Wu, D.C.; Fraser, J.W.; Ruan, B.; Cai, J.; Patterson, A.D.; Lai, Z.C.; Paulson, R.F. Gdf15 Regulates Murine Stress Erythroid Progenitor Proliferation and the Development of the Stress Erythropoiesis Niche. Blood Adv. 2019, 3, 2205–2217. [Google Scholar] [CrossRef]
- Bauer, A.; Tronche, F.; Wessely, O.; Kellendonk, C.; Reichardt, H.M.; Steinlein, P.; Schutz, G.; Beug, H. The Glucocorticoid Receptor is required for Stress Erythropoiesis. Genes Dev. 1999, 13, 2996–3002. [Google Scholar] [CrossRef]
- Obinata, M.; Yanai, N. Cellular and Molecular Regulation of an Erythropoietic inductive Microenvironment (Eim). Cell Struct. Funct. 1999, 24, 171–179. [Google Scholar] [CrossRef][Green Version]
- Porayette, P.; Paulson, R.F. Bmp4/Smad5 Dependent Stress Erythropoiesis is required for the Expansion of Erythroid Progenitors during Fetal Development. Dev. Biol. 2008, 317, 24–35. [Google Scholar] [CrossRef]
- Ganguli, G.; Back, J.; Sengupta, S.; Wasylyk, B. The P53 Tumour Suppressor inhibits Glucocorticoid-induced Proliferation of Erythroid Progenitors. EMBO Rep. 2002, 3, 569–574. [Google Scholar] [CrossRef]
- Kolbus, A.; Blazquez-Domingo, M.; Carotta, S.; Bakker, W.; Luedemann, S.; Von Lindern, M.; Steinlein, P.; Beug, H. Cooperative Signaling Between Cytokine Receptors and the Glucocorticoid Receptor in the Expansion of Erythroid Progenitors: Molecular Analysis by Expression Profiling. Blood 2003, 102, 3136–3146. [Google Scholar] [CrossRef]
- Lee, H.Y.; Gao, X.; Barrasa, M.I.; Li, H.; Elmes, R.R.; Peters, L.L.; Lodish, H.F. Ppar-Alpha and Glucocorticoid Receptor Synergize to Promote Erythroid Progenitor Self-Renewal. Nature 2015, 522, 474–477. [Google Scholar] [CrossRef]
- Varricchio, L.; Tirelli, V.; Masselli, E.; Ghinassi, B.; Saha, N.; Besmer, P.; Migliaccio, A.R. The Expression of the Glucocorticoid Receptor in Human Erythroblasts is Uniquely Regulated by Kit Ligand: Implications for Stress Erythropoiesis. Stem Cells Dev. 2012, 21, 2852–2865. [Google Scholar] [CrossRef]
- Von Lindern, M.; Zauner, W.; Mellitzer, G.; Steinlein, P.; Fritsch, G.; Huber, K.; Lowenberg, B.; Beug, H. The Glucocorticoid Receptor Cooperates With the Erythropoietin Receptor and C-Kit to Enhance and Sustain Proliferation of Erythroid Progenitors in Vitro. Blood 1999, 94, 550–559. [Google Scholar] [CrossRef]
- Wessely, O.; Deiner, E.M.; Beug, H.; Von Lindern, M. The Glucocorticoid Receptor is a Key Regulator of the Decision between Self-Renewal and Differentiation in Erythroid Progenitors. EMBO J. 1997, 16, 267–280. [Google Scholar] [CrossRef]
- Zhang, L.; Prak, L.; Rayon-Estrada, V.; Thiru, P.; Flygare, J.; Lim, B.; Lodish, H.F. Zfp36l2 is required for Self-Renewal of Early Burst-forming Unit Erythroid Progenitors. Nature 2013, 499, 92–96. [Google Scholar] [CrossRef]
- Flygare, J.; Rayon Estrada, V.; Shin, C.; Gupta, S.; Lodish, H.F. Hif1alpha Synergizes With Glucocorticoids to Promote Bfu-E Progenitor Self-Renewal. Blood 2011, 117, 3435–3444. [Google Scholar] [CrossRef]
- Diepstraten, S.T.; Hart, A.H. Modelling Human Haemoglobin Switching. Blood Rev. 2019, 33, 11–23. [Google Scholar] [CrossRef]
- Vinjamur, D.S.; Bauer, D.E.; Orkin, S.H. Recent Progress in Understanding and Manipulating Haemoglobin Switching for the Haemoglobinopathies. Br. J. Haematol. 2018, 180, 630–643. [Google Scholar] [CrossRef]
- Alter, B.P.; Rappeport, J.M.; Huisman, T.H.; Schroeder, W.A.; Nathan, D.G. Fetal Erythropoiesis Following Bone Marrow Transplantation. Blood 1976, 48, 843–853. [Google Scholar] [CrossRef]
- Alter, B.P.; Rosenberg, P.S.; Day, T.; Menzel, S.; Giri, N.; Savage, S.A.; Thein, S.L. Genetic Regulation of Fetal Haemoglobin in inherited Bone Marrow Failure Syndromes. Br. J. Haematol. 2013, 162, 542–546. [Google Scholar] [CrossRef]
- Weinberg, R.S.; Schofield, J.M.; Lenes, A.L.; Brochstein, J.; Alter, B.P. Adult ‘Fetal-Like’ Erythropoiesis Characterizes Recovery from Bone Marrow Transplantation. Br. J. Haematol. 1986, 63, 415–424. [Google Scholar] [CrossRef]
- Liu, N.; Hargreaves, V.V.; Zhu, Q.; Kurland, J.V.; Hong, J.; Kim, W.; Sher, F.; Macias-Trevino, C.; Rogers, J.M.; Kurita, R.; et al. Direct Promoter Repression by Bcl11a Controls the Fetal to Adult Hemoglobin Switch. Cell 2018, 173, 430–442.e17. [Google Scholar] [CrossRef]
- Menzel, S.; Garner, C.; Gut, I.; Matsuda, F.; Yamaguchi, M.; Heath, S.; Foglio, M.; Zelenika, D.; Boland, A.; Rooks, H.; et al. A Qtl influencing F Cell Production Maps to a Gene Encoding a Zinc-Finger Protein on Chromosome 2p15. Nat. Genet. 2007, 39, 1197–1199. [Google Scholar] [CrossRef]
- Sankaran, V.G.; Menne, T.F.; Xu, J.; Akie, T.E.; Lettre, G.; Van Handel, B.; Mikkola, H.K.; Hirschhorn, J.N.; Cantor, A.B.; Orkin, S.H. Human Fetal Hemoglobin Expression is Regulated by the Developmental Stage-Specific Repressor Bcl11a. Science 2008, 322, 1839–1842. [Google Scholar] [CrossRef]
- Weiss, G.; Ganz, T.; Goodnough, L.T. Anemia of inflammation. Blood 2019, 133, 40–50. [Google Scholar] [CrossRef]
- Sankaran, V.G.; Weiss, M.J. Anemia: Progress in Molecular Mechanisms and therapies. Nat. Med. 2015, 21, 221–230. [Google Scholar] [CrossRef]
- Prakash, D. Anemia in the Icu: Anemia of Chronic Disease Versus Anemia of Acute Illness. Crit. Care Clin. 2012, 28, 333–343. [Google Scholar] [CrossRef]
- Molica, S.; Mirabelli, R.; Molica, M.; Levato, L.; Mauro, F.R.; Foa, R. Clinical Relevance and Treatment of Nonautoimmune Anemia in Chronic Lymphocytic Leukemia. Cancer Manag. Res. 2011, 3, 211–217. [Google Scholar] [CrossRef]
- Jurado, R.L. Iron, infections, and Anemia of inflammation. Clin. Infect. Dis. 1997, 25, 888–895. [Google Scholar] [CrossRef]
- Cassat, J.E.; Skaar, E.P. Iron in infection and Immunity. Cell Host Microbe 2013, 13, 509–519. [Google Scholar] [CrossRef]
- Soares, M.P.; Weiss, G. The Iron Age of Host-Microbe interactions. EMBO Rep. 2015, 16, 1482–1500. [Google Scholar] [CrossRef]
- Libregts, S.F.; Gutierrez, L.; De Bruin, A.M.; Wensveen, F.M.; Papadopoulos, P.; Van Ijcken, W.; Ozgur, Z.; Philipsen, S.; Nolte, M.A. Chronic Ifn-Gamma Production in Mice induces Anemia by Reducing Erythrocyte Life Span and inhibiting Erythropoiesis Through An Irf-1/Pu.1 Axis. Blood 2011, 118, 2578–2588. [Google Scholar] [CrossRef]
- Papadaki, H.A.; Kritikos, H.D.; Valatas, V.; Boumpas, D.T.; Eliopoulos, G.D. Anemia of Chronic Disease in Rheumatoid Arthritis is associated With increased Apoptosis of Bone Marrow Erythroid Cells: Improvement Following Anti-Tumor Necrosis Factor-Alpha Antibody therapy. Blood 2002, 100, 474–482. [Google Scholar] [CrossRef]
- Rusten, L.S.; Jacobsen, S.E. Tumor Necrosis Factor (Tnf)-Alpha directly inhibits Human Erythropoiesis in Vitro: Role of P55 and P75 Tnf Receptors. Blood 1995, 85, 989–996. [Google Scholar] [CrossRef]
- Tsopra, O.A.; Ziros, P.G.; Lagadinou, E.D.; Symeonidis, A.; Kouraklis-Symeonidis, A.; Thanopoulou, E.; Angelopoulou, M.K.; Vassilakopoulos, T.P.; Pangalis, G.A.; Zoumbos, N.C. Disease-Related Anemia in Chronic Lymphocytic Leukemia is not due to intrinsic Defects of Erythroid Precursors: A Possible Pathogenetic Role for Tumor Necrosis Factor-Alpha. Acta Haematol. 2009, 121, 187–195. [Google Scholar] [CrossRef]
- Xiao, W.; Koizumi, K.; Nishio, M.; Endo, T.; Osawa, M.; Fujimoto, K.; Sato, I.; Sakai, T.; Koike, T.; Sawada, K. Tumor Necrosis Factor-Alpha inhibits Generation of Glycophorin A+ Cells by Cd34+ Cells. Exp. Hematol. 2002, 30, 1238–1247. [Google Scholar] [CrossRef]
- Zamai, L.; Secchiero, P.; Pierpaoli, S.; Bassini, A.; Papa, S.; Alnemri, E.S.; Guidotti, L.; Vitale, M.; Zauli, G. Tnf-Related Apoptosis-inducing Ligand (Trail) as a Negative Regulator of Normal Human Erythropoiesis. Blood 2000, 95, 3716–3724. [Google Scholar]
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A Red Carpet for Iron Metabolism. Cell 2017, 168, 344–361. [Google Scholar] [CrossRef]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. Il-6 Mediates Hypoferremia of inflammation by inducing the Synthesis of the Iron Regulatory Hormone Hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef]
- Aschemeyer, S.; Qiao, B.; Stefanova, D.; Valore, E.V.; Sek, A.C.; Ruwe, T.A.; Vieth, K.R.; Jung, G.; Casu, C.; Rivella, S.; et al. Structure-Function Analysis of Ferroportin Defines the Binding Site and An Alternative Mechanism of Action of Hepcidin. Blood 2018, 131, 899–910. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Hepcidin and Iron Homeostasis. Biochim. Biophys. Acta 2012, 1823, 1434–1443. [Google Scholar] [CrossRef]
- Guida, C.; Altamura, S.; Klein, F.A.; Galy, B.; Boutros, M.; Ulmer, A.J.; Hentze, M.W.; Muckenthaler, M.U. A Novel inflammatory Pathway Mediating Rapid Hepcidin-independent Hypoferremia. Blood 2015, 125, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Ludwiczek, S.; Aigner, E.; Theurl, I.; Weiss, G. Cytokine-Mediated Regulation of Iron Transport in Human Monocytic Cells. Blood 2003, 101, 4148–4154. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin Regulates Cellular Iron Efflux by Binding to Ferroportin and inducing Its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef]
- Theurl, I.; Aigner, E.; Theurl, M.; Nairz, M.; Seifert, M.; Schroll, A.; Sonnweber, T.; Eberwein, L.; Witcher, D.R.; Murphy, A.T.; et al. Regulation of Iron Homeostasis in Anemia of Chronic Disease and Iron Deficiency Anemia: Diagnostic and therapeutic Implications. Blood 2009, 113, 5277–5286. [Google Scholar] [CrossRef] [PubMed]
- Morceau, F.; Dicato, M.; Diederich, M. Pro-inflammatory Cytokine-Mediated Anemia: Regarding Molecular Mechanisms of Erythropoiesis. Mediat. Inflamm. 2009, 2009, 405016. [Google Scholar] [CrossRef]
- Lin, F.C.; Karwan, M.; Saleh, B.; Hodge, D.L.; Chan, T.; Boelte, K.C.; Keller, J.R.; Young, H.A. Ifn-Gamma Causes Aplastic Anemia by Altering Hematopoietic Stem/Progenitor Cell Composition and Disrupting Lineage Differentiation. Blood 2014, 124, 3699–3708. [Google Scholar] [CrossRef]
- Zhao, J.L.; Ma, C.; O’connell, R.M.; Mehta, A.; Diloreto, R.; Heath, J.R.; Baltimore, D. Conversion of Danger Signals into Cytokine Signals by Hematopoietic Stem and Progenitor Cells for Regulation of Stress-induced Hematopoiesis. Cell Stem Cell 2014, 14, 445–459. [Google Scholar] [CrossRef]
- Chavakis, T.; Mitroulis, I.; Hajishengallis, G. Hematopoietic Progenitor Cells as integrative Hubs for Adaptation to and Fine-Tuning of inflammation. Nat. Immunol. 2019, 20, 802–811. [Google Scholar] [CrossRef]
- Megias, J.; Yanez, A.; Moriano, S.; O’connor, J.E.; Gozalbo, D.; Gil, M.L. Direct Toll-Like Receptor-Mediated Stimulation of Hematopoietic Stem and Progenitor Cells Occurs in Vivo and Promotes Differentiation Toward Macrophages. Stem Cells 2012, 30, 1486–1495. [Google Scholar] [CrossRef]
- Nagai, Y.; Garrett, K.P.; Ohta, S.; Bahrun, U.; Kouro, T.; Akira, S.; Takatsu, K.; Kincade, P.W. Toll-Like Receptors On Hematopoietic Progenitor Cells Stimulate innate Immune System Replenishment. Immunity 2006, 24, 801–812. [Google Scholar] [CrossRef]
- La Ferla, K.; Reimann, C.; Jelkmann, W.; Hellwig-Burgel, T. inhibition of Erythropoietin Gene Expression Signaling involves the Transcription Factors Gata-2 and Nf-Kappab. FASEB J. 2002, 16, 1811–1813. [Google Scholar] [CrossRef]
- Bian, Z.; Shi, L.; Guo, Y.L.; Lv, Z.; Tang, C.; Niu, S.; Tremblay, A.; Venkataramani, M.; Culpepper, C.; Li, L.; et al. Cd47-Sirpalpha interaction and Il-10 Constrain inflammation-induced Macrophage Phagocytosis of Healthy Self-Cells. Proc. Natl. Acad. Sci. USA 2016, 113, E5434–E5443. [Google Scholar] [CrossRef] [PubMed]
- Akilesh, H.M.; Buechler, M.B.; Duggan, J.M.; Hahn, W.O.; Matta, B.; Sun, X.; Gessay, G.; Whalen, E.; Mason, M.; Presnell, S.R.; et al. Chronic Tlr7 and Tlr9 Signaling Drives Anemia Via Differentiation of Specialized Hemophagocytes. Science 2019, 363. [Google Scholar] [CrossRef] [PubMed]
- Pathak, V.A.; Ghosh, K. Erythropoiesis in Malaria infections and Factors Modifying the Erythropoietic Response. Anemia 2016, 2016, 9310905. [Google Scholar] [CrossRef] [PubMed]
- White, N.J. Anaemia and Malaria. Malar. J. 2018, 17, 371. [Google Scholar] [CrossRef]
- Gardenghi, S.; Renaud, T.M.; Meloni, A.; Casu, C.; Crielaard, B.J.; Bystrom, L.M.; Greenberg-Kushnir, N.; Sasu, B.J.; Cooke, K.S.; Rivella, S. Distinct Roles for Hepcidin and interleukin-6 in the Recovery from Anemia in Mice injected With Heat-Killed Brucella Abortus. Blood 2014, 123, 1137–1145. [Google Scholar] [CrossRef]
- Kim, A.; Fung, E.; Parikh, S.G.; Valore, E.V.; Gabayan, V.; Nemeth, E.; Ganz, T. A Mouse Model of Anemia of inflammation: Complex Pathogenesis with Partial Dependence on Hepcidin. Blood 2014, 123, 1129–1136. [Google Scholar] [CrossRef]
- Millot, S.; Andrieu, V.; Letteron, P.; Lyoumi, S.; Hurtado-Nedelec, M.; Karim, Z.; Thibaudeau, O.; Bennada, S.; Charrier, J.L.; Lasocki, S.; et al. Erythropoietin Stimulates Spleen Bmp4-Dependent Stress Erythropoiesis and Partially Corrects Anemia in A Mouse Model of Generalized inflammation. Blood 2010, 116, 6072–6081. [Google Scholar] [CrossRef]
- Brown, D.E.; Nick, H.J.; Mccoy, M.W.; Moreland, S.M.; Stepanek, A.M.; Benik, R.; O’connell, K.E.; Pilonieta, M.C.; Nagy, T.A.; Detweiler, C.S. increased Ferroportin-1 Expression and Rapid Splenic Iron Loss Occur With Anemia Caused by Salmonella Enterica Serovar Typhimurium infection in Mice. Infect. Immun. 2015, 83, 2290–2299. [Google Scholar] [CrossRef]
- Jackson, A.; Nanton, M.R.; O’donnell, H.; Akue, A.D.; Mcsorley, S.J. Innate Immune Activation during Salmonella infection initiates Extramedullary Erythropoiesis and Splenomegaly. J. Immunol. 2010, 185, 6198–6204. [Google Scholar] [CrossRef]
- Li, L.X.; Benoun, J.M.; Weiskopf, K.; Garcia, K.C.; Mcsorley, S.J. Salmonella infection Enhances Erythropoietin Production by the Kidney and Liver, Which Correlates With Elevated Bacterial Burdens. Infect. Immun. 2016, 84, 2833–2841. [Google Scholar] [CrossRef] [PubMed]
- Sasu, B.J.; Cooke, K.S.; Arvedson, T.L.; Plewa, C.; Ellison, A.R.; Sheng, J.; Winters, A.; Juan, T.; Li, H.; Begley, C.G.; et al. Antihepcidin Antibody Treatment Modulates Iron Metabolism and is Effective in A Mouse Model of inflammation-induced Anemia. Blood 2010, 115, 3616–3624. [Google Scholar] [CrossRef] [PubMed]
- Haldar, M.; Kohyama, M.; So, A.Y.; Kc, W.; Wu, X.; Briseno, C.G.; Satpathy, A.T.; Kretzer, N.M.; Arase, H.; Rajasekaran, N.S.; et al. Heme-Mediated Spi-C induction Promotes Monocyte Differentiation into Iron-Recycling Macrophages. Cell 2014, 156, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Arezes, J.; Foy, N.; Mchugh, K.; Sawant, A.; Quinkert, D.; Terraube, V.; Brinth, A.; Tam, M.; Lavallie, E.R.; Taylor, S.; et al. Erythroferrone inhibits the induction of Hepcidin by Bmp6. Blood 2018, 132, 1473–1477. [Google Scholar] [CrossRef] [PubMed]
- Kautz, L.; Jung, G.; Nemeth, E.; Ganz, T. Erythroferrone Contributes to Recovery from Anemia of inflammation. Blood 2014, 124, 2569–2574. [Google Scholar] [CrossRef]
- Kautz, L.; Jung, G.; Valore, E.V.; Rivella, S.; Nemeth, E.; Ganz, T. Identification of Erythroferrone as an Erythroid Regulator of Iron Metabolism. Nat. Genet. 2014, 46, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Heideveld, E.; Masiello, F.; Marra, M.; Esteghamat, F.; Yagci, N.; Von Lindern, M.; Migliaccio, A.R.; Van Den Akker, E. Cd14+ Cells from Peripheral Blood Positively Regulate Hematopoietic Stem and Progenitor Cell Survival Resulting in increased Erythroid Yield. Haematologica 2015, 100, 1396–1406. [Google Scholar] [CrossRef]
- Heideveld, E.; Hampton-O’neil, L.A.; Cross, S.J.; Van Alphen, F.P.J.; Van Den Biggelaar, M.; Toye, A.M.; Van Den Akker, E. Glucocorticoids induce Differentiation of Monocytes Towards Macrophages That Share Functional and Phenotypical Aspects With Erythroblastic island Macrophages. Haematologica 2018, 103, 395–405. [Google Scholar] [CrossRef]
- Falchi, M.; Varricchio, L.; Martelli, F.; Masiello, F.; Federici, G.; Zingariello, M.; Girelli, G.; Whitsett, C.; Petricoin, E.F., 3rd; Moestrup, S.K.; et al. Dexamethasone Targeted Directly to Macrophages induces Macrophage Niches That Promote Erythroid Expansion. Haematologica 2015, 100, 178–187. [Google Scholar] [CrossRef]
- Paulson, R.F. Epo Receptor Marks the Spot. Blood 2019, 134, 413–414. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Zhao, H.; Zhang, H.; Xu, Y.; Wang, S.; Guo, X.; Huang, Y.; Zhang, S.; Han, Y.; et al. Identification and Transcriptome Analysis of Erythroblastic island Macrophages. Blood 2019, 134, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Nairz, M.; Schroll, A.; Moschen, A.R.; Sonnweber, T.; Theurl, M.; Theurl, I.; Taub, N.; Jamnig, C.; Neurauter, D.; Huber, L.A.; et al. Erythropoietin Contrastingly Affects Bacterial infection and Experimental Colitis by inhibiting Nuclear Factor-Kappab-inducible Immune Pathways. Immunity 2011, 34, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hayashi, Y.; Yokota, A.; Xu, Z.; Zhang, Y.; Huang, R.; Yan, X.; Liu, H.; Ma, L.; Azam, M.; et al. Expansion of Epor-Negative Macrophages besides Erythroblasts by Elevated Epor Signaling in Erythrocytosis Mouse Models. Haematologica 2018, 103, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.; Huggins, M.; Ahmed, J.; Hashimoto, D.; Lucas, D.; Kunisaki, Y.; Pinho, S.; Leboeuf, M.; Noizat, C.; Van Rooijen, N.; et al. Cd169(+) Macrophages Provide A Niche Promoting Erythropoiesis Under Homeostasis and Stress. Nat. Med. 2013, 19, 429–436. [Google Scholar] [CrossRef]
- Ramos, P.; Casu, C.; Gardenghi, S.; Breda, L.; Crielaard, B.J.; Guy, E.; Marongiu, M.F.; Gupta, R.; Levine, R.L.; Abdel-Wahab, O.; et al. Macrophages Support Pathological Erythropoiesis in Polycythemia Vera and Beta-Thalassemia. Nat. Med. 2013, 19, 437–445. [Google Scholar] [CrossRef]
- Escoter-Torres, L.; Caratti, G.; Mechtidou, A.; Tuckermann, J.; Uhlenhaut, N.H.; Vettorazzi, S. Fighting the Fire: Mechanisms of inflammatory Gene Regulation by the Glucocorticoid Receptor. Front. Immunol. 2019, 10, 1859. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulson, R.F.; Ruan, B.; Hao, S.; Chen, Y. Stress Erythropoiesis is a Key Inflammatory Response. Cells 2020, 9, 634. https://doi.org/10.3390/cells9030634
Paulson RF, Ruan B, Hao S, Chen Y. Stress Erythropoiesis is a Key Inflammatory Response. Cells. 2020; 9(3):634. https://doi.org/10.3390/cells9030634
Chicago/Turabian StylePaulson, Robert F., Baiye Ruan, Siyang Hao, and Yuanting Chen. 2020. "Stress Erythropoiesis is a Key Inflammatory Response" Cells 9, no. 3: 634. https://doi.org/10.3390/cells9030634
APA StylePaulson, R. F., Ruan, B., Hao, S., & Chen, Y. (2020). Stress Erythropoiesis is a Key Inflammatory Response. Cells, 9(3), 634. https://doi.org/10.3390/cells9030634



