BMP-9 Modulates the Hepatic Responses to LPS
Abstract
1. Introduction
2. Materials and Methods
2.1. Purification and Culture of Monocytes from Human Blood
2.2. Cell Culture (LSEC, MF and J774A.1 Cells)
2.3. Affymetrix Arrays
2.4. RT-qPCR
2.5. Primary Antibodies
2.6. Preparation of Protein Lysates and Western Blot Immunoblotting
2.7. Mouse Experiments
2.8. Immunohistochemistry
2.9. ELISA Measurements
2.10. Statistical Analysis
3. Results
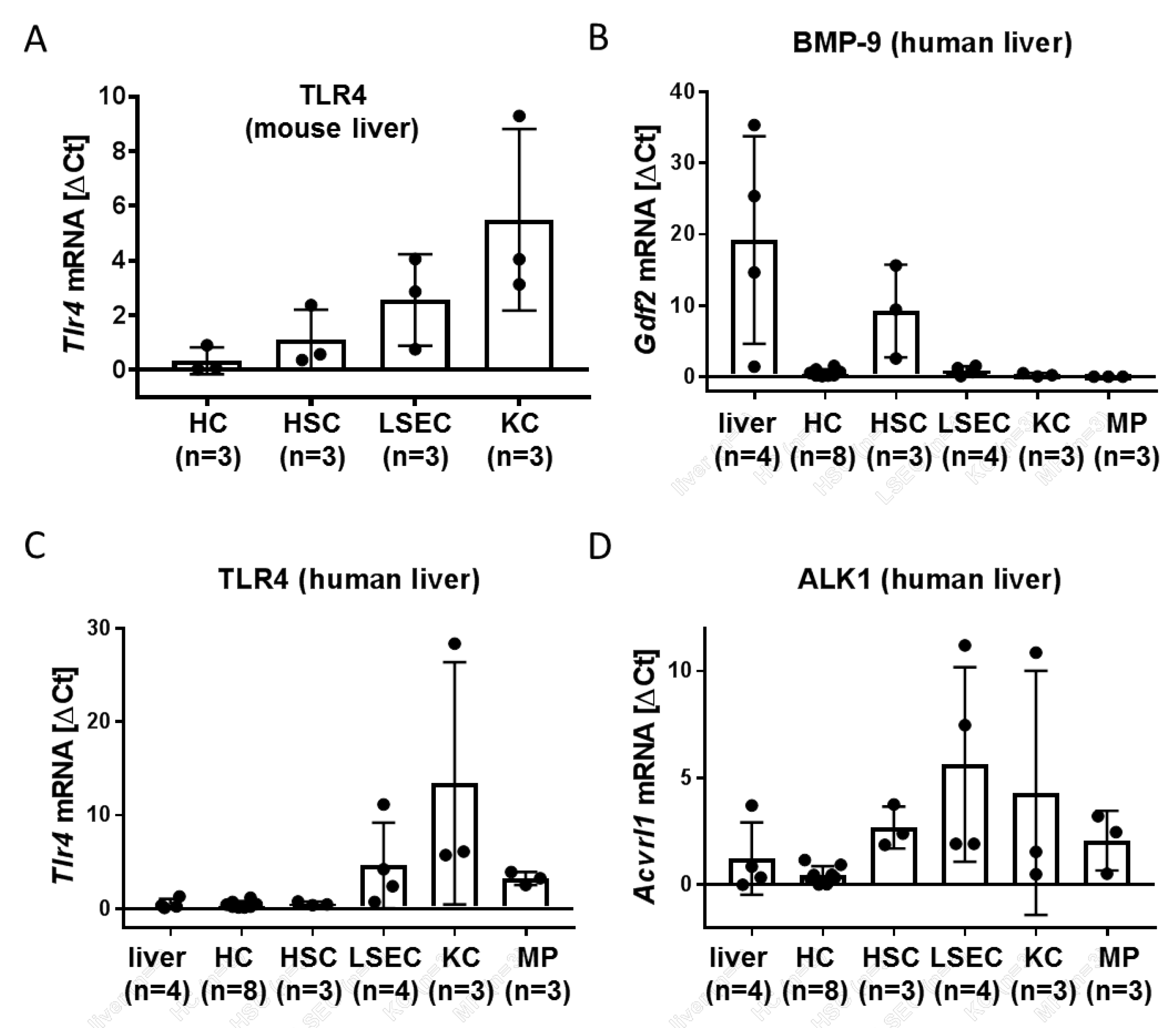
3.1. LSEC and Kupffer Cells (KC) Express Alk1 and TLR4
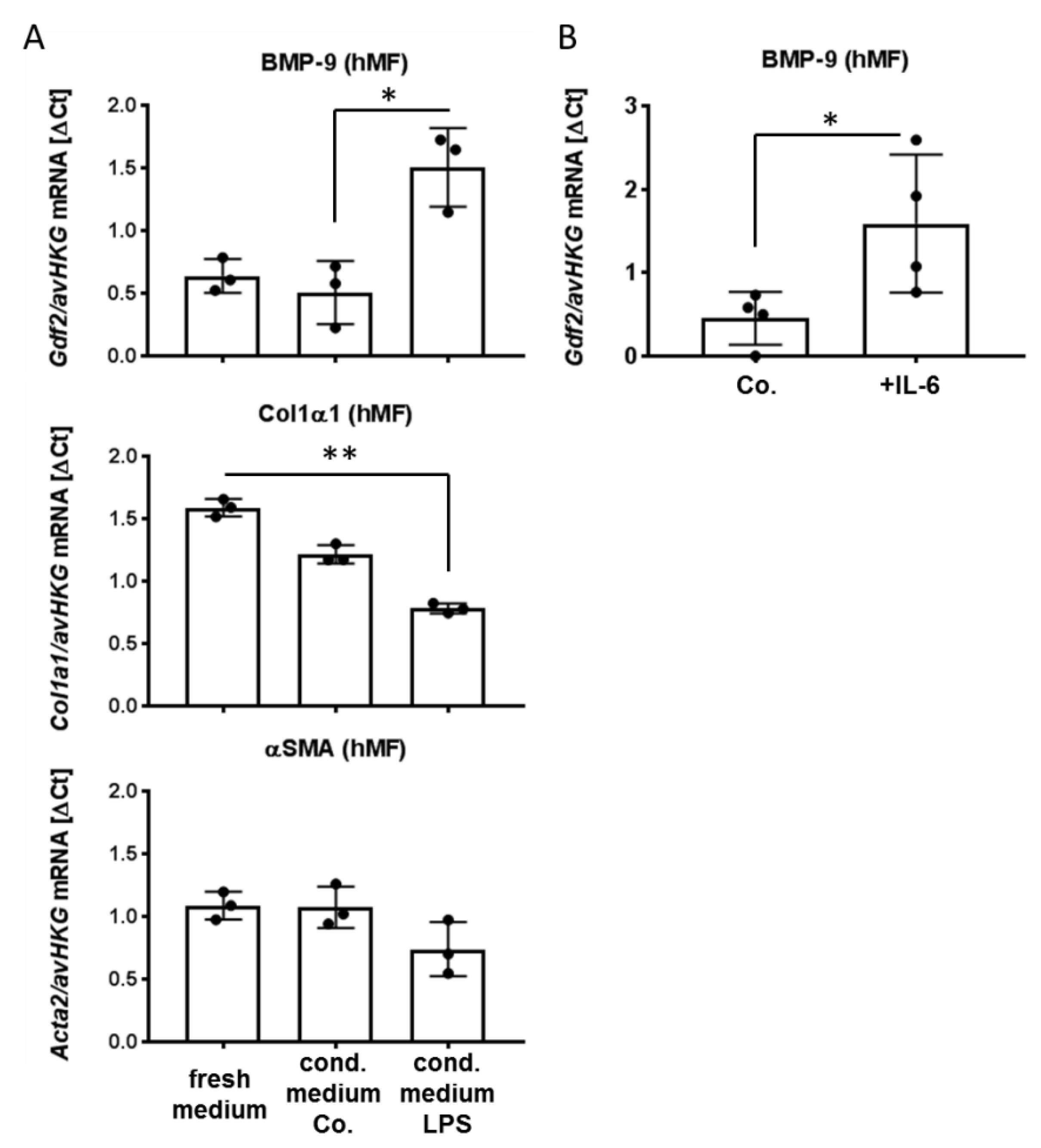
3.2. Factors Secreted from LSEC, Including IL-6, Induce BMP-9 Expression in MF
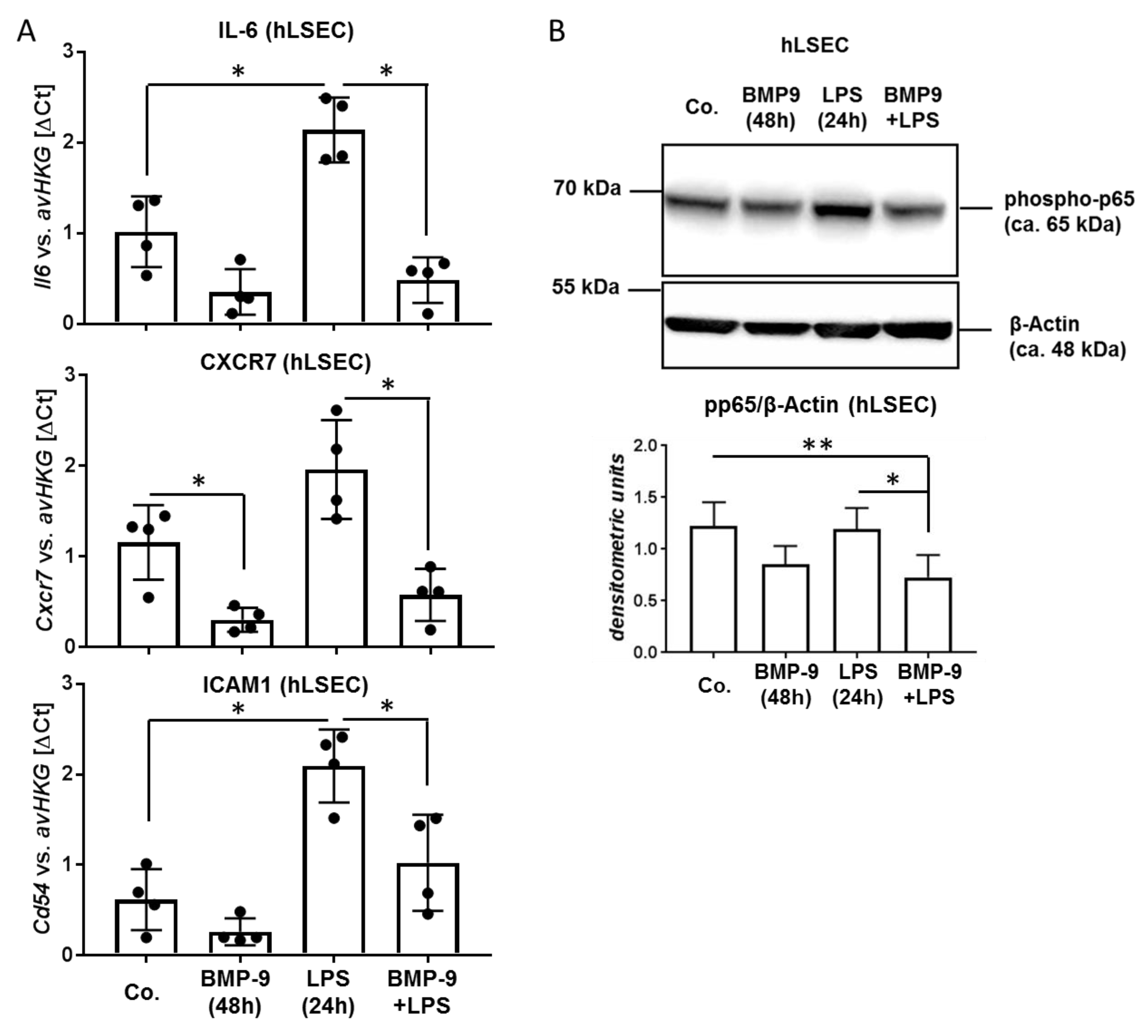
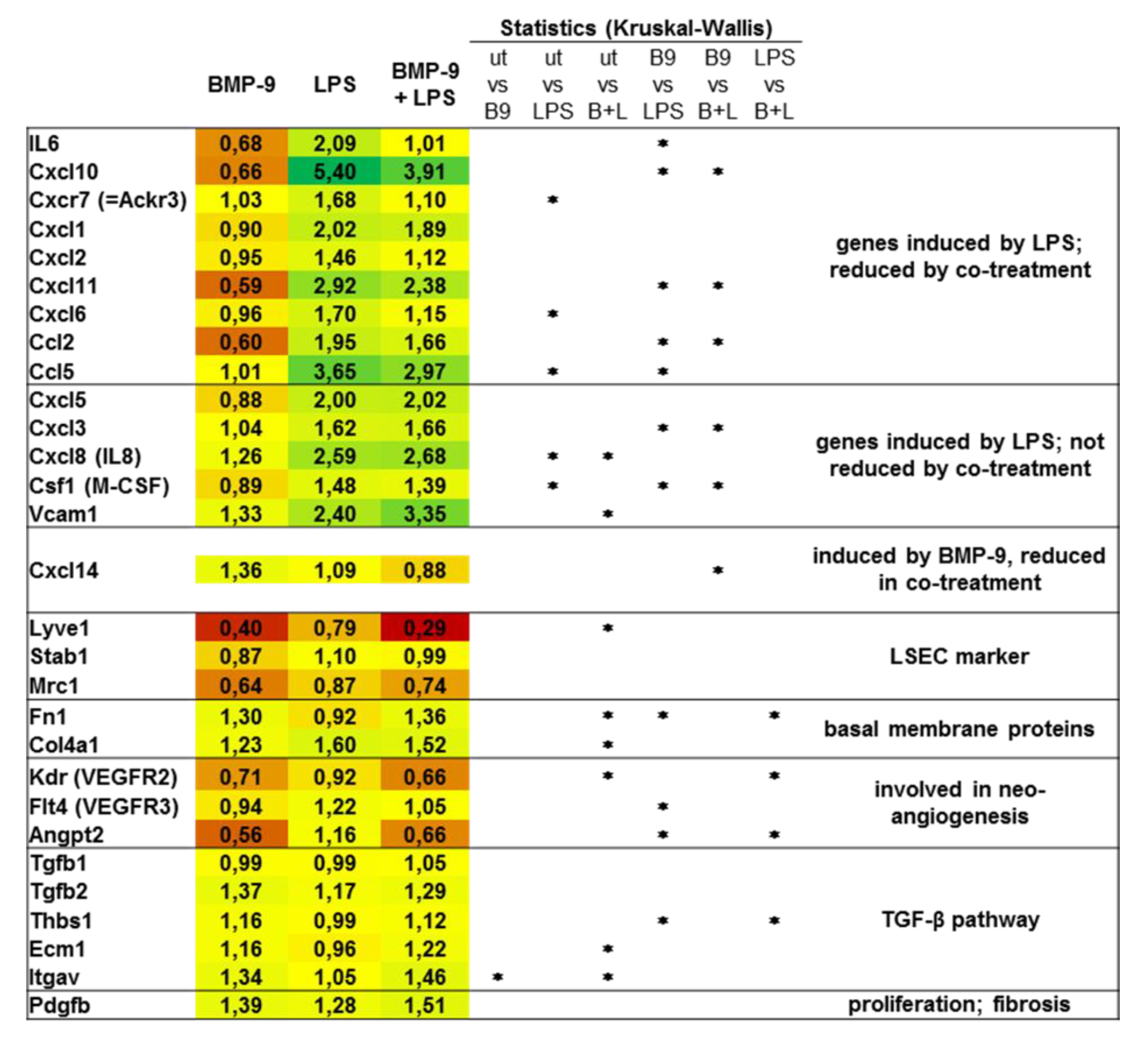
3.3. BMP-9 Antagonizes LPS Signaling in LSEC
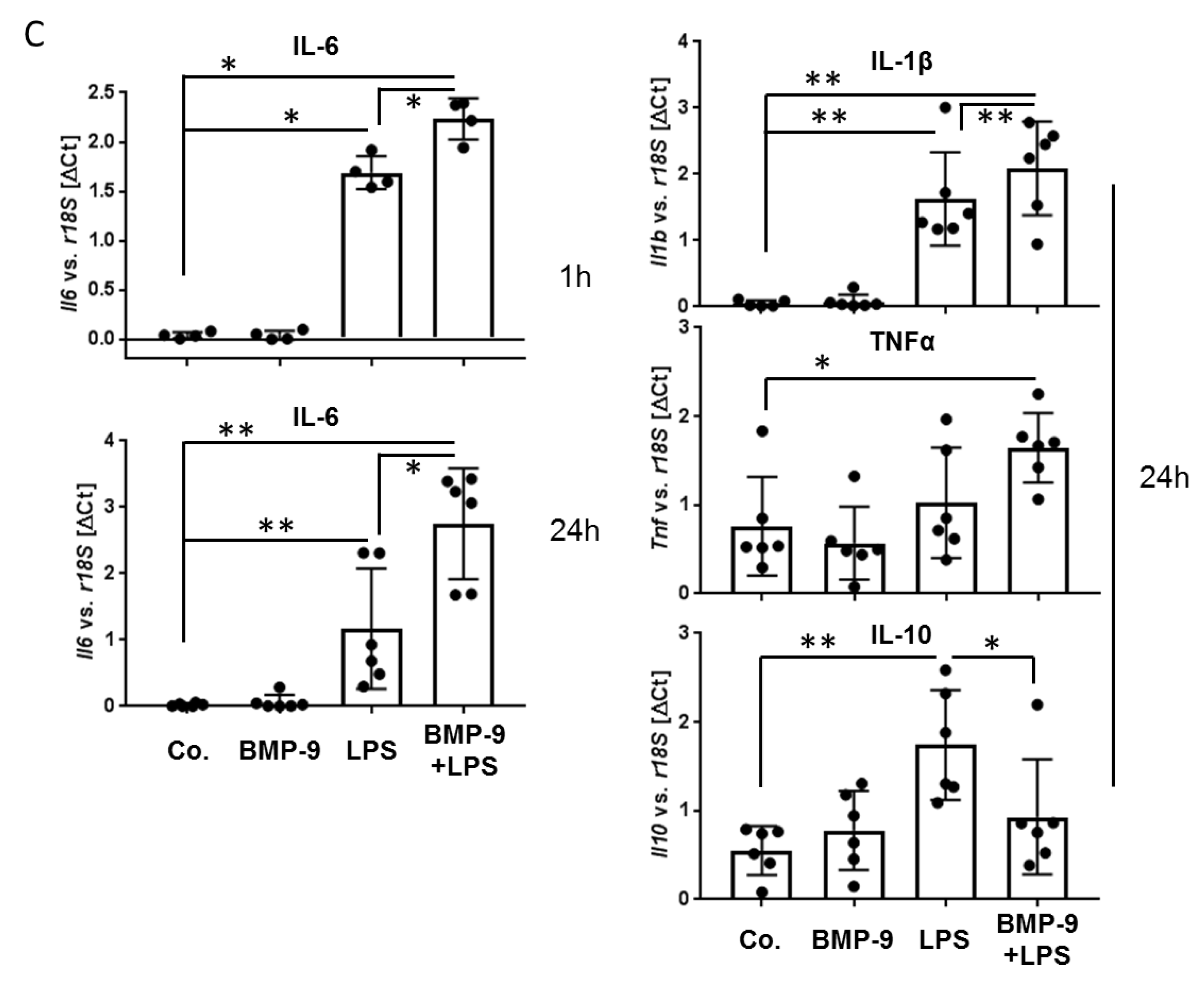
3.4. BMP-9 Modulates Macrophages Responses to LPS
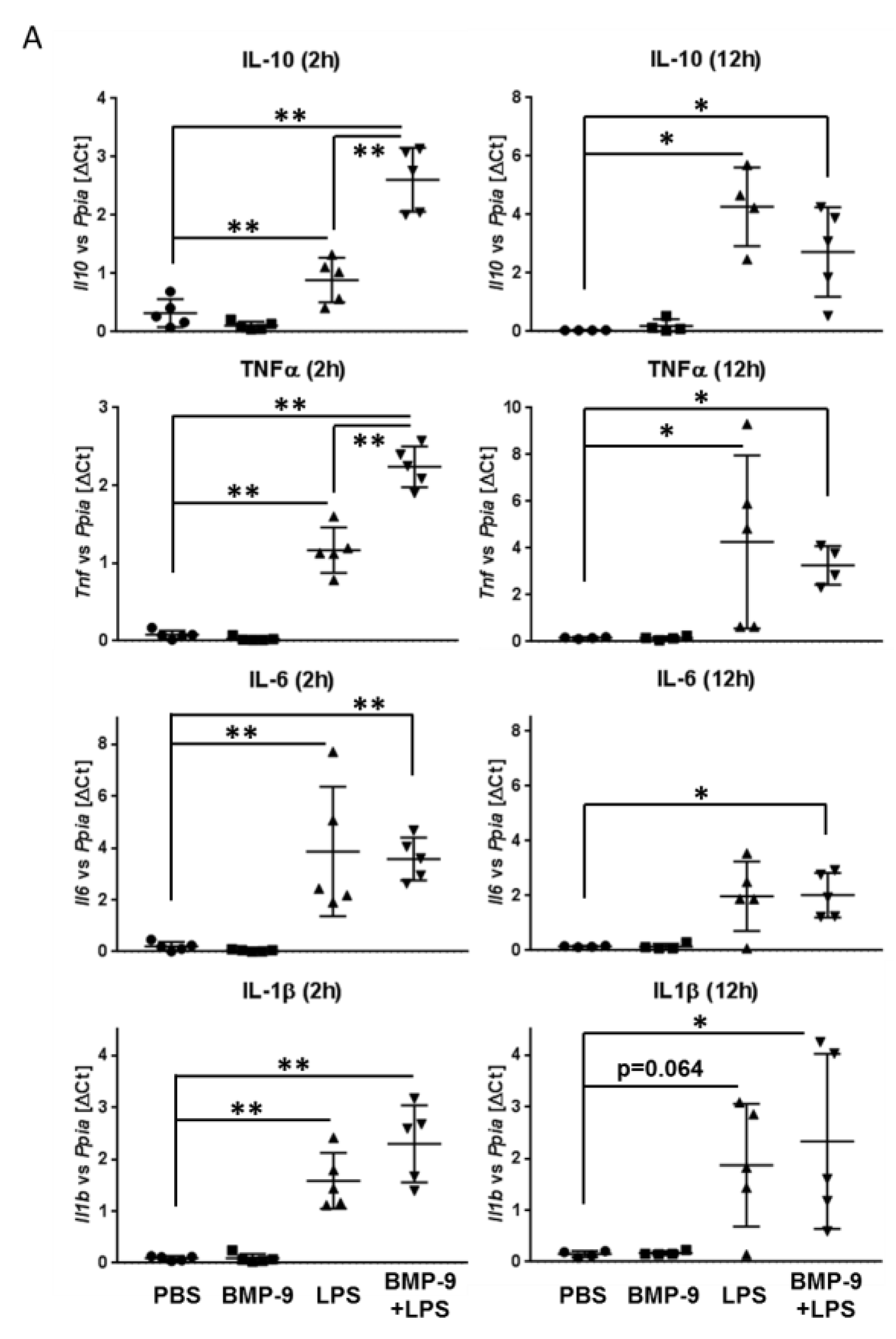
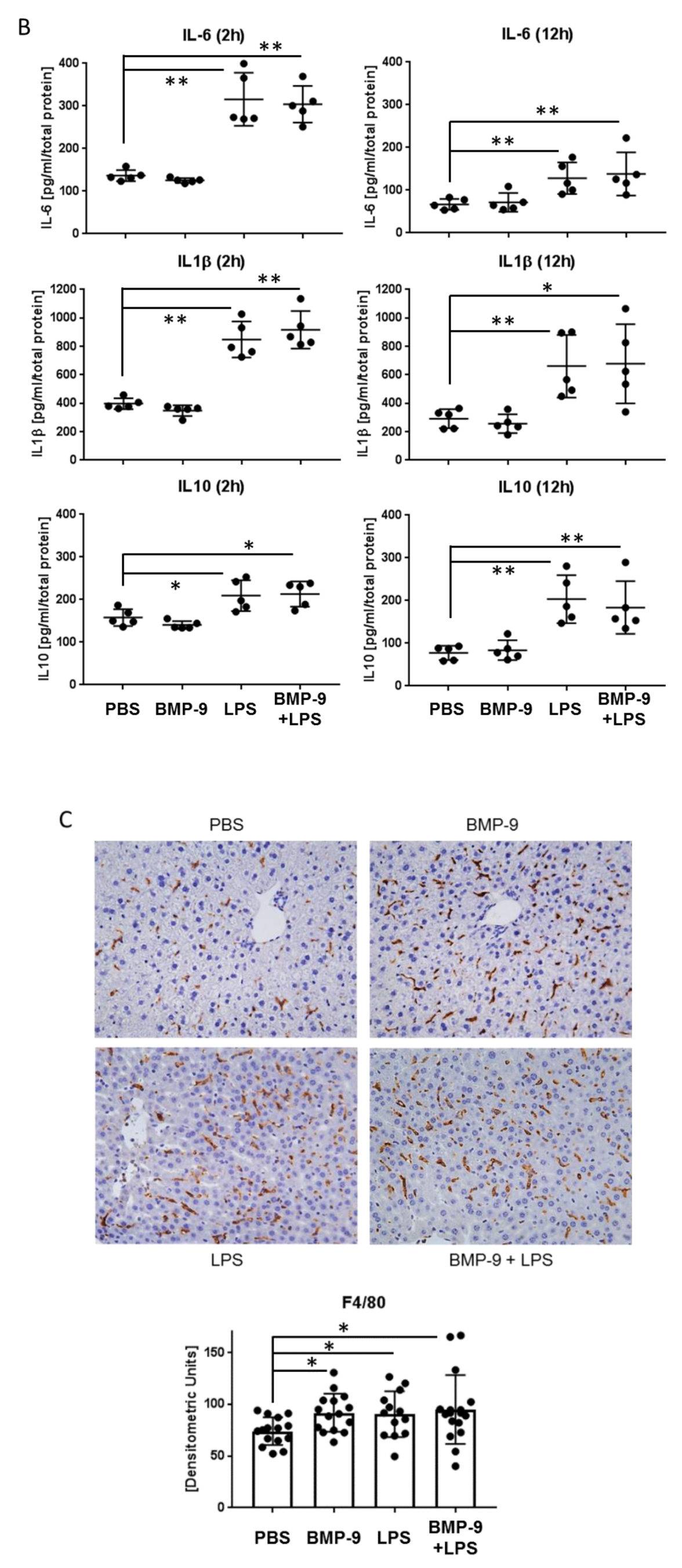
3.5. Effects of BMP-9 Plus LPS In Vivo in Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wakefield, L.M.; Hill, C.S. Beyond TGFbeta: Roles of other TGFbeta superfamily members in cancer. Nat. Rev. Cancer 2013, 13, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.F.; Harvey, S.A.; Thies, R.S.; Olson, M.S. Bone morphogenetic protein-9. An autocrine/paracrine cytokine in the liver. J. Biol. Chem. 2000, 275, 17937–17945. [Google Scholar] [CrossRef] [PubMed]
- Akatsu, Y.; Yoshimatsu, Y.; Tomizawa, T.; Takahashi, K.; Katsura, A.; Miyazono, K.; Watabe, T. Dual targeting of vascular endothelial growth factor and bone morphogenetic protein-9/10 impairs tumor growth through inhibition of angiogenesis. Cancer Sci. 2017, 108, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Bach, D.H.; Park, H.J.; Lee, S.K. The Dual Role of Bone Morphogenetic Proteins in Cancer. Mol. Ther. Oncolytics 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Breitkopf-Heinlein, K.; Meyer, C.; Konig, C.; Gaitantzi, H.; Addante, A.; Thomas, M.; Wiercinska, E.; Cai, C.; Li, Q.; Wan, F.; et al. BMP-9 interferes with liver regeneration and promotes liver fibrosis. Gut 2017, 66, 939–954. [Google Scholar] [CrossRef]
- Cao, J.; Wei, Y.; Lian, J.; Yang, L.; Zhang, X.; Xie, J.; Liu, Q.; Luo, J.; He, B.; Tang, M. Notch signaling pathway promotes osteogenic differentiation of mesenchymal stem cells by enhancing BMP9/Smad signaling. Int. J. Mol. Med. 2017, 40, 378–388. [Google Scholar] [CrossRef]
- Scharpfenecker, M.; van Dinther, M.; Liu, Z.; van Bezooijen, R.L.; Zhao, Q.; Pukac, L.; Lowik, C.W.; ten Dijke, P. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J. Cell Sci. 2007, 120, 964–972. [Google Scholar] [CrossRef]
- Li, Q.; Liu, B.; Breitkopf-Heinlein, K.; Weng, H.; Jiang, Q.; Dong, P.; Dooley, S.; Xu, K.; Ding, H. Adenovirusmediated overexpression of bone morphogenetic protein9 promotes methionine choline deficiencyinduced nonalcoholic steatohepatitis in nonobese mice. Mol. Med. Rep. 2019, 20, 2743–2753. [Google Scholar]
- Seki, E.; Schwabe, R.F. Hepatic inflammation and fibrosis: Functional links and key pathways. Hepatology 2015, 61, 1066–1079. [Google Scholar] [CrossRef]
- Wiest, R.; Garcia-Tsao, G. Bacterial translocation (BT) in cirrhosis. Hepatology 2005, 41, 422–433. [Google Scholar] [CrossRef]
- Seki, E.; De Minicis, S.; Osterreicher, C.H.; Kluwe, J.; Osawa, Y.; Brenner, D.A.; Schwabe, R.F. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat. Med. 2007, 13, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Mallet, C.; Keramidas, M.; Lamande, N.; Gasc, J.M.; Dupuis-Girod, S.; Plauchu, H.; Feige, J.J.; Bailly, S. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ. Res. 2008, 102, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Herrera, B.; Dooley, S.; Breitkopf-Heinlein, K. Potential roles of bone morphogenetic protein (BMP)-9 in human liver diseases. Int. J. Mol. Sci. 2014, 15, 5199–5220. [Google Scholar] [CrossRef] [PubMed]
- Mitrofan, C.G.; Appleby, S.L.; Nash, G.B.; Mallat, Z.; Chilvers, E.R.; Upton, P.D.; Morrell, N.W. Bone morphogenetic protein 9 (BMP9) and BMP10 enhance tumor necrosis factor-alpha-induced monocyte recruitment to the vascular endothelium mainly via activin receptor-like kinase 2. J. Biol. Chem. 2017, 292, 13714–13726. [Google Scholar] [CrossRef] [PubMed]
- Muhlbauer, M.; Bosserhoff, A.K.; Hartmann, A.; Thasler, W.E.; Weiss, T.S.; Herfarth, H.; Lock, G.; Scholmerich, J.; Hellerbrand, C. A novel MCP-1 gene polymorphism is associated with hepatic MCP-1 expression and severity of HCV-related liver disease. Gastroenterology 2003, 125, 1085–1093. [Google Scholar] [CrossRef]
- Carvalho, B.S.; Irizarry, R.A. A framework for oligonucleotide microarray preprocessing. Bioinformatics 2010, 26, 2363–2367. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- DeLeve, L.D. Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology 2015, 61, 1740–1746. [Google Scholar] [CrossRef]
- Wadkin, J.C.R.; Patten, D.A.; Kamarajah, S.K.; Shepherd, E.L.; Novitskaya, V.; Berditchevski, F.; Adams, D.H.; Weston, C.J.; Shetty, S. CD151 supports VCAM-1-mediated lymphocyte adhesion to liver endothelium and is upregulated in chronic liver disease and hepatocellular carcinoma. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G138–G149. [Google Scholar] [CrossRef]
- Cho, H.; Park, J.H.; Ahn, E.K.; Oh, J.S. Kobophenol A Isolated from Roots of Caragana sinica (Buc’hoz) Rehder Exhibits Anti-inflammatory Activity by Regulating NF-kappaB Nuclear Translocation in J774A.1 Cells. Toxicol. Rep. 2018, 5, 647–653. [Google Scholar] [CrossRef]
- Desroches-Castan, A.; Tillet, E.; Ricard, N.; Ouarne, M.; Mallet, C.; Belmudes, L.; Coute, Y.; Boillot, O.; Scoazec, J.Y.; Bailly, S.; et al. Bone Morphogenetic Protein 9 Is a Paracrine Factor Controlling Liver Sinusoidal Endothelial Cell Fenestration and Protecting Against Hepatic Fibrosis. Hepatology 2019, 70, 1392–1408. [Google Scholar] [CrossRef] [PubMed]
- Desroches-Castan, A.; Tillet, E.; Ricard, N.; Ouarne, M.; Mallet, C.; Feige, J.J.; Bailly, S. Differential Consequences of Bmp9 Deletion on Sinusoidal Endothelial Cell Differentiation and Liver Fibrosis in 129/Ola and C57BL/6 Mice. Cells 2019, 8, 1079. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Mallet, C.; Mazerbourg, S.; Feige, J.J.; Bailly, S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood 2007, 109, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Su, G.L.; Klein, R.D.; Aminlari, A.; Zhang, H.Y.; Steinstraesser, L.; Alarcon, W.H.; Remick, D.G.; Wang, S.C. Kupffer cell activation by lipopolysaccharide in rats: Role for lipopolysaccharide binding protein and toll-like receptor 4. Hepatology 2000, 31, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Seki, E.; Tsutsui, H.; Nakano, H.; Tsuji, N.; Hoshino, K.; Adachi, O.; Adachi, K.; Futatsugi, S.; Kuida, K.; Takeuchi, O.; et al. Lipopolysaccharide-induced IL-18 secretion from murine Kupffer cells independently of myeloid differentiation factor 88 that is critically involved in induction of production of IL-12 and IL-1beta. J. Immunol. 2001, 166, 2651–2657. [Google Scholar] [CrossRef] [PubMed]
- Uhrig, A.; Banafsche, R.; Kremer, M.; Hegenbarth, S.; Hamann, A.; Neurath, M.; Gerken, G.; Limmer, A.; Knolle, P.A. Development and functional consequences of LPS tolerance in sinusoidal endothelial cells of the liver. J. Leukoc. Biol. 2005, 77, 626–633. [Google Scholar] [CrossRef]
- Appleby, S.L.; Mitrofan, C.G.; Crosby, A.; Hoenderdos, K.; Lodge, K.; Upton, P.D.; Yates, C.M.; Nash, G.B.; Chilvers, E.R.; Morrell, N.W. Bone Morphogenetic Protein 9 Enhances Lipopolysaccharide-Induced Leukocyte Recruitment to the Vascular Endothelium. J. Immunol. 2016, 197, 3302–3314. [Google Scholar] [CrossRef]
- Kholodenko, I.V.; Kurbatov, L.K.; Kholodenko, R.V.; Manukyan, G.V.; Yarygin, K.N. Mesenchymal Stem Cells in the Adult Human Liver: Hype or Hope? Cells 2019, 8, 1127. [Google Scholar] [CrossRef]
- Karin, D.; Koyama, Y.; Brenner, D.; Kisseleva, T. The characteristics of activated portal fibroblasts/myofibroblasts in liver fibrosis. Differentiation 2016, 92, 84–92. [Google Scholar] [CrossRef]
- Iwaisako, K.; Jiang, C.; Zhang, M.; Cong, M.; Moore-Morris, T.J.; Park, T.J.; Liu, X.; Xu, J.; Wang, P.; Paik, Y.H.; et al. Origin of myofibroblasts in the fibrotic liver in mice. Proc. Natl. Acad. Sci. USA 2014, 111, E3297–E3305. [Google Scholar] [CrossRef]
- Stark, R.; Choi, H.; Koch, S.; Lamb, F.; Sherwood, E. Monophosphoryl lipid A inhibits the cytokine response of endothelial cells challenged with LPS. Innate Immun. 2015, 21, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Upton, P.D.; Davies, R.J.; Trembath, R.C.; Morrell, N.W. Bone morphogenetic protein (BMP) and activin type II receptors balance BMP9 signals mediated by activin receptor-like kinase-1 in human pulmonary artery endothelial cells. J. Biol. Chem. 2009, 284, 15794–15804. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Wang, X.; Wang, L.; Wang, L.; Atkinson, R.D.; Kanel, G.C.; Gaarde, W.A.; Deleve, L.D. Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats. Gastroenterology 2012, 142, 918–927. [Google Scholar] [CrossRef] [PubMed]

| Acta2 (αSMA) | human | CCCTGAAGTACCCGATAGAAC | GGCAACACGAAGCTCATT |
| Acvr1 (Alk2) | human | TCTCTGTAGTGTTCGCAGTATGT | CGTTCTTGGTTGCGCCTTTT |
| Acvrl1 (Alk1) | human | CATCGCCTCAGACATGACCTC | GTTTGCCCTGTGTACCGAAGA |
| Alb | human | CTTGAATGTGCTGATGACAGG | GCAAGTCAGCAGGCATCTCAT |
| Cd271 (NGFR) | human | AACCTCATCCCTGTCTATTG | GTTGGCTCCTTGCTTGTT |
| Cd34 | human | CTACAACACCTAGTACCCTTGGA | GGTGAACACTGTGCTGATTACA |
| Cd44 | human | GGAGCAGCACTTCAGGAGGTTAC | GGAATGTGTCTTGGTCTCTGGTAGC |
| Cd54 (ICAM1) | human | ATGCCCAGACATCTGTGTCC | GGGGTCTCTATGCCCAACAA |
| Cd68 | human | GCTACTGGCAGCCCCAGGG | GCTCTTGGTAGTCCTGTGG |
| Cd90 | human | ATGAACCTGGCCATCAGCA | GTGTGCTCAGGCACCCC |
| Col1a1 | human | GTGCGATGACGTGATCTGTGA | CGGTGGTTTCTTGGTCGGT |
| Col4a1 | human | TGGTGACAAAGGACAAGCAG | TAAGCCGTCAACACCTTTGG |
| Cxcr7 | human | CTATGACACGCACTGCTACATC | CTGCACGAGACTGACCACC |
| Fn1 | human | CGGTGGCTGTCAGTCAAAG | AAACCTCGGCTTCCTCCATAA |
| Gdf2 (BMP9) | human | pre-designed sequence: QT00210462; Qiagen | |
| Grem1 | human | TCATCAACCGCTTCTGTTACG | GGCTGTAGTTCAGGGCAGTT |
| Hamp | human | CTGACCAGTGGCTCTGTTTTC | GAAGTGGGTGTCTCGCCTC |
| Id1 | mouse | CTTCAGGAGGCAAGAGGAAA | CAAACCCTCTACCCACTGGA |
| Id1 | human | CTGCTCTACGACATGAACGG | GAAGGTCCCTGATGTAGTCGAT |
| Il10 | human | TCAAGGCGCATGTGAACTCC | GATGTCAAACTCACTCATGGCT |
| Il10 | mouse | CAGAGCCACATGCTCCTAGA | GTCCAGCTGGTCCTTTGTTT |
| Il1b | human | AGCTACGAATCTCCGACCAC | CGTTATCCCATGTGTCGAAGAA |
| Il1b | mouse | CTGCTGGTGTGTGACGTTCCCAT | GGTCCGACAGCACGAGGCTTT |
| Il6 | human | ACTCACCTCTTCAGAACGAATTG | CCATCTTTGGAAGGTTCAGGTTG |
| Il6 | mouse | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| Lyve1 | human | AGGCTCTTTGCGTGCAGAA | GGTTCGCCTTTTTGCTCACAA |
| Nnmt | human | GAGATCGTCGTCACTGACTACT | CACACACATAGGTCACCACTG |
| Tlr4 | mouse | AAGAGCCGGAAGGTTATTGTG | CCCATTCCAGGTAGGTGTTTC |
| Tlr4 | human | AGTTGATCTACCAAGCCTTGAGT | GCTGGTTGTCCCAAAATCACTTT |
| Tnf | human | CCTCTCTCTAATCAGCCCTCTG | GAGGACCTGGGAGTAGATGAG |
| Tnf | mouse | TCCCAGGTTCTCTTCAAGGGA | GGTGAGGAGCACGTAGTCGG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaitantzi, H.; Karch, J.; Germann, L.; Cai, C.; Rausch, V.; Birgin, E.; Rahbari, N.; Seitz, T.; Hellerbrand, C.; König, C.; et al. BMP-9 Modulates the Hepatic Responses to LPS. Cells 2020, 9, 617. https://doi.org/10.3390/cells9030617
Gaitantzi H, Karch J, Germann L, Cai C, Rausch V, Birgin E, Rahbari N, Seitz T, Hellerbrand C, König C, et al. BMP-9 Modulates the Hepatic Responses to LPS. Cells. 2020; 9(3):617. https://doi.org/10.3390/cells9030617
Chicago/Turabian StyleGaitantzi, Haristi, Julius Karch, Lena Germann, Chen Cai, Vanessa Rausch, Emrullah Birgin, Nuh Rahbari, Tatjana Seitz, Claus Hellerbrand, Courtney König, and et al. 2020. "BMP-9 Modulates the Hepatic Responses to LPS" Cells 9, no. 3: 617. https://doi.org/10.3390/cells9030617
APA StyleGaitantzi, H., Karch, J., Germann, L., Cai, C., Rausch, V., Birgin, E., Rahbari, N., Seitz, T., Hellerbrand, C., König, C., Augustin, H. G., Mogler, C., de la Torre, C., Gretz, N., Itzel, T., Teufel, A., Ebert, M. P. A., & Breitkopf-Heinlein, K. (2020). BMP-9 Modulates the Hepatic Responses to LPS. Cells, 9(3), 617. https://doi.org/10.3390/cells9030617





